Abstract
A type of breast cancer with a defect in three molecular markers such as the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor is called triple-negative breast cancer (TNBC). Many patients with TNBC have a lower survival rate than patients with other types due to a poor prognosis. In this study, we confirmed the anti-cancer effect of a natural compound, Gomisin G, in TNBC cancer cells. Treatment with Gomisin G suppressed the viability of two TNBC cell lines, MDA-MB-231 and MDA-MB-468 but not non-TNBC cell lines such as MCF-7, T47D, and ZR75-1. To investigate the molecular mechanism of this activity, we examined the signal transduction pathways after treatment with Gomisin G in MDA-MB-231 cells. Gomisin G did not induce apoptosis but drastically inhibited AKT phosphorylation and reduced the amount of retinoblastoma tumor suppressor protein (Rb) and phosphorylated Rb. Gomisin G induced in a proteasome-dependent manner a decrease in Cyclin D1. Consequently, Gomisin G causes cell cycle arrest in the G1 phase. In contrast, there was no significant change in T47D cells except for a mild decrease in AKT phosphorylation. These results show that Gomisin G has an anti-cancer activity by suppressing proliferation rather than inducing apoptosis in TNBC cells. Our study suggests that Gomisin G could be used as a therapeutic agent in the treatment of TNBC patients.
Keywords: AKT, Cell cycle, Cell proliferation, Cyclin D1, Gomisin G, Triple negative breast cancer
INTRODUCTION
Cancer is the second leading cause of morbidity and mortality worldwide. In 2015, around 17.5 million cancer cases prevailed with 8.7 million reported deaths. Globally, breast cancer is commonly diagnosed and the leading cause of cancer death among women (Fitzmaurice et al., 2017). Diagnosis and treatment of breast cancer is challenging and intricate due to its heterogeneous nature (Hutchinson, 2010). One such subtype of breast cancer is TNBC which is deficient in the expression of three molecular markers such as the estrogen receptor (ERα), progesterone receptor (PR), and human epidermal growth factor receptor (HER2) (Anders and Carey, 2009; Chavez et al., 2010; Foulkes et al., 2010; Pareja et al., 2016). TNBC has an aggressive clinical behavior and a mutation in breast cancer gene 1 (BRCA1) gene and accounts for 10–15% of breast cancers (Chavez et al., 2010; Anders and Carey, 2009). TNBC is extremely metastatic from the initial stage followed by mortality within 5 years of diagnosis (Anders and Carey, 2009; Pareja et al., 2016). Breast cancers expressing ER, PR and HER2 are treated with a combination of chemotherapy and target molecules that inhibit the production of hormones and their action mechanisms. Therefore, the prognosis of breast cancers expressing hormone receptors are advantageous over TNBC (Anders and Carey, 2009; Chavez et al., 2010). Large-scale research has been ongoing to comprehend the complexity and heterogeneity of TNBC. Moreover, intense efforts are ongoing to find molecular targets to refine prevailing treatments for TNBC (Collignon et al., 2016).
Gomisins, phytoestrogens, are dibenzocyclooctadiene lignans (type A) extracted from the fruits of the natural plant Schisandra chinesis (Opletal et al., 2004). These compounds have been essentially used in traditional medicine in Korea, China, Japan and Russia. S. chinesis also contains triterpenoids, polysaccharides, and sterols (Opletal et al., 2004; Choi et al., 2006; Min et al., 2008). Gomisins exhibit various therapeutic potential such as anti-inflammatory, anti-obesity, anti-oxidant, anti-cancer and liver protectant (Choi et al., 2006; Min et al., 2008; Oh et al., 2010; Park et al., 2014; Jang et al., 2017). One of the important components isolated from S. chinesis is Gomisin G, which has been reported to have anti-HIV, anti-liver cancer and anti-inflammatory activity (Chen et al., 1997; Ryu et al., 2011; Xiaoyang et al., 2015).
In this study, we evaluated the Gomisin G activity in the TNBC cell line MDA-MB-231. We show that Gomisin G inhibited the growth and proliferation of MDA-MB-231 cells but had no effect on T47D cells. Furthermore, we investigated its mechanism of action in MDA-MB-231 cells. Gomisin G induced cell cycle arrest at the G1 phase through an AKT-cyclin D1 dependent mechanism; however, apoptosis was not involved in the process. Our study shows that Gomisin G has potential as an anti-cancer therapeutic for TNBC.
