Abstract
Nanotechnology has illustrated significant potentials in biomolecular-sensing applications; particularly its introduction to anti-doping detection is of great importance. Illicit recreational drugs, substances that can be potentially abused, and drugs with dosage limitations according to the prohibited lists announced by the World Antidoping Agency (WADA) are becoming of increasing interest to forensic chemists. In this review, the theoretical principles of optical biosensors based on noble metal nanoparticles, and the transduction mechanism of commonly-applied plasmonic biosensors are covered. We review different classes of recently-developed plasmonic biosensors for analytic determination and quantification of illicit drugs in anti-doping applications. The important classes of illicit drugs include anabolic steroids, opioids, stimulants, and peptide hormones. The main emphasis is on the advantages that noble metal nano-particles bring to optical biosensors for signal enhancement and the development of highly sensitive (label-free) biosensors. In the near future, such optical biosensors may be an invaluable substitute for conventional anti-doping detection methods such as chromatography-based approaches, and may even be commercialized for routine anti-doping tests.
Keywords: Anti-doping, Drugs of abuse, Localized surface plasmon resonance, Noble metal nanoparticles, Optical biosensors
1. Introduction
The term “doping” refers to the use of any illicit substances and methods that have been prohibited by World Anti-Doping Agency (WADA), for enhancing athletic ability, training and performance [1]. Doping tests are needed for identification/quantification of hundreds of substances with a wide range of different chemical/biological properties.
The four most common classes of prohibited doping drugs include anabolic steroid, opioids (narcotics), stimulants and some peptide hormones [2–4]. Anabolic steroids, e.g., AKA, anabolic-androgenic steroids, AAS, as the largest class of the doping drugs have effects like increasing protein synthesis within skeletal muscles and overall strength [5–8]. Stimulants such as cocaine, amphetamine, modafinil, and ephedrine, as the second largest class, are drugs affecting the central nervous system to increase the feeling of excitement and diminishing tiredness [9]. Opioids such as morphine (MO), codeine, oxycodone, heroin, dextromethorphan, dextrorphan, noroxycodone, pentazocine, norpethidine, have effects on the μ- and κ-opioid receptors and decrease/inhibit pain by inhibiting release of neurotransmitters [4,10]. Peptide hormones like human growth hormone (GH), erythropoietin (EPO), insulin-like growth factors (IGF-1, etc.), work as performance-enhancing anabolic substances [11,12].
Such illicit drugs cause serious global issues with a great burden in healthcare such as damage to the health of athletes, and ethical issues like destroying the spirit of fair play lying at the bottom of sporting competition [13,14]. In this regard, due to the increasing consumption of illicit drugs, doping tests need to be able to identify and quantify hundreds of substances with a wide range of different chemical and biological properties. To this end, the development and improvement of analytical identification techniques in this area is a pressing concern.
The conventional technique for the determination of doping drugs was gas chromatography followed by mass spectrometry (GC–MS). Nowadays, due to technological improvements and the fact that the doping substances are often polar, the predominant technique has been altered to liquid chromatography–mass spectrometry (LC–MS) as well as high-resolution mass spectrometry (HRMS), as faster and sensitive technique [15–22]. It is notable that MS- and MS-based chromatographic techniques provide detailed structural information and enable qualitative as well as quantitative analysis at the trace level in a wide variety of sample types. However, beyond the appropriate selectivity, sensitivity and stability of GC–MS and LC–MS techniques, they are complex, costly, time consuming, and require sophisticated equipment, which usually limits them limited to the laboratory-level uses.
Considering the above-mentioned issues and an increasing need for routine, more user-friendly, and accessible point of care analyte detection techniques, in anti-doping researches, there have been many attempts to develop more advanced analytical instruments and sensors for the practical detection of the banned substances.
Biosensors owing to their high selectivity, reproducibility, stability, sensitivity and linearity of response have recently attracted a great deal of attention. Generally, a biosensor is a sensor that converts a chemical/biochemical event to a detectable signal (e.g., electrical, optical, and so forth), via a transduction process, within the specific interaction of a bio-recognition element with the target molecule. Biosensors can be classified based on the bio-recognition element (e.g., enzyme-based, antibody-based (immunosensors), DNA hybridization-based (genosensors), and apatamer-based (aptasensors)), or based on the transduction mechanism (e.g., optical, electrochemical, piezoelectric, and thermal) [15–19].
Recently, the use of metal nanoparticles (NPs) as the main building block of the plasmonic biosensors are becoming an appropriate identification approach. Noteworthy that plasmonic effect relies on the large enhancement of the local optical field because of the interaction of incident light with the free electrons in the conduction band of metal NPs. This induces the collective oscillation of the free electrons, known as localized surface plasmon resonance (SPR). Taken together, the mechanism of detection is based on the fact that any adsorbed molecule can cause a change in the local refractive index, SPR effect, and usually a red-shifting in the wavelength. Further, the resonance wavelength of NPs is tunable with changing their size, shape, and type. The wavelength shift is detectable using either absorption spectroscopy (if nano-particles are colloidal), or scattering spectroscopy (if nanoparticles are deposited on a substrate).
In this review, we mainly attempt to focus on the application of optical biosensors that are based on noble metal nanostructures in the anti-doping area and the detection of illicit drugs. The fundamental principles and properties of the optical biosensors, especially surface SPR, are presented. Different classifications of plasmonic biosensors are introduced as well as their operational and transduction mechanisms. Furthermore, an updated overview of anti-doping applications of various optical biosensors is taken into account. In addition, the advantages and disadvantages of the biosensors in comparison to other conventional methods such as chromatography-based approaches are discussed.
2. Optical features of plasmonic metal nanoparticles
2.1. Primary concept and fundamental principles of SPR
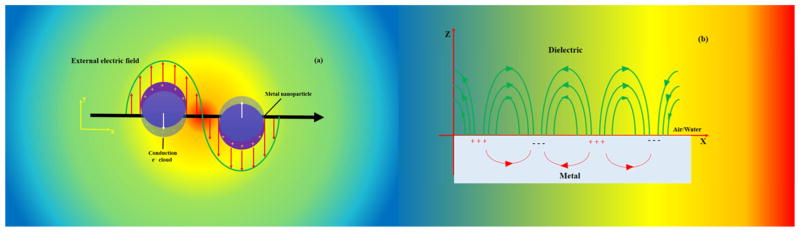
Plasmonic is a well-known subfield within nanophotonics and deals with the interaction between free electrons and electromagnetic fields in metallic materials. Consequently, free electrons throughout the metal have their collective oscillations as well as their excited levels affected by interaction with the electric field of incident light at the nanoscale level [20,21]. SPR is defined at the interface between a dielectric material and a metal (e.g. metal/air or metal/water) [20,22].
The SPR phenomenon applied to sensing applications can be classified into two main groups (as shown in Fig. 1: (a) propagating SPR (PSPR) and (b) localized SPR (LSPR) [23]. In PSPR the surface plasmon excitation occurs on surface of and propagates on noble metal thin films and along the metal/dielectric interface employing prism couplers or gratings [24]. In LSPR, the non-propagating surface plasmons are excited on the surface of the metal NPs [25].
Fig. 1.
Explanation of LSPR in metal nano particles as a) and b) PSPR on the surface of a metal nanoparticle.
LSPR and PSPR can also provide enhancement of fluorescence and SERS to detect biomolecules [26,27], and sense chemical species based on refractive index (RI) alterations [28].
2.2. The phenomenon of LSPR for noble metallic NPs
Theoretically, LSPR is conceivably possible for any semiconductor, metal or alloy, having a small imaginary or, a large negative dielectric constant. Both Ag and Au NPs have been by far the most often-employed metals [29–31]. By contrast, for other metals like copper, the LSPR is usually damped due to absorbing layers oxide on its surface, and to restore its SPR, the surface should be kept away from oxidation and completely cleaned [29]. Basically, the mean free pathway of an electron moving through a metallic material at room temperature (RT) is about 10–100 nm, thus, as the metallic particle shrinks to this dimension, different effects could be observed due to the rise of the quantum confinement effect with increasing surface-to-volume ratio characteristic of nanoscale materials [29–31]. Therefore, metal nanoparticles add plasmonics to their extra-ordinary properties (e.g. optical, mechanical, chemical, physical, etc.).
For example, besides their ability for sensing metal ions [32], AuNP plasmonic-based sensors can be used for determination of bio-molecules containing sulfur atoms that have a high affinity to gold, such as thiols [33], mercapto-containing biomolecules [34], prolactin hormone [35], carcinoembryonic antigen [36], glucose [37], and glutathione [38]. The invaluable properties of noble metals and their LSPR capacity make them promising for biosensor development based on near infrared (NIR), visible and even ultraviolet spectral regions.
Furthermore, in previous studies, the effect of NP’s properties, e.g., size, aspect ratio, and shape [39–42] on LSPR and its sensitivity have been indicated. Also, the optical properties of metallic NPs, especially LSPR, are tunable over the near infrared or visible part of the electromagnetic spectrum by varying NP’s shape and size, composition, or local dielectric medium as well as NP aggregation state [43,44].
2.3. Molecular sensing based on plasmonic phenomena
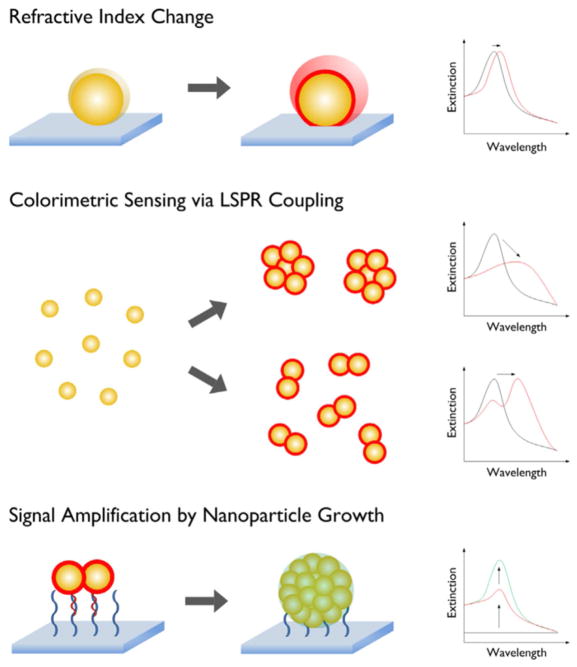
In SPR-based optical biosensors, desired bio-recognition elements are immobilized on a metallic sensor surface for the specific capture of analyte molecules contained in a liquid sample. The operating principles of a SPR biosensor is based on the fact that surface resonant oscillating plasmons (surface plasmon polaritons), induced by an electromagnetic field at the interface between a dielectric and a metal, form an evanescent electromagnetic field on the metal surface with deep penetration the in the range hundreds of nanometers, which changes the characteristics of the electromagnetic field (e.g., angle, wavelength, phase, etc.) through a refractive-index change of the surrounding medium. Thus, analyzing the characteristics of this electromagnetic field consequently leads to identifying the desired analyte molecules [45]. Chemical sensing using SPR dates back several decades and can be achieved by different approaches. The first SPR sensor was based on chemical modification of metal films using the PSPR concept and functioning as gas or pH sensors [46,47]. Another approach involves molecules in the surrounding medium binding to a functionalized film, thereby causing an observable shift in the incidence angle needed for SPR excitation in a variable coupling geometry [48]. In the same way, the LSPR excitation of metallic NPs is highly sensitive to the environment, because the resonance frequency is related to the changes in the local dielectric constant of the surrounding medium [49]. It is notable that the anisotropic property of non-spherical metallic NPs based on their plasmonic features makes them a favorable candidate for use in biosensors [50]. This strategy is useful for detecting molecules with different dielectric constants near the surface of NPs, because the responses of SPR are not chemically unique. This disadvantage can be overcome by using LSPR based on highly specific biological molecular recognition systems as exemplified by metal NPs conjugated to some systems e.g. biotin-streptavidin [27,51] or antibodies-antigens [52] which both have high selectivity for the target receptor-ligand binding. In order to overcome the disadvantages of chemical LSPR sensors, Kim et al. looked at methods for increasing the sensitivity of existing LSPR nanosensors [53]. Their suggested strategies (shown in Fig. 2) were divided into three different approaches: (1) detection of changes in the local dielectric medium (RI); (2), sensing via colorimetric (absorption) properties of noble metallic NPs with LSPR; and (3) employing the NPs growth method to amplify the detection sensitivity.
Fig. 2.
The representation of various strategies applied for signal enhancement and increasing the sensitivity of SPR nanosensors. Reprinted with permission from Ref. [53], Elsevier.
2.4. Fluorescence emission in biosensors
2.4.1. The principle of fluorescence emission
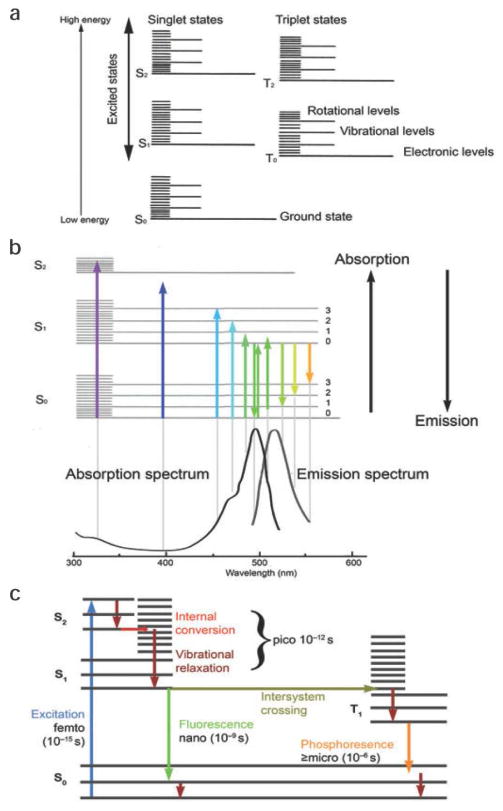
Luminescence is defined as the emission of light by any substance after returning from the electronically excited state. In fact, when the excitation has been induced by the absorption of light at particular wavelengths and the excited state is a singlet state, then the emission is called fluorescence. Initially, a higher electronic energy level in the S1 or S2 states is formed, then after a short time (picoseconds to a few nanoseconds) it relaxes to the lowest ground state by emitting a photon or by internal conversion to heat (vibrational energy). The Jablonski diagram illustrates transitions in the energy levels of a dye molecule after excitation by light. After internal conversion, fluorescence returns to the ground state in a time span about four orders of magnitude smaller compared to the lifetime of internal conversion (See Fig. 3).
Fig. 3.
(a) Jablonski diagram illustrating the energy states of a molecule during fluorescence excitation and emission. (b) The absorption and emission spectra for a fluorophore and their relation to the size of the energy steps required to transfer a molecule from one energy level to another. (c) The time durations for the various steps involved in fluorescence excitation and emission and phosphorescence. Reprinted with permission from Ref. [54], Nature Publishing Group.
A fluorophore is a molecule with the ability to absorb a specific wavelength of light photon and to reemit light at a longer wavelength [55]. The environment of a fluorophore affects the efficiency of the fluorescence emission. In other words, electromagnetic environment (EM) and both the excitation and the emission process are influenced by the type of employed dielectric interface in the proximity of the fluorophore. However, the internal conversion is insensitive to changes in the EM environment [56].
Nowadays, fluorescence plays a hugely important role in biology [56,57] and medical research [57,58], and is used for assays, measurements, and imaging on both microscopic and macroscopic scales. Therefore methods for improving its sensitivity have been widely explored.
2.4.2. The role of LSPR in enhancement of fluorescence near noble metal NPs
Using surface enhanced fluorescence (SEF) phenomenon [56], a surface can improve the sensitivity of fluorescence by applying modifications and controlling the local electromagnetic field. Near-field coupling between the fluorophore and the surface is the key phenomenon in this regard. Noble metal surfaces provide enhanced signal for fluorescence by resonance coupling with the surface plasmons. The energy transfer from the fluorophore to the surface plasmon modes usually results in SEF.
