Abstract
Background
Children are substantially affected by enteric fever in most settings with a high burden of the disease, including Nepal. However pathogen population structure and transmission dynamics are poorly delineated in young children, the proposed target group for immunization programs. Here we present whole genome sequencing and antimicrobial susceptibility data on 198 S. Typhi and 66 S. Paratyphi A isolated from children aged 2 months to 15 years of age during blood culture surveillance at Patan Hospital, Nepal, 2008–2016.
Principal findings
S. Typhi was the dominant agent and comprised several distinct genotypes, dominated by 4.3.1 (H58). The heterogeneity of genotypes in children under five was reduced compared to data from 2005–2006, attributable to ongoing clonal expansion of H58. Most isolates (86%) were non-susceptible to fluoroquinolones, associated mainly with S. Typhi H58 lineage II and S. Paratyphi A harbouring mutations in the quinolone resistance-determining region (QRDR); non-susceptible strains from these groups accounted for 50% and 25% of all isolates. Multi-drug resistance (MDR) was rare (3.5% of S. Typhi, 0 S. Paratyphi A) and restricted to chromosomal insertions of resistance genes in H58 lineage I strains. Temporal analyses revealed a shift in dominance from H58 Lineage I to H58 Lineage II, with the latter being significantly more common after 2010. Comparison to global data sets showed the local S. Typhi and S. Paratyphi A strains had close genetic relatives in other South Asian countries, indicating regional strain circulation. Multiple imports from India of ciprofloxacin-resistant H58 lineage II strains were identified, but these were rare and showed no evidence of clonal replacement of local S. Typhi.
Significance
These data indicate that enteric fever in Nepal continues to be a major public health issue with ongoing inter- and intra-country transmission, and highlights the need for regional coordination of intervention strategies. The absence of a S. Paratyphi A vaccine is cause for concern, given its prevalence as a fluoroquinolone resistant enteric fever agent in this setting.
Introduction
As in most developing countries, invasive bacterial infections account for a significant proportion of paediatric morbidity and mortality in Nepal[1,2]. Enteric fever, caused by Salmonella enterica serovars Typhi (S. Typhi) and Paratyphi A (S. Paratyphi A), is the most common cause of bloodstream infection in Nepal[1,2] and on a global scale causes an estimated 26 million cases of enteric fever annually of which a large proportion are in children[3,4]. In Nepal, it is estimated that 13% of febrile paediatric cases attending outpatient care are blood culture positive for S. Typhi or Paratyphi A[2]. Single nucleotide polymorphism (SNP) genotyping of S. Typhi isolated in a study of paediatric enteric fever cases at Patan Hospital in Kathmandu, Nepal during 2005 and 2006 suggested that, among children treated as inpatients, those aged ≤4 years were susceptible to a wider range of haplotypes due to immune naivety[5]. The most common genotype was H58 lineage II (70%), followed by H42 (19%)[5]. Another study of adults and children at Patan Hospital from 2005 to 2009 found that 26% of culture-positive cases were associated with S. Paratyphi A; the rest were caused by S. Typhi, mainly H58 lineage II (61%) or other H58 (3%), or H42 (15%)[6]. More recently, whole genome sequencing (WGS) was applied to study S. Typhi isolates collected during a randomized controlled trial of gatifloxacin vs ceftriaxone for treatment of blood culture confirmed enteric fever at Patan Hospital between 2011 and 2014, and found the H58 genotype continued to dominate the circulating S. Typhi population (83%)[7].
Multi-drug resistant (MDR) S. Typhi, defined as resistant to the first-line antibiotics ampicillin, chloramphenicol and co-trimoxazole, became common in the Indian subcontinent in the 1990s[8], driven by the spread of H58 carrying an IncHI1 plasmid harbouring a suite of antimicrobial resistance (AMR) genes[9]. These S. Typhi strains are still circulating in the region, including in India, Pakistan and Bangladesh. In this setting there is also evidence of migration of the MDR genes to the S. Typhi chromosome, and acquisition of resistance to fluoroquinolones and third-generation cephalosporins in MDR and non-MDR S. Typhi strain backgrounds, which further limits treatment options in the region[10]. However in Nepal, the MDR H58 S. Typhi appears to have been replaced by non-MDR H58 S. Typhi carrying the S83F mutation in gyrA and other mutations in the quinolone resistance determining region (QRDR) associated with reduced susceptibility to fluoroquinolones[5,6] and more recently the introduction of fluoroquinolone resistant H58 S. Typhi ‘triple mutants’ harbouring three QRDR mutations (gyrA S83F, gyrA D87V, and parC S80I), likely from India, resulting in failure of gatifloxacin treatment[7]. S. Paratyphi A in Nepal is generally not MDR, but frequently carries fluoroquinolone non-susceptibility alleles in gyrA and parC.[11–13]
Given the current treatment complexities of paediatric enteric fever, vaccination would seem the most feasible short-term strategy. There is no vaccine against S. Paratyphi A, which accounts for approximately a quarter of disease cases in Nepal. The Vi polysaccharide vaccine against S. Typhi is not effective in children under two years of age[14], and has therefore not been deployed as part of the national immunization schedule in Nepal and is only available privately. While the Vi conjugate vaccines have the potential to reduce the incidence of enteric fever in Nepal, the immunization approach and schedule needs to be clearly defined. This study sheds light on the age distribution of affected inpatient children at Patan Hospital, and the molecular structure and AMR determinants of circulating bacterial pathogen populations causing paediatric enteric fever from 2008 to 2016 in Nepal, with the view of informing preventive strategies including vaccine policy.
Methods
Ethics statement
Ethical approval was obtained from the Oxford Tropical Research Ethics Committee (OxTREC) as well as local institutional approval from the Nepal Health Research Council (R31579/CN007).
Study setting
Nepal is a low income[15], landlocked Himalayan nation with an under-five year old mortality rate of 35.8 per 1000 live births as of 2015[16]. Kathmandu Valley, the main urban centre of Nepal, has three districts and a population of 2.5 million[17] (average population density: 2,372/km2) of which 31% are between 0–14 years under age[18]. Over the course of the study, the Patan Academy of Health Sciences (PAHS) was one of only two large hospitals in Kathmandu Valley with referral and paediatric intensive care services. Patan Hospital accepts patients from all over the Valley. Annually the paediatric department cares for over 50,000 outpatients (21% of all hospital outpatient attendances) and accepts approximately 2,700 inpatient admissions. Only 10% of the patients reside outside Kathmandu Valley.
Surveillance of culture confirmed enteric fever amongst inpatients
Febrile children under 14 years of age, attending PAHS with clinical suspicion of invasive bacterial disease between January 2008 and December 2016 were included in an invasive bacterial disease database as described previously[19]. Inclusion criteria were: clinical presentation indicating an invasive bacterial infection requiring inpatient care with intravenous antibiotics. Blood culture was conducted as described below. Of the patients included in the database, all those that had blood cultures positive for S. Typhi or S. Paratyphi A were included in the present study, along with relevant demographic data. A total of 67/102 S. Typhi isolates and 17/27 S. Paratyphi A isolates from these patients had been stored, and were subjected to whole genome sequencing; these represent isolates associated with paediatric enteric fever presenting to the hospital, which may represent the more severe end of the spectrum of disease in the community.