MATERIALS AND METHODS
Gomisin G
Gomisin G was purchased from Biopurify Phytochemicals Ltd (Sichuan, China). Its purity was determined using an Agilent 1100 series high performance liquid chromatography (HPLC) system fitted with a RP-C18 column (Gemini, 5 µm, 4.6×250 mm; Phenomenex, Torrance, CA, USA) at room temperature. A UV/VIS detector (Agilent Technologies, Santa Clara, CA, USA) was used at 220 nm. The mobile phase was 65% aqueous acetonitrile, and the flow rate was 3.0 mL/min. Its chromatogram showed 98% purity; thus, it was used without further purification.
Cell culture
Human breast cancer cell lines MDA-MB-231 (TNBC cell line), MDA-MB-468 (TNBC cell line), MCF-7 (ERα and PR-positive cell line), T47D (ERα and PR-positive cell line) and ZR75-1 (ERα-positive cell line) were used in the study. MCF-7, MDA-MB-231, and MDA-MD-468 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). T47D and ZR75-1 cells were purchased from the Korean Cell Line Bank (Seoul, Korea). MDA-MB-231 and MDA-MB-468 cells were cultured in Leibovitz’s L-15 medium (Thermo Fisher Scientific, Waltham, MA, USA). T47D cells were maintained in RPMI-1640 medium (Thermo Fisher Scientific). MCF-7 cells were maintained in Eagle’s Minimum Essential Medium (ATCC) supplemented with 0.01 mg/mL human recombinant insulin. The media were complemented with 10% fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 µg/mL streptomycin. MDA-MB-468, MCF-7, T47D and ZR75-1 cells were incubated at 37°C in an atmosphere of 5% CO2, and MDA-MB-231 cells were cultured at 37°C in the absence of CO2.
MTT assay
The viability of MDA-MB231 and T47D cells were examined with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) (Kim et al., 2016). The cells were treated with Gomisin G (0, 1, 5, and 10 µM) and dimethyl sulfoxide (DMSO) as a control for 3 and 5 days. After the indicated period, MTT solution was added to each well and incubated for 4 h at 37°C. The media containing the MTT solution was suctioned out, and the formazan crystals were dissolved in DMSO. The absorbance was read at 570 nm with a spectrophotometer (Molecular Devices, Orleans, CA, USA).
Immunoblotting
Cells were lysed and centrifuged at 14,000 rpm for 15 min. at 4°C. The supernatant was separated with SDS-PAGE and transferred to a nitrocellulose membrane (NC). The membrane was blocked and incubated with various primary anti-bodies followed by HRP-conjugated secondary antibodies. Detection was carried out by applying chemiluminescence reagent (Thermo Fisher Scientific) and visualized with Chemi-Doc (Bio-Rad, Hercules, California, USA). Antibodies against poly-ADP ribose polymerase (PARP), caspase-3, phospho (p) ERK, ERK, pAKT, AKT, pP38, P38, pRB, RB, and cyclin D1 were purchased from Cell Signaling Technology (Danvers, MA, USA). For the inhibition of proteasomal degradation, MDA-MB-231 cells were treated with 10 µM of Gomisin G for 48 h followed by treatment with 10 µM of MG132 (Cayman Chemicals, Ann Arbor, Michigan, USA) for 6 h. Then, cell lysates were prepared followed by western blotting. Antibodies against ubiquitin and β-actin were purchased from Santa Cruz Biotechnology (Minneapolis, MN, USA) and Sigma-Aldrich, respectively.
Annexin V and propidium iodide (PI) staining
MDA-MB-231 and T47D cells were treated with or without 10 µM of Gomisin G for 24 h. The cells were detached by trypsin-EDTA (Thermo Fisher Scientific). The collected cells were washed with FACS buffer (1% FBS in PBS) followed by resuspension in buffer containing annexin V (eBioscience, San Diego, CA, USA) for 15 min at room temperature in the dark. Next, the cells were incubated with PI (eBioscience, San Diego, CA, USA). The cells were quickly analyzed with FAC-SCalibur (BD Biosciences, San Jose, CA, USA), and the data were analyzed with the Flowing software (Turku Centre for Biotechnology, Turun Yliopisto, Finland) (Kang et al., 2016).
