Abstract
Vitamin D3 (VD3) is an effective adjunctive agent, enhancing the destructive effects of freezing in prostate cancer cryoablation studies. We investigated whether dose escalation of VD3 over several weeks, to model the increase in physiological VD3 levels if an oral supplement were prescribed, would be as or more effective than a single treatment 1 to 2 days prior to freezing. PC-3 cells in log phase growth to model aggressive, highly metabolically active prostate cancer were exposed to a gradually increasing dose of VD3 to a final dose of 80 nM over a 4-week period, maintained for 2 weeks at 80 nM, and then exposed to mild sublethal freezing temperatures. Results demonstrate that both acute 24-hour exposure to 80 nM VD3 and dose escalation resulted in enhanced cell death following freezing at −15°C or colder, with no significant differences between the 2 exposure regimes. Apoptotic analysis within the initial 24-hour period postfreeze revealed that VD3 treatment induced both caspase 8- and 9-mediated cell death, most notably in caspase 8 at 8-hour postfreeze. These results indicate that both the intrinsic and extrinsic apoptotic pathways are involved in VD3 sensitization prior to freezing. Additionally, both acute and gradual dose escalation regimes of VD3 exposure increase prostate cancer cell sensitivity to mild freezing. Importantly, this study expands upon previous reports and suggests that the combination of VD3 and freezing may offer an effective treatment for both slow growth and highly aggressive prostate cancers.
Keywords: prostate cancer, vitamin D3, cryotherapy, apoptosis, cell death, freezing
Introduction
The development of adjunctive strategies for cryoablation is one of high interest due to the fact that simply freezing a given tumor may not completely destroy all cancerous cells. Reports have demonstrated that depending on the cancer type, cells can survive temperatures as low as −40°C. For instance, renal and liver cancer is destroyed at temperatures of −20°C or lower,1,2 whereas prostate cancer ranges from −25°C to −40°C depending on the molecular disposition of the particular cancer (ie, androgen-sensitive vs androgen-insensitive prostate cancer, respectively), as well as exposure intervals.3-6 Given this differential cancer specific response, it is critical to attain a specific target temperature (minimum lethal temperature) throughout a tumor to assure complete destruction. Due to the thermal gradients created within a frozen tissue mass, the necessity to freeze beyond the edge of a given tumor is often required. This positive freeze margin often results in the damage of nontargeted tissues, thus creating unwanted comorbidities. For instance, in prostate cryoablation, attainment of −40°C is often recommended to assure complete destruction.7-10 Attainment of −40°C at the tumor edge often requires the application of a positive freeze margin.11-14 With the application of a positive freeze margin in the prostate, physicians must be cognizant not to freeze the rectal wall or neurovascular bundle in an effort to avoid comorbidities. Given the balance between attaining a targeted minimal lethal temperature with a reduction of collateral damage associated with the positive freeze margin, the development of adjunctive strategies which can increase prostate cancer sensitivity to milder subfreezing temperatures is of great interest.
The objective of these strategies is to elevate the minimum lethal temperature through the use of low-dose, minimally toxic, adjunctive anticancer agents, thereby eliminating the need for a positive freeze margin while deploying a highly effective focal treatment strategy. Given the involvement of apoptotic cell death in freezing,4 agents that enhance apoptosis are of great interest. Combinatorial agents that have been studied range from cytotoxic chemotherapeutic agents2,15,16 to nutraceuticals,17,18 with the latter being the most attractive option due to typically decreased side effects. One such agent is calcitriol, the active metabolite of vitamin D3 (VD3).
Vitamin D3 has been reported to have positive anticancer properties in numerous in vitro and in vivo studies.1,17,19-24 Clinical studies investigating the correlation between VD3 status and an individual’s cancer risk have yielded conflicting results, particularly breast, colon, and prostate.25 Associations between vitamin D status, including single nucleotide polymorphisms in vitamin D binding protein and prostate cancer risk, have been reported.26-29 Although some clinical studies have found no significant differences between mortality rate and VD3 supplementation, many preclinical studies have shown the benefit that supplementation has on cancer prevention and treatment.30 One reason for this may be the body’s ability to retain tight homeostatic control over 25(OH)D3 to 1,25(OH)2D3 conversion (activation).31 As such, the measurable level of active 1,25(OH)2D3 has not been reported to be highly variable between individuals regardless of their 25(OH)D3 serum status.
For instance, a study of patients diagnosed with low-risk prostate cancer who took 4000 IU per day of VD3 revealed a potential reduction in cancer progression after 1 year as evidenced by a decrease in the number of positive cores or Gleason score (55%).21 The level of 25(OH) D3 in these patients was significantly higher after 1 year of supplementation (66.2 ± 14.5 vs 32.8 ± 13.3 at baseline). Interestingly, this study also noted that levels of 1,25(OH)2D3 were also significantly increased with this dosage,21 suggesting a continued role in the monitoring of calcitriol levels as more is learned about its significance.
One highly studied effect of VD3 is the ability to inhibit cell proliferation22,23 and induce apoptosis.24,32 This has made VD3 an attractive adjunctive agent for cancer therapies. Studies have shown that the use of VD3 in conjunction with various chemotherapeutic agents, radiation, and even cryoablation can yield improved cancer destruction.19,33-35 Studies have demonstrated that exposure of prostate cancer cells to high-dose (50-80 nM) VD3 in conjunction with mild freezing in the −15°C to −20°C range results in a significant increase in cell death.17,19,20 While effective, these studies focused on acute short-term (2 day) exposure of samples to VD3 followed by freezing. Further, these studies focused on plateau phase, highly confluent monolayers which correspond to slow growth, low metabolically active (low Ki67) prostate cancer models. This is significant given that the measurement of Ki67 antigen expression is widely used to determine growth fraction, and thus a logarithmically growing population of PC-3 cells express a higher percentage of Ki-67 positive cells than one that has reached high confluence and subsequent growth inhibition/plateau (unpublished results). A recent meta-analysis by Berlin et al strongly suggests the use of Ki67 as a prognostic marker for predicting overall poor prognosis in prostate cancer, as high Ki67 was consistently correlated with negative clinical outcomes. Low Ki67 was strongly correlated with better outcomes including disease free survival, lower rates of distant metastasis, and biochemical failure free survival among others.36
Given the importance of improved treatment studies for aggressive, highly active prostate cancers,37,38 we investigated the combination of VD3 exposure and freezing using a log-phase growth androgen-insensitive in vitro prostate cancer model. Studies were designed to compare the impact of gradual dose escalation of VD3 levels, such as would be attained via an oral dose regimen versus an acute high-dose exposure (utilized in previous studies). These studies expand upon our previous work to include a modified treatment to model clinical application, evaluation of an aggressive log-phase growth prostate cancer model, as well as a more in-depth analysis of the apoptotic pathways involved in VD3-mediated cell death.
