Abstract
Clavibacter michiganensis subsp. michiganensis (Cmm) is a seed-borne pathogen that causes bacterial canker disease of tomato. Cmm is typically detected in tomato seeds using quantitative real-time polymerase chain reaction (qPCR) combined with culture-based isolation. The viable but nonculturable (VBNC) state of Cmm may result in the underestimation or false negative detection of the pathogen. In the present study, propidium monoazide (PMA) and its improved structure PMAxx were used to pretreat Cmm prior to DNA extraction, followed by qPCR. Both PMA and PMAxx could bind to the chromosomal DNA of dead bacterial cells and therefore block DNA amplification by PCR. This effect, however, does not occur in living bacterial cells, as the chemicals cannot penetrate through the undamaged cell membrane. Both viable and dead Cmm cells were treated with PMA and PMAxx at various concentrations. With this treatment, the range of the cell population was determined for effective detection. PMAxx showed a better discrimination effect than PMA on the viable and dead cells of Cmm and was therefore used throughout the present study. VBNC cells of Cmm (108 CFU mL-1) was induced by 50 μM copper sulfate, which was detected at different sampling times up to a month by using both PMAxx-qPCR and flow cytometry assays. The optimal PMAxx concentration was 20 μM for detecting membrane-intact Cmm cells. High specificity and sensitivity were obtained at Cmm concentrations ranging from 103 to 107 CFU mL-1. The accurate and robust results of PMAxx-qPCR were confirmed by flow cytometry method to detect viable Cmm cells. Furthermore, the PMAxx-qPCR assay was successfully used in detecting VBNC Cmm cells in tomato seeds with as few as 10 seeds per set.
Introduction
Bacterial wilt and canker of tomato (Solanum lycopersicum) is caused by the Gram-positive bacterium Clavibacter michiganensis subsp. michiganensis (Cmm) [1,2]. It is a devastating disease and has caused serious economic losses [3,4]. Disease symptoms include wilting of the whole plant, cankers and necrosis on stems and petioles as well as the reduced quantity and quality of fruit yield [5,6]. Cmm is a typical seed-borne pathogen, which can remain in or on the seed and is spread over a long distance through seed transportation [4,7]. Therefore, the certification of pathogen-free seed is an effective strategy to prevent and manage the disease, and this strategy is highly dependent on a reliable detection method of seed assay for pathogens [8].
In detecting Cmm, a number of techniques that have been used, including immune fluorescence staining, enzyme-linked immunosorbent assay (ELISA), southern hybridization, direct PCR, immunomagnetic separation, and loop-mediated isothermal amplification (LAMP) [9–12]. PCR is considered a promising detection approach because of its high throughput, sensitivity, specificity and convenient operation. However, PCR per se does not distinguish the viability of bacterial cells. Bio-PCR improves the accuracy in which culturable bacteria can grow on selective agar media prior to PCR. This method is currently recommended for detecting Cmm in tomato seed samples [13]. The bio-PCR protocols have been developed by the International Seed Testing Association (ISTA), the European and Mediterranean Plant Protection Organization (EPPO) and other international organizations [4].
The challenge in detecting Cmm is that the bacterium enters a viable but nonculturable (VBNC) state under stress conditions [14]. VBNC is defined as metabolically active but without the ability to grow on conventional media [15]. This characteristic is shared by many species of nonsporulating bacteria [16]. Studies have suggested that the ability to enter a VBNC state may be a survival strategy for bacteria to retain a low level of metabolic activity and maintain their cellular structure and virulence in adversity, and resuscitate under favorable conditions [17–19]. If VBNC occurs in Cmm from a commercial seed lot, then it is likely that the bacterial population can be underestimated by a culture-based method. Thus, the above detection method should be modified.
The VBNC state of bacteria is determined by examining cell viability. This examination can be accomplished by using techniques such as the LIVE/DEAD BacLight Bacterial Viability Kit, cyanoditolyl tetrazolium chloride (CTC) and direct viable count (DVC), which is based on membrane integrity, respiration and responsiveness to nutritional stimuli. The LIVE/DEAD BacLight Bacterial Viability Kit has been widely used to distinguish live cells [16,20]. Jiang et al. used this chemical treating bacteria prior to measuring the Cmm population with the flow cytometry method to count viable Cmm cells, combined with agar plating assay to enumerate VBNC cells [14]. However, this method cannot be used to detect the VBNC Cmm cells from a real sample, such as seed extract, because the flow cytometry method cannot differentiate between Cmm and other bacterial cells.