Thus, in SEF, the main point is the spatial localization of the light. The EM field can be affected and enhanced in the close vicinity of metal particles and surfaces, provided that the size of the metal particle is remarkably smaller than the wavelength of the emitted fluorescence. Under these circumstances, the EM field in close vicinity of the metal surface will assume an inhomogeneous structure. These inhomogeneities are one of the main reasons for enhancement of fluorescence.
Many localized surfaces and NPs have been studied revealing that the geometry and size of the NPs and even the gap between two adjacent NPs can affect the enhancement of fluorescence; of course, these effects are heavily dependent on wavelength.
As a whole, SEF effects can modify the fluorescence lifetime, fluorescence quantum yield, photobleaching, as well as amplifying the fluorescence emission [56,58]. Improving the electromagnetic environment of the fluorophore increases the detection efficiency in fluorescence assays, and this amplification is much more impressive in presence of surfaces with plasmonic properties [56].
2.4.3. Plasmon-enhanced fluorescence biosensors
As discussed above, the plasmonic property of NP surfaces can strongly confine the EM field, which causes a great improvement of the field intensity and consequently causes enhancement of quantum yield, increases excitation rate, and controls the far field angular distribution of the fluorescence emitted by organic dyes and quantum dots.
Plasmon-enhanced fluorescence (PEF) combines fluorescent labels together with enhancement due to the electromagnetic field generated on the surface of metal films due to the collective oscillation of surface plasmons. Taking advantage of this interaction can intensively enhance the intensity of the emitted fluorescent light, allowing the detection of minute amounts of analytes with lower LOD (i.e. higher detection ability) and shorter analysis time [55].
PEF is directly dependent on the strength of the field E generated in the vicinity of metallic surfaces, as well as the feature of SP-mediated fluorescence excitation. This enables the development of new surface plasmon enhanced fluorescence based biosensors with benefits such as ultra-high sensitivity (even at sub-femtomolar concentration), simplicity, and easy integration with SPR effect [55,58].
Hence, the fluorophores interaction with surface plasmons which leads to their amplified fluorescence signals can be employed as a label for sensing of various chemical and biological agents as is well-known for regular fluorescence assays (See Fig. 4). Particularly, in the last few years, applications of plasmon-enhanced fluorescence (PEF) have been widely developed due to the potential to be integrated with established immunoassays for sensing of various analytes [57]. PEF is based on amplifying the fluorescence signal by SPR at the excitation wavelength λab of the fluorophores. An optical set up with the angular interrogation of SPR and another module for detection of the emitted fluorescence signal is used.
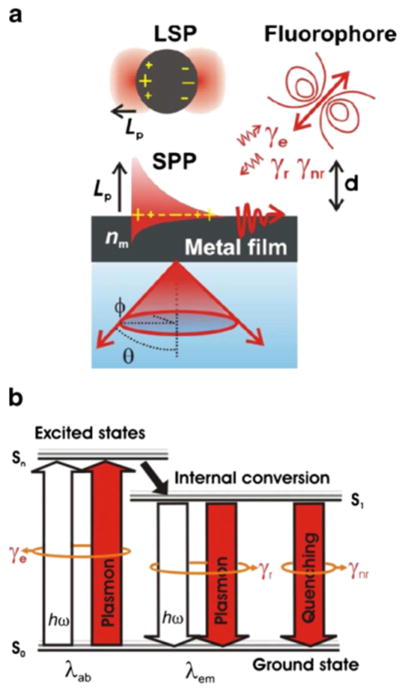
Fig. 4.
A schematic representation of (a) the confinement of the electromagnetic field intensity, due to the coupling of light with localized surface plasmons (LSPs) from metallic NPs and surface plasmon polaritons (SPPs) originated from bulk metal films and their interaction with fluorophores. (b) The interaction of fields with fluorophores at their absorption λ ab and emission λem wavelengths can alter respective transitions between the ground state and excited states. Reprinted with permission from Ref. [55], Springer.
Typically, analytes are allowed to flow over the SPR-active surface of the biosensor, which has biomolecules attached to it, and when the analyte is captured as a result of binding to the bio-molecules, the intensity of fluorescence emitted from the surface can be recorded and SPR can be assigned [57]. The amplification of fluorescence by labeling with NPs such as AuNPs has been investigated using surface plasmon-enhanced fluorescence (SPEF) spectroscopy [58]. It has been shown that PEF biosensors, can amplify the intensity of fluorescence up to 1000-fold (>103 times), so this technique can lower the limit of detection compared to other assay read-outs [57].
2.5. Plasmonic antibody-based immunosensors, and aptasensors
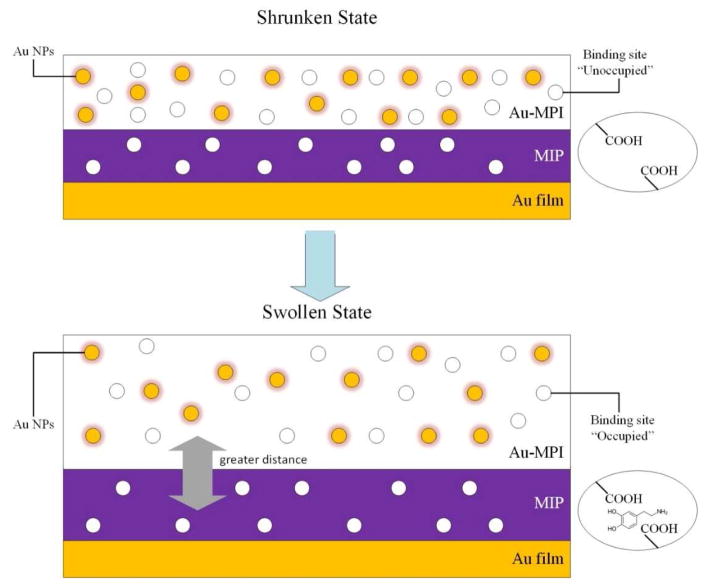
The use of optical transduction mechanisms (or combinations thereof) including FL, CL, ECL, SPR and SERS to detect the antibody-antigen binding reaction is a popular bioanalytical approach, offering a variety of advantages, such as utilizing a visible light source, nondestructive procedure, and a rapid signal transduction. Noble metal plasmonic have been used in immunoassays as labels, to increase the sensitivity either by attaching to proteins (enzyme, antibody, etc.) or by catalyzing the optical process [59]. The challenge of determination of small molecules with the SPR technique is due to relatively marginal changes in the dielectric constants of the medium occurring after the binding event. This drawback can be improved by employing a label-free approach, for instance, via using molecular imprinted polymers (MIP) with inlaid AuNPs, and non-sandwich labeling formats. In one study, an AuNP-embedded polymer layer (separated from an Au film by an AuNP-free MIP layer) acted as the detection layer, and as a matrix for signal enhancement. In the presence of a target molecule (i.e. analyte), its binding was shown to cause swelling and expansion of the AuNP-modified polymer layer, which changed the inter-particle distance of AuNPs (i.e. making a greater distance between the individual AuNPs); this resulted in the alteration of the dielectric constant and the electromagnetic coupling between the AuNPs in the film, and thus a change in LSPR signals. Thereafter, a coupling between LSPR of the AuNPs and surface plasmon polarization of the Au surface allowed a change in SPR signal and enhanced the SPR peak shift. Therefore, the target molecules present in the environment could be detected. Recently, this method has been reported to be able to detect dopamine (as the analyte) [60–62]. A schematic of the SPR sensor chip using the MIP embedded with AuNPs for analyte detection is shown in Fig. 5.
Fig. 5.
Schematic of SPR sensor chip using a MIP embedded with AuNPs for analyte detection.
The MIP offers the advantage of low detection limits, however, the leakage of the template, low binding capacity, and weak detection in solution are possible limitations of MIP [63].
While SPR provides a label-free detection approach, the use of labels is also being investigated to increase the sensitivity and small molecule detection. Despite this, very few reports can be found on the plasmonic-based multiplex detection of small molecules. Small molecules possess only a few functional groups in comparison to proteins, making this issue a crucial challenge in the design of cross-reactive biosensing devices [64]. The inhibition assay format is based on stabilizing a preformed analyte on the sensor surface, and a known concentration of analyte-specific antibody is pre-incubated with the analyte-containing assay sample. Next, the solution containing a mixture of analyte-antibody complex and remaining free antibody is added to the SPR-sensor surface, so that the free antibodies bind to the analyte conjugate immobilized on the sensor surface. As expected, the resulting signal is reversely proportional to the concentration of the target analyte in the sample [65].
The inhibition immunoassay has been mostly applied due to high sensitivity requirements for these substances, because of their often-small molecular size, and the presence of only trace levels of these substances in biological fluids. Recently, aptasensors based on RNA apatamer molecular recognition are becoming popular because of some advantages of aptamers over antibodies. However, plasmonic aptasensors have been less often developed for other groups of prohibited substances [66].
Aptamers are synthetic sequences of RNA, DNA or peptides exhibiting high affinity and specific binding to a wide range of target molecules. Despite having several advantages, in order to compete with well-established optical immunosensors, novel amplification strategies exploiting metallic nanostructures are being developed for aptamers. However, approaches to enhance their sensitivity are still required to make them suitable for analysis of real biological samples, because of the presence of possible interfering substances in complex biological samples and the likely low concentration of the analyte [66].
3. Anti-doping detection by plasmonic biosensors
3.1. SPR biosensors
In optical biosensing based on SPR, the electromagnetic field-induced surface plasmons (polaritons) are generated and employed in order to detect interactions that an analyte in solution has with another biomolecular recognition element immobilized on the sensor surface [67]. Due to their unique capabilities in characterizing and quantifying low molecular weight molecules, SPR biosensors have widespread applications in theranostics, pharmaceutics, food safety, environmental monitoring and homeland security [68,69].
SPR biosensors potentially can be used for real-time monitoring of illicit drugs and doping agents with the prominent feature of being label-free. A detectable shift in the resonance angle in sensorgram provides various information about the interaction rate, kinetic rate constants and quantification of analyte concentration, and also affinity constants [70].
Recently, the need for identification of peptide hormones in complex biological fluids has been challenging, because of their low-abundance and possible wide dynamic range of concentration that could theoretically span 10 to 12 orders of magnitude for abundant and rare proteins. Proteomic approaches i.e. a large-scale analysis and quantification of the proteome (the entire or a specific set of proteins) expressed by a specific genome, cell, tissue or organism, and their biological functions are cumbersome, resulting in assays that require several antibody incubations, that involve multiple measurements, suggesting that novel techniques for doping control, such as SPR that can measure interactions directly, could lead to substantial improvements [71].
Human growth hormone (hGH), is a protein-based hormone secreted by the pituitary gland, and several reports of abuse of recombinant hGH by athletes leading to performance-boosting effects have led to its being ranked on the prohibited substances list announced by the WADA agency. Several cases of abuse of this substance have been reported and two common analytical approaches are currently being applied for the determination of hGH in body fluids. One is based on immunoassay differentiation of the multiple isoforms of hGH (22-kDa, 20 kDa and 17.5 kDa forms), because natural hGH is present as a combination of isoforms in serum, and the proportion of these isoforms is constant in the absence of administration of exogenous recombinant human growth hormone (rhGH). Another approach is based on detecting hGH biomarkers, due to its influence on the expression of different proteins in the body [72]. Insulin-like growth factor-I (IGF-I) and procollagen type III peptide (P-III-P) have been reported to be prominent biomarkers of rhGH administration and their concentration in plasma can be measured to confirm possible abuse. These biomarkers have been measured employing various immunoassays, such as enzyme linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) and have so far yielded satisfactory results [73].
Juan-Franco et al. [74] reported an immunosensor for simultaneous detection of the hGH isoforms, 22K and 20K hGH, in serum samples by incorporating two different isoform-specific anti-hGH monoclonal antibodies, with detection based on SPR using a Kretschmann configuration. The results of the study showed good sensitivity and reproducibility with a 0.9 ng/mL detection limit for both isoforms, however the sensitivity of the system needed to be improved due to the extremely low concentration of the 20K isoform (below 0.05 ng/mL), observed after exogenous administration of hGH, which was below the detection range of the system. Several nanoplasmonic biosensors have been reported in recent years for determination of bovine growth hormone or bovine somatotropin (bST) in dairy products due to their implication for public health issues [75]. Some of these assays have been integrated into microfluidic chip platforms [76–78]. These miniaturized sensitive approaches could be implemented to develop highly efficient anti-doping LSPR immunosensors to allow doping laboratories to measure rhGH level in serum samples.
Determination of the peptide hormone, insulin requires highly sensitive analytical approaches because of its relatively low molecular weight (5800 Da). Since insulin produces only a small SPR signal, LSPR-based immunosensors have been explored for determination of insulin for diagnostic applications in diabetes [79]. Besides diabetes diagnostics, insulin has significance for sports medicine and doping control. Insulin is regarded as a prohibited doping substance because of its performance-enhancing effects. It can increase lean muscle mass by boosting glycogen stores in the muscles. Hiep et al. developed a microchip with an anti-insulin antibody immobilized on the surface for determination of insulin, exploiting the LSPR characteristics of AuNPs. The reported detection limit was 100 ng/mL and linear detection range of the immunosensor was 102–104 ng/mL [80].
Frasconi et al. developed an SPR immunosensor chip by encapsulating AuNPs in a fourth-generation polyamidoamine dendrimer (G4-PAMAM) and then immobilizing it on a modified gold sensor surface. The surface modification provided high selectivity toward insulin and minimized non-specific protein binding. Insulin was stabilized on the sensor surface and upon introducing a mixture of insulin antibody and antibody-bound complex, an SPR shift was observed due to binding of free antibody in a competitive immunoassay format. A detection limit of 0.5 × 10−3 nM was obtained, and a comparison with a reference radioimmunoassay demonstrated the high performance of the proposed system [81]. The results of this study were considered to be competitive with results obtained by a recently-developed electrochemical impedance spectroscopy (EIS)-based immunosensor without utilizing any nanoparticle amplification that had a detection limit of 1.2 × 10−3 nM [82].
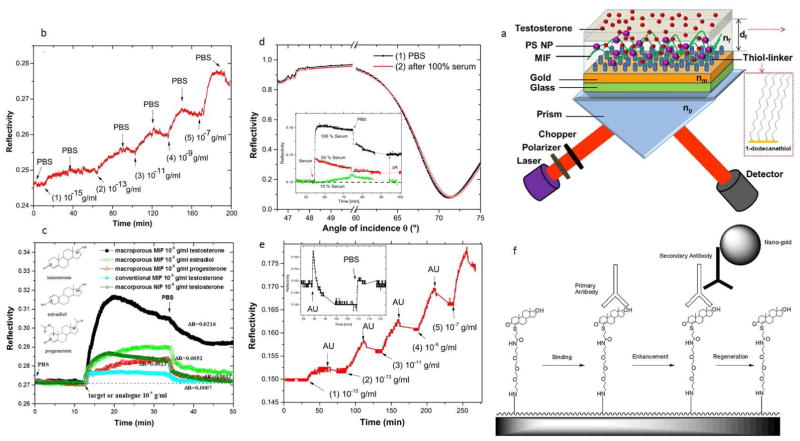
Analysis of testosterone levels (the male steroid sex hormone) is highly important, as it is not only a biomarker of some pathologic conditions such as prostate cancer, but is also frequently used as a doping agent due to its role in increasing muscle mass and boosting power when administered to athletes. A molecularly imprinted film (MIF) used testosterone templates to obtain a macroporous structure and combined with the SPR transduction pathway provided ultra-high sensitivity (femtomolar) without the disadvantages of poor thermal stability and a requirement for multiple incubation and washing steps. Fig. 6-a illustrates the schematic of such SPR sensor setup. Here, the (polystyrene) PS–MIF functionalized sensor chip was designed. In this sense, LOD of 10−15 g/ml for detection of testosterone was achieved, which was reported to be among the lowest LODs in SPR sensors for small molecule detection. Moreover, this SPR sensor using macroporous MIF indicated appropriate accessibility, selectivity and sensitivity for testosterone binding, enhanced stability and reproducibility, about 8 months (see Fig. 6-b and c). Also, the unspecific adsorption of serum and artificial urine on macroporous MIF was investigated to assess the potential of the analysis regarding testosterone detection. Hence, the macroporous MIF sensor chip revealed the possibility for testosterone detection in human urine and insignificant non-specific binding of artificial urine in the macroporous MIF, while unfeasibility for testosterone detection in 50 or 100% serum (see Fig. 6-d, and -e) [83,84]. Another interesting approach was proposed by Mitchell et al., in which the testosterone and specific linker conjugate were immobilized on the surface of the dextran chip, and a monoclonal primary antibody and AuNP-conjugated secondary antibody were used to detect the ligand in a competitive sandwich format. The enhancement due to plasmon coupling of AuNPs increased the sensitivity 12.5 fold compared with using a primary antibody alone, giving a lower detection limit of 3.7 × 10−3 ng/mL in the buffer and 1.54 × 10−2 ng/mL in male saliva. Fig. 6-f illustrates the Schematic of the binding process of a SPR-based immunosensor assay using nanogold labeling [85]. A very similar sensing scheme based on ligand immobilization on the sensor surface and AuNP-labeled secondary antibody signal enhancement were introduced for determination of progesterone with an SPR immunosensor [86].