Materials and Methods
Cell Culture
PC-3 (ATCC, CRL-1435) were cultured in T-75 flasks (Cell Treat, Shirley, Massachusetts) in Roswell Park Memorial Institute (RPMI) 1640 medium (Caisson, North Logan, Utah) supplemented with 10% fetal bovine serum (Peak Serum, Colorado) and 1% Penicillin/Streptomycin (Corning/Mediatech, Manassas, Virginia). Cells were lifted using TrypLE Express (Gibco/Life Technologies, Grand Island, New York), centrifuged, and plated into Costar stripwell plates (Corning, Tewksbury, Massachusetts) at 3.2 × 103 cells/cm2 for 24 hours prior to VD3 treatment or 48 hours prior to freezing.
Vitamin D3 Treatment
Calcitriol (1,25α(OH)2D3; Calbiochem/EMD Millipore, Billerica, Massachusetts) was reconstituted in ethanol to a (100 µM) stock solution and stored at −80°C. Stock solutions were diluted to final working concentrations in media immediately prior to application. For single applications, cells were exposed to 80 nM for 24 hours prior to freezing and returned to normal media for the freeze and recovery.
Dose Escalation
PC-3 cultures in T-75 flasks were exposed to gradually increasing concentrations of calcitriol during the course of treatment. Beginning at 5 nM, exposure was increased by 5 nM every 2 days until the final concentration of 80 nM was achieved. Dose-escalated cultures were maintained at 80 nM for 2 weeks prior to experimentation with media replenished with fresh calcitriol every 2 days. At 1-week dose-escalated, a subculture was performed and a “recovery” condition established. This culture was returned to normal media conditions for 1 week prior to experimentation.
Freezing Protocol
PC-3 samples in log-phase growth in Costar 8-well strips (75 µL medium/well) were exposed to freezing temperatures of −10°C, −15°C, −20°C, or −25°C in a refrigerated circulating bath (Neslab/Thermo Scientific, Waltham, Massachusetts) for 10 minutes. Ice nucleation was initiated at −2°C using liquid nitrogen vapor to prevent supercooling. Sample temperature was recorded using a type T thermocouple (Omega HH806AU; Omega, Stamford, Connecticut). Samples were thawed passively at room temperature for 10 minutes before recovery incubation at 37°C.
Viability Assessment
The metabolic activity indicator alamarBlue (Invitrogen, Carlsbad, California) was utilized to assess cell viability diluted 1:20 in Hank balanced salt solution (Corning/Mediatech) and applied to samples for 60 minutes (±1 minutes) at 37°C. Raw fluorescent units were obtained using a TECAN SpectraFluor Plus plate reader (excitation 530 nm and emission 590 nm; Tecan Austria GmBH, Grodig, Austria) and analyzed using Microsoft Excel. Raw fluorescence units were converted to percentages based upon prefreeze control values. A minimum of 3 experimental repeats with an intraexperimental repeat of 7 wells was performed in each condition (n ≥ 21). Assessments were repeated on alternating days for at least 5 days of recovery.
Caspase Inhibition Assays
Caspase 8 inhibitor II or Caspase 9 Inhibitor I (Calbiochem, #218759 and 218761) were diluted to 10 µM in culture medium and applied 30 minutes prior to freezing. Inhibitors remained on cultures for 24 hours postfreeze.
Immunofluorescence
Samples were fixed in Costar strips with ice-cold 100% methanol for 15 minutes at −20°C, followed by 3 × 5-minute washes in 1× phosphate-buffered serum (PBS; Corning). Samples were blocked in PBS containing 5% normal goat serum (Sigma-Aldrich, St. Louis, Missouri) and 0.03% Triton X-100 (VWR, Radnor, Pennsylvania) for 60 minutes at room temperature. Immunostaining with primary antibodies (cleaved Caspase 3, CST#9664; cleaved Caspase 8, CST#9496; cleaved Caspase 9, CST#20750) at 4°C overnight was followed by secondary antibody binding (goat anti-rabbit IgG Alexa Fluor 594 conjugate, CST #8889) for 60 minutes at room temperature. Both primary and secondary antibodies were diluted in PBS containing 1% bovine serum albumin (VWR) and 0.03% Triton X-100 (VWR). Samples were then rinsed in 1× PBS, counterstained with 1 µg/mL Hoechst 33342 (Molecular Probes), and imaged using a Zeiss Axiovert 200 (Zeiss, Thornwood New York). Three images per well at 10× magnification were obtained and ImageJ software (National Institutes of Health, Bethesda, MD) analysis was performed to quantitate positive staining. All experiments were performed at least in triplicate.