Isolates collected from outpatients
Children with milder clinical presentations who are usually treated with oral antibiotics as outpatients were not included in the invasive bacterial disease database; however they are subjected to the same microbiological diagnostic procedures as inpatients (as detailed below). A total of 1283 S. Typhi and 926 S. Paratyphi A isolates from paediatric outpatients were stored between 2008 and 2016; every 10th S. Typhi isolate and every 5th S. Paratyphi A isolate were included in this current study, representing isolates associated with milder presentation of paediatric enteric fever at the hospital.
Blood culture processing
Aerobic blood culture bottles were used to culture 3–5 mL of blood, which were then incubated in a BD Bactec FX 40 incubator at 37°C for a maximum of 5 days. Turbid samples were then inoculated directly onto MacConkey agar and incubated for maximum of 5 days at 37°C to identify potential S. Typhi and S. Paratyphi A colonies. Candidate S. Typhi and S. Paratyphi A isolates were further subjected to standard biochemical tests for additional confirmation[20].
Antimicrobial susceptibility testing
Antimicrobial susceptibility profiles were gauged by Kirby-Bauer disk diffusion tests. The CLSI (Clinical and Laboratory Standards Institute) guidelines were used to evaluate zones of inhibition for chloramphenicol, co-amoxiclav, co-trimoxazole, cefexime, ceftriaxone, azithromycin, nalidixic acid, and ciprofloxacin[21]. Isolates displaying sensitivity to the tested antimicrobials as per the cut-off values in the CLSI guidelines were designated as susceptible and those that were intermediate (I) or resistant (R) to the tested antimicrobials were designated as non-susceptible.
Genome sequencing and SNP analysis
Briefly, DNA was extracted using the Wizard Genomic DNA Extraction Kit (Promega, Wisconsin, USA), according to manufacturer’s instructions. Genomic DNA was then subjected to indexed whole genome sequencing on an Illumina Hiseq 2500 platform at the Wellcome Trust Sanger Institute to generate paired-end reads of 100–150 bp in length.
For analysis of SNPs in S. Typhi, Illumina reads were mapped to the reference genome sequence of strain CT18[22] (accession AL515582) using the RedDog (V1beta.10.3) mapping pipeline, available at https://github.com/katholt/RedDog. RedDog uses Bowtie (v2.2.9)[23] to map reads to the reference sequence; uses SAMtools (v1.3.1)[24] to identify SNPs with phred quality scores above 30; filters out those supported by <5 reads or with >2.5 times the average read depth (representing putative repeated sequences), or with ambiguous consensus base calls. For each SNP that passed these criteria in any one isolate, consensus base calls for the SNP locus were extracted from all genomes (ambiguous base calls and those with phred quality scores less than 20 were treated as unknowns and represented with a gap character). These SNPs were used to assign isolates to previously defined lineages according to an extended S. Typhi genotyping framework[25] (code available at https://github.com/katholt/genotyphi). For phylogenetic analyses, SNPs with confident homozygous allele calls (i.e. phred score >20) in >95% of the S. Typhi genomes (representing a ‘soft’ core genome of common S. Typhi sequences) were concatenated to produce an alignment of alleles at 233,527 variant sites. SNPs called in phage regions, repetitive sequences (354 kb; ~7.4% of bases in the CT18 reference chromosome, as defined previously) or in recombinant regions identified using Gubbins (v2.0.0)[26] were excluded, resulting in a final set of 2,187 SNPs identified in an alignment length of 4,809,037 bp for the 198 novel Nepali S. Typhi isolates. SNP alleles from S. Paratyphi A strain AKU_12601[27] (accession FM200053) were also included as an outgroup to root the tree.
To provide regional context, genome data from: (i) a published study of mainly Nepali adults[7] (n = 95), (ii) a global S. Typhi genome collection[25] (n = 1,221); were subjected to SNP calling and genotyping, resulting in an alignment of 12,216 SNPs for a total of 1,514 isolates. Details and accession numbers of sequence data included in our analysis have been included in S1 & S2 Tables. An additional analysis of all 261 H58 (genotype 4.3.1) from Nepal was carried out in the same manner, resulting in an alignment of 631 SNPs.
To characterize and analyse the genomes of the 66 S. Paratyphi A strains, a similar bioinformatic process was adopted using S. Paratyphi A AKU_12601[27] (accession no: FM200053) as the reference genome to create an alignment with another selected 176 isolates from previous studies[28–30], for global context resulting in an alignment of 5,277 SNPs in a total of 242 S. Paratyphi isolates, with alleles from S. Typhi CT18[22] (accession no: AL515582) included as an outgroup to root the tree.
Phylogenetic analysis
Maximum likelihood (ML) phylogenetic trees were inferred from SNP alignments using RAxML (v8.1.23)[31], with the generalized time-reversible model, a Gamma distribution to model site-specific rate variation (the GTR+ Γ substitution model; GTRGAMMA in RAxML), and 100 bootstrap pseudo-replicates to assess branch support. The resulting trees were visualized using Microreact[32] and the R package ggtree[33]. For visualization purposes, S. Typhi isolates representing ‘outbreaks’ (defined as members of the same monophyletic clade, isolated from the same study location in the same year) were manually thinned to a single representative.
Temporal analysis
To investigate the temporal signal and emergence dates of antimicrobial resistance determinants for Nepali S. Typhi 4.3.1, we used several methods. First, we used TempEst (v1.5)[34] to assess temporal structure (i.e. whether the data have clocklike behaviour) by conducting a regression of the root-to-tip branch distances of the Nepal H58/4.3.1 ML tree as a function of the sampling time, using the heuristic residual mean squared method with the best-fitting root selected. The resultant data were then visualized in R[35]. To estimate divergence times we analysed the sequence data in BEAST2 (v2.4.7)[36]. We used both constant-coalescent population size and Bayesian skyline tree priors, in combination with either a strict molecular clock model or a relaxed (uncorrelated lognormal distribution) clock model to identify the model that best fits the data. For the BEAST2 analysis the GTR+Γ substitution model was selected, and the sampling times (tip dates) were defined as the year of isolation to calibrate the molecular clock. For all model and tree prior combinations, a chain length of 100,000,000 steps sampling every 5000 steps was used[37]. The relaxed (uncorrelated lognormal) clock model, which allows evolutionary rates to vary among branches of the tree together with the skyline demographic model, proved to be the best fit for the data. To assess the signal of these Bayesian estimates we conducted a date-randomization test whereby sampling times were assigned randomly to the sequences, and the analysis re-run 20 times[37,38]. These randomization tests were conducted with the same ‘best fit’ models (uncorrelated lognormal clock and skyline demographic). This test suggested that the data display ‘strong’ temporal structure[37].
For the final analysis reported here, 5 independent runs conducted with a chain length of 600,000,000 states, sampling every 300,000 iterations, were combined using LogCombiner (v2.4.7)[36] following removal of the first 10% of steps from each as burn-in. Maximum-clade credibility (MCC) trees were generated with ‘keep target heights’ specified for node heights using TreeAnnotator (v2.4.7)[36,39]. The effective sample sizes from the combined runs were estimated to be >200 for all reported parameters.