Several chemicals, such as ethidium monoazide (EMA) and propidium monoazide (PMA), are able to permeate bacterial cells with damaged membranes and covalently bind to double-stranded DNA upon exposure to bright visible light. The bounded DNA cannot be amplified in PCR and results in the amplification of only viable cells [21,22]. A quantitative real-time PCR (qPCR) combined with EMA (EMA-qPCR) or PMA (PMA-qPCR) has been developed to detect and quantify VBNC cells of Listeria monocytogenes and Campylobacter spp. [23,24]. PMA has been demonstrated as more effective than EMA in many PCR-based molecular assays [25], and PMA-qPCR was the most appropriate method for counting viable cells compared to acridine orange direct counts (AODC), DVC, and qPCR [26]. EMA-qPCR has also been used to quantify viable Cmm cells from pure Cmm microcosm [27].
The PMA-qPCR method is widely applied in the detection of viable cells in food-borne and environmental bacteria [26,28–32], while the application in plant pathogenic bacteria is less documented, particularly in plant seed health test. Both TaqMan real-time PCR assay and LAMP dilution endpoint assay combined with PMA have been used to detect the viable cells of Xanthomonas hortorum pv. carotae in carrot seeds [33] and Acidovorax citrulli in watermelon seeds [34]. However, no other studies have been reported by using the PMA-qPCR method to detect VBNC plant pathogenic bacterial cells. PMAxx, developed by Biotium in 2015, is an improved structure of PMA and can be used for viability PCR. It functions in a manner similar to PMA but has much greater activity and ability to distinguish between live and dead bacteria [35]. The objectives of the present study are to develop a protocol by using PMA-qPCR or PMAxx-qPCR, combined with a culture-based approach, for detecting Cmm cells in a VBNC state and apply the method for a tomato seed test.
Materials and methods
Ethics statement
The present study was conducted at Seed Health Centre of China Agricultural University (SHC-CAU). The microorganism used in the present study was isolated from diseased plants, and no endangered or protected species were involved.
Bacterial strains and growing conditions
The Clavibacter michiganensis subsp. michiganensis strain BT0505 was isolated from an infected tomato plant in Inner Mongolia, China [27]. Bacterial cells were initially stored in 20% (v/v) glycerol at -80°C and streaked onto Luria-Bertani (LB) agar [27] at 28°C for 72 h. A single colony was selected to inoculate in 10 mL liquid LB and shaken at 120 rpm at 28°C for 24 h (log phase). Prior to use, the fresh live cells were washed three times with a 0.85% (w/v) NaCl solution, followed by centrifugation at 10000 ×g for 3 min. To assess the number of culturable cells, the cell suspension was tenfold diluted serially with sterilized distilled water. One hundred microliters of each diluted suspension was evenly spread onto a LB agar plate with three replicates. After incubation at 28°C for 72 h, plates with colony populations ranging from 30 to 300 were selected for bacterial enumeration.
Preparation and counting of VBNC and dead cells of Cmm
Cmm cells at log phase (108 CFU mL-1) were treated with 50 μM copper sulfate (CuSO4) at 28°C for 20 h to induce a VBNC state [14]. To detect VBNC cells, the induced microcosm was concentrated 100 times by centrifugation, subsequently plated onto LB agar to estimate the number of culturable cells, and analyzed with a flow cytometer to determine cell viability using the FACSCalibur system (BD Biosciences, San Jose, California, USA) according to a published protocol [14]. To obtain a VBNC Cmm suspension at 108 CFU mL-1, the supernatant of copper-induced microcosm was collected, centrifuged and adjusted to OD580 = 0.5 with a sterile 0.85% (w/v) NaCl solution. The LIVE/DEAD BacLight Bacterial Viability kit (Invitrogen, Carlsbad, California, USA) was used for bacterial staining prior to flow cytometry measurement [14]. Cmm cells treated with copper sulfate for 0, 3, 24, 72, 144, 240, 360, 480 and 720 h were measured with a flow cytometer for viability and on LB plates for culturability, respectively. If no colonies were observed on LB plates, then all viable cells determined by a flow cytometry were in a VBNC state.
