Abstract
In a previous study, we have demonstrated that S-methylmethionine sulfonium (SMMS) confers wound-healing and photoprotective effects on the skin, suggesting that SMMS can be used as a cosmetic raw material. However, it has an unpleasant odor. Therefore, in the present study, we synthesized odor-free SMMS derivatives by eliminating dimethyl sulfide, which is the cause of the unpleasant odor and identified two derivatives that exhibited skin-protective effects: one derivative comprised (2S,4S)- and (2R,4S)-2-phenylthiazolidine-4-carboxylic acid and the other comprised (2S,4R)-, (2S,4S)-, (2R,4R)-, and (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid. We performed in vitro proliferation assays using human dermal fibroblasts (hDFs) and an immortalized human keratinocyte cell line (HaCaT). The two SMMS derivatives were shown to increase hDF and HaCaT cell proliferation as well as improve their survival by protecting against ultraviolet exposure. Moreover, the derivatives regulated the expression of collagen type I and MMP mRNAs against ultraviolet exposure in hDFs, suggesting that these derivatives can be developed as cosmetic raw materials.
Keywords: S-methylmethionine derivatives, Proliferation, UVA/B protection, Reactive oxygen species
INTRODUCTION
S-Methylmethionine sulfonium (SMMS) is a methionine derivative found most commonly in plant sources, such as cabbage, kohlrabi, turnip, tomatoes, and celery (Turner and Shapiro, 1961; Skodak et al., 1965; Samson, 1971; Hattula and Granroth, 1974). SMMS is widely referred to as vitamin U because of its potent therapeutic effect in preventing gastrointestinal ulceration (Cheney, 1949; Samson, 1971; Kopinski et al., 2007). For example, rapid healing of peptic ulcers has been reported in patients consuming fresh cabbage juice (Cheney, 1949). Recently, protective effects of SMMS in valproic acid-induced liver and kidney injury have been reported (Sokmen et al., 2012; Gezginci-Oktayoglu et al., 2016). In addition, hypolipidemic effects and inhibitory effects of SMMS on differentiation of 3T3-L1 pre-adipocytes have been reported (Matsuo et al., 1980a, 1980b; Nakamura et al., 1981; Lee et al., 2012).
Previously, we have demonstrated that SMMS confers wound-healing and photoprotective effects on skin and can thus be used as a cosmetic raw material (Kim et al., 2010, 2015). Animal experiments have shown that topical administration of SMMS to both physical and chemical wounds facilitates wound closure and promotes re-epithelialization (Kim et al., 2010, 2015). SMMS treatment is sufficient to promote the growth and migration of human dermal fibroblasts (hDFs); the promotion of hDF proliferation and migration is caused by significant activation of the ERK1/2 pathway (Kim et al., 2010). In addition, SMMS protects skin from UVB irradiation by scavenging reactive oxygen species. SMMS treatment has also been shown to decrease the UVB radiation-induced erythema index and depletion of Langerhans cells in an animal experiment (Kim et al., 2015). Moreover, SMMS protects keratinocyte progenitor cells and hDFs against UVB irradiation (Kim et al., 2015).
Although SMMS is effective in skin regeneration, its unpleasant odor is a major disadvantage (Kovacheva, 1974; Kovatscheva and Popova, 1977). The unpleasant odor may be caused by the sulfonium functional group (Hattula and Granroth, 1974; Loscos et al., 2008); reportedly, heat causes SMMS breakdown and dimethyl sulfide formation during the malting process (Loscos et al., 2008). Similarly, cosmetics containing SMMS have an unpleasant odor, which intensifies over time. As mentioned above, SMMS has wound-healing and UVB-protective effects on skin; however, the unpleasant odor limits its application as a cosmetic raw material. Therefore, in the present study, we synthesized 50 SMMS derivatives to reduce its odor. Using in vitro activity assays with hDFs and an immortalized human keratinocyte cell line (HaCaT), we identified two SMMS derivatives that exhibited skin-protective effects and can thus be used as cosmetic raw materials.