Cell cycle analysis
MDA-MB-231 and T47D cells were treated with or without 10 µM of Gomisin G for 72 h. The cells were collected and washed with FACS buffer (1% FBS in PBS) followed by fixing with 70% ethanol in PBS overnight at 4°C. Next, the fixed cells were washed with FACS buffer and treated with RNase (Sigma-Aldrich) for 30 min at 37°C. The cells were incubated with PI and immediately analyzed with FACSCalibur (BD Biosciences).
RESULTS
Growth inhibitory action of Gomisin G is not through apoptosis in MDA-MB-231 cells
To investigate possible applications of Gomisin G in the treatment of breast cancer, we first analyzed its inhibitory effect on growth in several breast cancer cell lines using the MTT assay and apoptosis assay. Gomisin G selectively reduced the growth of MDA-MB-231 and MDA-MB-468 cells (TNBC cells) but not the other breast cancer cell lines (Fig. 1). Therefore, we used T47D cells as a negative control in this study. We next determined whether the Gomisin G-induced poor cell viability was prompted by apoptosis. Annexin V and PI staining in MDA-MB-231 cells exposed to Gomisin G showed no apoptosis (Fig. 2A). Furthermore, no changes were observed in immunoblotting detecting for apoptosis markers PARP and caspase-3 (Fig. 2B). Our results suggest the inhibitory action of Gomisin G is not through apoptosis in MDA-MB-231 cells.
Fig. 1.
Effect of Gomisin G on the proliferation of breast cancer cell lines. (A) MDA-MB-231, (B) MDA-MD-468, (C) MCF-7, (D) T47D, and (E) ZR75-1 cells were untreated (−), or treated with DMSO (D) and 1, 5, or 10 µM of Gomisin G for 3 and 5 days followed by the MTT assay.
Fig. 2.
Apoptosis analysis of MDA-MB-231 and T47D cells. (A) Apoptosis was measured using an annexin V and PI detection kit and analyzed with FACSCalibur. MDA-MB-231 cells were treated with 10 µM of Gomisin G for 24 h. (B) MDA-MB-231 and T47D cells were treated with 10 µM of Gomisin G for 24, 48, and 72 h and analyzed with immunoblotting. Blots were detected with PARP, caspase-3 and β-actin antibodies.
Gomisin G inhibits AKT phosphorylation
AKT is involved in regulating cell proliferation and is found to be overexpressed in various tumors (Chang et al., 2003; Arcaro and Guerreiro, 2007). Therefore, we next explored its involvement in the Gomisin G-mediated decrement of cell proliferation. The treatment of Gomisin G in MDA-MB-231 cells led to a remarkable reduction in the level of AKT phosphorylation at various time points (Fig. 3A). There was no change in the protein level of total AKT. We further investigated the effect on signaling molecules such as ERK and P38. The Gomisin G treatment also lowered the ERK phosphorylation level; however, no difference in the level of P38 phosphorylation was seen in Fig. 3A. Because Rb regulates the expression of cell cycle related genes, we checked the protein levels of Rb. The Gomisin G treatment significantly reduced Rb phosphorylation and reduced the total Rb levels in MDA-MB-231 cells. In contrast, treatment of Gomisin G in T47D cells did not result in any significant changes in the signaling molecules except for AKT phosphorylation (Fig. 3B). It is likely that the inhibitory effect of Gomisin G on AKT phosphorylation alone is not enough to induce growth suppression in T47D cells. Therefore, these results suggest that the Gomisin G-mediated decrease in proliferation of MDA-MB-231 cells is through the down-regulation of AKT and Rb phosphorylation.
Fig. 3.
Gomisin G mediated alteration of various signaling mediators in MDA-MB-231 and T47D cells. (A) MDA-MB-231 and (B) T47D cells were treated with 10 µM of Gomisin G for 24, 48, and 72 h and lysed. Equal concentrations of proteins were resolved, electrically blotted and visualized with pAKT, AKT, pERK, ERK, pP38, P38, pRB, and RB antibodies. For a loading control, β-actin was used.