In silico resistance plasmid and AMR gene analysis
The mapping based allele typer SRST2[40] was used to detect plasmid replicons and acquired AMR genes and determine their precise alleles, by comparison to the ARG-Annot[41] and ResFinder[42] databases (for AMR genes) and PlasmidFinder[41] (for plasmid replicons). Where AMR genes were observed without evidence of a known resistance plasmid, raw read data was assembled de novo with SPAdes (v3.7.1)[43] and Unicycler (v0.3.0b)[44] and examined visually using the assembly graph viewer Bandage (0.8.1)[45] to inspect the composition and insertion sites of resistance-associated transposons. These putative transposon sequences were annotated using Prokka (v1.11)[45] followed by manual curation, and visualized using the R package genoPlotR[45]. SNPs in the QRDR of gyrA, gyrB, parC and parE genes, which are associated with reduced susceptibility to fluoroquinolones in S. Typhi, S. Paratyphi A and other species[7], were extracted from the whole genome SNP alignments.
Nucleotide sequence and read data accession numbers
Raw sequence data have been deposited in the European Nucleotide Archive under project PRJEB14050; and individual accession numbers are listed in S1 and S2 Tables. Genome assemblies for isolates RN2293 and RN2370 were deposited in GenBank.
Results
Paediatric enteric fever surveillance
Blood cultures were performed on 11,430 children with a suspected invasive bacterial infection and requiring inpatient care with intravenous antibiotics and supportive care. Of these, 129 had blood cultures positive for the enteric fever agents S. Typhi (n = 102, 79%) or S. Paratyphi A (n = 27, 21%). Relevant patient characteristics are reported in Table 1. Most cases of culture-confirmed enteric fever (n = 83, 64%) occurred between the hot and rainy months of May and October. However, a substantial proportion (36%) of cases also occurred in colder months, indicating perennial transmission. Children under 5 years of age accounted for 45% of the disease burden among inpatients, with children under 2 years of age accounting for 18% (Table 1). Clinical suspicion of enteric fever at presentation was significantly lower amongst children under 2 years with culture-confirmed infection (13% vs. 52%, p = 0.0005 using Fisher’s exact test; Table 1), highlighting the undifferentiated febrile nature of the disease even in an endemic setting such as Nepal.
Table 1. Hospital based (inpatient) paediatric enteric fever surveillance.
| Total blood cultures performed | 11430 | |||
| Total number of significant cultures | 1048 (9.2%) | |||
| Total number of enteric fever pathogens | 129 (1.1%) | |||
| S. Typhi | 102 (0.9%) | |||
| S. Paratyphi A | 27 (0.2%) | |||
| Age stratified characteristics of blood-culture positive enteric fever patients | ||||
| Age groups | <2 y | 2–4 y | 5–9 y | 10–14 y |
| Number | 23 | 35 | 39 | 31 |
| Median age (years) | 1.2 | 3.3 | 6.8 | 11.8 |
| Male (%) | 16 (70%) | 23 (66%) | 24 (62%) | 18 (58%) |
| Median temperature at admission (C°) (range) | 37.2 (36.7–38.9) | 38.3 (36.5–39.9) | 38.9 (36.1–40.5) | 37.2 (36.5–39.2) |
| Median duration of admission (days) (range) | 6 (2–23) | 6.5 (1–19) | 8 (2–36) | 7.5 (3–20) |
| Enteric fever suspicion on admission (%) | 3 (13%) | 17 (49%) | 19 (49%) | 19 (61%) |
Phylogenetic structure of paediatric isolates from Nepal
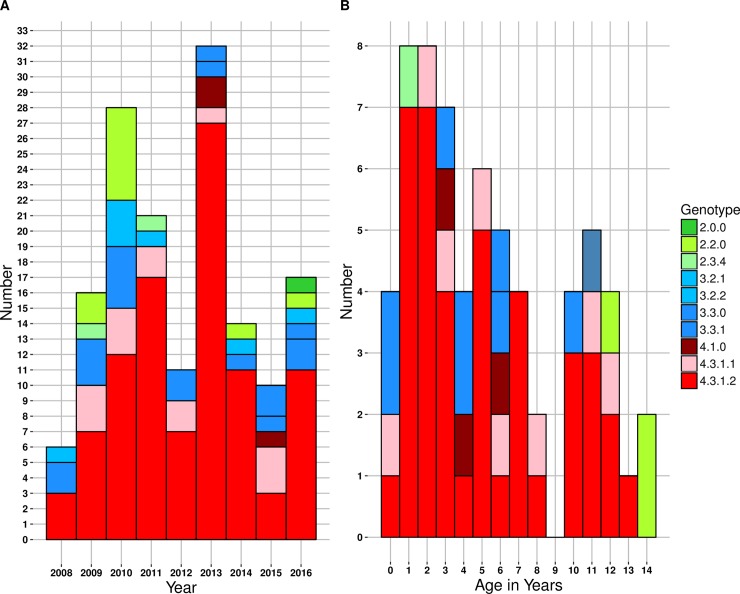
The genomes of S. Typhi isolated from inpatient surveillance (n = 67) and a random selection (every 10th isolate of S. Typhi and every 5th isolate of S. Paratyphi A) of isolates from outpatients (n = 131) were sequenced and subjected to SNP genotyping and phylogenomic analysis as described in Methods. The resulting phylogeny (S1 Fig) revealed the presence of 8 distinct genotypes, each corresponding to a different subclade including 2.0.0 (N = 1, 0.5%) 2.2.0 (N = 10, 5%), 2.3.4 (N = 2, 1%), 3.2.2 (N = 6, 3%), 3.3.0 (N = 19, 9.6%), 3.3.1 (N = 3, 1.5%), 4.1.0 (N = 3, 1.5%), and 4.3.1 (N = 154, 77.8%). There was no significant association between genotype and treatment status (outpatient vs. inpatient), clinical characteristics, period of isolation (Fig 1A) or patient age (Fig 1B).
Fig 1. Nepal paediatric S. Typhi genotypes.
(A) Genotypes observed per annum. (B) Genotypes observed per age in years. Individual S. Typhi genotypes are coloured as described in the inset legend.
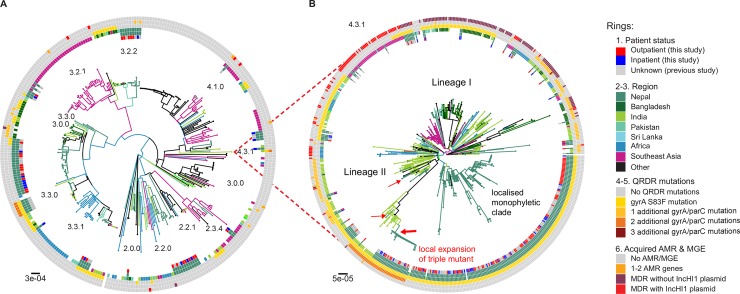
To place the novel paediatric isolates in context, we constructed a whole genome phylogeny including other S. Typhi previously sequenced from adults in Nepal, and a global collection of S. Typhi (Fig 2; an interactive version of the phylogeny and associated geographical data are also available for exploration online at https://microreact.org/project/SJmU6dhlz). The novel paediatric isolates clustered together with the adult isolates from Nepal, with no evidence of certain genotypes circulating in children more so than adults. In comparison to global isolates, Nepali isolates clustered with those from other regions in the Indian subcontinent, suggesting ongoing transmission within the region (Fig 2); indeed 14% of the novel Nepali paediatric isolates and 15% of the previously sequenced Nepali isolates were closest to an isolate from outside Nepal (majority from neighbouring India, Bangladesh or Pakistan), indicating frequent pathogen transfer within the region.