MATERIALS AND METHODS
Synthesis of compounds 15 and 16
Benzaldehyde (0.74 mM) was added to a stirred solution of L-cysteine or DL-homocysteine (0.74 mM) in ethanol (4 mL) and distilled water (1 mL). After 3 days of stirring at room temperature, the precipitate was suction-filtered, washed with ether, and dried under vacuum to yield the corresponding products as white solids.
Cell culture
hDFs and HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; low, high glucose; Hyclone, Thermo Scientific, Logan, UT, USA) with 10% fetal bovine serum (FBS; Gibco, Invitrogen, Carlsbad, CA, USA), 1% penicillin, and streptomycin (Gibco) at 37°C in 5% CO2 in a humidified atmosphere (Kim et al., 2015, 2016). The hDF and HaCaT media were replaced with fresh media every 2 days.
Cell proliferation assay using InCu safe system
HaCaT cells (4×104/well) and hDFs (3×104/well) were seeded into six-well plates in DMEM with 10% FBS and cultured for 24 h; subsequently, they were starved with serum-free DMEM media for 16 h. Next, cells were treated with SMMS and its derivatives and then incubated for 3–5 days in InCu safe (Panasonic, Osaka, Japan) to automatically analyze cell proliferation index. Proliferation percentage was analyzed using the InCu Cyte zoom2014A program.
UV irradiation
Cells were seeded in plates, and after 24 h, they were washed with phosphate-buffered saline (PBS) and covered with a thin layer of PBS prior to UV exposure. The culture plate lid was removed, and cells were irradiated (UVA: 7 J/cm2; UVB: 120 mJ/cm2) in a dark box. As the UVA irradiation source, a UVA lamp (TUV 15W/G15 T8) purchased from Philips (Groningen, The Netherlands) was used. The UVB irradiation apparatus (BLE-1T158) was obtained from Spectronics (Westbury, NY, USA). The incident dose of UVA or UVB was measured using a Waldmann UV meter (model No. 585100; Waldmann Co., Villingen-Schwenningen, Germany). After UV irradiation, PBS was replaced with culture medium, and then cells were incubated under standard conditions for 24 h prior to analysis.
MTT assay
UV-irradiated cells were incubated in DMEM in the presence or absence of SMMS and its derivatives for 48 h, and then an MTT assay was performed (Kim et al., 2016). The MTT solution (5 μg/mL in PBS) was added to each well at 5% of the medium volume. The cells were incubated at 37°C for 2 h and the supernatant was removed. Dimethyl sulfoxide was then added in order to dissolve the formazan crystals and the absorbance was measured at 595 nm using an ELISA reader (TECAN, Grodig, Austria).
BrdU labeling assay
For the 5-bromo-2′-deoxyuridine (BrdU) labeling assay of hDFs or HaCaT cells, 5×103 cells were seeded in six-well plates and were irradiated with 120 mJ/cm2 of UVB. Next, the cells were incubated in DMEM in the presence or absence of SMMS derivatives for 48 h, and then BrdU labeling was performed. BrdU (Sigma-Aldrich, St. Louis, MO, USA) was added to the cell culture media at a final concentration of 100 μM and incubated for 2 h at 37°C in 5% CO2. The cells were fixed with 4% paraformaldehyde, incubated with mouse anti-BrdU (1:500) (Abcam, Cambridge, MA, USA) overnight at 4°C, and then incubated with Alexa Fluro 488 goat anti-mouse IgG. Immunofluorescence staining was imaged using a ZEISS LSM710 confocal microscope (Invitrogen, NY, USA).
RT-polymerase chain reaction (PCR)
Total RNA was extracted from hDFs using TRIzol reagent, and reverse transcription was performed to obtain cDNA. The following oligonucleotides were used as primers: collagen type I (5′-TAGG-GTCTAGACATGTTCAGCTTTGT-3′ and 5′-GTGATTGG TGGGATGTCTTCGT-3′), MMP-1 (5′-AGAT-GTGGAGTGCCTGATGT-3′ and 5′-AGCTA GGGTACATCAAAGCC-3′), and the control glyceraldehyde 3-phosphate dehydro genase (GAPDH) (5′-CGAGA-TCCCTCCAAAATCAA-3′ and 5′-TGTGGTCATGAGTCCTCCCA-3′). PCR amplification of cDNA was performed in a total volume of 30 μL under the following conditions: an initial denaturation at 95°C for 5 min; 35 cycles of 95°C for 30 s, 54°C for 20 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. The gapdh mRNA level was used for sample standardization.