Gomisin G causes G1 phase arrest and regulates the cyclin D1 level in MDA-MB-231 cells
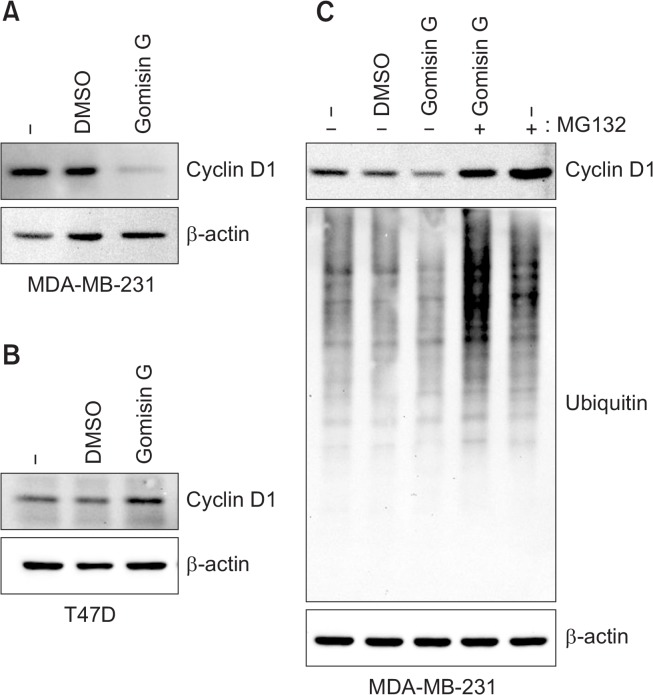
AKT phosphorylation down-regulates glycogen synthase kinase 3β (GSK3β) resulting in the sustained activity of cyclin D1 (VanArsdale et al., 2015). Therefore, we evaluated the effect of Gomisin G on the level of cyclin D1 using immunoblotting analysis. We found that the treatment of Gomisin G caused a significant decrease in the cyclin D1 level in the MDA-MB-231 cells; however, no such difference was observed in the T47D cells (Fig. 4A and B). To illustrate the mechanism behind the decrease in the cyclin D1 level, we pretreated the MDA-MB-231 cells with Gomisin G followed by MG132, a proteasome inhibitor, and then examined the cyclin D1 expression by immunoblotting. MG132 totally restored the Gomisin-G induced reduction of cyclin D1 (Fig. 4C). Cyclin D1 is a vital mediator of cell cycle progression from the G1 to the S phase in response to stimulation by growth factors (Musgrove et al., 2011; Casimiro et al., 2012; Qie and Diehl, 2016). We evaluated the effect of Gomisin G on cell cycle distribution using PI staining. The FACS data showed an increase in cell percentage at the G1 phase and a marked decrease at the S and G2/M phase in the MDA-MB-231 cells, whereas no such difference was observed in the T47D cells (Fig. 5). These results indicate that the cell cycle arrest at the G1 phase is responsible for the decrease in cell proliferation induced by Gomisin G. Additionally, the Gomisin G-mediated down-regulation of the cyclin D1 level is due to proteasomal degradation.
Fig. 4.
Gomisin G induced cyclin D1 degradation in MDA-MB-231 cells. (A) MDA-MB-231 and (B) T47D cells were treated with 10 µM of Gomisin G for 72 h. The expression of cyclin D1 was determined with immunoblotting. β-actin was used as a loading control. (C) MDA-MB-231 cells were treated with 10 µM of Gomisin G for 42 h followed by 10 µM of MG132 for 6 h. The expression of cyclin D1 and ubiquitin was determined with immunoblotting. β-actin was used as a loading control.
Fig. 5.
Gomisin G induced cell cycle arrest at the G1 phase in the MDA-MB-231 cells. MDA-MB-231 and T47D cells were treated with 10 µM of Gomisin G for 72 h. Cells were stained with PI, and cell cycles were examined with FACSCalibur.
DISCUSSION
TNBC has a characteristic aggressive behavior and occurs at a young age. It is also renowned for early relapse after diagnosis and additional aggressive metastases to the lungs, brain and bone. At present, surgery and chemotherapy are the only available mode for the treatment of TNBC patients. The foremost challenge in the treatment of TNBC patients is the deficit of obvious target molecules. Furthermore, the diversity of the disease has restricted the progression for successful treatment. However, numerous efforts are ongoing to identify, develop and improve therapies targeted specifically to TNBC (Dent et al., 2007; Criscitiello et al., 2012; Collignon et al., 2016).