Fig. 6.
(a) Schematic of such SPR sensor setup: a (polystyrene) PS–MIF functionalized sensor chip, (b) SPR reflectivity alterations (in terms of time) because of the affinity binding of testosterone (at concentrations equal to 10−15 g/ml (1), 10−13 g/ml (2), 10−11 g/ml (3), 10−9 g/ml (4), 10−7 g/ml (5) in PBS buffer. Between each binding cycle, a rinse process with pure PBS was conducted, (c) Reflectivity alterations (in terms of time) for the macroporous MIF regarding the injection of progesterone, estradiol and testosterone (at 10−6 g/ml concentration in PBS), and conventional MIF and macroporous non-imprinted film (NIF) incubated with testosterone (at 10−6 g/ml concentration), (d) angular reflectivity of the macroporous MIF before (1) and after (2) incubation with 100% serum (measured in PBS). The inset indicates the reflectivity signal regarding injecting 10, 50, and 100% serum (measured at light incident angle of 70°), (e) the kinetics of reflectivity regarding testosterone binding on the macroporous MIF at concentrations ranging between 10−15–10−7 g/ml in artificial urine. The inset indicates the reflectivity signal regarding injecting the artificial urine (measured at light incident angle equal to 70°). Reprinted with permission from Ref. [83], Elsevier, (f) Schematic illustrating the binding process of a SPR-based immunosensor assay using nanogold labeling. Reprinted with permission from Ref. [85], Elsevier.
Anabolic androgenic steroids, have historically been the most frequently detected illicit substances reported in doping analysis, and selective and sensitive new assays are particularly required due to the large numbers of different chemical compounds fall into to this category, and their structural similarity to naturally occurring endogenous steroids. The complexity of the usual sample type (urine), presence of steroids only at low levels in urine, and the sensitivity requirements defined by WADA, the minimum required performance level (MRPLs) of 2–10 ng/mL, means that precise determination of these compounds is critical, hence developing sensitive and high throughput assays that work with a small urine volume requirement, is desirable [87]. Several SPR biosensors have been introduced for determination of non-endogenous (exogenous or synthetic) anabolic steroids, however, immunosensors based on LSPR of metal NPs have been only rarely explored for this purpose. Kruezer et al. developed an LSPR sensor for detection of stanozolol, a synthetic derivative of dihydrotestosterone, which has been widely abused in sports competitions and horse racing. The bio-sensing scheme was based on immobilizing AuNPs to a glass surface via sulfhydryl groups and attaching anti-stanozolol antibodies onto the gold surface. A 20 nM detection limit was recorded for the biosensor and the group underlined some advantages of LSPR sensing over traditional SPR, such as a more practical optical configuration (eliminating the need for total internal reflection illumination providing simple integration of the system) short experimental time, and inexpensive instrumentation [88]. The same group reported a very similar LSPR biosensor with some modifications, such as optimization of the gold colloid density on the glass substrates, and optimization of the glass substrate through chemical silanization. The results of the study showed a 2.4 nM detection limit for stanozolol, and according to their report, the obtained LOD was lower than MRPL defined by IOC, determined by chromatographic methods [89].
A literature review regarding detection of prohibited hormonal substances employing SPR biosensors is represented in Table 1.
Table 1.
Various SPR biosensors for determination of some prohibited hormonal substances and the biological function of these substances.
| Analyte | Sensing scheme | Biological function | Biorecognition element | Range of detection (ng/mL) | Low detection limit (ng/mL) | MRPLp (ng/mL) | Ref. |
|---|---|---|---|---|---|---|---|
| Cortisol | Direct immunoassayc | Steroid hormoneh | antibody | 9–132m 3–125n |
3m 3n |
30 | [90] |
| hFSHa hLHb | Inhibition immunoassay formatd | Gonadotropins include (FSH), (LH), and chorionic gonadotropin (CG)i | antibody | hFSH 11–546 hLH 14–546 |
1 | 20 | [91] |
| Estradiol | Samples containing estradiole | anabolic agentj | MIP filml | 10−10–10−6o | 10−10o | 5 | [92] |
| hGH | The antigen immobilizedf | Steroid hormonek | hGH-27 antibody | 30–100 | 6 | 5 | [93] |
| 17β-estradiol | Steroid-BSA targetg | Aptamer | – | – | 5 | [94] |
Follicle stimulating hormone.
Luteinizing hormone.
Based on immobilizing specific antibody on the sensor chip, followed by adding the sample.
In which the antibody was added in a constant amount to the immobilized antigen.
Pumped through the modified sensor chip to allow estradiol to interact with the imprinted film.
The antigen was immobilized on sensor surface and the pre-incubated sample and antibody was introduced.
Steroid-BSA target was immobilized on a Biacore chip, the aptamer flowed through the chip and the binding between aptamer-target was monitored.
Glucocorticoids are prohibited according to WADA due to non-physiological boosting of sports performance.
Stimulate endogenous testosterone production. Can potentially be misused by athletes, for muscle building either in a direct way, or indirectly to normalize production of down-regulated testosterone after administration of anabolic steroids (Prohibited for males) [95].
Steroid and estrogen sex hormone, and the primary female sex hormone.
Prohibited in and out of competition because of contributing to anabolic effects, such as muscle mass increase and the reduction of body fat as well as its tissue-repairing effects (recovery).
Molecular imprinted film with specific recognition sites.
For urine sample.
For saliva sample.
Molar.
WADA general Minimum Required Performance Limit (MRPL) values.
Detection and quantification of opiates, such as MO and cocaine have also been studied by means of SPR-based immunosensor techniques. The SPR technique for detection of MO was first employed about two decades ago and could quantify MO in the ppb concentration range. The immunoassay was based on immobilizing MO-BSA and introducing a mixture of free and bound anti-MO monoclonal antibody [96], which is still a very common approach for detecting molecules with low molecular weight. In 2002, Dillon et al. developed an SPR immunosensor for determination of MO-3-glucuronide (M3G, the main metabolite of heroin and MO which is evidence of drug abuse) that used two polyclonal antibodies and in a competitive ELISA immunoassay format [97]. In another SPR study, a microarray gold chip was developed for imaging a group of illicit drugs, i.e. cocaine, ecstasy, heroin and amphetamine, which was based on indirect immunoassay and addition of antibodies to the surface-immobilized drug analogs [98].
Despite their therapeutic importance in medicinal applications, such as pain relieving agents, opioid narcotics probably have the most negative reputation among all classes of drugs of abuse. Opioid abuse goes beyond doping for sports competitions, having immense implication for law enforcement and forensic investigations. A few efforts have been made to develop efficient plasmonic biosensors for determination of these prominent substances. In one recent forensic study directed toward determination of cocaine, aptamer-bound Au nanoparticles (AuNPs) were used as dual imaging and recognition probes. Dark-field microscopy (DFM) images were used to record the LSPR of AuNPs that had been attached to latent finger-prints (LFPs) detected at crime scenes. Recognition of cocaine by the aptamer, caused the aggregation of the AuNPs providing a colorful image of the LFPs [99].
B2-Adrenergic agonists and their derivatives, such as mabuterol (MBL), salbutamol and clenbuterol have therapeutic applications for bronchial diseases in humans and animals, but since they can considerably enhance the muscle-to-fat ratio, several cases of abuse of these drugs have been reported in farm animals [100]. In a recent study, a highly sensitive SPR immunosensor was developed for β-agonists via an indirect competitive inhibition format, with very low detection limits of 0.01 ng/mL and 5 × 10−3 ng/mL for ractopamine and salbutamol, respectively [101]. Apart from abuse of these drugs in agriculture by addition to livestock feedstuffs, β-2 agonists with their proven ergogenic properties in exercise have been prohibited by WADA. However, due to legitimate therapeutic applications of these drugs, salbutamol and formoterol with maximum concentrations of 1600 and 54 μg, respectively over 24 h delivered by inhalation, and salmeterol administered according to the manufacturers’ guidelines, are not considered as cases of doping [102].
Fortunately, varieties of immunosensors have been developed in recent years for quantification of β2-agonists, taking advantage of the LSPR features of metal NPs. Although these studies have been mainly directed towards control in livestock, similar procedures can be adapted for detecting doping substances in human urine samples. Table 2 summarizes some plasmonic biosensors that have been fabricated for the determination of β2-agonists in different biological samples.
Table 2.
Some plasmonic immunosensors developed for veterinary control applications of various β2-agonists i.e. clenbuterol, salbutamol, zilpaterol and ractopamine, in animal feedstuffs, and biological samples.
| Sensing pathway | Transducer | LOD (ng/mL) | Linear range (ng/mL) | Analyte/sample | MRPLp (ng/mL) | Ref. |
|---|---|---|---|---|---|---|
| Direct immunoassaya | LSPR | 20 | 50–800 | MA/human urine | 500 | [103] |
| Direct immunoassayb | LSPR | 10 | 20–800 | Salbutamol/pig complex feed and pork liver | 20 | [104] |
| Antibody-based inhibitionc | SPR | 10k | – | Clenbuterol/bovine hair | 0.2 | [105] |
| Indirect competitive immunoassayd | SPR | 0.12 | 0.28–4.29 | Ractopamine/pork liver | 3 | [106] |
| Antibody-based inhibitione | SPR | – | 2–8 | Zilpaterol/sheep urine | 2 | [107] |
| Competitive inhibition formatf | SPR | – | 90–9325n 1.8–7515o |
Ractopamine/sheep & Cattle urine | 3 | [108] |
| Competitive inhibition formatg | SPR | 0.27 | – | Clenbuterol/bovine urine | 0.2 | [109] |
| Indirect competitive inhibition immunoassayh | SPR | 3 × 10−3 | – | Clenbuterol/meat from food producing animals | 0.2 | [110] |
| Two different inhibition assays based on either immobilization of anti-clenbuterol or clenbuterol-BSAi | SPR | 6.7 × 103l 4.5 × 103m |
6.25 × 103–105 | Clenbuterol | 0.2 | [111] |
| Competitive inhibition formatj | SPR | 6.32 × 103 | 6.25 × 103–5 × 104 | Clenbuterol | 0.2 | [112] |
Antibody specific for salbutamol was immobilized on hollow gold nanoparticles (HGNs), which were deposited on transparent indium tin oxide (ITO) film.
Salbutamol specific antibodies were immobilized on triangular silver nanoparticles deposited on transparent ITO film on glass.
CM5 chip surface was coated with clenbuterol and first antibody and second antibody were sequentially applied.
Ractopamine–ovalbumin (RCT–OVA) conjugate was immobilized onto an Au-thiolate sensor chip and the antigen (ractopamine) was detected upon adding the antibody.
CM-5 sensor chip was used to immobilize zilpaterol conjugate and the antibody was added.
The antibody is bound to the ractopamine derivative immobilized on the sensor chip.
Clenbuterol antibody was added to capture the drug stabilized on the sensor chip surface.
Clenbuterol was immobilized on the surface treated with succinimidyl-modified propanethiol monolayer on a gold chip.
1) immobilization of anti-clenbuterol antibody on the sensor chip as a probe (the target site compensation method) 2) immobilization of clenbuterol-BSA conjugate on the sensor chip with a competition assay.
Sensing surface modified with 3D bioprobe consisting of BSA-CLEN conjugate.
ng/g hair.
For target site compensation method.
For the solution competition method.
For sheep urine.
For cattle urine.
WADA general Minimum Required Performance Limit (MRPL) values.
As can be concluded from Table 2, the competitive indirect inhibition immunoassay offers lower detection limits, which can be enhanced using the LSPR features of noble metal NPs as immobilization platforms achieving even higher sensitivities.
Stimulants are another category of drugs that have been widely abused by athletes in sports competitions. Methamphetamine (a central nervous system stimulant) is the most widely abused drug in the amphetamine class. This drug and its derivatives are regarded as illicit substances; hence, their determination is of great importance in doping control tests. Immunoassays have been widely utilized for determination of amphetamines in urine samples, followed by GC-MS confirmation when positive test results have been obtained by immunoassay. However, GC-MS analyses are not convenient for use in routine control [113]. In this regard, over the last two decades, several studies have been conducted to develop optical immunoassay platforms, such as SPR immunosensors to substitute for the accurate but costly and time-consuming analysis using GC-MS [103,113,114].
3.2. Luminescence and electrochemiluminescence nanoparticle-based biosensors
CL involves the emission of light produced by an electronically excited intermediate or product of a chemical reaction [115].
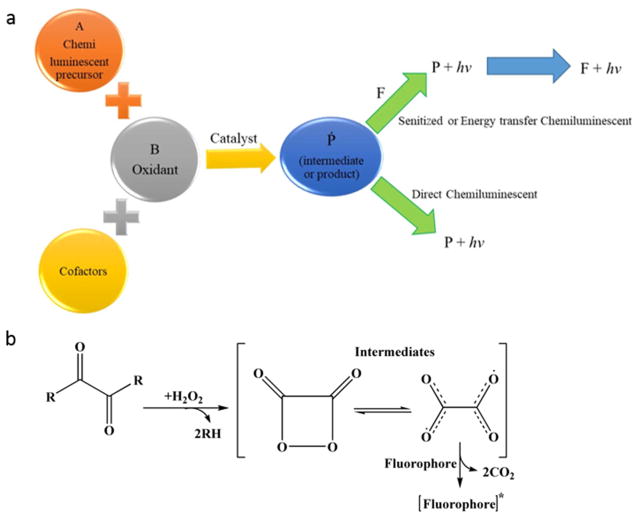
Basically, all CL reactions can be categorized into two main mechanisms, i.e. direct and indirect reactions. In both direct and indirect reactions, two precursors, one acting as an oxidant and another acting as a substrate, in the presence of cofactors and occasionally a catalyst, react to form an intermediate or product. Moreover, a fraction of the intermediate or product is then transformed into an electronically excited state and returns to the ground state via emission of a photon. It is notable that the catalyst, e.g., metal ions or enzymes, moderates the activation energy and provides an adequate environment for generating significant CL efficacy (Fig. 7-a) [116].
Fig. 7.
Schematic illustrating a) various types of CL reaction (F stands for fluorescing agent and P for product), and b) a simplified mechanism showing peroxyoxalate-CL reactions, applicable for nanobiosensing.
ECL, however, is a method in which the advantages of electro-chemistry and chemiluminescence are combined so as to increase the width of the linearity response range, providing higher selectivity and sensitivity while retaining simplicity [117]. ECL describes light emission produced from a chemical reaction occurring during electrolysis with at least one product generated on an electrode where reactions can be tuned by altering the applied potential [115,118].