Results
Baseline Viability of Treatment Regimes in Combination With Freezing
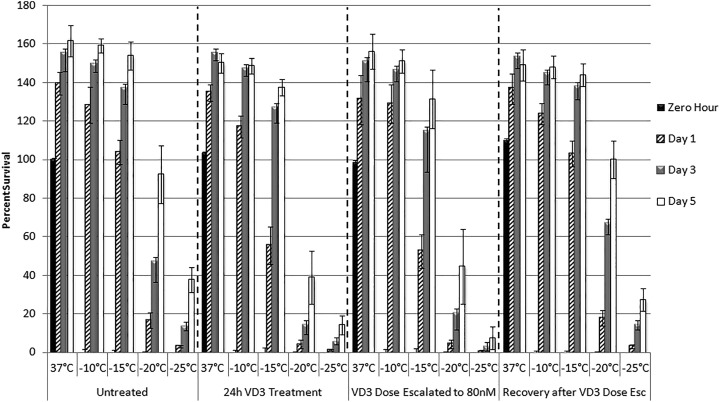
Sample viability was assessed at 24 hours following a single 10-minute exposure to a range of sublethal temperatures associated with the periphery of a cryosurgical iceball. At temperatures associated with those near the iceball edge (−10°C to −15°C), minimal cell death was observed in freeze alone samples (128.1% ± 9.3% of prefreeze control at −10°C and 103.8% ± 6.4% at −15°C, compared to 139.5% ± 5.8% in nonfrozen samples, Figure 1). Following exposure to −20°C and −25°C, viability was found to decrease to 16.6% (±4.0%) and 3.2% (±0.6%), respectively. Vitamin D3 pretreatment, either a single 24-hour exposure or the dose escalation regime, significantly increased cell death following freeze exposure compared to nontreated samples frozen to −15°C, −20°C, and −25°C. Specifically, 24-hour VD3 pretreatment decreased viability by 46.4%, 75.4%, and 66.3% following freezing to −15°C, −20°C, and −25°C, respectively. Dose-escalated samples exposed to the same temperatures decreased by 49.7%, 73.9%, and 81.2%, respectively.
Figure 1.
Viability of human prostate cancer cells following treatment with vitamin D3 and freezing. Cells were either untreated or treated with 80 nM calcitriol for 24 hours, dose escalated to 80 nM, or recovery from dose escalation and exposed to freezing temperatures of −10°C, −15°C, −20°C, or −25°C. Error bars represent standard deviation and each condition is compared to the prefreeze value of the individual 37°C control sample.
Comparison of VD3 single exposure or dose-escalated samples revealed no significant difference in the level of cell death following freezing. Both 24-hour VD3 exposure and dose-escalated VD3 samples exposed to −15°C resulted in similar levels of posttreatment viability, both significantly lower than −15°C alone samples (55.6% ± 9.8% vs 52.5% ± 8.6%, P = .07 compared to 103.8% ± 6.4%; Figure 1). Similar results were seen with samples exposed to −20°C (4.1% ± 2.2% vs 4.3% ± 2.1%, P = .9 compared to 16.6% ± 4.0%). While similar increases in cell death were observed, a trend toward increased cell death was observed in dose-escalated samples compared to single treatment in the −25°C samples (0.61% ± 0.4% vs 1.1% ± 0.5%, P = .001). Interestingly, even under ideal recovery conditions created in vitro, sample exposure to VD3 dose escalation followed by freezing to −25°C demonstrated a much lower level of repopulation than that of either freeze alone or single VD3 exposure + −25°C samples.
Analysis of the VD3 dose-escalated recovery samples (2 weeks of culture without VD3 prior to freezing) revealed no significant difference in cell death from that of untreated freeze alone samples at any temperature (Figure 1 and Table 1).
Table 1.
Assessment of the Percent Change in 1-Day Postfreeze Viability Following VD3 Exposure in Comparison to Matched Freeze Only Samples.
| % Change in Day 1 Postfreeze Viability From Freeze Only Matched Controls | |||||
|---|---|---|---|---|---|
| 37°C | −10°C | −15°C | −20°C | −25°C | |
| Dose escalated | −4.19 | 2.55 | −54.31 | −73.39 | −82.79 |
| 24-hour Vitamin D3 | −3.42 | −8.73 | −42.10 | −75.42 | −62.45 |
| Recovery from escalation | −10.53 | −3.52 | −0.65 | −0.65 | −3.93 |
Membrane-Mediated Cell Death Is Increased in VD3-Treated Cells
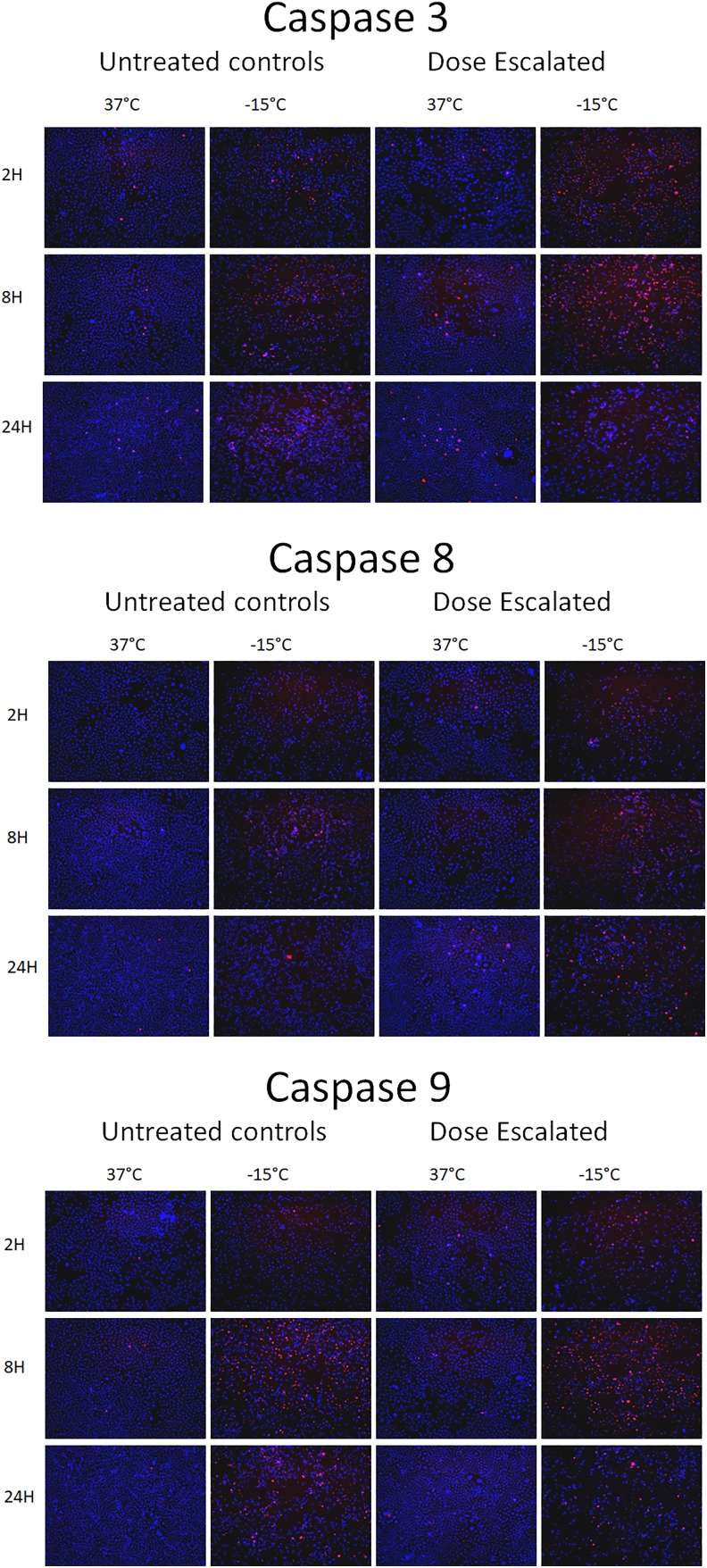
Given the significant increase in cell death observed with VD3 pretreatment, a series of investigations were conducted to determine (1) the level of apoptotic involvement and (2) if either mitochondrial or membrane-mediated apoptosis was most prominently involved. To this end, samples were frozen to −15°C with and without VD3 dose escalation and then assessed for caspase 8 (membrane), caspase 9 (mitochondrial), and caspase 3 (downstream execution) activity (caspase cleavage) at 2, 8, and 24 hours following freezing and compared to nonfrozen matched controls. Immunofluorescent staining revealed minimal changes in VD3 dose escalation nonfrozen control samples; however, in VD3 exposed/−15°C samples elevated levels of all active caspases were found following freezing.