Statistical analysis
All data are representative of independent experiments performed in triplicate. The statistical significance of differences among groups was tested using a Student’s t-test, and p<0.05, p<0.01, and p<0.001 were considered as significant.
RESULTS
Screening of SMMS derivatives using hDFs
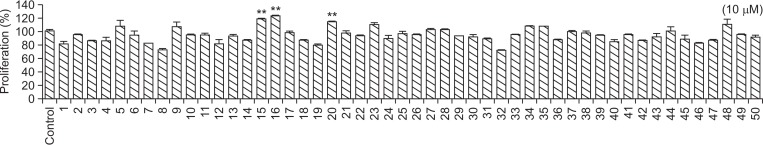
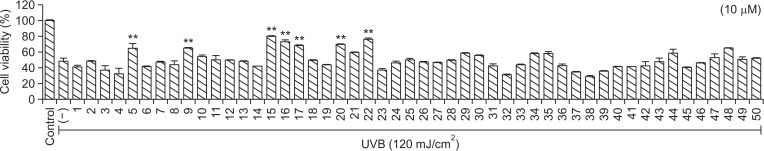
To increase SMMS stability, we synthesized 50 SMMS derivatives and screened their proliferative and UVB-protective effects on hDFs. As shown in Fig. 1, compounds 15, 16, and 22 increased hDF proliferation at a concentration of 10 μM (Fig. 1). UVB irradiation (120 mJ/cm2) significantly reduced hDF survival (Fig. 2). However, treatment with compounds 5, 9, 15, 16, 17, 20, and 22 following UVB irradiation increased hDF survival (Fig. 2). Although compounds 5 and 9 were effective in protecting hDFs against UVB irradiation (Fig. 2), they are degradable and have an unpleasant odor (data not shown). The structures of compounds 17, 20, and 22 are similar to those of compounds 15 and 16 (Supplementary Fig. 1). However, the proliferative and protective effects of compounds 17, 20, and 22 were not as good as those of 15 and 16 (Fig. 1, 2). Therefore, we further examined the skin-protective effects of compounds 15 and 16 in hDFs and HaCaT cells.
Fig. 1.
Screening of S-methylmethionine sulfonium (SMMS) derivatives in human dermal fibroblasts (hDFs). Effect of SMMS derivatives (10 μM) on hDF proliferation. **p<0.01, n=3. All error bars indicate SEM.
Fig. 2.
Effect of S-methylmethionine sulfonium (SMMS) derivatives on the survival of human dermal fibroblasts (hDFs) after UVB irradiation. UVB irradiation (120 mJ/cm2) reduced the viability of hDF cells (−); however, many SMMS derivatives (10 μM) recovered the viability of hDFs reduced by UVB. **p<0.01, n=3. All error bars indicate SEM.