Recently, various chemiluminescence reactions based on different substrates (e.g. Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), tris(2,2-bipyridine) ruthenium(II)) and oxidants (e.g., acidic KMnO4, Mn(IV)) or oxidation of sulfite as well as peroxyoxalate CL systems have been employed for analysis and detection of both illicit along with therapeutics. For example, a schematic of peroxyoxalate CL reaction, which includes several oxamide and oxalate compounds with H2O2 in the presence of fluorescent composites, is illustrated in Fig. 7-b [119].
Various methods have been employed to enhance the signal amplification in immunosensors using CL and ECL assays, one of which has taken advantage of nanomaterials. Saydack et al. [120] reviewed efforts employing nanoparticles to improve the performance of optical immunosensors.
Optical immunosensors including electrochemiluminescence and chemiluminescence transduction pathways equipped with nanomaterials for the detection of doping substances have attracted attentions recently.
Nanoparticle-based systems can play a critical role in the detection of illicit drugs because of advantages such as easy operation, sensitivity, selectivity, low cost, ability to be miniaturized and automated [119]. The use of noble metal NPs to augment the efficiency of ECL and CL sensors for various prohibited drugs have been studied recently. In addition, competitive ECL immunosensors based on quantum dots and noble metal NPs have been developed.
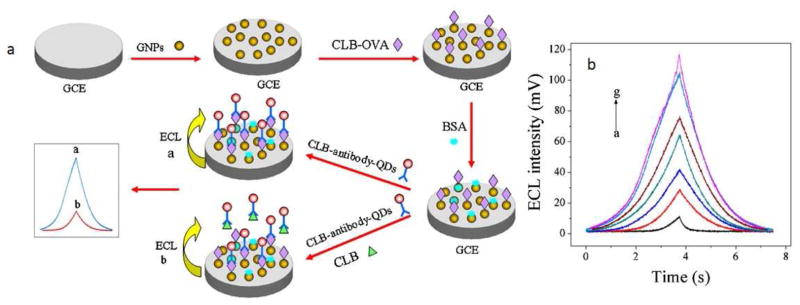
Yan et al. [121] reported an ultrasensitive ECL detection system composed of AuNPs as a substrate and CdSe quantum dots as an ECL probe to measure clenbuterol (Fig. 8-a). This nanobiosensor had a limit of detection of 8.4 × 103 ng/mL and the AuNPs played an important role in accelerating electron transport between the surface of the electrode and the quantum dot, which led to a significant improvement in sensitivity. Fig. 8-b shows the ECL curves for detection of clenbuterol at concentrations of 1000, 50, 10, 1, 0.5, 0.02, and 0 ng/ml. This nanoparticle-aided label-free detection of clenbuterol was able to show much higher sensitivity as compared to other methods reported in Table 3. A possible mechanism for ECL immunosensing based on CdSe QDs with an AuNP substrate for the determination of clenbuterol could be explained as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Fig. 8.
(a) Schematic demonstrating the designing and the detection mechanisms of the ECL-based immunosensor, (b) ECL curves regarding detection of clenbuterol at concentrations equal to a. 1000, b. 50, c. 10, d. 1, e. 0.5, f. 0.02, and g. 0 ng/ml, in pH = 7.4 PBS buffer encompassing 0.1 M K2S2O8 and 0.1 M KCl. Reprinted with permission from Ref. [121], Elsevier.
Table 3.
NP-based clenbuterol detection systems.
| Immunoassay type | Immunoassay based | Biosensing element | Analyte | LOD (ng/mL) | MRPc (ng/mL) | Ref. |
|---|---|---|---|---|---|---|
| ECL | Au NPs & CdSe QDs | Polyclonal antibody | Clenbuterol | 8.4 | 0.2 | [121] |
| ECL | CdSe QDs | Polyclonal antibody | Clenbuterol | 20 | 0.2 | [122] |
| CL | MIP | – | Clenbuterol | 30 | 0.2 | [123] |
| CEa | – | – | 0.7 | [124] | ||
| Electrochemical | r-GO&Ag-Pd alloy NPsb | Monoclonal antibody | Clenbuterol | 1.38 × 103 | 0.2 | [125] |
| CL enzyme Immunoassay | – | Polyclonal antibody | Clenbuterol | 0.17 | 0.2 | [126] |
Capillary Electrophoresis.
Reduced graphene oxide and silver–palladium alloy nanoparticles.
WADA general Minimum Required Performance Limit (MRPL) values.
In studies reported by Yifeng’s group [127,128], an indium tin oxide-coated glass substrate was used for determination of meth-amphetamine and MO. AuNPs mediated direct binding of antibodies to model drugs resulting in ultrahigh sensitivity with detection limits of 0.3 ng/mL and 0.82 ng/mL for methamphet-amine and MO, respectively. For comparison, ECL biosensors modified with silicate films [129] exhibited a lower sensitivity of 30 nM for MO, thus demonstrating the enhancing effect of NPs [128]. The authors attributed the high amplification obtained to optical field enhancement generated by AuNPs. Table 4 shows other recent reports of monitoring prohibited drugs in sports using AuNPs to enhance sensitivity in ECL and CL immunosensors. It is noteworthy that different results achieved by CL and ECL methods can be compared with other analysis approaches; therefore, the following Table 4 also aims to illustrate a comparison between ECL and CL using noble metal nanoparticles with some of other detection methods. According to the data shown in the table, it is not clear that CL/ECL biosensing approaches are demonstrably superior compared to conventional analytical techniques, and the LODs of both types of techniques are mostly in a similar order of magnitude. Despite this, for some specific compounds in the table, such as insulin, growth hormone and MO, SPR technique LODs have been shown to have smaller values, indicating a better performance of CL/ECL biosensing approaches for the detection of such illicit drugs. In addition, for the aforementioned substances, the LODs meet the standard requirements, i.e. MRPL, set by WADA, even better than chromatography and mass spectroscopy-based techniques.
Table 4.
ECL and CL noble metal NPs-aided sensing compared to other analytical techniques such as chromatographic and mass spectroscopy-based approaches in anti-doping applications.
| Drug | Method | LOD (ng/mL) | Linear range (ng/mL) | MRPLi (ng/mL) | Ref. |
|---|---|---|---|---|---|
| Trimetazidine | CL combined with flow injection | 6.7 | 10–5000 | – | [130] |
| GC-MSa | 15–50 | – | – | [131] | |
| LC-MSb | 0.5 | – | – | [131] | |
| Protein kinase A | ECL | 0.09h | 0.1–10h | 20 | [132] |
| Electrochemical | 0.014h | 0.05–100h | [133] | ||
| ECL aided by MOFsc | 0.005h | 0.01–20h | [134] | ||
| Insulin | ECL | 3.5*10−5 | 5.8*10−5–2.9 | 0.05 | [135] |
| LC-IMMSd | 0.2 | – | [136] | ||
| Growth hormone | CL | 0.036 | 0.2–50 | 1–2 | [137] |
| MS-IAe | 1.5 | – | [138] | ||
| MO | ECL | 0.067 | 0.2–180 | 50 | [117] |
| TLCf | 300 | – | [139] | ||
| HMSg | 3 | – | [140] |
Gas chromatography-mass spectrometry.
Liquid chromatography–tandem mass spectrometry.
Metal organic frameworks.
Liquid chromatography-ion mobility mass spectrometry.
Mass Spectrometric Immunoassay.
Thin layer chromatography.
High resolution mass spectrometric.
Units per milliliter.
WADA general Minimum Required Performance Limit (MRPL) values.
To our knowledge, only AuNPs among other types of noble metal nanomaterials has been widely employed in the luminescence-based analysis of doping drugs. However, a recent work [141] has investigated different noble metal nanoparticles including Pt for optical CL detection of gonadotropin. It was stated that this nano-biosensor was able to provide a 103 fold increase in sensitivity compared to the conventional rapid analysis without Pt NPs.
3.3. Plasmon-enhanced fluorescence biosensors
Recently, several fluorescence-based biosensors have been developed for detection of doping drugs, such as cocaine, stanozolol, insulin and various kinds of platelet-derived growth factor using fluorophore and quencher pairs incorporated in the biosensor design. This technique is based on the “signal-on” mode, where a fluorophore is displaced from its quencher as a consequence of aptamer or antibody binding to the analyte [142]. The detection of insulin using AgNPs based SPEF immunoassay has been developed based on a competitive assay format. In this study, the surfaces of silver colloids were used as platform to immobilize the anti-insulin-antibodies giving a detection limit of 250 nM for F-insulin. When a fluorophore labeled antigen such as insulin binds to its cognate antibody, the fluorescence on the colloid surface increases sufficiently to allow real time monitoring of the binding. In this system, FITC-labeled insulin, murine monoclonal anti-insulin-antibodies and F(ab)2 anti-mouse Immunoglobulin G (IgG), were used to link the anti-insulin-antibody to the surface of silver colloid. The working detection range of insulin in serum was 10–250 nM [143].
The “signal-on” aptamer-based biosensors involves different strategies, as an example, using a label-free aptamer, which can displace fluorophores that were either deliberately quenched or, had only low fluorescence, when aptamer bound to its target [142].
A study designed oligodeoxynucleotide (ODN) heterodimeric aptamers for cocaine detection using fluorescence. In this detection strategy, one subunit was labeled with 5′-6-carboxy-fluorescein and the other with a 3′-dabcyl quencher. Dabcyl is characterized by an efficient π-orbital overlap with fluorescein when the ODN strands are hybridized, while this quenching almost disappears in the non-hybridized state due to cocaine binding. These two subunits acted as a self-assembled fluorescent biosensor for detection of cocaine with a limit of detection of 103 nM under optimum conditions [144].
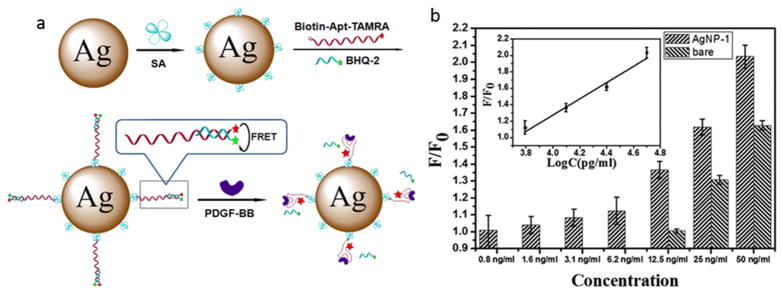
Platelet-derived growth factor (PDGF) is another example of a banned substance in sports that can be detected using an LSPR technique. In chemical terms, PDGF is a dimeric glycoprotein consisting of two A (-AA) or two B (-BB) polypeptide chains, or a combination of the two (-AB) [145]. LSPR based on AuNPs or AgNPs combined with different aptamers can be used for detection of various isoforms of PDGF. Different aptamers can be designed for detection of different PDGF isoforms. For example, a recent report described metal NP-based fluorescence biosensor using fluorophore-functionalized aptamers containing the Black Hole Quencher-2 (BHQ-2) for the detection of PDGF-BB. The fluorophore-quencher pair aptamer was coupled to AgNPs (Fig. 9-a). After introducing PDGF-BB, the BHQ-2 was displaced resulting in the disruption of quenching fluorescence resonance energy transfer (FRET) effect and a sharp increase in fluorescence intensity. According to the results, AgNP based FRET sensing provided enhanced target specificity, and a better increase in fluorescence intensity compared with bare FRET sensing (Fig. 9-b) and AuNP-based sensing, leading to a detection limit of 0.8 ng/mL for PDGF-BB (a 16-fold increase in the sensitivity was observed compared to the bare FRET sensor) [142].
Fig. 9.
a) Fluorophore-functionalized aptamer coupled to AgNPs and containing BHQ-2, used for the bio-sensing of PDGF-BB, as a banned substance in sports, b) F/F0 (the ratio of fluorescence intensity of PDGF-BB to fluorescence intensity of BSA) of the FRET sensing nanoplatform in presence and absence of AgNP-1; the inset indicates the linearity of F/F0 and concentration when the concentration of PDGF-BB is between 6.2 and 50 ng/mL. Reprinted with permission from Ref. [142], Copyright 2013 the American Chemical Society.
In another study, an aptamer attached to AuNPs was tested for detection of PDGF-AA, with a detection limit of 8 × 10−3 nM [146]. With the advantages of simplicity and specificity, this approach holds great potential for protein analysis and cancer diagnosis, but pre-concentration of the target proteins (such as PDGF) from biological samples such as blood by using ion-exchange chromatography or ligand-coupled magnetic microbeads may be required [146].
3.4. Surface enhanced Raman spectroscopy (SERS)
One of the promising features of SERS technique is its ability to identify a variety of drugs including illicit drugs and doping agents. In the following, some investigations into the application of the SERS technique for detection of illicit drugs are summarized in Table 5. Cocaine, methamphetamine (MA), known as “ice”), 3,4-methylenedioxy methamphetamine (MDMA, known as “ecstasy”) are the most commonly abused psychoactive drugs. In a comprehensive study, 80 illicit drugs and metabolites were satisfactorily characterized using Au-NP and Ag-NP doped sol–gels as SERS substrates [147]. Some drugs that have been assayed using SERS and their corresponding LOD are listed in Table 5.
Table 5.
Detection of illicit drugs in body fluids by SERS technique.
| Type of analyte | Category | Type of substrate | LOD (ng/mL) | MRPLd (ng/mL) | Ref. |
|---|---|---|---|---|---|
| APa | Stimulant | Au-doped sol-gel | 1000 | 500 | [147] |
| Diazepam | Depressant | Au-doped sol-gel | 1000 | [147] | |
| Methadone | Narcotics | Au-doped sol-gel | 1000 | 50 | [147] |
| PCPb | Hallucinogen | Au-doped sol-gel | 1000 | [147] | |
| Cocaine | Stimulant | Au and Ag sol-gels | 50 | 100 | [148] |
| MAc | Stimulant | Ag NPs in suspension | 1.49 | 500 | [149] |
| Benzoylecgonine | Stimulant | Fe3O4 dotted with Au | 29 | 500 | [150] |
| Cotinine | Stimulant | Fe3O4 dotted with Au | 8.8 | 100 | [150] |
| MA/APd | Stimulant | Ag nanorods | 50 | 500 | [151] |
| MDMA | Stimulant | Au nanorods | 100 | 500 | [152] |
Amphetamine.
1(1phenylcyclohexyl)piperidine.
Methamphetamine.
WADA general Minimum Required Performance Limit (MRPL) values.
The U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) has published adjusted cut-off limits for drug control purposes determined in saliva in the range of 10–50 ng/mL [147]. Clearly, these regulatory requirements can be determined by the SERS technique in most cases, especially in saliva samples. Due to the importance of the type and shape of the noble metal substrate in the SERS effect, several different types of nanostructures comprising Au and AgNPs deposited on a glass capillary, colloidal Au and AgNPs, and also Au nanorods have been tested.
Finally, a few researchers have investigated the effectiveness of SERS technique for identification of drugs that are abused in sports doping (Table 6). β2-adrenergic agonist (βAA) type drugs including clenbuterol (CB), salbutamol (SB), and terbutaline (TB), as described above, cause a βAA effect, and despite having perfectly legal uses, are often illegally used by athletes to increase their performance. For this reason, WADA has regulated (SB, TB) and even prohibited (CB) consumption by athletes to control abuse of these drugs. Lorenzo et al. investigated the adsorption of βAA drugs (CB, SB and TB) onto noble metal NPs with the aim of testing whether SERS could act as an alternative antidoping assay method. Their results indicated that the AuNPs (mean diameter 15 nm) and an acidic pH were optimal conditions for accurate and effective detection of these drugs [153]. It is worth noting that their obtained LOD (details are given in Table 3) were significantly lower than other values reported in the literature using capillary electrophoresis (CE) coupled with UV–visible detection (LOD = 0.5 × 106–2 × 106 ng/L) [154], and comparable to use of ion chromatography (IC) with direct conductivity detection (CD) (LOD = 2 ng/mL, 10 ng/mL for SB and CB, respectively) [155], and high-speed gas chromatography (HSGC) (LOD = 1.5 ng/mL, 20 ng/mL for CB and SB, respectively) [156].
Table 6.