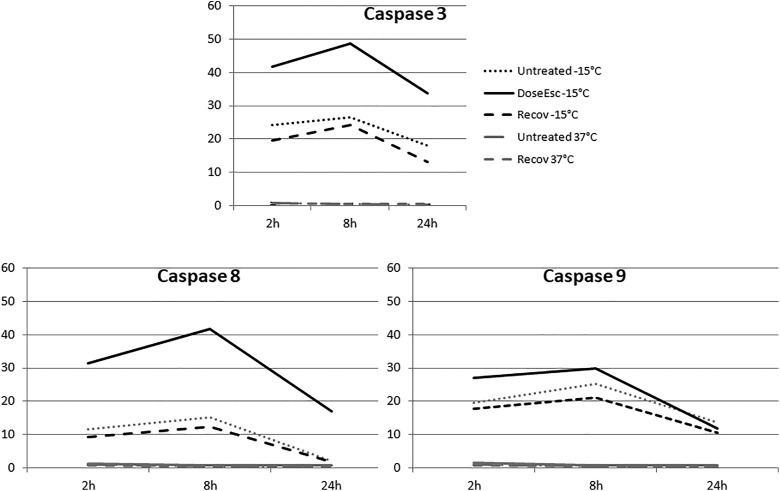
Within 2 hours following −15°C freezing in VD3 samples, 42% of cells stained positive for cleaved caspase 3, which increased slightly at 8 hours (49%) before decreasing to 34% at 24-hour postfreeze. This represented an approximated doubling in caspase 3 activity in the VD3 dose-escalated samples from that of −15°C freeze alone samples at all 3 time points (Table 2). Analysis of the levels of 2 initiator caspases revealed that activated caspase 8 was detected in 32% of cells at 2 hours postfreeze, peaked at 8 hours (42%), and decreased to 17% by 24 hours. The 32% caspase 8 staining at 2-hour postfreeze was a 166% increase in activity compared to non-VD3 exposed freeze samples (32% vs 12%, respectively). Similarly, at 8- and 24-hour VD3/−15°C sample caspase 8 levels were significantly elevated compared to either VD3 exposure or freeze alone samples (42% vs 15% at 8 hours and 17% vs 3% at 24 hours).
Table 2.
Quantification of the Percent Positively Labeled Cells in Immunofluorescent Micrographs for VD3-Treated Samples Following Freezing to −15°C.a
| % Positive Staining Cells per Well | ||||
|---|---|---|---|---|
| 2-Hour PF | cl C3 | cl C8 | cl C9 | |
| Ctrl | 37°C | 0.73 (0.1) | 1.04 ( 0.5) | 1.05 (0.2) |
| Ctrl | −15°C | 24.26 (2.1) | 11.5 (5.4) | 19.7 (3.2) |
| ESC | 37°C | 1.37 (0.3) | 1.44 (0.8) | 1.52 (0.1) |
| ESC | −15°C | 41.71 (8.5) | 31.5 (17.8) | 26.96 (6.2) |
| Recovery | 37°C | 0.87 (0.4) | 0.8 (0.5) | 0.76 (0.3) |
| Recovery | −15°C | 19.54 (7.6) | 9.37 (9.9) | 17.88 (7.2) |
| 8-Hour PF | cl C3 | cl C8 | cl C9 | |
| Ctrl | 37°C | 0.43 (0.1) | 0.52 (0.3) | 0.56 (0.1) |
| Ctrl | −15°C | 26.47 (9.2) | 15.27 (5.6) | 25.34 (10.5) |
| ESC | 37°C | 0.82 (0.2) | 0.9 (0.6) | 0.91 (0.3) |
| ESC | −15°C | 48.74 (15.8) | 41.79 (19.2) | 29.97 (18.9) |
| Recovery | 37°C | 0.61 (0.2) | 0.40 (0.3) | 0.62 (0.03) |
| Recovery | −15°C | 24.2 (10.7) | 12.26 (11.5) | 21.09 (6.9) |
| 24-Hour PF | cl C3 | cl C8 | cl C9 | |
| Ctrl | 37°C | 0.34 (0.2) | 0.28 (0.2) | 0.31 (0.2) |
| Ctrl | −15°C | 18.13 (4.9) | 2.50 (2.4) | 13.65 (5.2) |
| ESC | 37°C | 0.97 (0.6) | 0.73 (0.7) | 0.86 (0.7) |
| ESC | −15°C | 33.72 (14.0) | 16.95 (6.0) | 11.75 (2.7) |
| Recovery | 37°C | 0.51 (0.2) | 0.40 (0.2) | 0.53 (0.2) |
| Recovery | −15°C | 13.18 (5.1) | 1.73 (2.8) | 11.0 (6.3) |
Abbreviations: cl C, cleaved caspase; VD3, vitamin D3; PF, postfreeze; ESC, dose escalated.
aStandard deviation is represented.