Characterization of compounds 15 and 16
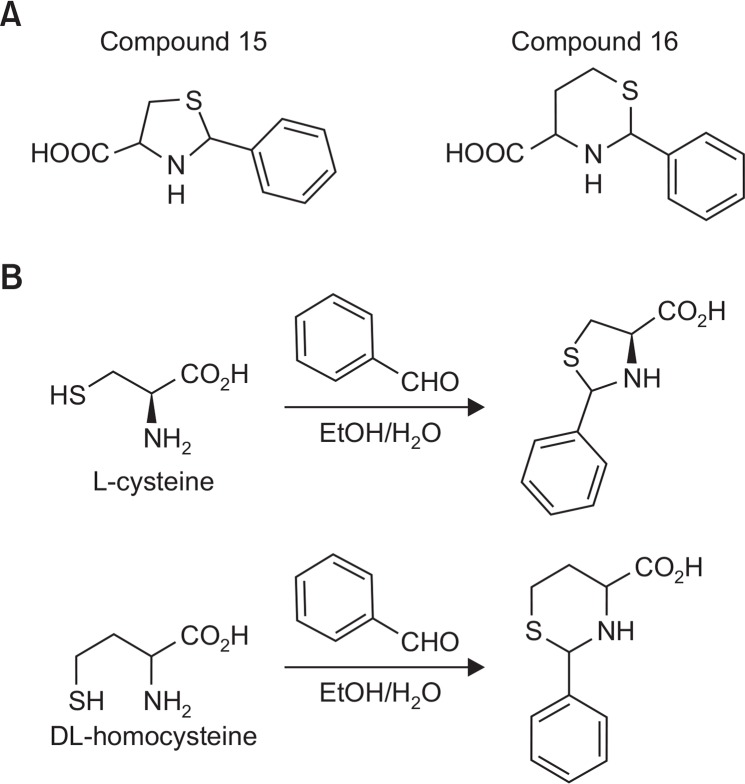
Compound 15 (Fig. 3A) comprises (2S,4S)- and (2R,4S)-2-phenylthiazolidine-4-carboxylic acid and appears as a white solid; 1H NMR (400 MHz, DMSO-d6) δ 7.25–7.52 (m, 5H), 5.67 (s, 0.6H), 5.50 (s, 0.4H), 4.23 (dd, J=4.8, 4.8 Hz, 0.6H), 3.90 (dd, J=7.6, 8.0 Hz, 0.4H), 3.28–3.40 (m, 1H), 3.06–3.16 (m, 1H) (Fig. 3B). Compound 16 (Fig. 3A) comprises (2S,4R)-, (2S,4S)-, (2R,4R)-, (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid and appears as a white solid; 1H NMR (400 MHz, DMSO-d6) δ 7.28–7.45 (m, 5H), 5.27 (s, 1H), 3.60 (dd, J=3.2, 12 Hz, 1H), 3.19–3.26 (m, 1H), 2.88–2.93 (m, 1H), 2.04–2.08 (m, 1H), 1.50 (m, 1H) (Fig. 3B).
Fig. 3.
Structure (A) and synthesis (B) of S-methylmethionine sulfonium (SMMS) derivatives.
Proliferative and protective effects of SMMS derivatives on hDFs
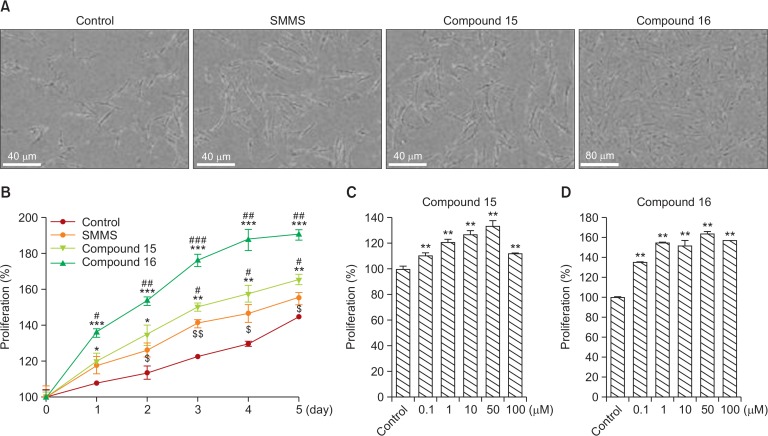
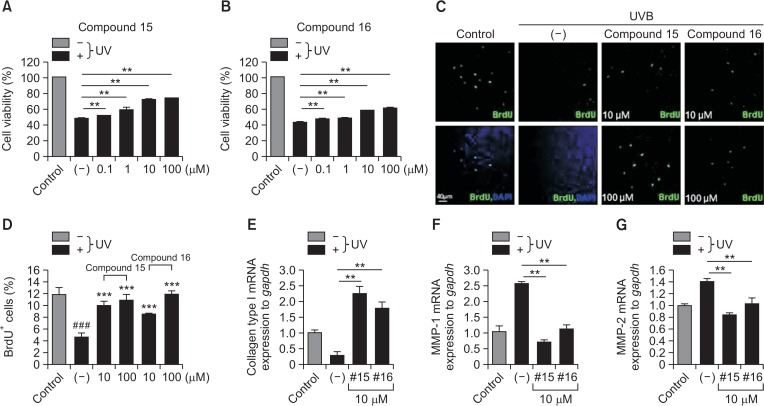
As we initially measured the proliferative and protective effects of compounds 15 and 16 at a concentration of 10 μM (Fig. 1, 2), we further examined the protective effects of compounds 15 and 16 on hDFs in a time- or dose-dependent manner. SMMS and its derivatives, compounds 15 and 16, increased the proliferation ratio in a time-dependent manner for 5 days (Fig. 4A, 4B). Compounds 15 and 16 increased hDF proliferation in a dose-dependent manner up to 100 μM concentration (Fig. 4C, 4D). Compounds 15 and 16 restored the reduced hDF survival and proliferation following UVB irradiation up to 100 μM concentration (Fig. 5A–5D). In addition, in hDFs, these compounds recovered the expression of collagen type I mRNA that had been downregulated by UVB irradiation (Fig. 5E). In hDFs, these compounds also restored the expression of metalloproteinases, MMP-1, and