To prepare dead bacterial cells, Cmm at log phase (108 CFU mL-1) was transferred to Eppendorf tubes (1.5 mL) and heated at 80°C for 20 min in a dry bath. All dead cells were confirmed with a flow cytometer and dilution plating as described above. All the experiments were performed three times with two biological replicates.
Optimization of photoactivatable dye for Cmm detection
One milligram of PMA (Biotium, Hayward, California, USA) was dissolved in 98 μL of sterile water with 20% dimethyl sulfoxide (DMSO) to obtain a 20 mM solution, and 20 mM PMAxx (a new and improved chemical of PMA) was purchased from Biotium Co., Ltd. Both dyes were stored in the dark at -20°C. To optimize the rate of photoactivatable dye for treating Cmm cells in quantitative real-time PCR, 1 mL of either culturable Cmm cells at the exponential phase or heat-killed cells (107 CFU mL-1) was treated with PMA and PMAxx at a final concentration of 0, 2, 5, 10, 20, 30, 40 and 50 μM in Eppendorf tubes in the dark at room temperature (20 to 25°C) for 8 min. The tubes were placed in an ice bath with lids removed and exposed to the light of a halogen bulb (300 W) for 10 min at a distance of 20 cm to ensure the DNA free dye to be photolyzed. To ensure homogeneous exposure to light, the samples were shaken every 3 min during the light treatment. After photoactivatable dye treatments, the cells were collected for DNA extraction and purification using the E.Z.N.A. Bacterial DNA Kit (Omega Bio-tek, Norcross, Georgia, USA) according to the manufacturer's instructions. The purified DNA was resuspended in 50 μL DNA elution buffer and stored at -20°C for subsequent use.
In a PCR assay, Cmm-specific primers Spm4f/Spm2r were used, which targets the internal transcribed spacer (ITS) of ribosomal DNA [27]. The reaction for qPCR contained 2 μL of template DNA, 10 μL of 2 × SYBR Premix Ex Taq, 0.4 μL of 50 × ROX Reference Dye II (TaKaRa, Kusatsu, Shiga, Japan), 0.4 μM of each forward and reverse primer, and ultrapure water to bring up the final reaction volume to 20 μL. The qPCR was performed by using the ABI 7500 Fast fluorescence system (Applied Biosystems, Carlsbad, California, USA), and the following program setting: 1 min at 95°C; 40 cycles of 10 s at 95°C, 30 s at 60°C and 34 s at 72°C. All qPCR assays were performed three times.
To select the optimized concentrations of PMA and PMAxx for Cmm treatment, the quantification evaluation was calculated by dCt and ddCt values according to Randazzo [35]. Briefly, the dCt value was calculated by subtracting the Ct value of no-dye-treated samples from Ct values of dye-treated samples, while the ddCt value was calculated by subtracting the dCt of viable cells from the dCt of dead cells.
Determination of viable Cmm cells in pure culture using PMAxx-qPCR
Viable cells of Cmm were measured by qPCR. Cmm cells at log phase were prepared as two groups: culturable cells (not treated) and dead cells (heat-killed), which were diluted by tenfold serial dilution to obtain a range from 108 to 102 CFU mL-1. The culturable and heat-killed cells were either treated with 20 μM PMAxx or without treatment for testing the influence of DNA amplification and the detection limit for PMAxx treatment prior to DNA extraction and qPCR assay. The number of culturable bacteria at each concentration was counted by dilution plating. A linear regression of standard curve was established between colony counts on plates and Ct values of PMAxx-qPCR.
To determine the effectiveness of the PMAxx-qPCR method in detecting VBNC Cmm cells, bacterial cells in log phase induced by 50 μM CuSO4 up to 30 days was used as the sample for analysis by PMAxx-qPCR and flow cytometry method. Cmm cells treated with copper for 0, 3, 24, 72, 144, 240, 360, 480 and 720 h were collected and used for plate count and PMAxx-qPCR as described above. The culturable Cmm cells were calculated by counting colonies on LB plates, and the viable cells were counted from the Ct value of qPCR. The number of VBNC cells was calculated by subtracting the number of culturable cells from the number of viable cells. Each assay was performed three times, and the experiments were conducted with two biological replicates.