Analysis of caspase 9 activity in dose-escalated samples revealed that caspase 9 cleavage products were observed in 27% of cells at 2 hours postfreeze, increasing to 30% at 8 hours followed by a decrease to 12% by 24 hours. When comparing caspase 9 levels in VD3/−15°C samples to −15°C samples, both the 2- and 8-hour time points were found to be elevated in the VD3 exposed conditions. By 24 hours there were no differences in caspase 9 levels between freeze alone and VD3/−15°C samples (11% vs 13%).
In addition to non-VD3 exposed and VD3/−15°C samples, VD3 exposure recovery samples were analyzed for caspase 3, 8, and 9 activities. Analysis of the recovery samples revealed a slight decrease in overall caspase activity at all time points postthaw in comparison to both the VD3/−15°C and −15°C alone samples (Figure 2 and Figure 3, Table 2).
Figure 2.
Immunofluorescence micrographs of dose-escalated VD3 samples following freezing to −15°C. Immunofluorescence was performed on methanol fixed samples using caspase 3, 8, or 9 as primary antibody followed by secondary antibody staining with an AlexaFluor 594 conjugate. Samples were counterstained with Hoechst 33342 for DNA visualization and fluorescence images were obtained at 2, 8, and 24 hours postfreeze at 10× magnification.
Figure 3.
Time course graphical analysis of positively labeled caspase 3, 8, and 9 following VD3 treatment and/or freezing to −15°C. Number of cells staining positive for each antibody were divided by the total number of Hoechst staining cells to determine the percentage of positively staining cells in each population. Three separate images per well were obtained and data from 3 separate immunofluorescence experiments were combined.
Caspase Inhibition Improves Cell Viability Postfreeze
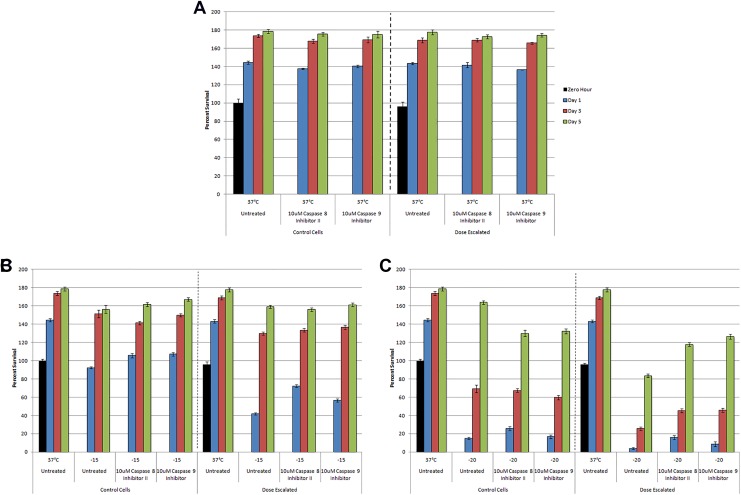
With the observed increase in caspase activity in VD3 pretreated/frozen samples, studies were conducted to determine if the increase in caspase 8 and 9 activity resulted in the observed increase in cell death or if it was simply a nonlethal cellular response. To test this, samples with or without VD3 exposure were frozen to −15°C or −20°C with either a caspase 8 or 9 inhibitor. Addition of either inhibitor had minimal effect on nonfrozen and VD3-treated control samples (Figure 4A).
Figure 4.
Assessment of the effect of caspase inhibition on postfreeze viability in VD3-treated and/or frozen PC-3 samples. Metabolic activity of untreated or VD3 dose-escalated PC-3 cells was obtained in response to caspase inhibition and freezing to −15°C and −20°C. A, Normothermic controls; B, −15°C; C, −20°C.
Addition of 10 µM caspase 8 inhibitor prior to freezing to −15°C resulted in an increase in postfreeze viability at 24 hours by 14.3% (105.7% ± 5.5% vs 92.5% ± 12.6%, Figure 4B). When caspase 8 inhibitor was applied to VD3 dose-escalated samples frozen to −15°C, postfreeze viability increased significantly (71%) compared to nontreated dose-escalated samples (72.0% ± 7.2% vs 42% ± 5.5%; Figure 4B). Similar outcomes were observed in samples frozen to −20°C, where postfreeze viability increased by 73.4% in non-VD3-treated samples (25.8% ± 8.5% vs 14.9% ± 4.3%, Figure 4C). Caspase inhibition in VD3 dose-escalated samples frozen to −20°C also yielded a significant increase (300%) compared to noninhibited VD3 dose-escalated samples (16% ± 4.3% vs 4% ± 0.8%, Figure 4C).
Analysis of the effect of caspase 9 inhibition on various samples revealed a similar yet lower impact as caspase 8 inhibition following both −15°C and −20°C exposure in both the untreated and VD3 dose-escalated samples (VD3 dose escalation/−15°C: 56.7% ± 8.6% vs 42% ± 5.5%; VD3 dose esc/−20°C: 8.7% ± 1.4% vs 4% ± 0.8%; Figure 4 B and C).
Discussion
This study investigated the impact of a dose escalation regime of VD3 on the cryosensitization of prostate cancer cells in vitro. This study was designed to model the bioavailability of VD3 in vivo following a 1-month prescription of a moderately high oral dose. The effects of the dose escalation regime were compared to that of a single acute high-dose exposure as well as non-VD3-treated samples. The impact of VD3 exposure was assessed in conjunction with freezing to −15°C and −20°C. These temperatures were selected as they represent nonlethal subfreezing temperatures for prostate cancer which are associated with the periphery of the iceball.6 This thermal region (≥−20°C) is of interest, given that in prostate cancer cryosurgery, concerns of potential disease recurrence are associated with incomplete death if a temperature of −40°C isn’t attained at the tumor edge. As such, efforts to increase cell death within the periphery thereby increasing the critical isotherm for prostate cancer from the reported −40°C to −20°C or warmer are of great interest. This would reduce the necessity for tissue overfreezing and associated collateral damage.