Fig 2. Global population structure of Nepalese S. Typhi genotypes.
(A) Global population structure of Non-H58 (4.3.1) Nepalese genotypes. (B) Global population structure of H58 (4.3.1). Inner ring shows patient status. Branch colours indicate country/region of origin, as do the second ring and third rings from the inside. Fourth ring from the inside indicates QRDR gyrA S83F mutation. Fifth ring from the inside indicates the number of additional gyrA and parC QRDR mutations. Outer ring describes the presence of MDR. All rings and branches are coloured as per the inset legend. Branch lengths are indicative of the estimated number of substitutions rate per variable site, and the tree was outgroup rooted with a S. Paratyphi A strain AKU_12601.
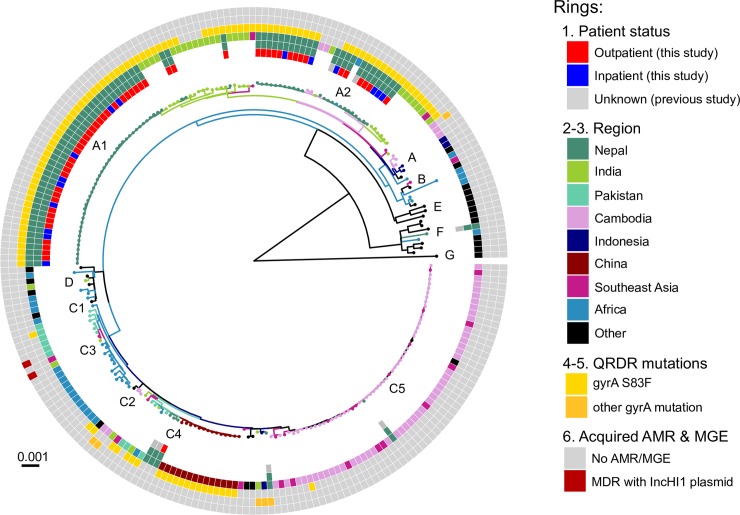
We used the same approach to investigate genome variation amongst 66 S. Paratyphi A isolated from inpatients (n = 17) and outpatients (n = 49) in Nepal, in the context of globally representative genome diversity (Fig 3; interactive version available at https://microreact.org/project/rk2ec5mWM). The Nepali S. Paratyphi A population was far less diverse than that of S. Typhi; most belonged to lineage A and clustered into two distinct subgroups, which we designated sublineages A1 and A2 (see Fig 3). Akin to S. Typhi, the global context of S. Paratyphi A also revealed close clustering with isolates from other regions in the Indian subcontinent and China, which where S. Paratyphi A infections occur at high prevalence.
Fig 3. Global population structure of S. Paratyphi A.
Inner ring indicates patient status. Branch colours indicate country/region of origin, as do the second and third rings from the inside. Fourth ring from the inside indicates QRDR gyrA S83F mutation. Fifth ring from the inside indicates the number of additional gyrA and parC QRDR mutations. Outer ring describes the presence of MDR. All rings and branches are.
Antimicrobial resistance (AMR)
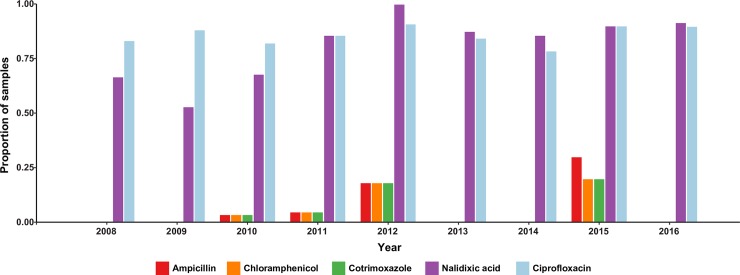
Amongst the paediatric isolates analysed in this study, most S. Typhi isolates (96%) and all S. Paratyphi A were susceptible to traditional first-line antibiotics co-trimoxazole, ampicillin and chloramphenicol (Fig 4). Most (86%) of S. Typhi and all the S. Paratyphi A of isolates were non-susceptible to the fluoroquinolone ciprofloxacin (assessed by disk diffusion; Fig 4). MDR was observed in six S. Typhi (3%) and no S. Paratyphi A. There were no differences in the frequency of MDR or fluoroquinolone non-susceptibility between the paediatric inpatients and outpatients (OR for MDR = 0.97, 95% CI 0.23–4.00; and OR for fluoroquinolone non-susceptibility = 1.23, 95% CI 0.62–2.44).
Fig 4. Bars are coloured as described in the inset legend.
Susceptibility to Ampicillin, Chloramphenicol, Ciprofloxacin, Cotrimoxazole, Nalidixic acid, Cephalosporin, Ceftriaxone, Cefixime, and Azithromycin were tested. No resistance to Azithromycin, Ceftriaxone, and Cefixime was observed.
Genetic determinants of AMR detected in the paediatric isolates are summarized in Table 2. All S. Paratyphi A (besides the single lineage C4 isolate) carried the gyrA S83F mutation responsible for nalidixic acid resistance and fluoroquinolone non-susceptibility. S. Typhi isolates displaying fluoroquinolone non-susceptibility harboured known QRDR SNPs (Table 2); these included isolates of genotypes 4.3.1 (gyrA SNPs), 3.3.0 (parE SNPs), and 3.3.1 (gyrA and parE SNPs, see S1 Fig). Sixteen S. Typhi isolates (all genotype 4.3.1) were QRDR ‘triple mutants’, which are associated with failure to respond to fluoroquinolone therapy[7]. All MDR isolates (n = 6) belonged to S. Typhi genotype 4.3.1 and harboured the acquired AMR genes catA, dfrA7, sul1, sul2, strA, strB and blaTEM-1, conferring resistance to chloramphenicol, co-trimoxazole, streptomycin and ampicillin. An additional genotype 4.3.1 isolate carried a subset of four of these genes (sul2, strA, strAB and blaTEM-1) and displayed resistance to ampicillin but was sensitive to co-trimoxazole and chloramphenicol (consistent with the lack of dfr and cat genes). Acquired AMR genes were not detected amongst the S. Paratyphi A.