MMP-2 that had been upregulated by UVB irradiation (Fig. 5F, 5G). Furthermore, SMMS and compounds 15 and 16 protected hDFs from UVA irradiation (Supplementary Fig. 2; UVA dose: 7 J/cm2). These results suggested that SMMS derivatives increased hDF survival and proliferation and protected them against UV irradiation by regulating the expression of collagen type I and MMPs.
Fig. 4.
Effect of compounds 15 and 16 on human dermal fibroblasts (hDFs). (A) Cell photography and (B) cell proliferation after treatment with S-methylmethionine sulfonium (SMMS), compound 15, and compound 16 (10 μM). Compounds 15 and 16 increased hDF proliferation in a time-dependent manner. $control vs. SMMS; #SMMS vs. compound 15 or 16; *control vs. compound 15 or 16. $p<0.05, $$p<0.01; #p<0.05, ##p<0.01, ###p<0.001; *p<0.05, **p<0.01, ***p<0.001; n=3. All error bars indicate SEM. (C, D) Compounds 15 and 16 increased hDF proliferation in a dose-dependent manner. **p<0.01, n=3. All error bars indicate SEM.
Fig. 5.
Effect of compounds 15 and 16 on human dermal fibroblasts (hDFs). (A) Compound 15 recovered the reduced survival of hDFs following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. (B) Compound 16 recovered the reduced survival of hDFs following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. (C, D) Compounds 15 and 16 recovered the reduced proliferation index (% of BrdU+ cells) of hDFs following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. #control vs. (−); *(−) vs. compound 15 or 16. ***p<0.001, ###p<0.001; n=5–8. All error bars indicate SEM. (E–G) Compounds 15 and 16 restored the downregulated expression of collagen type I mRNA (E) and upregulated expression of MMP-1 and MMP-2 mRNAs (F, G) following UVB irradiation in hDFs. **p<0.01, n=3. All error bars indicate SEM.