Given the substantial benefit of VD3 reported in earlier studies coupled with similar outcomes reported in a murine in vivo model,20 we elected to expand our line of investigation to determine the impact of gradual dose escalation of VD3 levels, as would be associated with an oral dose prescription model, on prostate cancer cell response to freezing. Further, with the growing interest in the use of adjunctive therapies to treat aggressive fast growing (elevated Gleason and Ki-67) prostate cancer, we utilized a log-phase growth in vitro model to determine if VD3 sensitization in conjunction with freezing at sublethal temperatures had a similar impact on fast growing highly metabolically active samples as were found in slow growth models.
Another interesting aspect is as prostate cancer progresses from an androgen-sensitive (early stage) to androgen-insensitive state, the ability of VD3 to halt cell cycle progression is lost, thereby negating many of its antiproliferative/anticancer benefits.39 This shift in androgen responsiveness has also been reported to be associated with increased prostate cancer resistance to a number of treatment regimes including various chemotherapeutic agents, radiation, and even impact cancer cell tolerance to cold ablation.5,40,41 While there is some debate within the literature on the extent of the antiproliferative responsiveness of PC-3 cells to calcitriol, many studies report little to negligible proliferation inhibition.42-45 These studies are in agreement with our observation of minimal impact of VD3 on actively dividing log phase PC-3 cells. While the overall impact of VD3 on androgen-insensitive prostate cancer is altered, the ability of VD3 pretreatment to increase cell sensitivity to freezing injury does not seem to be impacted.17,19
In this study, we used the active form of VD3, calcitriol (1,25(OH)2 D3) due to the lack of adequate hydroxylase activity in vitro. We chose the active metabolite to model a regime of gradually rising VD3 availability and conversion, which would result from an oral dose prescription. Due to the short half-life of 1,25(OH)2 D3, it is rarely used as a reliable measurement of VD3 status in vivo. More typically, the precursor metabolite 25(OH) D3 is measured as serum levels; however, studies suggest that there is not a linear conversion.46
The data presented herein suggest that VD3 pretreatment, whether applied as a short acute dose or in a dose escalation model, significantly improves cell death following freezing to temperatures associated with the iceball periphery. Further, the benefit of VD3 pretreatment in conjunction with cryoablation was also found in an actively dividing, highly metabolically active androgen-insensitive prostate cancer model.
To this end, the combination of VD3 exposure coupled with −25°C freezing resulted in near complete cell destruction 1 day following freezing with minimal culture recovery (under ideal recovery conditions). This was significantly different from −25°C freeze alone samples which demonstrated substantial repopulation following freezing. Similar outcomes were observed following VD3 pretreatment combined with freezing to −20°C. Following exposure to −15°C, VD3-treated samples yielded a significant decrease in viability; however, they were able to repopulate. This differed from previous reports where near complete ablation was observed following exposure to −15°C and 48-hour VD3 treatment.19 The differences in the critical isotherm following VD3 pretreatment in this study (−20°C to −25°C range) and previously reported −15°C are believed to be a result of the log phase, highly metabolic nature of the current model versus the plateau phase, low metabolic activity utilized in previous studies. Further, in this study, we utilized the androgen-insensitive PC-3 prostate cancer cell line (late stage, aggressive cancer) versus the LNCaP-HP (late-stage slow growth cancer) cell line. While different, both these studies illustrate the benefits of VD3 pretreatment in combination with cryoablation which results in a shift in the minimal lethal isotherm for late-stage androgen-insensitive prostate cancer from the previously established −40°C to the −20°C (±5°C) region, regardless of the growth fraction of the cancer.
While the benefit of the combination of VD3 pretreatment and freezing was found to be significant, it was also found that once VD3 was removed (within 2 weeks of recovery), this positive effect was negated thereby suggesting that active exposure to VD3 immediately prior to freezing is necessary. Further, unpublished observations indicate a pretreatment interval of 24 hours or longer is required to yield the positive synergistic effect.
Previous studies from our laboratory have shown the involvement of both extrinsic and intrinsic apoptotic signaling pathways in freezing-induced cell death. Specifically, membrane-mediated cell death was most prominent in severe freeze insults (<−30°C), and the mitochondrial pathway in a milder freeze event (−15°C).47 Further, in vitro studies on plateau phase slow growth LNCaP-HP prostate cancer cells have suggested that VD3 pretreatment results in an increase in apoptotic cell death via the mitochondrial-mediated caspase 9 pathway following exposure to −15°C.19 With the observed continued benefit of VD3 pretreatment in our rapidly dividing aggressive prostate cancer model, yet an observed shift in the minimum lethal isotherm for −15°C to the −20°C to −25°C range, we investigated if this was due to an alteration in the apoptotic response following combination treatment. To this end, our results indicate that VD3 pretreatment in the actively dividing log-phase growth prostate cancer model result in increased activity in both the mitochondrial and membrane-mediated apoptotic pathways. Inhibition studies suggested that in the highly active model, the extrinsic membrane-mediated apoptotic induction pathway (caspase 8) played a greater role in increasing cell death following the combination of VD3 pretreatment and freezing. These results suggest that while the typical effect of VD3 on halting cell cycle activity is negated in aggressive androgen-insensitive PC-3 cells, at the molecular level the impact of VD3 pretreatment still results in the upregulation of the apoptotic cell death machinery which when combined with a sublethal freeze insult yields an increase in cell death. Further, these results clearly establish a role for membrane-mediated cell death in VD3-induced cryosensitization, which previously has been seen in more severe freezing insults. The 72% improvement in viability following inhibition of caspase 8 in dose-escalated cells further confirmed the large role of the extrinsic cell death pathway.