Table 2. Genetic determinants of antimicrobial resistance in paediatric isolates from Nepal.
| S. Typhi | S. Paratyphi A | |
|---|---|---|
| Total isolates | 198 | 66 |
| QRDR | 164 (82.8%) | 65 (98%) |
| gyrA S83F | 143 (72%) | 65 (98%) |
| gyrA S83F only | 15 (7.6%) | 0 |
| gyrA S83F, gyrA D87N | 16 (8.1%) | 0 |
| gyrA S83F, gyrA D87N, parC S80I | 15 (7.6%) | 0 |
| gyrA S83F, gyrA D87N, parC E84G | 1 (0.5%) | 0 |
| gyrA S83F, parC E84G | 1 (0.5%) | 0 |
| gyrA S83F, parE A364V | 5 (2.5%) | 0 |
| gyrA S83Y only | 6 (3%) | 0 |
| parE A364V only | 15 (7.6%) | 0 |
| Acquired AMR genes | 7 (3.5%) | 0 |
| blaTEM-1, strAB, sul2 | 1 (0.5%) | 0 |
| catA1, dfrA7, sul1, blaTEM-1, strAB, sul2 (+ gyrA S83F) | 4 (2%) | 0 |
| catA1, dfrA7, sul1, blaTEM-1, strAB, sul2 (+ gyrA S83Y) | 2 (1%) | 0 |
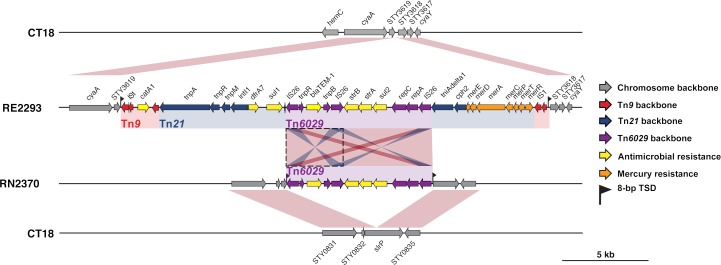
The full suite of seven acquired AMR genes are common amongst S. Typhi globally and are typically located within a composite transposon, comprising Tn6029 (sul2, strA, strAB and blaTEM-1) and Tn21 (dfrA7, sul1) inserted within Tn9 (catA), which is most often carried on IncHI1 plasmids[9]. Here, all MDR isolates carried this typical composite transposon, inserted in the chromosome between genes STY3618 and STY3619 and associated with an 8 bp target site duplication (GGTTTAGA), consistent with integration mediated by the flanking IS1 transposases of Tn9 (see Fig 5). The additional ampicillin resistant isolate carried only transposon Tn6029, which was inserted directly into the chromosomal pseudogene slrP and associated with an 8 bp target site duplication (TAGCTGAT), consistent with integration mediated by the flanking IS26 transposases of Tn6029.
Fig 5. Insertion sites of transposons observed in S. Typhi from Nepal.
Genes and transposons are indicated according to the inset legend. TSD indicates target site duplication, and Tn indicates transposon.
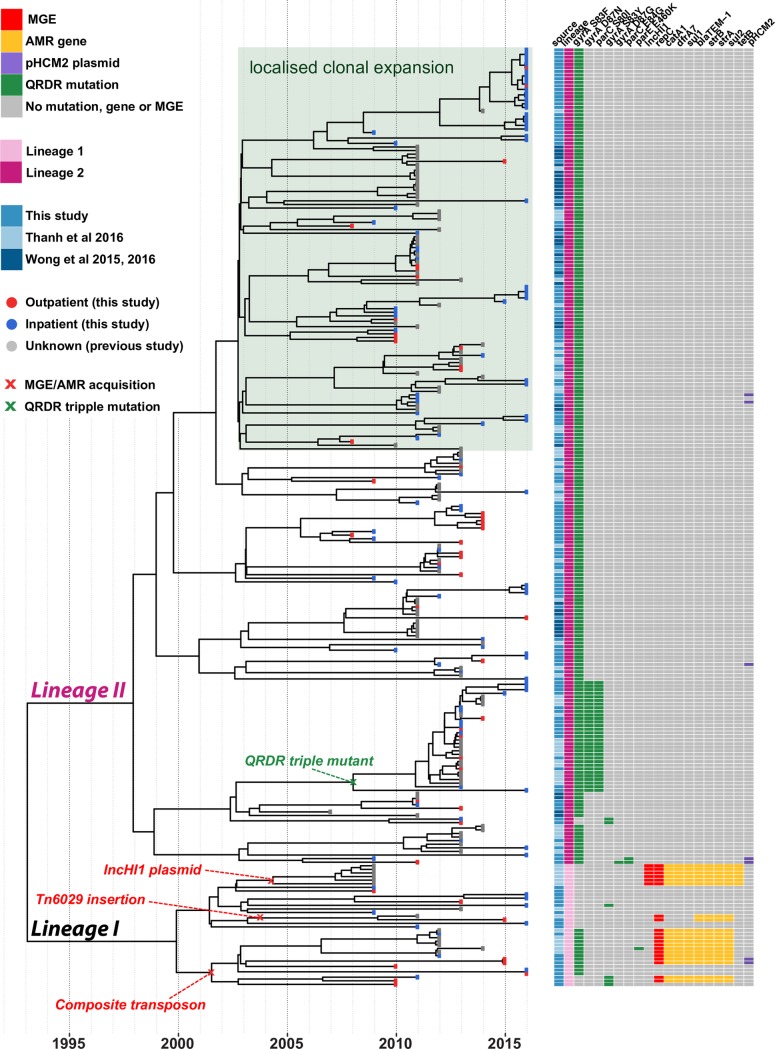
Evolutionary history of AMR S. Typhi 4.3.1 in Nepal
We constructed a dated phylogeny of all available S. Typhi 4.3.1 from Nepal, using BEAST2 (Fig 6, interactive version available at https://microreact.org/project/rJnfyOGxG). This analysis yielded a local substitution rate of 0.8 SNPs per genome per year (95% highest posterior density (HPD), 0.5–1.1) or 1.7x10-7 genome-wide substitutions per site per year (95% HPD, 1.1x10-7–2.4x10-7). The data showed strong temporal structure to support these results (see Methods and S2 Fig), which were consistent with previous estimates for global S. Typhi 4.3.1[10]. We estimated the most recent common ancestor (mrca) for all S. Typhi 4.3.1 in Nepal existed circa 1993, similar to the mrca estimated globally for S. Typhi 4.3.1, which is predicted to have emerged in neighbouring India[10].
Fig 6. BEAST2 maximum-clade credibility phylogenetic tree of Nepalese genotype 4.3.1 (H58) S. Typhi.
Tips colours indicate outpatient (red) vs. inpatient (blue) isolates. Acquisitions of molecular determinants of resistance are labelled.
Both of the previously described sublineages of S. Typhi 4.3.1 (I and II) were present amongst the Nepali isolates, however (i) lineage II was far more common (67% vs. 10% of paediatric isolates from this study; 68% vs 10% of isolates from other studies); and (ii) the lineages were associated with different AMR patterns (Fig 6): lineage I was associated with MDR (59% of lineage I vs 0 lineage II, p<1x10-15), while lineage II was associated with QRDR mutations (99% of lineage II vs 50% of lineage I, p<1x10-15). The majority of isolates formed a local monophyletic clade that was not detected in other countries in the global collection, indicative of local clonal expansion in Nepal. The relative proportion of local S. Typhi infections caused by lineage II increased after 2010 (40% pre-2010 vs 74% from 2011 onwards, p = 1x10-7), suggesting clonal replacement of the MDR-associated lineage I with the expansion of the quinolone resistance-associated Lineage II over time.
Most of the ciprofloxacin resistant triple mutant isolates harboured gyrA S83F, gyrA D87N, and parC S80I and formed a monophyletic subclade of lineage II, together with those previously reported as associated with gatifloxacin failure during the treatment trial in 2013–2014[7]. We dated the mrca of this subclade to 2008 (95% HPD, 1998–2011; see Fig 6), and comparison to the global tree confirmed it most likely originated in India[7] and was introduced to Nepal at least twice (see Fig 2B). We also identified a distinct ciprofloxacin resistant triple mutant (harbouring gyrA S83F, gyrA D87G, and parC E84G) that was isolated from a five-year old girl in 2011. This was also S. Typhi 4.3.1 lineage II but shared no particularly close relatives in the Nepali or global collections (Fig 2B, Fig 6).