Previous studies have demonstrated the anti-HIV, anti-liver cancer and anti-inflammatory capacity of Gomisin G, a wellknown natural compound extracted from Schisandra chinesis (Chen et al., 1997; Ryu et al., 2011; Xiaoyang et al., 2015). In this study, we explored the potential activity of Gomisin G as a novel and effective compound against TNBC. We found that Gomisin G suppressed the proliferation of MDA-MB-231 and MDA-MB-468 cells. We thought that these effects were related to apoptosis. However, Gomisin G did not induce apoptosis in MDA-MB-231 cells as shown by annexin V and PI staining. In addition, the western blotting data showed no differences in the PARP and caspase-3 activity.
Cancer is depicted by uncontrolled proliferation of tumor cells. AKT signaling has a central role in cell proliferation, death and cell cycle and is an important molecule regulating various cancers (Chang et al., 2003; Arcaro and Guerreiro, 2007). AKT overexpression is linked to a low prognosis of cancer (Altomare and Testa, 2005). Here, we found that treatment of the MDA-MB-231 cells with Gomisin G significantly diminished the AKT phosphorylation level at various time intervals which implies its inhibition of proliferation in TNBC. The abnormal activity of cell cycle proteins contributes to irrepressible tumor cell proliferation. Cyclin D1 is often dysregulated in cancers (Otto and Sicinski, 2017). Cyclin D1 is required for the transition of the cell from the G1 to the S phase and is overexpressed in several human cancers (Bartkova et al., 1994; Caputi et al., 1999). Prior numerous in vitro studies have reported on therapeutic molecules that cause the degradation of cyclin D1 (Alao, 2007). Studies have shown that PI3K-AKT regulates the cyclin D1 level. GSK3β is known to mediate the ubiquitination and subsequent proteasomal degradation of Cyclin D1 (Alao, 2007; VanArsdale et al., 2015). AKT phosphorylation suppresses the GSK3 β activity resulting in high levels of cyclin D1 in cells (VanArsdale et al., 2015). In agreement with those findings, we showed that the Gomisin G treatment led to a pronounced down-regulation of cyclin D1 protein which was rescued by the MG132 treatment. These results might explain the inability of the cells to proceed through the cell cycle. PI staining of the cells also validates this interpretation as shown by the increased cell population in the G1 phase and decreased population in the S and G2/M phase. Gomisin G was ineffective in the T47D cells suggesting a TNBC specific inhibition.
Rb protein is another key regulator of cell cycle progression. Rb binds and sequesters the E2F transcription factor preventing the synthesis of cell cycle genes including cyclins. Phosphorylation of Rb releases the E2F resulting in the transcription of cell cycle associated genes (VanArsdale et al., 2015). Our results showed that the Rb phosphorylation level is down-regulated along with the total Rb level. A previous study showed that Rb deficient TNBCs are more susceptible to radiation therapy compared to Rb expressing TNBCs (Robinson et al., 2013). Considering that the Gomisin G treatment reduced the Rb level in the cells, combination therapy that included radiation and Gomisin G might be more effective in the treatment of TNBCs. However, more experiments are necessary to reach this conclusion.
In summary, this study demonstrated the growth inhibitory action of Gomisin G on a TNBC cell line and its underlying mechanisms. Furthermore, this study also shows that Gomisin G could be a potential therapeutic for the treatment of TNBCs.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in the Republic of Korea (2009-0093812) and the Agri-Bio Industry Technology Development Program (316028-3, Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries). Byoung Kwon Park was supported by the Hallym University Postdoctoral Fellowship Program of 2017 (HLM-PF-2017-0001).