Detection of some banned doping agents in sports by SERS assay using noble metal NPs.
| Type of analyte | Category | Type of substrate | LOD (ng/mL) | MRPLi (ng/mL) | Ref. |
|---|---|---|---|---|---|
| CB | Beta-2 agonist | Au colloid | 35 | 0.2 | [153] |
| SB | Beta-2 agonist | Au colloid | 765 | 20 | [153] |
| TB | Beta-2 agonist | Au colloid | 55 | 20 | [153] |
| PB | Diuretic and masking agent | Ag NPs | 1.2 × 103 | 200 | [157] |
| AGI | Hormone and metabolic modulator | Au NPs | 85 | 20 | [159] |
| AGI | Hormone and metabolic modulator | Au NPsc | 85 | 20 | [162] |
| Ag NPsd | 5.1 | ||||
| Ag NPse | 0.13 | ||||
| 17 β-Estradiol (E2) | Anabolic agent | Au NPsf | 0.65 × 10−3 | 2 | [164] |
| rHuEPOa | Peptide hormone, growth factor, | Magnetic core Au NPs (Au@ iron oxide core shell) | 10−3h | 2 | [165] |
| hCGb | Glycoprotein hormone | Ag NPs | 0.05–20 | 0.05 | [166] |
| Propranolol | Beta-blocker | Ag NPs | 2.36 | 100 | [167] |
| PB | Diuretic and masking agent | Ag NPsg | 51.3 | 200 | [163] |
Recombinant human erythropoietin.
Human chorionic gonadotropin (banned in men only).
Spherical shape with 20 nm in diameter.
Spherical shape with 50 nm in diameter.
Triangular shape.
36.7 ± 5.5 nm.
Star-shaped.
Nano molar (nM).
WADA general Minimum Required Performance Limit (MRPL) values.
Probenecid (p-dipropylsulphamyl) benzoic acid (PB) was assayed for the first time by Lorenzo et al. using SERS-active AgNPs (average diameter 60 nm). They reported the LOD of PB determined by SERS as low as 1.2 × 103 ng/mL [157] which was in the range of the published value using GC-MS (50 ng/mL) [158]. This sensitivity was lower compared to the one resulted with the fast-GC and fast-GC/MS techniques (10 ng/mL) [156]. In spite of their reported relatively high LOD, they claimed their method could open new horizons to find more efficient SERS-based methods for PB detection.
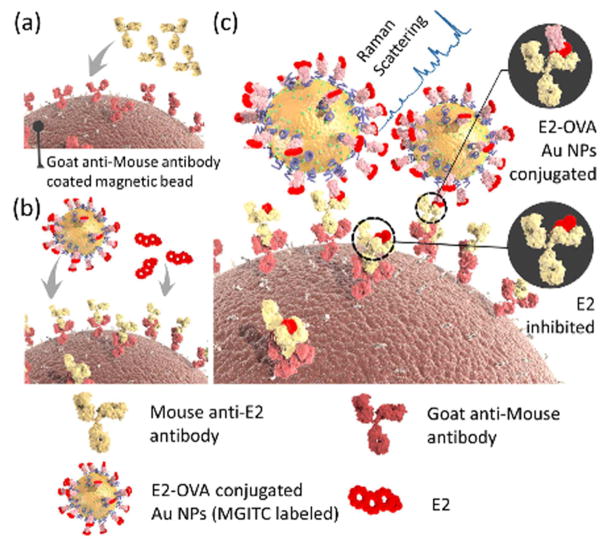
Aminoglutethimide (AGI) as an aromatase inhibitor, which is therapeutically used to block estrogen production for treatment of advanced breast cancer and Cushing’s syndrome. However, some bodybuilders and other athletes have illegally used it to increase testosterone concentration and to suppress the circulating levels of cortisol in the body and prevent muscle loss. Lorenzo et al. utilized SERS for detection of AGI through its adsorption on AuNPs at different pH and analyte concentrations [159]. They found optimal pH value = 5.0 with corresponding LOD = 85 ng/mL, which were lower compared to other common techniques such as GC/MS (100 ng/mL) [160], and LC/MS/MS analysis (100 ng/mL) [161]. In a recent study, the effects of different AgNP and AuNP morphology including spherical (average diameter of 20 and 50 nm) and triangular (36 and 6 nm length and width) on adsorption and detection of AGI was investigated. Their results showed the higher efficiency of triangular AgNPs for AGI detection and the lowest ever reported LOD value for this drug was given as 0.13 ng/mL [162]. Novel star-shaped AgNPs were used as plasmonic substrates giving very sharp and intense SERS spectra for detection of probenecid (PB), with a LOD of 51.3 ng/mL [163]. Table 7 summarizes different classes of performance-enhancing drugs that have been characterized by means of SERS biosensors. Fig. 10 shows the schematic of a competitive sandwich SERS immunoassay for the detection of 17 β-Estradiol (E2) using (primary and secondary) antibodies immobilized-magnetic beads and AuNP-based SERS nano-tags [164].
Table 7.
Colorimetric biosensors developed for detecting illicit drugs.
| Type of analyte | Type of substrate | LOD (nM) | MRPLc (ng/mL) | Ref. |
|---|---|---|---|---|
| CBa | Ag NPs | 2.8 × 10−2 | 0.2 | [173] |
| CB | Au NPs | 50 | 0.2 | [172] |
| CB and Ractopamine | Ag NPs | 5.7 × 104a and 6.1 × 105b | 0.2 & 3 | [153] |
| PBc | Ag NCs | 4.205 × 103 | 250 | [157] |
| Cocaine | Au NPs | 1 | 100 | [177] |
| MA & MDMA | Au NPs | Low micromolar range | 500 | [178] |
WADA general Minimum Required Performance Limit (MRPL) values.
For CB.
For Ractopamine.
Probenecid.
Fig. 10.
Schematic showing the steps involved in competitive sandwich SERS immunoassay for the detection of E2. (a) goat anti-mouse antibodies (secondary antibodies) are immobilized on the surfaces of magnetic beads. (b), mouse anti-E2 monoclonal antibodies (primary antibodies) attach to the secondary antibodies via an antibody-antibody interaction. (c) The competitive interaction of the free target E2 and E2-OVA-conjugated SERS nano-tags mixed with magnetic beads. Raman signals are recorded after the formation of immunocomplexes, which can be assessed for E2 quantification. Reprinted with permission from Ref. [164], the American Chemical Society.
3.5. Colorimetric biosensor
In this section, we focus on the application of colorimetric noble metal NPs based assays for the detection of doping substances. The aggregation-dependent color change seen in noble metal NPs have been increasingly applied in solution-based sensors for detection of a large variety of targets such as biological toxins [168], nucleic acids, proteins, small molecules and metal ions [169]. Colorimetric assays have received considerable attention due to the excellent LSPR enhancement provided by Ag and Au NPs including deep colors and easy visualization of color changes, selectivity, sensitivity, as well as simplicity and low cost [170].
Zhang et al. reported a colorimetric assay to detect β-agonists, such as clenbuterol (CLB), ractopamine and isoproterenol [171]. As a result of the exposure of soluble HAuCl4 salt to β-agonists, reduction of Au(III) occurs and elemental gold with a red visible color was formed. However, this method had low specificity and selectivity compared to the methods that were developed later on [172]. Another study reported the detection of CLB in human urine samples, using a colorimetric assay involving AuNPs [173]. In this method, melamine was employed as a cross-linking agent between the AuNPs surface and CLB, thorough amine group hydrogen-bonding. The binding led to AuNP aggregation and changed the color of the sample from wine-red to blue in response to CLB concentration, which was visible by the naked eye. Moreover, the results were confirmed by UV–vis spectrometry indicating that absorption ratio (A670/A520) was correlated with the CLB concentration. There was no change in the performance of assay in presence of potentially interfering substances such as DL-epinephrine, phenylalanine, tryptophan, alanine, uric acid, glycine, glycerol, glucose, MgCl2, CaCl2 and NaCl and the limit of detection was reported to be 2.8 × 10−2 nM. A one-step alternative for the detection of CLB in real blood samples was provided by cysteamine-modified AuNPs, developed by Kang et al. [172]. In the presence of CLB, aggregation of AuNPs occurred due to hydrogen bond formation between –NH2 groups of cysteamine and the –OH, –Cl and –NH2 groups of clenbuterol, which changed the color of the sample from red to blue (LOD 50 nM). Potentially interfering substances such as alanine, phenylalanine, glycerol, vitamin C, threonine, urea, exogenous cysteamine, glucose, glycine, NaCl, and CaCl2 did not make any difference to the results. Recently, Luo et al. [174] demonstrated a highly sensitive technique for direct visual detection of CLP and ractopamine in pig urine samples using AuNPs without employing any bio-recognition agent. The electrostatic interaction between AuNPs and targets (CLP or ractopamine) caused aggregation of AuNPs and produced a blue color. The sensitivity of the technique could be improved by adding an optimized amount of NaHSO4, which caused destabilization of the AuNPs. This technique was able to detect CLB in the range of 0.1 × 103 to 4 × 103 ng/mL with a LOD of 15.8 ng/mL, and ractopamine in the range of 1 × 103 to 9 × 103 ng/mL with a LOD of 22.9 ng/mL. Zhao et al. [175] reported an ultrasensitive method to detect methamphetamine (MA) exploiting Au nanoclusters (AuNCs) which demonstrated an intrinsic non-enzymatic peroxidase activity comparable to that of horseradish peroxidase (HRP) [176]. This method was based on the sandwich immunoassay principle. An antibody was anchored to the solid phase and another antibody was attached to the AuNCs which in presence of analyte formed the sandwich structure. Next, they immersed the complex in a solution of HAuCl4 and H2O2, which prevented any reduction of HAuCl4. However, in the presence of analyte, the AuNCs present in the sandwich exerted their peroxidase mimetic activity allowing the H2O2 to oxidize the peroxidase substrate 3,3,5,5-tetramethylbenzidine producing a blue color. The LOD of this method was 2.3 × 10−12 ng/mL and it has the potential for detection of other drugs by changing the antibodies.
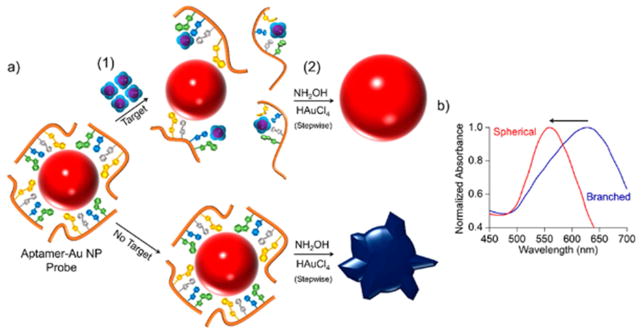
Soh et al. [177] developed an aptamer-functionalized AuNP-based strategy to detect cocaine in synthetic urine and saliva. The cocaine-specific aptamer was adsorbed on the surface of AnNPs, but was detached from the particles surface after introducing its target. In the presence of the ingredients needed for growing AuNPs (HAuCl4 and NaOH), the AuNPs with less aptamer on their surface (meaning a high concentration of cocaine) preferentially grew in a branched shape, and a blue color was observed, while the AuNPs with aptamer present adopted a red color. The LOD measured using the peak shift in UV–vis spectra was 1 nM [177] (See Fig. 11). Another application of aptamer based assay was reported for detection of MA and MDMA. In this study, the aptamers against MA were attached to AuNPs, which stabilized the AuNPs against salt-induced aggregation. When aptamers were exposed to their targets (MA or MDMA), they formed a different tertiary structure which had lower affinity to AuNPs, therefore, by adding the salt to samples, AuNPs without aptamers had a tendency to aggregate and the color of the sample changed from red to blue. It should be mentioned that this method demonstrated less sensitivity for detecting MDMA than MD [178]. See Table 7 for the varieties of colorimetric biosensors developed against doping agents.
Fig. 11.
Schematic for the mechanism of colorimetric detection of small molecules including cocaine, by means of gold nanoparticles. (a) Aptamer–Au NP probes are generated through adsorption of aptamers to the surface of AuNP by Au–nucleoside interaction. (1) By introducing target small molecules, the interaction between aptamer and target leads to dissociation of aptamer strands from the Au NP surface, which increases with the increase in the target concentration. (2) Au NPs were grown by adding hydroxylamine (NH2 OH) and hydrogen tetrachloroaurate (III) (HAuCl4). The Au NPs holding low amounts of aptamer grown with spherical morphology and red color, while those with increased amounts of adsorbed aptamer indicated a branched morphology and blue color. (b) A blue shift in UV–Vis spectra of Au NPs was recorded when morphology changed and the color change from blue to red was observed. Reprinted with permission from Ref. [177], the American Chemical Society.
4. Pros and cons of plasmonic biosensors
One of the important research priorities in WADA is the development of novel biosensing techniques able to identify illicit recreational drugs as early as possible.
MS and MS-based chromatographic detection methods have the ability to extract detailed structural information and qualitative/quantitative analysis at the trace level, particularly in anti-doping applications. Undoubtedly GC–MS, LC-MS and similar techniques can be employed for simultaneous detection of several analytes such as steroids and their metabolites, but these methods are costly and also they require pre-treatment of samples and derivatization of compounds, e.g. converting the analytes into volatile derivatives, or requiring solid phase extraction, hydrolysis along with liquid–liquid extraction, in GC and HPLC, respectively. Hence, conducting analysis via chromatographic approaches is difficult, laborious, and has a need for highly skilled analytical staff (e.g. multi-step procedures and laborious fluid handling), where the analysis is performed in a laboratory usually far from the site of the competition [179].
In addition, using chromatographic methods, a comparatively large sample volume is usually needed due to pre-treatment requirements, while biosensing approaches mostly need significantly smaller sample volumes, and generally have no requirement for pre-treatment or sample derivatization [179].
In the area of anti-doping testing, there is an urgent demand to fore more easy-to-use, inexpensive, and precise techniques that also are rapid, portable and can be used on-site in the field to detect analytes (e.g., illicit drugs). In addition, the design of novel bio-sensing tools for the identification of miscellaneous illicit recreational drugs with a significantly higher reliability, sensitivity, and selectivity is also necessary and could be a crucial step for law enforcement. Thus major efforts and researches studies have been devoted to the fabrication of diverse optical biosensors based on plasmonic transduction for anti-doping detection, including SPR, LSPR, SERS, FRET, and so forth.
Plasmonic biosensors have the advantage of being faster, smaller and cheaper, use harmless visible light, have a nondestructive nature of operation, and demonstrate a rapid signal generation and read-out [180].
SPR biosensors show certain advantages over conventional detection techniques, especially in anti-doping applications. Although, electrochemical sensors are commonly used due to their ease of use, low costs, miniaturizability, fast response, they require a labeling method, usually through ELISA technique. Employing SPR biosensors for doping analysis, affords advantages over electrochemical biosensors; a most important example is the label-free real-time monitoring of antibody–antigen interaction, without the need for labeling, merely by utilizing the refraction index change resulting from the biorecognition event. So, this method reduces nonspecific binding phenomena and matrix effect onto SPR biosensors, which can affect the accuracy of the measurement. Mazzei et al. [1] summarized the comparative advantages and drawbacks of SPR and electrochemical biosensors and concluded that SPR had overall advantages compared to the electrochemical transduction pathway. Generally, the advantages of SPR biosensors compared with other electrochemical sensors are: high quick and rich data acquisition, label free detection, high surface sensitivity, high sensitive (pM concentrations), low amounts of analyte required, and commercial availability of modified surfaces. But, disadvantages of SPR biosensors compared with electrochemical sensors are: low miniaturizability, complication of usage, difficulty in the measurements of low molecular weight substance, immobilization effect, and steric hindrance [1].