The overall findings of this study support the conclusion that VD3 pretreatment sensitizes prostate cancer cells via priming of the apoptotic pathway, thereby making the cell more susceptible to freezing injury. The data suggest that the combination of VD3 pretreatment and mild freezing increases the minimal lethal temperature from −40°C to the −20°C to −25°C range when applied to aggressive, rapidly dividing, highly metabolically active prostate cancer. As described, similar results have also been reported in slow growth cancer models as well as in an in vivo murine study. In the murine study, the increase in cell death as measured by % necrotic tissue was found to be 25% in the VD3 freeze combination over the freeze alone condition.20 This increase correlates with the reported differential between the volume of tissue found within the −20°C and −40°C isotherms following a given freeze interval.11 This suggests that the lethal isotherm in the Kimura study was around −20°C following VD3 sensitization combined with freezing, which is in agreement with the findings of this study.
While the current findings support the benefit of the combination of VD3 and freezing, there are several limitations. Firstly, the study was conducted in an in vitro prostate cancer cell model. The in vitro nature of the model provides ideal culture conditions for cell survival and recovery and thus the results presented herein may not directly reflect a clinical scenario. As such, future studies in vivo are necessary to elucidate the clinical potential of this approach.
Another limitation is the use of the active form of VD3, calcitriol, and final concentrations utilized may be higher than levels attained in vivo via an oral dose regimen. To overcome this, an intratumoral injection (ITIJ) of calcitriol prior to cryoablation, as used in the Kimura study, may provide for a more effective means of delivering high doses of active VD3 than a systemic dose escalation approach. To this end, the results of this study suggest that the duration of VD3 pretreatment does not significantly affect postfreeze outcomes. Accordingly, a patient could potentially receive a dose of VD3 1 to 2 days before a cryosurgical procedure to significantly improve treatment success. However, little research exists into the distribution of calcitriol or other drugs following ITIJ, but studies in rats indicate successes in ITIJ of paclitaxel,48 the β-emitting radionuclide (166)Ho,49 and zinc-acetate50 in models of prostate cancer. Intratumoral injection of calcitriol has been utilized as a treatment in Japan for hyperparathyroidism,51 and a phase I/II clinical trial was conducted using ITIJ of inactivated sendai virus particles (Hemagglutinating virus of Japan Envelope, or HVJ-E) for prostate cancer treatment.52 Though promising, further studies are needed to determine the efficacy of calcitriol ITIJ in human prostate cancer treatment.
In conclusion, our findings suggest that VD3 pretreatment in combination with cryoablation results in a significant increase in the level of cell death. The data suggest that both acute (1 day) and dose escalation–based exposure of actively dividing androgen insensitive prostate cancer cells to elevated VD3 levels provided benefit. The data suggest that the combination results in a shift of the minimum lethal isotherm for aggressive prostate cancer from −40°C to the −20°C to −25°C range. Extrapolating these in vitro findings to an in vivo scenario, the data suggest the strategy of VD3 pretreatment may increase the lethality of the iceball. If correct, this could be critical as in a typical cryosurgical procedure, the volume of iceball between the −20°C and −40°C isotherms represents ∼20% of the frozen mass.11 Importantly, an increase in the lethal isotherm to −20°C would represent an overall doubling of the ablation volume within the frozen mass (12.67 cm3 vs 6.4 cm3, respectively) following a standard 10/5/10 minutes double freeze procedure using an argon-based cryosurgical device.11 This in turn has the potential to improve outcome while reducing comorbidities associated with overfreezing (positive freeze margins) to assure cancer destruction. In combination with previous in vitro and in vivo reports, these data suggest VD3 sensitization combined with freezing may provide an improved path for the treatment of prostate cancer at any stage.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by CPSI Biotech (Owego, NY, USA) and the National Institutes of Health Grant # 1R43CA183265-01.
References
- 1. Clarke DM, Robilotto AT, Rhee E, et al. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6(2):69–79. [DOI] [PubMed] [Google Scholar]
- 2. Clarke DM, Hollister WR, Baust JG, Van Buskirk RG. Cryosurgical modeling: sequence of freezing and cytotoxic agent application affects cell death. Mol Urol. 1999;3(1):25–31. [PubMed] [Google Scholar]
- 3. Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95(9):1187–1191. [DOI] [PubMed] [Google Scholar]
- 4. Hollister WR, Mathew AJ, Baust JG, et al. The effects of freezing on cell viability and mechanisms of cell death in an in vitro human prostate cancer cell line. Mol Urol 1998;2(1):13–18. [Google Scholar]
- 5. Klossner DP, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 6. Klossner DP, Robilotto AT, Clarke DM, et al. Cryosurgical technique: assessment of the fundamental variables using human prostate cancer model systems. Cryobiology. 2007;55(3):189–199. [DOI] [PubMed] [Google Scholar]
- 7. Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993–2004. [DOI] [PubMed] [Google Scholar]
- 8. Larson TR, Rrobertson DW, Corica A, Bostwick DG. In vivo interstitial temperature mapping of the human prostate during cryosurgery with correlation to histopathologic outcomes. Urology. 2000;55(4):547–552. [DOI] [PubMed] [Google Scholar]
- 9. Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205(2):342–356. [DOI] [PubMed] [Google Scholar]
- 10. Baust JG, Bischof JC, Jiang-Hughes S, et al. Re-purposing cryoablation: a combinatorial ‘therapy’ for the destruction of tissue. Prostate Cancer Prostatic Dis. 2015;18(2):87–95. [DOI] [PubMed] [Google Scholar]
- 11. Baust JM, Robilotto A, Snyder KK, et al. Assessment of cryosurgical device performance using a 3D tissue-engineered cancer model. Technol Cancer Res Treat. 2017:1533034617708960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20(10):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplan SA, Greenberg R, Baust JG. A comparative assessment of cryosurgical devices: application to prostatic disease. Urology. 1995;45(4):692–699. [DOI] [PubMed] [Google Scholar]