REFERENCES
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer. 2009;9(Suppl 2):S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics. 2007;8:271–306. doi: 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- Caputi M, Groeger AM, Esposito V, Dean C, De Luca A, Pacilio C, Muller MR, Giordano GG, Baldi F, Wolner E, Giordano A. Prognostic role of cyclin D1 in lung cancer. Relationship to proliferating cell nuclear antigen. Am J Respir Cell Mol Biol. 1999;20:746–750. doi: 10.1165/ajrcmb.20.4.3366. [DOI] [PubMed] [Google Scholar]
- Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and cell cycle control in cancer and disease. Genes Cancer. 2012;3:649–657. doi: 10.1177/1947601913479022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DF, Zhang SX, Xie L, Xie JX, Chen K, Kashiwada Y, Zhou BN, Wang P, Cosentino LM, Lee KH. Anti-AIDS agents--XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg Med Chem. 1997;5:1715–1723. doi: 10.1016/S0968-0896(97)00118-1. [DOI] [PubMed] [Google Scholar]
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod. 2006;69:356–359. doi: 10.1021/np0503707. [DOI] [PubMed] [Google Scholar]
- Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscitiello C, Azim HA, Jr, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann. Oncol. 2012;23(Suppl 6):vi13–vi18. doi: 10.1093/annonc/mds188. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;7:669–670. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- Jang MK, Yun YR, Kim JH, Park MH, Jung MH. Gomisin N inhibits adipogenesis and prevents high-fat diet-induced obesity. Sci Rep. 2017;7:40345. doi: 10.1038/srep40345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Hong JY, Choi JS, Lee SK. Columbianadin inhibits cell proliferation by inducing apoptosis and necroptosis in HCT116 colon cancer cells. Biomol Ther (Seoul) 2016;24:320–327. doi: 10.4062/biomolther.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kang JI, Tung NH, Kim YH, Hyun JW, Koh YS, Chang WY, Yoo ES, Kang HK. The effect of (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-Olide (LS-1) from lobophyyum sp. on the apoptosis induction of SNU-C5 human colorectal cancer cells. Biomol Ther (Seoul) 2016;24:623–629. doi: 10.4062/biomolther.2016.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HY, Park EJ, Hong JY, Kang YJ, Kim SJ, Chung HJ, Woo ER, Hung TM, Youn UJ, Kim YS, Kang SS, Bae K, Lee SK. Antiproliferative effects of dibenzocyclooctadiene lignans isolated from Schisandra chinensis in human cancer cells. Bioorg Med Chem Lett. 2008;18:523–526. doi: 10.1016/j.bmcl.2007.11.082. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY, Lee HJ, Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci Biotechnol Biochem. 2010;74:285–291. doi: 10.1271/bbb.90597. [DOI] [PubMed] [Google Scholar]
- Opletal L, Sovova H, Bartlova M. Dibenzo[a, c]cyclooctadiene lignans of the genus Schisandra: importance, isolation and determination. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:357–371. doi: 10.1016/S1570-0232(04)00646-4. [DOI] [PubMed] [Google Scholar]
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareja F, Geyer FC, Marchio C, Burke KA, Weigelt B, Reis-Filho JS. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer. 2016;2:16036. doi: 10.1038/npjbcancer.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee SJ, Song Y, Jang SH, Ko YG, Kang SN, Chung BY, Kim HD, Kim GS, Cho JH. Schisandra chinensis prevents alcohol-induced fatty liver disease in rats. J Med Food. 2014;17:103–110. doi: 10.1089/jmf.2013.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TJ, Liu JC, Vizeacoumar F, Sun T, Maclean N, Egan SE, Schimmer AD, Datti A, Zacksenhaus E. RB1 status in triple negative breast cancer cells dictates response to radiation treatment and selective therapeutic drugs. PLoS ONE. 2013;8:e78641. doi: 10.1371/journal.pone.0078641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EY, Park SY, Kim SG, Park DJ, Kang JS, Kim YH, Seetharaman R, Choi YW, Lee SJ. Anti-inflammatory effect of heme oxygenase-1 toward Porphyromonas gingivalis lipopolysaccharide in macrophages exposed to gomisins A, G, and J. J Med Food. 2011;14:1519–1526. doi: 10.1089/jmf.2011.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular Pathways: Targeting the Cyclin D-CDK4/6 Axis for Cancer Treatment. Clin Cancer Res. 2015;21:2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- Xiaoyang L, Chenming N, Chengqing L, Tao L. Drug-drug interation prediction between ketoconazole and anti-liver cancer drug Gomisin G. Afr Health Sci. 2015;15:590–593. doi: 10.4314/ahs.v15i2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]