SPR biosensors can also detect biomolecules that do not produce a complete monolayer, with real-time data, and greater sensitivity to changes in the bulk refractive index compared to LSPR sensors. However, they have disadvantages such as a poorer ability to miniaturize the instrumentation, lack simplicity, and possess insufficient spatial imaging resolution. On the other hand, the advantages of LSPR biosensors include having more sensitivity to local changes in refractive index, especially in the nanometer range (in close proximity to the nanoparticles), better spectral tunability (e.g. modification of the nanoparticle surface according to different biomolecules), possibility of miniaturization, easy integration with detection chips, and shooter detection times [45,181–183]. In addition, LSPR has very little sensitivity to interference from fluctuations in RI in solutions, and is more sensitive to the changes on the surface of nanostructures. Also LSPR biosensing provides other advantages including possibility for multiplexed sensing, more versatility in signal transduction [184], and provides real-time and portable detection systems [185].
The major drawback of SPR label-free technique is that it requires expensive tools or difficult sensor fabrication. LSPR technique has easy sensor fabrication and inexpensive instrumentation with all of the advantages of label-free surface sensing; for example, field decay length for LSPR is shorter than SPR [184].
Alterations in the local dielectric environment influence both SPR and LSPR; the sensing mechanism for both approaches relies on the measurement of small changes in RI as a result of analyte-ligand binding at the surface or within a very close distance from the noble metal and are used to obtain thermodynamic and real-time kinetic information for the binding [43]. However, unlike SPR (e.g. PSPR sensors), LSPR is not sensitive to temperature fluctuations and offers advantages of the short and tunable distance for electromagnetic field decay, which allows competitive sensitivity and minimizes the sensing region allowing low cost instrumentation [61].
Although SPR biosensors have been widely commercialized and marketed as routine analytical devices for a variety of applications with easy, fast, real-time and rather sensitive detection capabilities, advancements in material science, nanotechnology and developments in nanofabrication technology have still been required to provide improvements and overcome some limitations of SPR biosensors such as sensitivity, high-throughput capacity and better miniaturization [186]. Considering the promising potential of modern LSPR techniques over conventional SPR, and the investigations and advancements that have occurred in recent years, the translation of LSPR biosensors for routine biomedical applications, such as environmental control, food analysis, forensics and clinical diagnostics is highly desirable [187].
LSPR biosensors can detect a wide range of biomolecular interactions because almost all the occurred interactions can change local RI. This can be a negative point, as the sensor cannot identify between capturing the target analyte and the sediment surface. In addition, other analytes with similar performance cannot be detected by LSPR measurements alone. A possible solution to this problem is combining LSPR with MS. In this method, the analyte is detected by MS, and then a quantitative analysis is done by LSPR. This method is useful for nonspecific binding to the surface as well for multiple analytes. Excellent properties offered by LSPR, greatly enhances its capability for detection of multiple analytes [184].
An innovative platform that could be an alternative to conventional SPR-based detection systems is called “ordered plasmonic nanoarrays”. They provide advantages including affordability, controllable sizes, uniform structure, and easier production, have a large surface area for surface functionalization (e.g. antibody immobilization to enhance sensitivity and specificity), facile integration with microfluidic systems for real-time monitoring, and provide a significant amplification of the 3D local field [185]. In this context, these nanoarray structures could be deployed in the design of novel biosensors for detection of illicit drugs.
Noble metal NPs possess a large surface-to-volume ratio, good biocompatibility, electrical conductivity, high electrolytic activity, the ability to preserve the activity of biomolecules by controlling their microenvironment, ability to facilitate electron transfer between redox-active biomolecules and the electrode surface. Thus, in ECL sensing, together with the ability to be conjugated to various biomolecules, these considerations have made nanomaterials a useful tool to provide signal transduction and amplification [188–191]. Quantum dot-based ECL immunoassay sensors have been reported to have advantages such as high sensitivity and selectivity, wide linearity range, and excellent LOD, compared to SPR biosensors [117].
Generally, the advantages of the SERS technique include an ultra-trace level detection capability (possibly even single molecules), a wide range of target molecules, smaller sample volume, ability to analyze at very low concentrations of target analyte in a complex chemical mixture, simultaneous discriminating ability between different biomarkers via their specific molecular signatures using Raman scattering or intensity, rapid analysis, nondestructive nature, no need for fluorescence labels, and exact knowledge about the adsorption mechanism. Despite all these unique features, some drawbacks and challenges of SERS exist, including a relatively complicated synthetic process for the substrates, high cost associated with highly efficacious and stable SERS substrates, low affinity of some molecules toward SERS substrates (particularly in complex fluids containing multiple chemical and biological species), low repeatability and reproducibility of SERS spectra, and a high dependency on the size and morphology of the substrate. Future studies should work towards overcoming these drawbacks, and challenges [185,192,193]. Furthermore, to in order to translate SERS biosensing into real-world applications, efforts need to be made to achieve optimized surface modification of metal nanostructures to more efficiently and specifically capture target biomarkers by NP surface-attached bioreceptors especially in complex biological milieux [185].
Affinity-based biosensing approaches, which can be integrated with optical transduction mechanisms in illicit analyte detection, are regarded as inexpensive, and user-friendly platforms with good selectivity. In addition, they have demonstrated a good match with WADA requirements in terms of limits of detection. Despite these advantages, probably because of insufficient selectivity (e.g., for detection of pseudo-endogenous compositions), and not being able to provide complete data regarding probable matrix effects on real analytes, affinity-based biosensors have not yet become acceptable candidates to be substitutes for traditional antidoping analytical tests [1].
5. Conclusion
Nanotechnology has brought almost revolutionary changes to the field of analytical chemistry. Noble metal NPs have been at the forefront of this revolution, largely due to their amazing plasmonic properties. Among the many analytes whose detection has been improved by plasmonic sensors, illicit chemicals, banned substances and performance-enhancing drugs have been predominant. This review has provided a summary of various sensing strategies employing the powerful LSPR effect to enhance a variety of optical techniques that could improve the efficiency, sensitivity and specificity of traditional methods in drug biosensing. Highly sensitive and specific biosensors based on noble metals NPs have continued to improve the versatility of diagnostic platforms for detection and quantitative assays of drugs of abuse. The advantages of LSPR devices in comparison to conventional SPR devices lie in improved detection of low molecular weight compounds, sensitivity to small changes in the absorption wavelength, reduced complexity, robust resistance to noise and vibration, and affordable manufacturing costs.
Surface-enhanced optical approaches, such as SERS, SEF, colorimetric, and electrochemical sensing have been introduced up to now. However, the translation of these biosensors to real-life samples, and routine use for screening large numbers of sports competitors, or participants in drug rehabilitation programs has yet to materialize. High sensitivity and selectivity at minimal cost and real-time monitoring are the key elements, which need a concrete demonstration. In future years, the plasmonic property of noble metal nanostructures will find great application in biology and medicine, and significant advances will be made to realize, faster, smaller and cheaper plasmonic-based biosensors.
Highlights.
Analysis of doping substances and drugs of abuse requires improvement.
Plasmonics is the local optical field enhancement at metal surfaces.
Localized surface plasmonic resonance detects binding to noble metal nanoparticles.
Immunosensors employ gold nanoparticles to improve detection limits.
Surface enhanced Raman spectroscopy, fluorescence and chemiluminescence.
Acknowledgments
Michael R Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
References
- 1.Mazzei F, et al. Affinity-based biosensors in sport medicine and doping control analysis. Bioanalysis. 2014;6(2):225–245. doi: 10.4155/bio.13.308. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Guidi G. Doping and sports. Minerva Med. 1999;90(9):345–357. [PubMed] [Google Scholar]
- 3.Thieme D, et al. Analytical strategy for detecting doping agents in hair. Forensic Sci Int. 2000;107(1):335–345. doi: 10.1016/s0379-0738(99)00177-2. [DOI] [PubMed] [Google Scholar]
- 4.Thevis M, et al. Sports drug testing using complementary matrices: advantages and limitations. J Pharmaceut Biomed Anal. 2016 doi: 10.1016/j.jpba.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich HP, Hunt TK. The effects of cortisone and anabolic steroids on the tensile strength of healing wounds. Ann Surg. 1969;170(2):203. doi: 10.1097/00000658-196908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb DR. Anabolic steroids in athletics: how well do they work and how dangerous are they? Am J Sports Med. 1983;12(1):31–38. doi: 10.1177/036354658401200105. [DOI] [PubMed] [Google Scholar]
- 7.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kicman A. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deventer K, et al. Prevalence of legal and illegal stimulating agents in sports. Anal Bioanal Chem. 2011;401(2):421–432. doi: 10.1007/s00216-011-4863-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, et al. Simultaneous determination of 18 abused opioids and metabolites in human hair using LC–MS/MS and illegal opioids abuse proven by hair analysis. J Pharmaceut Biomed Anal. 2014;89:99–105. doi: 10.1016/j.jpba.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Ferro P, et al. Structure, Äêactivity relationship for peptidic growth hormone secretagogues. Drug Test Anal. 2016 doi: 10.1002/dta.1947. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls AR, Holt RI. Sports Endocrinology. Karger Publishers; 2016. Growth hormone and insulin-like growth factor-1; pp. 101–114. [DOI] [PubMed] [Google Scholar]
- 13.Jha SK, Hayashi K, Yadava R. Drugs of abuse and their detection methodologies: contribution of chemical sensor. Curr Org Chem. 2015;19(12):1191–1201. [Google Scholar]
- 14.WADA. The 2016 Prohibited List-international Standard. 2016. [Google Scholar]
- 15.Thévenot DR, et al. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron. 2001;16(1):121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 16.Gerard M, Chaubey A, Malhotra B. Application of conducting polymers to biosensors. Biosens Bioelectron. 2002;17(5):345–359. doi: 10.1016/s0956-5663(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra P. Biosensors and their applications, ÄìA review. J Oral Biol Craniofac Res. 2016;6(2):153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhalla N, et al. Introduction to biosensors. Essays Biochem. 2015 doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon J-Y. Introduction to Biosensors. Springer; 2016. Glucose sensors; pp. 213–228. [Google Scholar]
- 20.Maier SA. Plasmonics: Fundamentals and Applications. Springer Science & Business Media; 2007. [Google Scholar]
- 21.Butet J, Brevet PF, Martin OJ. Optical second harmonic generation in plasmonic nanostructures: from fundamental principles to advanced applications. ACS Nano. 2015;9(11):10545–10562. doi: 10.1021/acsnano.5b04373. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Ming H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sensors. 2012;2(1):37–49. [Google Scholar]
- 23.Stewart ME, et al. Nanostructured plasmonic sensors. Chem Rev. 2008;108(2):494–521. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 24.Zeng S, et al. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sensors Actuators B Chem. 2013;176:1128–1133. [Google Scholar]
- 25.Zeng S, et al. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics. 2011;6(3):491. [Google Scholar]
- 26.Bardhan R, et al. Fluorescence enhancement by Au nanostructures: nano-shells and nanorods. ACS Nano. 2009;3(3):744–752. doi: 10.1021/nn900001q. [DOI] [PubMed] [Google Scholar]
- 27.Marinakos SM, Chen S, Chilkoti A. Plasmonic detection of a model analyte in serum by a gold nanorod sensor. Anal Chem. 2007;79(14):5278–5283. doi: 10.1021/ac0706527. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, et al. Surface plasmon resonance refractive sensor based on silver-coated side-polished fiber. Sensors Actuators B Chem. 2016;230:206–211. [Google Scholar]
- 29.El-Sayed MA. Some interesting properties of metals confined in time and nanometer space of different shapes. Accounts Chem Res. 2001;34(4):257–264. doi: 10.1021/ar960016n. [DOI] [PubMed] [Google Scholar]
- 30.Fedlheim DL, Foss CA. Metal Nanoparticles: Synthesis, Characterization, and Applications. CRC Press; 2001. [Google Scholar]
- 31.Daniel M-C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 32.Ding L, Gao Y, Di J. A sensitive plasmonic copper(II) sensor based on gold nanoparticles deposited on ITO glass substrate. Biosens Bioelectron. 2016;83:9–14. doi: 10.1016/j.bios.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Ghasemi F, Hormozi-Nezhad MR, Mahmoudi M. A colorimetric sensor array for detection and discrimination of biothiols based on aggregation of gold nanoparticles. Anal Chim Acta. 2015;882:58–67. doi: 10.1016/j.aca.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Lan M, et al. A recyclable carbon nanoparticle-based fluorescent probe for highly selective and sensitive detection of mercapto biomolecules. J Mater Chem B. 2015;3(1):127–134. doi: 10.1039/c4tb01354a. [DOI] [PubMed] [Google Scholar]
- 35.Faridli Z, et al. Development of a localized surface plasmon resonance-based gold nanobiosensor for the determination of prolactin hormone in human serum. Anal Biochem. 2016;495:32–36. doi: 10.1016/j.ab.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Li G, et al. Electrochemical biosensor based on nanocomposites film of thiol graphene-thiol chitosan/nano gold for the detection of carcinoembryonic antigen. Electroanalysis. 2015;27(5):1245–1252. [Google Scholar]
- 37.El-Ads EH, Galal A, Atta NF. Electrochemistry of glucose at gold nano-particles modified graphite/SrPdO3 electrode – towards a novel non-enzymatic glucose sensor. J Electroanal Chem. 2015;749:42–52. [Google Scholar]
- 38.Devadoss A, et al. Single-step electrospun TiO2-Au hybrid electrodes for high selectivity photoelectrocatalytic glutathione bioanalysis. J Mater Chem B. 2016;4(2):220–228. doi: 10.1039/c5tb01740h. [DOI] [PubMed] [Google Scholar]
- 39.Mock J, et al. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys. 2002;116(15):6755–6759. [Google Scholar]
- 40.Sun Y, Xia Y. Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes. Anal Chem. 2002;74(20):5297–5305. doi: 10.1021/ac0258352. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, et al. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir. 2008;24(10):5233–5237. doi: 10.1021/la800305j. [DOI] [PubMed] [Google Scholar]