All isolates with acquired AMR genes belonged to Lineage I: one cluster of IncHI1 plasmid-containing isolates (from a previous study conducted by Thanh et al 2016) with a mean tmrca of 2004 (95% HPD, 1996–2007); two related clusters with the composite transposon inserted in the chromosome after STY3618, with mean tmrca 2001 (95% HPD, 1995–2009); and one cluster with Tn6029 inserted in the chromosome, with mean tmrca 2003 (95% HPD, 1997–2010) (see Fig 6).
Discussion
These data show that enteric fever is a significant cause of illness amongst children in this study (Table 1), the majority of which (86%) is non-susceptible to fluoroquinolones (Table 2). Genomic analysis revealed substantial diversity within the local pathogen population (Figs 1 & S1), with evidence of transfer of S. Typhi and S. Paratyphi A between Nepal and neighbouring countries in South Asia (Figs 2 and 3), and intermingling of isolates from adults and children consistent with transmission across age groups (Fig 2). Data from 2005–2006 suggested that younger children were more susceptible to a wider range of genotypes, a phenomenon attributed to a naïve immune response[5]. A decade later this tendency seems to have shifted towards a more pathogen attributable trend as seen in Fig 1, which shows the S. Typhi 4.3.1 genotype is dominant regardless of the age and admission status of the host. This is consistent with recent mathematical modelling of historical enteric fever patterns in this setting, which identified the introduction of AMR 4.3.1, as well as an increase in migration of immunologically naïve 15–25 year olds from outside the Kathmandu Valley, as key drivers of the local typhoid problem[46].
The high frequency of fluoroquinolone non-susceptibility is attributable to indiscriminate and uncontrolled use of antimicrobials, which since the turn of the century have been used to treat a range of infections common in the tropics in addition to enteric fever. Fluoroquionolone non-susceptibility has been observed locally[7], associated with mutations in gyrA and parC. Our data show that the problem of fluoroquinolone non-susceptible enteric fever in Nepali children is mainly driven by two locally established pathogen variants, namely S. Typhi 4.3.1 (H58) lineage II harbouring the gyrA-S83F mutation (accounting for 50% of all enteric fever, 57% of non-susceptible cases, and 66% of all S. Typhi) and S. Paratyphi A clade A harbouring the gyrA-S83F mutation (accounting for 25% of enteric fever, 28% of non-susceptible cases, and 98% of all S. Paratyphi A). These strains have been present since the increase in local case numbers began in 1997, and their arrival likely contributed to the increased disease burden[46]. The universal fluroquinolone resistance demonstrated by the S. Paratyphi A population is of great concern particularly since a vaccine against paratyphoid fever is still in development.
Notably, the fully fluoroquinolone resistant triple mutant S. Typhi strain that was first detected in local adults in 2013 and halted the gatifloxacin treatment trial was still causing in disease in Nepali children in 2015–2016, but was rare (2.5% of cases in 2015–16) and showed no signs of displacing the wider population that carries only the gyrA-S83F mutation (65% of cases in 2015–16). This lack of clonal replacement is consistent with the presence of a single, distinct, triple mutant S. Typhi strain isolated from a 5-year old girl in 2011, which had no descendant strains detected amongst the 126 cases examined from 2012–16, suggesting it has not spread within the local human population. The lack of fully resistant S. Paratyphi A is also notable. It has been shown that the gyrA-S83F mutation is not associated with a fitness cost in S. Typhi and can be maintained in the absence of direct selection from fluoroquinolones; however our data suggest the same is not true of the triple mutants, hence limiting exposure to fluoroquinolones may at least control the spread of highly resistant strains.
Acquired resistance to other antimicrobials was rare, and in the paediatric population was associated only with S. Typhi 4.3.1 lineage I strains carrying chromosomally integrated AMR genes (Fig 6). This has not been reported previously in the local population, where MDR S. Typhi has typically been associated with plasmids[47]. Here we identified at least two distinct AMR gene integration events, that we estimate occurred contemporaneously with the MDR plasmid circulating in the early 2000s (Fig 6). Although similar findings have also been reported from S. Typhi strains in other neighbouring countries of India and Bangladesh[10], this is the first description in strains from Nepal. Notably, in addition to the integration of the typical S. Typhi MDR composite transposon mediated by IS1 transposase genes of Tn9, we identified for the first time direct integration of Tn6029 into the S. Typhi chromosome (Fig 5), mediated by IS26 and conferring ampicillin resistance in the absence of resistance to chloramphenicol or co-trimoxazole.
The findings of this study supplement our understanding of enteric fever in an endemic setting. The occurrence of disease in the <5 years population is in agreement with the other multi-centre data from South Asia, underscoring the importance of understanding the disease transmission dynamics and preventive strategies in the vulnerable population. The magnitude of disease occurrence in this age group is still an underestimation for several reasons; clinical suspicion of enteric fever in this age group is generally low as evidenced in these data and this trend has also been reported in other endemic regions[48]. The lack of clinical suspicion leads to a lack of diagnostic testing, which is in itself, fraught with impediments to reliable results. Blood culture, which is the feasible gold standard diagnostic performs poorly in this population owing to the difficulty in obtaining the required amount of blood and due to pre-treatment with antimicrobials prior to obtaining a blood sample. Despite the unique challenges associated with diagnosing enteric fever in this population and the supposed lack of exposure, reports from various endemic regions continue to reiterate the enormous burden of enteric fever in pre-school children. Coupled with the problem of antimicrobial non-susceptibility once a diagnosis is made, these difficulties highlight the urgent need for enteric fever vaccines in children under 5. However vaccination options for these children are limited due to the poor immunogenicity of the Vi polysaccharide vaccine in infants and the difficulty in administering the Ty21a vaccine. Until the Vi conjugate vaccines are rolled out, in addition to improving sanitation and providing clean water, antimicrobial treatment remains the only short-term option for containing the disease in this age group.
Cephalosporins are currently the first-line treatment for enteric fever in Nepal. We did not detect any cephalosporin non-susceptibility in these isolates, however it is anticipated that this will emerge via the acquisition of plasmid-encoded extended-spectrum beta-lactamase genes, as has recently been observed among S. Typhi isolates from neighbouring India and Pakistan[49–52]. Given the re-emergence of antimicrobial sensitivity to chloramphenicol and co-trimoxazole as evidenced in this study, it may be logical to shift to these first-line drugs for treating enteric fever; indeed there has already been a case report demonstrating efficacy of co-trimoxazole treatment in the treatment of fluoroquinolone resistant H58 S. Typhi in this setting[53]. We acknowledge the possibility that typhoidal Salmonella strains will acquire resistance to these antibiotics when re-introduced and the cycling of antimicrobials is seldom sufficient to effectively prevent MDR in the long-term. However we propose this short-term strategy might be commissioned until the typhoid conjugate vaccines are deployed, in order to conserve cephalosporins and macrolides for the treatment of other tropical infections which require higher-end antibiotics.