- 14. Shah TT, Arbel U, Foss S, et al. Modeling cryotherapy ice ball dimensions and isotherms in a novel gel-based model to determine optimal cryo-needle configurations and settings for potential use in clinical practice. Urology. 2016;91:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42(4):274–285. [DOI] [PubMed] [Google Scholar]
- 16. Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49(1):45–61. [DOI] [PubMed] [Google Scholar]
- 17. Santucci KL, Snyder KK, Baust JM, et al. Use of 1,25alpha dihydroxyvitamin D3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis. 2011;14(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol. 2016;40-41:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baust JM, Klossner DP, Robilotto A, et al. Vitamin D(3) cryosensitization increases prostate cancer susceptibility to cryoablation via mitochondrial-mediated apoptosis and necrosis. BJU Int. 2012;109(6):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimura M, Rabbani Z, Mouraviev V, et al. Role of vitamin D(3) as a sensitizer to cryoablation in a murine prostate cancer model: preliminary in vivo study. Urology. 2010;76(3):764 e714-720. [DOI] [PubMed] [Google Scholar]
- 21. Marshall DT, Savage SJ, Garrett-Mayer E, et al. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab. 2012;97(7):2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao XY, Peehl DM, Navone NM, Feldman D. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology. 2000;141(7):2548–2556. [DOI] [PubMed] [Google Scholar]
- 23. Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59(3):243–251. [DOI] [PubMed] [Google Scholar]
- 24. Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276(12):9101–9107. [DOI] [PubMed] [Google Scholar]
- 25. Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39(1):28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilbert R, Bonilla C, Metcalfe C, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control. 2015;26(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gilbert R, Metcalfe C, Fraser WD, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer. 2012;131(5):1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy AB, Nyame Y, Martin IK, et al. Vitamin D deficiency predicts prostate biopsy outcomes. Clin Cancer Res. 2014;20(9):2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nyame YA, Murphy AB, Bowen DK, et al. Associations between serum vitamin D and adverse pathology in men undergoing radical prostatectomy. J Clin Oncol. 2016;34(12):1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ness RA, Miller DD, Li W. The role of vitamin D in cancer prevention. Chin J Nat Med. 2015;13(7):481–497. [DOI] [PubMed] [Google Scholar]
- 31. Hollis BW. Assessment of circulating 25(OH)D and 1,25(OH)2D: emergence as clinically important diagnostic tools. Nutr Rev. 2007;65(8 pt 2): S87–S90. [DOI] [PubMed] [Google Scholar]
- 32. Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1(9):667–677. [PubMed] [Google Scholar]
- 33. Beer TM, Hough KM, Garzotto M, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in advanced prostate cancer. Semin Oncol. 2001;28(4 suppl 15):49–55. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Yang W, Uytingco MS, Christakos S, Wieder R. 1,25-Dihydroxyvitamin D3 and all-trans-retinoic acid sensitize breast cancer cells to chemotherapy-induced cell death. Cancer Res. 2000;60(7):2040–2048. [PubMed] [Google Scholar]
- 35. Sundaram S, Sea A, Feldman S, et al. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res. 2003;9(6):2350–2356. [PubMed] [Google Scholar]
- 36. Berlin A, Castro-Mesta JF, Rodriguez-Romo L, et al. Prognostic role of Ki-67 score in localized prostate cancer: a systematic review and meta-analysis. Urol Oncol. 2017;35(8):499–506. [DOI] [PubMed] [Google Scholar]
- 37. Sulik M, Maruszak K, Puchalska J, Misiukiewicz-Poć M. Expression of Ki-67 as a proliferation marker in prostate cancer. Polish Ann Med. 2011;18(1):12–19. [Google Scholar]
- 38. Verma R, Gupta V, Singh J, et al. Significance of p53 and ki-67 expression in prostate cancer. Urol Ann. 2015;7(4):488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao XY, Ly LH, Peehl DM, Feldman D. 1alpha,25-dihydroxyvitamin D3 actions in LNCaP human prostate cancer cells are androgen-dependent. Endocrinology. 1997;138(8):3290–3298. [DOI] [PubMed] [Google Scholar]
- 40. Lin HP, Lin CY, Hsiao PH, et al. Difference in protein expression profile and chemotherapy drugs response of different progression stages of lncap sublines and other human prostate cancer cells. PLoS One. 2013;8(12):e82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan X, Cai C, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2014;33(22):2815–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ting HJ, Hsu J, Bao BY, Lee YF. Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1alpha,25-dihydroxyvitamin D3. Cancer Lett. 2007;247(1):122–129. [DOI] [PubMed] [Google Scholar]
- 43. Sha J, Pan J, Ping P, et al. Synergistic effect and mechanism of vitamin A and vitamin D on inducing apoptosis of prostate cancer cells. Mol Biol Rep. 2013;40(4):2763–2768. [DOI] [PubMed] [Google Scholar]
- 44. Muindi JR, Yu WD, Ma Y, et al. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology. 2010;151(9):4301–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7(4):1043–1051. [PubMed] [Google Scholar]
- 46. Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59:14–19. [PubMed] [Google Scholar]
- 47. Robilotto AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Temperature-dependent activation of differential apoptotic pathways during cryoablation in a human prostate cancer model. Prostate Cancer Prostatic Dis. 2013;16(1):41–49. [DOI] [PubMed] [Google Scholar]
- 48. Shikanov S, Shikanov A, Gofrit O, Nyska A, Corn B, Domb AJ. Intratumoral delivery of paclitaxel for treatment of orthotopic prostate cancer. J Pharm Sci. 2009;98(3):1005–1014. [DOI] [PubMed] [Google Scholar]
- 49. Kwak C, Hong SK, Seong SK, Ryu JM, Park MS, Lee SE. Effective local control of prostate cancer by intratumoral injection of (166)Ho-chitosan complex (DW-166HC) in rats. Eur J Nucl Med Mol Imaging. 2005;32(12):1400–1405. [DOI] [PubMed] [Google Scholar]
- 50. Shah MR, Kriedt CL, Lents NH, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. 2009;28:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitaoka M, Fukagawa M, Fukuda N, Yi H, Ogata E, Kurokawa K. Direct injections of calcitriol into enlarged parathyroid glands in chronic dialysis patients with severe parathyroid hyperfunction. Nephrology. 1995;1(6):563–567. [Google Scholar]
- 52. Fujita K, Nakai Y, Kawashima A, et al. Phase I/II clinical trial to assess safety and efficacy of intratumoral and subcutaneous injection of HVJ-E in castration-resistant prostate cancer patients. Cancer Gene Ther. 2017;24(7):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]