Proliferative and protective effects of SMMS derivatives on HaCaT cells
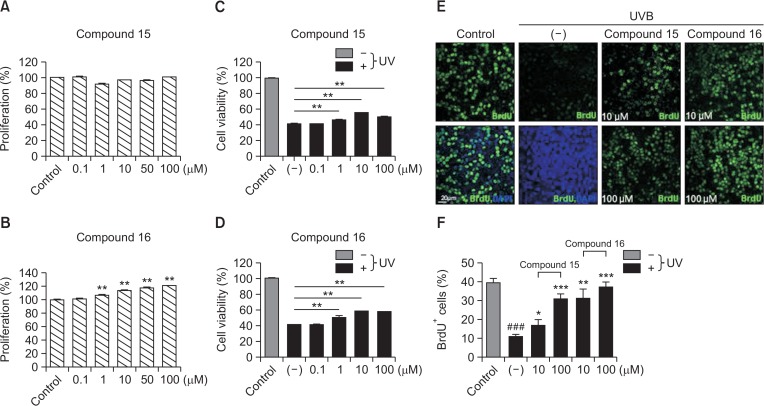
We next examined the proliferative and protective effects of SMMS derivatives on HaCaT cells. Compound 15 did not increase HaCaT cell proliferation (Fig. 6A); however, it recovered their survival and proliferation that had been decreased by UVB irradiation (Fig. 6C, 6E, and 6F). Compound 16 increased the HaCaT cell proliferation (Fig. 6B) and also recovered their survival and proliferation that had been decreased by UVB irradiation (Fig. 6D–6F). These results suggested that SMMS derivatives increased HaCaT cell proliferation and survival by protecting them against UVB irradiation.
Fig. 6.
Effect of compounds 15 and 16 in an immortalized human keratinocyte cell line (HaCaT). (A) Compound 15 did not increase HaCaT cell proliferation. (B) Compound 16 increased HaCaT cell proliferation in a dose-dependent manner. (C) Compound 15 recovered the reduced survival of HaCaT following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. (D) Compound 16 recovered the reduced survival of HaCaT following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. **p<0.01, n=3. All error bars indicate SEM. (E, F) Compounds 15 and 16 recovered the reduced proliferation index (% of BrdU+ cells) of HaCaT cells following UVB irradiation (120 mJ/cm2) in a dose-dependent manner. #control vs. (−); *(−) vs. compound 15 or 16. *p<0.05, **p<0.01, ***p<0.001; ###p<0.001; n=4–5. All error bars indicate SEM.
DISCUSSION
In the present study, we synthesized 50 SMMS derivatives and investigated whether these compounds exhibited increased stability and reduced unpleasant odor. We then performed in vitro activity assays using hDFs and HaCaT cells and identified two SMMS derivatives (compounds 15 and 16) that increased their proliferation and survival. Compound 15 comprises (2S,4S)- and (2R,4S)-2-phenylthiazolidine-4-carboxylic acid, and compound 16 comprises (2S,4R)-, (2S,4S)-, (2R,4R)-, and (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid. These compounds protect skin cells against UVB irradiation. These SMMS derivatives are easy to synthesize and are effective for skin repair and regeneration; thus, they can be developed as cosmetic raw materials.
We first synthesized linear vitamin U derivatives with structures similar to vitamin U containing a sulfonium ion (i.e., compounds 5 and 9 that were effective in protecting hDFs against UVB). However, these compounds were degradable and had an unpleasant odor at room temperature. Therefore, we cyclized the vitamin U derivatives and found that compounds 15 and 16 also increased the proliferation and survival of hDFs and HaCaT cells. Compared with the sulfonium ion-containing vitamin U derivatives, compounds 15 and 16 are odor-free. Although compounds 17, 20, and 22 slightly increased hDF survival following UVB irradiation, these compounds were not as potent as compounds 15 and 16 in terms of hDF proliferation and protection.
In addition to skin-protective effects, compound 15 and 16 do not have an unpleasant odor in either solid or liquid form. As the unpleasant odor is caused by the degradation of the sulfonium functional group and the formation of dimethyl sulfide, we synthesized SMMS derivatives containing a cyclic ring to avoid degradation and dimethyl sulfide formation. Therefore, compounds 15 and 16 do not have an unpleasant order in either solid or liquid and are promising as cosmetic raw materials.