Our study has limitations as all isolates examined were from a single hospital based passive surveillance programme and thus may not be representative of the disease trends in the wider community. For inpatient isolates only stored samples were available (67 out of 102 for S. Typhi and 17 out of 27 for S. Paratyphi A) for retrospective sequencing analysis, preventing the use of a more formal random sampling technique; however we consider this is unlikely to introduce sampling bias with respect to genotype, as genotypes were not known at the time of storage and there is no reason to suspect particular genotypes would be more likely to have been stored. Consistent with this expectation, the population structure observed here is comparable to that observed in previous studies suggesting that our opportunistic sampling is reasonably representative of the population structure of paediatric typhoid in Nepal from 2008–2016. Outpatient isolates were randomly sampled and expected to be representative, but these were unable to be stratified by age, gender and admission information as outpatient data was limited and did not include any patient level information.
Conclusion
These data highlight the burden of enteric fever in children in Nepal while demonstrating the importance of laboratory and molecular surveillance in endemic regions. Those under the age of 5 years contributed most to the burden of enteric fever among inpatients who represent the severe spectrum of disease. The substantial contribution of those less than 2 years emphasize the urgent need for the Vi conjugate vaccine in regions such as Nepal where antimicrobial therapy is currently the main modality against enteric fever. Antimicrobial non-susceptibility continues to complicate management protocols and calls for prudent strategies aimed at conserving the currently effective drugs while buying time for vaccine deployment. Finally, the control of enteric fever in Nepal and South Asia requires a coordinated strategy given the inter-country transmission that occurs with the Indian subcontinent. The Vi conjugate vaccines offer the real possibility of controlling enteric fever but eradication will only become a possibility when the immunization strategy is supplemented by the provision of clean water and improved sanitation.
Supporting information
Paratyphi A outgroup rooted maximum likelihood phylogeny. Branch colours indicate the genotype (as labelled). Branch lengths are indicative of the estimated number of substitution rate per variable site. The adjacent heatmap shows the presence of AMR genes (yellow) and QRDR mutations (green) present in each isolate as described in the inset legend.
(TIF)
(A) Tempest regression of root-to-tip distance as (in the SNP alignment) a function of sampling time, with the root of the tree selected using heuristic residual mean squared (each point represents a tip of the maximum likelihood tree). The slope is a crude estimate of the substitution rate for the SNP alignment, the x-intercept corresponds to the age of the root node, and the R2 is a measure of clocklike behaviour (B) Date randomisation test with the left most box plot showing the posterior substitution rate estimate from the SNP alignment of the data with the correct sampling times, and the remaining 20 boxplots showing the posterior distributions of the rate from replicate runs using randomised dates. The data are considered to have strong temporal structure if the estimate with the correct sampling times does not overlap with those from the randomisations.
(TIF)
Simpson’s diversity of S. Typhi genotypes is printed on each plot.
(TIF)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CDB is a Rhodes scholar, class of 2015 funded by the Rhodes trust. ZAD was supported by the Wellcome Trust of Great Britain (106158/Z/14/Z). SD was supported by a McKenzie Fellowship from the University of Melbourne. KEH was supported by the NHMRC of Australia (Fellowship #1061409). GD is supported by the NIHR Cambridge, BRC and the Wellcome trust. AJP is funded the NIHR Oxford, BRC and Wellcome trust. The authors also wish to acknowledge the Gates foundation, which support enteric fever studies conducted by our group in Nepal. The sequencing of isolates in this study was funded by the Wellcome Trust Strategic Award: “A strategic vision to drive the control of enteric fever through vaccination” Strategic Award (106158/Z/14/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kelly DF, Thorson S, Maskey M, Sandeep M, Umesh S, Hamaluba M, et al. The burden of vaccine-preventable invasive bacterial infections and pneumonia in children admitted to hospital in urban Nepal. Int J Infect Dis. 2011;15: e17–e23. http://dx.doi.org/10.1016/j.ijid.2010.05.021 doi: 10.1016/j.ijid.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 2.Pradhan R, Shrestha U, Gautam SC, Thorson S, Shrestha K, Yadav BK, et al. Bloodstream Infection among Children Presenting to a General Hospital Outpatient Clinic in Urban Nepal. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckle GC, Walker CLF, Black RE. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2: 10401 doi: 10.7189/jogh.02.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azmatullah A, Qamar FN. Systematic review of the global epidemiology, clinical and laboratory profile of enteric fever. J Glob Health. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt KE, Baker S, Dongol S, Basnyat B, Adhikari N, Thorson S, et al. High-throughput bacterial SNP typing identifies distinct clusters of Salmonella Typhi causing typhoid in Nepalese children. BMC Infect Dis. 2010;10: 144 doi: 10.1186/1471-2334-10-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker S, Holt KE, Clements ACA, Karkey A, Arjyal A, Boni MF, et al. Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open Biol. Royal Society Journals; 2011;1: 110008 doi: 10.1098/rsob.110008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanh DP, Karkey A, Dongol S, Thi NH, Thompson CN, Rabaa MA, et al. A novel ciprofloxacin-resistant subclade of H58. Salmonella typhi is associated with fluoroquinolone treatment failure. Elife. 2016;5: 1–13. doi: 10.7554/eLife.14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugboko H, De N. Mechanisms of Antibiotic resistance in Salmonella typhi. IntJCurrMicrobiolAppSci. 2014;3: 461–476. [Google Scholar]
- 9.Holt KE, Phan MD, Baker S, Duy PT, Nga TVT, Nair S, et al. Emergence of a globally dominant inchi1 plasmid type associated with multiple drug resistant typhoid. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet. 2015;47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau TT, Campbell JI, Galindo CM, Van Minh Hoang N, Diep TS, Nga TTT, et al. Antimicrobial drug resistance of Salmonella enterica serovar typhi in asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob Agents Chemother. American Society for Microbiology; 2007;51: 4315–23. doi: 10.1128/AAC.00294-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Wang H, Lu J, Wu J, Chen M, Xu Y, et al. Genetic Diversity of Salmonella enteric serovar Typhi and Paratyphi in Shenzhen, China from 2002 through 2007. BMC Microbiol. BioMed Central; 2010;10: 32 doi: 10.1186/1471-2180-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharat P, Tuladhar NR. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: surveillance of resistance and a search for newer alternatives. Int J Infect Dis. Elsevier; 2006;10: 434–438. doi: 10.1016/j.ijid.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Date KA, Bentsi-Enchill A, Marks F, Fox K. Typhoid fever vaccination strategies. Vaccine. 2015;33: C55–C61. doi: 10.1016/j.vaccine.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank. datahelpdesk.worldbank.org. In: Available:https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 16.Available at. UNICEF. In: https://data.unicef.org/topic/child-survival/under-five-mortality/.
- 17.World Bank. Available at. In: http://www.worldbank.org/en/news/feature/2013/04/01/managing-nepals-urban-transition.
- 18.IndexMundi. Nepal Age Structure. IndexMundi.com. 2013.