- 42.Jain PK, et al. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 43.Willets KA, Van Duyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem. 2007;58:267–297. doi: 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]
- 44.Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35(3):209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 45.Zeng S, et al. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem Soc Rev. 2014;43(10):3426–3452. doi: 10.1039/c3cs60479a. [DOI] [PubMed] [Google Scholar]
- 46.Chen KJ, Lu CJ. A vapor sensor array using multiple localized surface plasmon resonance bands in a single UV–vis spectrum. Talanta. 2010;81(4):1670–1675. doi: 10.1016/j.talanta.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Mack NH, et al. Optical transduction of chemical forces. Nano Lett. 2007;7(3):733–737. doi: 10.1021/nl0629759. [DOI] [PubMed] [Google Scholar]
- 48.Haynes CL, Van Duyne RP. Plasmon-sampled surface-enhanced Raman excitation spectroscopy. J Phys Chem B. 2003;107(30):7426–7433. [Google Scholar]
- 49.Mock JJ, Smith DR, Schultz S. Local refractive index dependence of plasmon resonance spectra from individual nanoparticles. Nano Lett. 2003;3(4):485–491. [Google Scholar]
- 50.Sau TK, et al. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater. 2010;22(16):1805–1825. doi: 10.1002/adma.200902557. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, et al. Ultrahigh sensitivity made simple: nanoplasmonic label-free biosensing with an extremely low limit-of-detection for bacterial and cancer diagnostics. Nanotechnology. 2009;20(43):434015. doi: 10.1088/0957-4484/20/43/434015. [DOI] [PubMed] [Google Scholar]
- 52.Mayer KM, et al. A single molecule immunoassay by localized surface plasmon resonance. Nanotechnology. 2010;21(25):255503. doi: 10.1088/0957-4484/21/25/255503. [DOI] [PubMed] [Google Scholar]
- 53.Guo L, et al. Strategies for enhancing the sensitivity of plasmonic nano-sensors. Nano Today. 2015;10(2):213–239. [Google Scholar]
- 54.Lichtman JW, Conchello JA. Fluorescence microscopy. Br J Pharmacol. 2005;2(12):910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 55.Bauch M, et al. Plasmon-enhanced fluorescence biosensors: a review. Plasmonics. 2014;9(4):781–799. doi: 10.1007/s11468-013-9660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fort E, Grésillon S. Surface enhanced fluorescence. J Phys Appl Phys. 2008;41(1):013001. [Google Scholar]
- 57.Bauch M, et al. Plasmon-enhanced fluorescence biosensors: a review. Plasmonics. 2013;9(4):781–799. doi: 10.1007/s11468-013-9660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dostalek J, Knoll W. Biosensors based on surface plasmon-enhanced fluorescence spectroscopy (Review) Biointerphases. 2008;3(3):FD12. doi: 10.1116/1.2994688. [DOI] [PubMed] [Google Scholar]
- 59.Tang D, Cui Y, Chen G. Nanoparticle-based immunoassays in the biomedical field. Analyst. 2013;138(4):981–990. doi: 10.1039/c2an36500f. [DOI] [PubMed] [Google Scholar]
- 60.Matsui J, et al. SPR sensor chip for detection of small molecules using molecularly imprinted polymer with embedded gold nanoparticles. Anal Chem. 2005;77(13):4282–4285. doi: 10.1021/ac050227i. [DOI] [PubMed] [Google Scholar]
- 61.Haes AJ, Van Duyne RP. A unified view of propagating and localized surface plasmon resonance biosensors. Anal Bioanal Chem. 2004;379(7–8):920–930. doi: 10.1007/s00216-004-2708-9. [DOI] [PubMed] [Google Scholar]
- 62.Storhoff JJ, et al. One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc. 1998;120(9):1959–1964. [Google Scholar]
- 63.Bedford EE, et al. Surface plasmon resonance biosensors incorporating gold nanoparticles. Macromol Biosci. 2012;12(6):724–739. doi: 10.1002/mabi.201100435. [DOI] [PubMed] [Google Scholar]
- 64.Chávez JL, et al. Plasmonic aptamer–gold nanoparticle sensors for small molecule fingerprint identification. Analyst. 2014;139(23):6214–6222. doi: 10.1039/c4an01376j. [DOI] [PubMed] [Google Scholar]
- 65.Abdulhalim I, Zourob M, Lakhtakia A. Surface plasmon resonance for bio-sensing: a mini-review. Electromagnetics. 2008;28(3):214–242. [Google Scholar]
- 66.Feng C, Dai S, Wang L. Optical aptasensors for quantitative detection of small biomolecules: a review. Biosens Bioelectron. 2014;59:64–74. doi: 10.1016/j.bios.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Homola J. Present and future of surface plasmon resonance biosensors. Anal Bioanal Chem. 2003;377(3):528–539. doi: 10.1007/s00216-003-2101-0. [DOI] [PubMed] [Google Scholar]
- 68.Situ C, et al. Advances in surface plasmon resonance biosensor technology towards high-throughput, food-safety analysis. TrAC Trends Anal Chem. 2010;29(11):1305–1315. [Google Scholar]
- 69.Szunerits S, et al. Recent advances in the development of graphene-based surface plasmon resonance (SPR) interfaces. Anal Bioanal Chem. 2013;405(5):1435–1443. doi: 10.1007/s00216-012-6624-0. [DOI] [PubMed] [Google Scholar]
- 70.Tanious FA, Nguyen B, Wilson WD. Methods in Cell Biology. Academic Press; 2008. Biosensor-surface plasmon resonance methods for quantitative analysis of biomolecular interactions; pp. 53–77. [DOI] [PubMed] [Google Scholar]
- 71.Gutiérrez-Gallego R, et al. Surface plasmon resonance in doping analysis. Anal Bioanal Chem. 2011;401(2):389–403. doi: 10.1007/s00216-011-4830-9. [DOI] [PubMed] [Google Scholar]
- 72.Barroso O, Schamasch P, Rabin O. Detection of GH abuse in sport: past, present and future. Growth Horm IGF Res. 2009;19(4):369–374. doi: 10.1016/j.ghir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Abellan R, et al. Evaluation of immunoassays for the measurement of insulin-like growth factor-I and procollagen type III peptide, indirect bio-markers of recombinant human growth hormone misuse in sport. Clin Chem Lab Med. 2005;43(1):75–85. doi: 10.1515/CCLM.2005.012. [DOI] [PubMed] [Google Scholar]
- 74.de Juan-Franco E, et al. Implementation of a SPR immunosensor for the simultaneous detection of the 22K and 20K hGH isoforms in human serum samples. Talanta. 2013;114:268–275. doi: 10.1016/j.talanta.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 75.SadAbadi H, et al. Rapid microwave-induced synthesis of gold-polydimethylsiloxane nanocomposites for biosensing of proteins. J Nanosci Nanotechnol. 2013;13(10):6880–6887. doi: 10.1166/jnn.2013.7755. [DOI] [PubMed] [Google Scholar]
- 76.SadAbadi H, et al. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens Bio-electron. 2013;44:77–84. doi: 10.1016/j.bios.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Ozhikandathil J, Badilescu S, Packirisamy M. Plasmonic gold decorated MWCNT nanocomposite for localized plasmon resonance sensing. Sci Rep. 2015;5 doi: 10.1038/srep13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozhikandathil J, Packirisamy M. Nano-islands integrated evanescence-based lab-on-a-chip on silica-on-silicon and polydimethylsiloxane hybrid platform for detection of recombinant growth hormone. Biomicrofluidics. 2012;6(4):046501. doi: 10.1063/1.4757968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gobi KV, Iwasaka H, Miura N. Self-assembled PEG monolayer based SPR immunosensor for label-free detection of insulin. Biosens Bioelectron. 2007;22(7):1382–1389. doi: 10.1016/j.bios.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 80.Hiep HM, et al. A microfluidic chip based on localized surface plasmon resonance for real-time monitoring of antigen–antibody reactions. Jpn J Appl Phys. 2008;47(2S):1337. [Google Scholar]
- 81.Frasconi M, et al. Multifunctional Au nanoparticle dendrimer-based surface plasmon resonance biosensor and its application for improved insulin detection. Anal Chem. 2010;82(17):7335–7342. doi: 10.1021/ac101319k. [DOI] [PubMed] [Google Scholar]
- 82.Xu M, Luo X, Davis JJ. The label free picomolar detection of insulin in blood serum. Biosens Bioelectron. 2013;39(1):21–25. doi: 10.1016/j.bios.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Q, et al. Surface plasmon resonance sensor for femtomolar detection of testosterone with water-compatible macroporous molecularly imprinted film. Anal Biochem. 2014;463:7–14. doi: 10.1016/j.ab.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 84.Tan Y, et al. A novel double-layer molecularly imprinted polymer film based surface plasmon resonance for determination of testosterone in aqueous media. Appl Surf Sci. 2015;342:84–91. [Google Scholar]
- 85.Mitchell JS, Lowe TE. Ultrasensitive detection of testosterone using conjugate linker technology in a nanoparticle-enhanced surface plasmon resonance biosensor. Biosens Bioelectron. 2009;24(7):2177–2183. doi: 10.1016/j.bios.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 86.Yuan J, et al. Sensitivity enhancement of SPR assay of progesterone based on mixed self-assembled monolayers using nanogold particles. Biosens Bio-electron. 2007;23(1):144–148. doi: 10.1016/j.bios.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 87.Brun EM, Puchades R, Maquieira Á. Analytical methods for anti-doping control in sport: anabolic steroids with 4, 9, 11-triene structure in urine. TrAC Trends Anal Chem. 2011;30(5):771–783. [Google Scholar]
- 88.Kreuzer MP, et al. Quantitative detection of doping substances by a localised surface plasmon sensor. Biosens Bioelectron. 2006;21(7):1345–1349. doi: 10.1016/j.bios.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 89.Kreuzer MP, et al. Colloidal-based localized surface plasmon resonance (LSPR) biosensor for the quantitative determination of stanozolol. Anal Bioanal Chem. 2008;391(5):1813–1820. doi: 10.1007/s00216-008-2022-z. [DOI] [PubMed] [Google Scholar]
- 90.Frasconi M, et al. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal Bioanal Chem. 2009;394(8):2151–2159. doi: 10.1007/s00216-009-2914-6. [DOI] [PubMed] [Google Scholar]
- 91.Treviño J, et al. Surface plasmon resonance immunoassay analysis of pituitary hormones in urine and serum samples. Clin Chim Acta. 2009;403(1):56–62. doi: 10.1016/j.cca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Jing L, et al. Determination of estradiol by surface plasmon resonance using a molecularly imprinted film. Anal Lett. 2016;49(11):1696–1710. [Google Scholar]
- 93.Treviño J, et al. Determination of human growth hormone in human serum samples by surface plasmon resonance immunoassay. Talanta. 2009;78(3):1011–1016. doi: 10.1016/j.talanta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Svobodová M, et al. The characterization and validation of 17 β-estradiol binding aptamers. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 95.van den Broek I, et al. Current trends in mass spectrometry of peptides and proteins: application to veterinary and sports-doping control. Mass Spectrom Rev. 2015;34(6):571–594. doi: 10.1002/mas.21419. [DOI] [PubMed] [Google Scholar]
- 96.Sakai G, et al. A surface plasmon resonance-based immunosensor for highly sensitive detection of morphine. Sensors Actuators B Chem. 1998;49(1):5–12. [Google Scholar]
- 97.Dillon PP, et al. Immunoassay for the determination of morphine-3-glucuronide using a surface plasmon resonance-based biosensor. Biosens Bioelectron. 2003;18(2):217–227. doi: 10.1016/s0956-5663(02)00182-3. [DOI] [PubMed] [Google Scholar]
- 98.Klenkar G, Liedberg B. A microarray chip for label-free detection of narcotics. Anal Bioanal Chem. 2008;391(5):1679–1688. doi: 10.1007/s00216-008-1839-9. [DOI] [PubMed] [Google Scholar]
- 99.Li K, et al. Nanoplasmonic imaging of latent fingerprints and identification of cocaine. Angew Chem Int Ed. 2013;52(44):11542–11545. doi: 10.1002/anie.201305980. [DOI] [PubMed] [Google Scholar]
- 100.Traynor I, et al. Detection of multi-β-agonist residues in liver matrix by use of a surface plasma resonance biosensor. Anal Chim Acta. 2003;483(1):187–191. [Google Scholar]
- 101.Suherman, Morita K, Kawaguchi T. Highly selective and sensitive detection of β-agonists using a surface plasmon resonance sensor based on an alka-nethiol monolayer functionalized on a Au surface. Biosens Bioelectron. 2015;67:356–363. doi: 10.1016/j.bios.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 102.Mie G. Articles on the optical characteristics of turbid tubes, especially colloidal metal solutions. Ann Phys. 1908;25(3):377–445. [Google Scholar]
- 103.Sakai G, et al. Highly selective and sensitive SPR immunosensor for detection of methamphetamine. Electrochim Acta. 1999;44(21):3849–3854. [Google Scholar]
- 104.Wu Y, et al. Development of a label-free and reagentless plasmonic immunosensor for the detection of salbutamol. Anal Methods. 2013;5(19):5222–5226. [Google Scholar]
- 105.Johansson MA, Hellenäs KE. Immunobiosensor analysis-of clenbuterol in bovine hair. Food Agric Immunol. 2003;15(3–4):197–205. [Google Scholar]
- 106.Liu M, et al. Development of indirect competitive immunoassay for highly sensitive determination of ractopamine in pork liver samples based on surface plasmon resonance sensor. Sensor Actuators B Chem. 2012;161(1):124–130. [Google Scholar]
- 107.Shelver WL, et al. Development of an immunobiosensor assay for the beta-adrenergic compound zilpaterol. Food Agric Immunol. 2005;16(3):199–211. [Google Scholar]
- 108.Shelver WL, Smith DJ. Determination of ractopamine in cattle and sheep urine samples using an optical biosensor analysis: comparative study with HPLC and ELISA. J Agric Food Chem. 2003;51(13):3715–3721. doi: 10.1021/jf021175q. [DOI] [PubMed] [Google Scholar]
- 109.Haughey SA, et al. Determination of clenbuterol residues in bovine urine by optical immunobiosensor assay. J AOAC Int. 2001;84(4):1025–1030. [PubMed] [Google Scholar]
- 110.Suherman, Morita K, Kawaguchi T. Surface plasmon resonance for detecting clenbuterol: influence of monolayer structure. Appl Surf Sci. 2015;332:229–236. [Google Scholar]
- 111.Wu Y, et al. Clenbuterol assay by spectral imaging surface plasmon resonance biosensor system. Appl Biochem Biotechnol. 2015;177(6):1327–1337. doi: 10.1007/s12010-015-1817-6. [DOI] [PubMed] [Google Scholar]
- 112.Yao M, et al. Spectral surface plasmon resonance imaging for the detection of clenbuterol via three-dimensional immobilization of bioprobes. Anal Biochem. 2015;475:40–43. doi: 10.1016/j.ab.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 113.Harvey BR, et al. Engineering of recombinant antibody fragments to methamphetamine by anchored periplasmic expression. J Immunol Methods. 2006;308(1):43–52. doi: 10.1016/j.jim.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Smith JP, et al. Measurement of methamphetamine on surfaces using surface plasmon resonance. Toxicol Mech Methods. 2009;19(6–7):416–421. doi: 10.1080/15376510903114959. [DOI] [PubMed] [Google Scholar]
- 115.Adcock JL, et al. Chemiluminescence and electrochemiluminescence detection of controlled drugs. Drug Test Anal. 2011;3(3):145–160. doi: 10.1002/dta.236. [DOI] [PubMed] [Google Scholar]
- 116.Fereja TH, Hymete A, Gunasekaran T. A recent review on chemiluminescence reaction, principle and application on pharmaceutical analysis. ISRN Spectrosc. 2013;2013 [Google Scholar]
- 117.Fei W, et al. Ultrasensitive electrochemiluminescent immunoassay for morphine using a gold electrode modified with CdS quantum dots, polyamidoamine, and gold nanoparticles. Microsc Acta. 2013;181(3–4):419–425. [Google Scholar]
- 118.McGeehan J, Dennany L. Electrochemiluminescent detection of methamphetamine and amphetamine. Forensic Sci Int. 2016;264:1–6. doi: 10.1016/j.forsciint.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 119.Lad AN, Pandya A, Agrawal YK. Overview of nano-enabled screening of drug-facilitated crime: a promising tool in forensic investigation. TrAC Trends Anal Chem. 2016;80:458–470. [Google Scholar]
- 120.Seydack M. Nanoparticle labels in immunosensing using optical detection methods. Biosens Bioelectron. 2005;20(12):2454–2469. doi: 10.1016/j.bios.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Yan P, et al. Ultrasensitive detection of clenbuterol by quantum dots based electrochemiluminescent immunosensor using gold nanoparticles as substrate and electron transport accelerator. Sensors Actuators B Chem. 2014;191:508–515. [Google Scholar]