Compound 15 comprises (2S,4S)- and (2R,4S)-2-phenylthiazolidine-4-carboxylic acid. It was originally synthesized to evaluate its anticancer activities. Compound 15 is active as a cysteine precursor; however, it has been shown to have no effect on the growth of a methionine-dependent tumor in rats (Recasens et al., 1992). In addition, a series of substituted 2-phenylthiazole-4-carboxamide derivatives have been synthesized as potential cytotoxic agents and evaluated against three human cancer cell lines (Aliabadi et al., 2010). However, in the present study, we found that compound 15 increased the proliferation and survival of hDFs and HaCaT cells. In addition, 2-substituted thiazolidine-4(R)-carboxylic acids act as prodrugs of L-cysteine and protect mice against acetaminophen hepatotoxicity (Nagasawa et al., 1984), indicating that compound 15 is cytoprotective. Furthermore, (2R/S,4R)-2-(2,4-dimethoxyphenyl) thiazolidine-4-carboxylic acid inhibits the L-DOPA oxidase activity of mushroom tyrosinase and can be used to inhibit melanin synthesis. Taken together, these findings indicate that compound 15 is a promising candidate for use as a skin-rejuvenating agent.
Compound 16 comprises (2S,4R)-, (2S,4S)-, (2R,4R)-, and (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid. To date, no report on the direct pharmacological effect of compound 16 has been published. To our knowledge, the present study is the first to demonstrate that compound 16 increases skin cell proliferation and protects skin cells against UVB irradiation. In addition, compound 16 is more effective than compound 15 in promoting HaCaT cell proliferation and is easy to synthesize without degradation at room temperature. Therefore, compound 16 can be developed as a more promising cosmetic raw material than compound 15.
Compounds 15 and 16 also regulate collagen type I, MMP-1, and MMP-2 expression. They restore collagen type I expression downregulated by and MMP expression upregulated by UVB exposure (Fig. 5E–5G). The collagenous component of the dermal extracellular matrix is responsible for the strength and resiliency of skin and is intimately involved in the pathology of photoaging. In photoaged human skin, collagen expression is substantially reduced in the papillary dermis; this reduction results from reduced procollagen biosynthesis and increased enzymatic breakdown via the action of MMPs (Kim et al., 2007). Thus, compounds 15 and 16 may help in the recovery of photoaged skin by regulating collagen and MMP expression.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HN14C0084). Jong-Hyuk Sung was partly supported by Yonsei University (2014-11-1658).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare and have not received any payment for the preparation of this manuscript.
REFERENCES
- Aliabadi A, Shamsa F, Ostad SN, Emami S, Shafiee A, Davoodi J, Foroumadi A. Synthesis and biological evaluation of 2-phenylthiazole-4-carboxamide derivatives as anticancer agents. Eur J Med Chem. 2010;45:5384–5389. doi: 10.1016/j.ejmech.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Cheney G. Rapid healing of peptic ulcers in patients receiving fresh cabbage juice. Calif Med. 1949;70:10–15. [PMC free article] [PubMed] [Google Scholar]
- Gezginci-Oktayoglu S, Turkyilmaz IB, Ercin M, Yanardag R, Bolkent S. Vitamin U has a protective effect on valproic acid-induced renal damage due to its anti-oxidant, anti-inflammatory, and anti-fibrotic properties. Protoplasma. 2016;253:127–135. doi: 10.1007/s00709-015-0796-3. [DOI] [PubMed] [Google Scholar]