- 19.Williams EJ, Thorson S, Maskey M, Mahat S, Hamaluba M, Dongol S, et al. Hospital-based surveillance of invasive pneumococcal disease among young children in urban Nepal. Clin Infect Dis. 2009;48 doi: 10.1086/596488 [DOI] [PubMed] [Google Scholar]
- 20.Services M. UK Standards for Microbiology Investigations. Bacteriology. 2015;B 55: 1–21. ID 7 [Google Scholar]
- 21.Matuschek E, Brown DFJ, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20 doi: 10.1111/1469-0691.12373 [DOI] [PubMed] [Google Scholar]
- 22.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413: 848–52. doi: 10.1038/35101607 [DOI] [PubMed] [Google Scholar]
- 23.Langmead BSS. Fast gapped-read alignment with Bowtie 2. Nat Commun. 2012;9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25: 2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong VK, Baker S, Connor TR, Pickard D, Page AJ, Dave J, et al. An extended genotyping framework for Salmonella enterica serovar Typhi, the cause of human typhoid. Nat Commun. 2016; 1–11. doi: 10.1038/ncomms12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. Oxford University Press; 2015;43: e15–e15. doi: 10.1093/nar/gku1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt K.Thomson E, Wain N.R, Langridge J.,Hasan G.C, Bhutta R,Quail Z.A,Norbertczak MA,Walker H.,Simmonds D,White M,Bason B,Mungall N,Dougan K,G Parkhill J. Salmonella enterica subsp. enterica serovar Paratyphi A str. AKU_12601 complete genome, strain AKU_12601. In: Bioinformatics [Internet]. 15 August 2009. [cited 11 Aug 2017] pp. 2078–2079. doi: 10.1093/bioinformatics/btp35219505943 [Google Scholar]
- 28.Zhou Z, McCann A, Weill F- X, Blin C, Nair S, Wain J, et al. Transient Darwinian selection in Salmonella enterica serovar Paratyphi A during 450 years of global spread of enteric fever. Proc Natl Acad Sci U S A. National Academy of Sciences; 2014;111: 12199–204. doi: 10.1073/pnas.1411012111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan M, Yang B, Wang Z, Wang S, Zhang X, Zhou Y, et al. A Large-Scale Community-Based Outbreak of Paratyphoid Fever Caused by Hospital-Derived Transmission in Southern China. Ryan ET, editor. PLoS Negl Trop Dis. Public Library of Science; 2015;9: e0003859 doi: 10.1371/journal.pntd.0003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuijpers LMF, Le Hello S, Fawal N, Fabre L, Tourdjman M, Dufour M, et al. Genomic analysis of Salmonella enterica serotype Paratyphi A during an outbreak in Cambodia, 2013–2015. Microb genomics. Microbiology Society; 2016;2: e000092. 10.1099/mgen.0.000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. England; 2006;22: 2688–2690. doi: 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 32.Argimón S, Abudahab K, Goater RJE, Fedosejev A, Bhai J, Glasner C, et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb genomics. Microbiology Society; 2016;2: e000093 doi: 10.1099/mgen.0.000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. McInerny G, editor. Methods Ecol Evol. 2017;8: 28–36. doi: 10.1111/2041-210X.12628 [Google Scholar]
- 34.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. Oxford University Press; 2016;2: vew007 doi: 10.1093/ve/vew007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Core Team R. A language and environment for statistical computing. R Found Stat Comput Vienna, Austria. [Google Scholar]
- 36.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, et al. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. Prlic A, editor. PLoS Comput Biol. Public Library of Science; 2014;10: e1003537 doi: 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duchêne S, Holt KE, Weill F- X, Le Hello S, Hawkey J, Edwards DJ, et al. Genome-scale rates of evolutionary change in bacteria. doi: 10.1099/mgen.0.000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firth C, Kitchen A, Shapiro B, Suchard MA, Holmes EC, Rambaut A. Using Time-Structured Data to Estimate Evolutionary Rates of Double-Stranded DNA Viruses. doi: 10.1093/molbev/msq088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo X, Zhao B. A Statistical Tree Annotator and Its Applications. 2011; 1230–1238. [Google Scholar]
- 40.Inouye M, Dashnow H, Raven L, Schultz MB, Pope BJ, Tomita T, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. 2014; 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 2014;58: 212–20. doi: 10.1128/AAC.01310-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. doi: 10.1093/bioinformatics/btv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saad NJ, Bowles CC, Grenfell BT, Basnyat B, Arjyal A, Dongol S, et al. The impact of migration and antimicrobial resistance on the transmission dynamics of typhoid fever in Kathmandu, Nepal: A mathematical modelling study. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamang MD, Oh JY, Seol SY, Kang HY, Lee JC, Lee YC, et al. Emergence of multidrug-resistant Salmonella enterica serovar Typhi associated with a class 1 integron carrying the dfrA7 gene cassette in Nepal. Int J Antimicrob Agents. Elsevier; 2007;30: 330–5. doi: 10.1016/j.ijantimicag.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 48.Britto C, Pollard AJ, Voysey M, Blohmke CJ. An Appraisal of the Clinical Features of Pediatric Enteric Fever: Systematic Review and Meta-analysis of the Age- Stratified Disease Occurrence. Clin Infect Dis. 2017; doi: 10.1093/cid/cix229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigues C, Kapil A, Sharma A, Ragupathi N, Inbabathan F, Veeraraghavan B, et al. Whole-Genome Shotgun Sequencing of Cephalosporin-Resistant Salmonella enterica Serovar Typhi. Genome Announc. 2017;5: e01639–16. doi: 10.1128/genomeA.01639-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qamar FN, Saleem K, Shakoor S, Yousufzai T, Kazi M, Lohana H, et al. Proceedings of 10th International Conference on Typhoid and other Invasive Salmonelloses. Kampala, Uganda; 2017. p. 40.
- 51.Munir T, Lodhi M, Ansari JK, Andleeb S, Ahmed M. Extended Spectrum Beta Lactamase producing Cephalosporin resistant Salmonella Typhi, reported from Rawalpindi, Pakistan. J Pak Med Assoc. 2016;66: 1035–6. [PubMed] [Google Scholar]
- 52.Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Shankar BA, Munusamy E, Anandan S, Veeraraghavan B. Draft genome sequence of blaTEM-1-mediated cephalosporin-resistant Salmonella enterica serovar Typhi from bloodstream infection. J Glob Antimicrob Resist. 2016;7: 11–12. doi: 10.1016/j.jgar.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 53.Karki M, Pandit S, Baker S, Basnyat B. Cotrimoxazole treats fluoroquinolone-resistant Salmonella typhi H58 infection. BMJ Case Rep. England; 2016;2016 doi: 10.1136/bcr-2016-217223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Paratyphi A outgroup rooted maximum likelihood phylogeny. Branch colours indicate the genotype (as labelled). Branch lengths are indicative of the estimated number of substitution rate per variable site. The adjacent heatmap shows the presence of AMR genes (yellow) and QRDR mutations (green) present in each isolate as described in the inset legend.
(TIF)
(A) Tempest regression of root-to-tip distance as (in the SNP alignment) a function of sampling time, with the root of the tree selected using heuristic residual mean squared (each point represents a tip of the maximum likelihood tree). The slope is a crude estimate of the substitution rate for the SNP alignment, the x-intercept corresponds to the age of the root node, and the R2 is a measure of clocklike behaviour (B) Date randomisation test with the left most box plot showing the posterior substitution rate estimate from the SNP alignment of the data with the correct sampling times, and the remaining 20 boxplots showing the posterior distributions of the rate from replicate runs using randomised dates. The data are considered to have strong temporal structure if the estimate with the correct sampling times does not overlap with those from the randomisations.
(TIF)
Simpson’s diversity of S. Typhi genotypes is printed on each plot.
(TIF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.