- 122.Yao X, et al. Quantum dots based electrochemiluminescent immunosensor by coupling enzymatic amplification for ultrasensitive detection of clenbuterol. Anal Chim Acta. 2013;798:82–88. doi: 10.1016/j.aca.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 123.Zhou H, et al. Flow chemiluminescence sensor for determination of clenbuterol based on molecularly imprinted polymer. Anal Chim Acta. 2004;523(2):237–242. [Google Scholar]
- 124.Zhou J, Xu X, Wang Y. Competitive immunoassay for clenbuterol using capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Anal Technol Biomed Life Sci. 2007;848(2):226–231. doi: 10.1016/j.jchromb.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 125.Wang H, et al. A silver-palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens Bioelectron. 2013;49:14–19. doi: 10.1016/j.bios.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 126.Wu Y, et al. Determination of salbutamol, clenbuterol, and brombuterol in urine by a highly sensitive chemiluminescence enzyme immunoassay. Anal Lett. 2014;47(16):2761–2773. [Google Scholar]
- 127.Jiang L, Yang Y, Tu Y. A new strategy to develop the disposable label-free immunosensor with electrochemiluminescent probing. J Electroanal Chem. 2015;747:136–142. [Google Scholar]
- 128.Shen WJ, et al. Cu-based metal–organic frameworks as a catalyst to construct a ratiometric electrochemical aptasensor for sensitive lipopolysaccharide detection. Anal Chem. 2015;87(22):11345–11352. doi: 10.1021/acs.analchem.5b02694. [DOI] [PubMed] [Google Scholar]
- 129.Qiu B, et al. Electrochemiluminescence determination of codeine or morphine with an organically modified silicate film immobilizing Ru(bpy) 3(2+) Luminescence. 2007;22(3):189–194. doi: 10.1002/bio.947. [DOI] [PubMed] [Google Scholar]
- 130.Li J, et al. Chemiluminescence determination of trimetazidine via inducing the aggregation of gold nanoparticles. Spectrochim Acta Mol Biomol Spectrosc. 2013;114:33–37. doi: 10.1016/j.saa.2013.04.097. [DOI] [PubMed] [Google Scholar]
- 131.Sigmund G, et al. Doping control analysis of trimetazidine and characterization of major metabolites using mass spectrometric approaches. Drug Test Anal. 2014;6(11–12):1197–1205. doi: 10.1002/dta.1680. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, et al. Multiple signal amplification electrogenerated chemiluminescence biosensors for sensitive protein kinase activity analysis and inhibition. Biosens Bioelectron. 2015;68:771–776. doi: 10.1016/j.bios.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Zhou Y, et al. Electrochemical biosensor for protein kinase A activity assay based on gold nanoparticles-carbon nanospheres, phos-tag-biotin and β-galactosidase. Biosens Bioelectron. 2016;86:508–515. doi: 10.1016/j.bios.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 134.Zhang G-Y, et al. Zirconium-metalloporphyrin frameworks as a three-in-one platform possessing oxygen nanocage, electron media, and bonding site for electrochemiluminescence protein kinase activity assay. Nanoscale. 2016;8(22):11649–11657. doi: 10.1039/c6nr01206j. [DOI] [PubMed] [Google Scholar]
- 135.Li T, et al. Gold nanoparticles/Orange II functionalized graphene nanohybrid based electrochemical aptasensor for label-free determination of insulin. RSC Adv. 2016;6(36):30732–30738. [Google Scholar]
- 136.Thomas A, Schänzer W, Thevis M. Determination of human insulin and its analogues in human blood using liquid chromatography coupled to ion mobility mass spectrometry (LC-IM-MS) Drug Test Anal. 2014;6(11–12):1125–1132. doi: 10.1002/dta.1710. [DOI] [PubMed] [Google Scholar]
- 137.Hasanpour F, et al. A chemiluminescent metalloimmunoassay based on copper-enhanced gold nanoparticles for quantification of human growth hormone. Luminescence. 2013;28(5):780–784. doi: 10.1002/bio.2438. [DOI] [PubMed] [Google Scholar]
- 138.Oran PE, et al. Parallel workflow for high-throughput (>1,000 samples/day) quantitative analysis of human insulin-like growth factor 1 using mass spectrometric immunoassay. PloS One. 2014;9(3):e92801. doi: 10.1371/journal.pone.0092801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Timcheh-Hariri A, et al. Comparison of ELISA and TLC methods for the morphine detection in urine of drug abusers. Iran J Toxicol. 2016;10(3):47–50. [Google Scholar]
- 140.Jagerdeo E, Schaff JE. Rapid screening for drugs of abuse in biological fluids by ultra high performance liquid chromatography/Orbitrap mass spectrometry. J Chromatogr B. 2016;1027:11–18. doi: 10.1016/j.jchromb.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 141.Park JM, et al. Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Anal Chim Acta. 2015;853:360–367. doi: 10.1016/j.aca.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 142.Li Hui, Wang M, Wang Chongzhi, Li Wei, Qiang Weibing, Xu Danke. Silver nanoparticle-enhanced fluorescence resonance energy transfer sensor for human platelet-derived growth factor-BB detection. Anal Chem. 2013;85:4492–4499. doi: 10.1021/ac400047d. [DOI] [PubMed] [Google Scholar]
- 143.Lochnera Nina, Lobmaier C, Wirtha Michael, Leitnerb Alfred, Pittnerc Fritz, Gabor Franz. Silver nanoparticle enhanced immunoassays: one step real time kinetic assay for insulin in serum. Eur J Pharm Biopharm. 2003;56:469–477. doi: 10.1016/s0939-6411(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 144.Stojanovic Milan N, de Prada Paloma, Landry Donald W. Aptamer-based folding fluorescent sensor for cocaine. J Am Chem Soc. 2001;123:4928–4931. doi: 10.1021/ja0038171. [DOI] [PubMed] [Google Scholar]
- 145.Donoghue DJ, Hannink M. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta. 1989;989:1–10. doi: 10.1016/0304-419x(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 146.Huang Chih-Ching, Chiu S-H, Huang Yu-Fen, Chang Huan-Tsung. Aptamer-functionalized gold nanoparticles for turn-on light switch detection of platelet-derived growth factor. Anal Chem. 2007;79:4798–4804. doi: 10.1021/ac0707075. [DOI] [PubMed] [Google Scholar]
- 147.Farquharson S, et al. Rapid detection and identification of overdose drugs in Saliva by surface-enhanced Raman scattering using fused gold colloids. Pharmaceutics. 2011;3(3):425–439. doi: 10.3390/pharmaceutics3030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dana K, et al. Rapid analysis of cocaine in saliva by surface-enhanced Raman spectroscopy. J Anal Bioanal Tech. 2015;6(6):1. doi: 10.4172/2155-9872.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andreou C, et al. Rapid detection of drugs of abuse in saliva using surface enhanced Raman spectroscopy and microfluidics. ACS Nano. 2013;7(8):7157–7164. doi: 10.1021/nn402563f. [DOI] [PubMed] [Google Scholar]
- 150.Yang T, et al. Magnetically optimized SERS assay for rapid detection of trace drug-related biomarkers in saliva and fingerprints. Biosens Bioelectron. 2015;68:350–357. doi: 10.1016/j.bios.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 151.Nuntawong N, et al. Detection of methamphetamine/amphetamine in human urine based on surface-enhanced Raman spectroscopy and acidulation treatments. Sensors Actuators B Chem. 2017;239:139–146. [Google Scholar]
- 152.Dong R, et al. Detection and direct readout of drugs in human urine using dynamic surface-enhanced Raman spectroscopy and support vector machines. Anal Chem. 2015;87(5):2937–2944. doi: 10.1021/acs.analchem.5b00137. [DOI] [PubMed] [Google Scholar]
- 153.Izquierdo-Lorenzo I, Sánchez-Cortés S, García-Ramos JV. Adsorption of beta-adrenergic agonists used in sport doping on metal nanoparticles: a detection study based on surface-enhanced Raman scattering. Langmuir. 2010;26(18):14663–14670. doi: 10.1021/la102590f. [DOI] [PubMed] [Google Scholar]
- 154.Sirichai S, Khanatharana P. Rapid analysis of clenbuterol, salbutamol, procaterol, and fenoterol in pharmaceuticals and human urine by capillary electrophoresis. Talanta. 2008;76(5):1194–1198. doi: 10.1016/j.talanta.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 155.Shen S, et al. Determination of β2-agonists by ion chromatography with direct conductivity detection. J Pharmaceut Biomed Anal. 2005;38(1):166–172. doi: 10.1016/j.jpba.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 156.Brunelli C, et al. High-speed gas chromatography in doping control: fast-GC and fast-GC/MS determination of β-adrenoceptor ligands and diuretics. J Sep Sci. 2006;29(18):2765–2771. doi: 10.1002/jssc.200500387. [DOI] [PubMed] [Google Scholar]
- 157.Izquierdo-Lorenzo I, García-Ramos JV, Sanchez-Cortes S. Vibrational characterization and surface-enhanced Raman scattering detection of probenecid doping drug. J Raman Spectrosc. 2013;44(10):1422–1427. [Google Scholar]
- 158.Zaporozhets O, Tsyrulneva I, Ischenko M. Determination of 8 diuretics and probenecid in human urine by gas chromatography-mass spectrometry: confirmation procedure. Am J Anal Chem. 2012;03(04):8. [Google Scholar]
- 159.Izquierdo-Lorenzo I, Sánchez-Cortés S, García-Ramos JV. Trace detection of aminoglutethimide drug by surface-enhanced Raman spectroscopy: a vibrational and adsorption study on gold nanoparticles. Anal Methods. 2011;3(7):1540–1545. [Google Scholar]
- 160.Mareck U, et al. Identification of the aromatase inhibitor aminoglutethimide in urine by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2002;16(24):2209–2214. doi: 10.1002/rcm.838. [DOI] [PubMed] [Google Scholar]
- 161.Kang MJ, et al. Validation and application of a screening method for β2-agonists, anti-estrogenic substances and mesocarb in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(2):252–264. doi: 10.1002/rcm.2834. [DOI] [PubMed] [Google Scholar]
- 162.Izquierdo-Lorenzo I, et al. Adsorption and detection of sport doping drugs on metallic plasmonic nanoparticles of different morphology. Langmuir. 2012;28(24):8891–8901. doi: 10.1021/la300194v. [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Leis A, Garcia-Ramos JV, Sanchez-Cortes S. Silver nanostars with high SERS performance. J Phys Colloid Chem. 2013;117(15):7791–7795. [Google Scholar]
- 164.Wang R, et al. Highly sensitive detection of hormone estradiol E2 using surface-enhanced Raman scattering based immunoassays for the clinical diagnosis of precocious puberty. ACS Appl Mater Interfaces. 2016;8(17):10665–10672. doi: 10.1021/acsami.5b10996. [DOI] [PubMed] [Google Scholar]
- 165.Agoston R, et al. Rapid isolation and detection of erythropoietin in blood plasma by magnetic core gold nanoparticles and portable Raman spectroscopy. Nanomed Nanotechnol Biol Med. 2016;12(3):633–641. doi: 10.1016/j.nano.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 166.Wen G, et al. A novel nanocatalytic SERS detection of trace human chorionic gonadotropin using labeled-free Vitoria blue 4R as molecular probe. Biosens Bioelectron. 2016;85:450–456. doi: 10.1016/j.bios.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 167.Levene C, et al. Enhancing surface enhanced Raman scattering (SERS) detection of propranolol with multiobjective evolutionary optimization. Anal Chem. 2012;84(18):7899–7905. doi: 10.1021/ac301647a. [DOI] [PubMed] [Google Scholar]
- 168.Upadhyayula VK. Functionalized gold nanoparticle supported sensory mechanisms applied in detection of chemical and biological threat agents: a review. Anal Chim Acta. 2012;715:1–18. doi: 10.1016/j.aca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 169.Zhao W, Brook MA, Li Y. Design of gold nanoparticle-based colorimetric biosensing assays. ChemBioChem. 2008;9(15):2363–2371. doi: 10.1002/cbic.200800282. [DOI] [PubMed] [Google Scholar]
- 170.Vilela D, González MC, Escarpa A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: chemical creativity behind the assay. A review. Anal Chim Acta. 2012;751:24–43. doi: 10.1016/j.aca.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 171.He P, et al. Direct detection of beta-agonists by use of gold nanoparticle-based colorimetric assays. Anal Chem. 2011;83(18):6988–6995. doi: 10.1021/ac200769f. [DOI] [PubMed] [Google Scholar]
- 172.Kang J, et al. A rapid colorimetric sensor of clenbuterol based on cysteamine-modified gold nanoparticles. ACS Appl Mater Interfaces. 2016;8(1):1–5. doi: 10.1021/acsami.5b09079. [DOI] [PubMed] [Google Scholar]
- 173.Zhang X, et al. Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine. Biosens Bioelectron. 2012;34(1):112–117. doi: 10.1016/j.bios.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 174.Luo Y, et al. Visual screening and colorimetric determination of clenbuterol and ractopamine using unmodified gold nanoparticles as probe. J Nanosci Nanotechnol. 2016;16(1):548–554. doi: 10.1166/jnn.2016.11682. [DOI] [PubMed] [Google Scholar]
- 175.Zhao Q, et al. Strategy to fabricate naked-eye readout ultrasensitive plasmonic nanosensor based on enzyme mimetic gold nanoclusters. Anal Chem. 2016;88(2):1412–1418. doi: 10.1021/acs.analchem.5b04089. [DOI] [PubMed] [Google Scholar]
- 176.Jv Y, Li B, Cao R. Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. Chem Commun (Camb) 2010;46(42):8017–8019. doi: 10.1039/c0cc02698k. [DOI] [PubMed] [Google Scholar]
- 177.Soh JH, et al. Colorimetric detection of small molecules in complex matrixes via target-mediated growth of aptamer-functionalized gold nanoparticles. Anal Chem. 2015;87(15):7644–7652. doi: 10.1021/acs.analchem.5b00875. [DOI] [PubMed] [Google Scholar]
- 178.Yarbakht M, Nikkhah M. Unmodified gold nanoparticles as a colorimetric probe for visual methamphetamine detection. J Exp Nanosci. 2015;11(7):593–601. [Google Scholar]
- 179.Yadav SK, et al. A review on determination of steroids in biological samples exploiting nanobio-electroanalytical methods. Anal Chim Acta. 2013;762:14–24. doi: 10.1016/j.aca.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 180.Englebienne P. Use of colloidal gold surface plasmon resonance peak shift to infer affinity constants from the interactions between protein antigens and antibodies specific for single or multiple epitopes. Analyst. 1998;123(7):1599–1603. doi: 10.1039/a804010i. [DOI] [PubMed] [Google Scholar]
- 181.Parvez Arnob MM, Shih W-C. 3D plasmonic nanoarchitecture as an emerging biosensing platform. Future Med. 2017 doi: 10.2217/nnm-2017-0258. [DOI] [PubMed] [Google Scholar]
- 182.Ge C, Tao Y, Guo Z. Biosensors based on plasmonics. Biosens J. 2015;4(123):2. [Google Scholar]
- 183.Jatschka J, et al. Propagating and localized surface plasmon resonance sensing—a critical comparison based on measurements and theory. Sens Bio-Sens Res. 2016;7:62–70. [Google Scholar]
- 184.Sagle LB, et al. Advances in localized surface plasmon resonance spectroscopy biosensing. Nanomedicine. 2011;6(8):1447–1462. doi: 10.2217/nnm.11.117. [DOI] [PubMed] [Google Scholar]
- 185.Focsan M, et al. Flexible and tunable 3D gold nanocups platform as plasmonic biosensor for specific dual LSPR-SERS immunodetection. Sci Rep. 2017;7(1):14240. doi: 10.1038/s41598-017-14694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Estevez MC, et al. Trends and challenges of refractometric nanoplasmonic biosensors: a review. Anal Chim Acta. 2014;806:55–73. doi: 10.1016/j.aca.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 187.Brolo AG. Plasmonics for future biosensors. Nat Photon. 2012;6(11):709–713. [Google Scholar]
- 188.Tian Y, Tatsuma T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem Commun (Camb) 2004;16:1810–1811. doi: 10.1039/b405061d. [DOI] [PubMed] [Google Scholar]
- 189.Tian Y, Tatsuma T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J Am Chem Soc. 2005;127(20):7632–7637. doi: 10.1021/ja042192u. [DOI] [PubMed] [Google Scholar]
- 190.Wang J. Electrochemical biosensing based on noble metal nanoparticles. Microchim Acta. 2012;177(3–4):245–270. [Google Scholar]
- 191.Yu S, et al. Label-free immunosensor for the detection of kanamycin using Ag@Fe(3)O(4) nanoparticles and thionine mixed graphene sheet. Biosens Bioelectron. 2013;48:224–229. doi: 10.1016/j.bios.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 192.Han Z, et al. Portable kit for identification and detection of drugs in human urine using surface-enhanced Raman spectroscopy. Anal Chem. 2015;87(18):9500–9506. doi: 10.1021/acs.analchem.5b02899. [DOI] [PubMed] [Google Scholar]
- 193.Cialla D, et al. Surface-enhanced Raman spectroscopy (SERS): progress and trends. Anal Bioanal Chem. 2012;403(1):27–54. doi: 10.1007/s00216-011-5631-x. [DOI] [PubMed] [Google Scholar]