- Hattula T, Granroth B. Formation of dimethyl sulphide from S-methylmethionine in onion seedlings (Allium cepa). J Sci Food Agric. 1974;25:1517–1521. doi: 10.1002/jsfa.2740251212. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park SH, Park SG, Choi JS, Xia Y, Sung JH. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011;20:1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Kim I, Kim WK, Choi JY, Kim DY, Moon SG, Min HK, Song MK, Sung JH. Mitochondria-targeted vitamin E protects skin from UVB irradiation. Biomol Ther (Seoul) 2016;24:305–311. doi: 10.4062/biomolther.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Kim WS, Seo HM, Kim WK, Choi JS, Kim I, Sung JH. The Photoprotective effect of S-methylmethionine sulfonium in skin. Int J Mol Sci. 2015;16:17088–17100. doi: 10.3390/ijms160817088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Yang YJ, Min HG, Song MG, Lee JS, Park KY, Kim JJ, Sung JH, Choi JS, Cha HJ. Accelerated wound healing by S-methylmethionine sulfonium: evidence of dermal fibroblast activation via the ERK1/2 pathway. Pharmacology. 2010;85:68–76. doi: 10.1159/000276495. [DOI] [PubMed] [Google Scholar]
- Kopinski JS, Fogarty R, McVeigh J. Effect of s-methylmethionine sulphonium chloride on oesophagogastric ulcers in pigs. Aust Vet J. 2007;85:362–367. doi: 10.1111/j.1751-0813.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Kovacheva EG. A method of determining S-methylmethionine in natural products. Prikladnaia Biokhimiia I Mikrobiologiia. 1974;10:129–135. [PubMed] [Google Scholar]
- Kovatscheva EG, Popova JG. S-methylmethionine content in plant and animal tissues and stability during storage. Nahrung. 1977;21:465–472. doi: 10.1002/food.19770210602. [DOI] [PubMed] [Google Scholar]
- Lee NY, Park KY, Min HJ, Song KY, Lim YY, Park J, Kim BJ, Kim MN. Inhibitory effect of vitamin U (S-methylmethionine sulfonium chloride) on differentiation in 3T3-L1 pre-adipocyte cell lines. Ann Dermatol. 2012;24:39–44. doi: 10.5021/ad.2012.24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscos N, Segurel M, Dagan L, Sommerer N, Marlin T, Baumes R. Identification of S-methylmethionine in Petit Manseng grapes as dimethyl sulphide precursor in wine. Anal Chim Acta. 2008;621:24–29. doi: 10.1016/j.aca.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Seri K, Kato T. Comparative effects of S-methylmethionine (vitamin U) and methionine on choline-deficient fatty liver in rats. Arzneimittelforschung. 1980a;30:68–69. [PubMed] [Google Scholar]
- Matsuo T, Seri K, Kato T. A possible activation of cholesterol 7-hydroxylation by S-methylmethionine (vitamin U). Arzneimittelforschung. 1980b;30:258–259. [PubMed] [Google Scholar]
- Nagasawa HT, Goon DJ, Muldoon WP, Zera RT. 2-Substituted thiazolidine-4(R)-carboxylic acids as prodrugs of L-cysteine. Protection of mice against acetaminophen hepatotoxicity. J Med Chem. 1984;27:591–596. doi: 10.1021/jm00371a006. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Uzawa H, Kanazawa K, Tamai Y, Tashiro Y, Koide M. Hypolipidemic effect of L-form S-methylmethionine sulfonium chloride in man. Arzneimittelforschung. 1981;31:725–729. [PubMed] [Google Scholar]
- Recasens MA, Possompes B, Astre C, Saint Aubert B, Joyeux H. Thiazolidine-4-carboxylate and 2-phenylthiazolidine-4-carboxylate are active as cysteine precursors but have no effect on growth of a methionine-dependent tumor in rats. J Nutr. 1992;122:19–27. doi: 10.1093/jn/122.1.19. [DOI] [PubMed] [Google Scholar]
- Samson EI. The effect of S-methylmethionine (vitamin U) on the acid-forming function of the stomach in peptic ulcer. Vrach Delo. 1971;12:33–35. [PubMed] [Google Scholar]
- Skodak FI, Wong FF, White LM. Determination of S-methylmethionine ion in plant materials by automated amino acid analysis. Anal Biochem. 1965;13:568–571. doi: 10.1016/0003-2697(65)90354-4. [DOI] [PubMed] [Google Scholar]
- Sokmen BB, Tunali S, Yanardag R. Effects of vitamin U (S-methyl methionine sulphonium chloride) on valproic acid induced liver injury in rats. Food Chem Toxicol. 2012;50:3562–3566. doi: 10.1016/j.fct.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Turner JE, Shapiro SK. S-methylmethionine- and S-adenosylmethionine-homocysteine transmethylase in higher plant seeds. Biochim Biophys Acta. 1961;51:581–584. doi: 10.1016/0006-3002(61)90617-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.