Detection of VBNC Cmm from tomato seed using PMAxx-qPCR
Culturable, VBNC and dead Cmm cells were prepared and verified as described above. Solanum lycopersicum ‘908’ (Changzhong Corp., Shanghai, China) was assayed by incubation on semi-selective medium mSCM [36], and quantitative real-time PCR to confirm the seeds as Cmm free. These seeds were artificially inoculated with culturable, VBNC and heat-killed Cmm cells (108 CFU mL-1, OD580 = 0.5) for different treatments. The tomato seeds were inoculated artificially by vacuum processing [37,38]. An aspirator connected to a vacuum box was used to inoculate the Cmm cells on or in tomato seeds and vacuumed at -100 kpa for 5 min using SHB-III-type multi-use of recycled water vacuum pump (Great wall scientific industry, Zhengzhou, Henan, China). The inoculated seed was collected and placed on sterilized filter paper to air dry. Ten inoculated seeds from each treatment were randomly selected and ground by using a ball mill instrument (Retsch, Haan, Germany) in an Eppendorf tube. Five hundred microliters of 0.85% (w/v) NaCl solution was added to the broken seeds and incubated at room temperature (20 to 25°C) for 4 h for bacterial extraction. The seed extract was diluted 20 times with 0.85% (w/v) NaCl solution prior to PMAxx treatment. One milliliter of diluted seed extract was used for PMAxx pretreatment, DNA extraction, and qPCR as described above. Each assay was performed three times.
Statistical analysis
Data analysis was performed by using the SPSS statistical program (Version 17.0, International Business Machines Corp., Armonk, New York, USA). The mean values were compared using Student’s t-test at a significance level α = 0.05.
Results
Optimization of photoactivatable dye for Cmm detection
The Ct values of viable cells treated with either PMA or PMAxx showed no significant difference compared with the control when PMA was less than 10 μM or PMAxx less than 20 μM. At 20 μM PMA, the Ct value of the viable cells was significantly higher than that with 20 μM PMAxx (Table 1).
Table 1. Cycle threshold (Ct) of quantitative real-time polymerase chain reaction for the detection of Clavibacter michiganensis subsp. michiganensis (107 CFU mL-1) treated with propidium monoazide (PMA) and PMAxx (an improved PMA).
| Photoactivatable dye | Concentration (μM) | Viable cells | Dead cells | ddCtz |
|---|---|---|---|---|
| Ctx | Cty | |||
| PMA/ PMAxx | 0 | 17.04 ± 0.12 a | 16.85 ± 0.02 a | — |
| PMA | 2 | 17.05 ± 0.19 a | 29.16 ± 0.05 b | 12.30 |
| 5 | 17.16 ± 0.18 a | 31.58 ± 0.17 cd | 14.61 | |
| 10 | 17.39 ± 0.06 ab | 34.41± 0.27 ef | 17.21 | |
| 20 | 17.93 ± 0.28 c | 34.28 ± 0.28 ef | 16.54 | |
| 30 | 18.53 ± 0.24 d | 33.08± 0.18 de | 14.74 | |
| 40 | 18.82 ± 0.25 e | 34.34 ± 0.32 ef | 15.71 | |
| 50 | 19.22 ± 0.19 f | 35.96± 0.38 f | 16.93 | |
| PMAxx | 2 | 17.29 ± 0.13 a | 29.87 ± 0.14 bc | 12.77 |
| 5 | 17.47 ± 0.11 ab | 31.14± 0.27 bcd | 13.86 | |
| 10 | 17.35 ± 0.09 ab | 33.98 ± 0.14 ef | 16.82 | |
| 20 | 17.42 ± 0.01 ab | 35.37 ± 0.24 ef | 18.14 | |
| 30 | 17.66 ± 0.08 bc | 34.64 ± 0.35 ef | 17.17 | |
| 40 | 17.70 ± 0.38 bc | 35.71± 0.32 ef | 18.20 | |
| 50 | 18.00 ± 0.11 c | 35.01± 0.44 ef | 17.20 |
x and y = mean value ± standard deviation (SD).
zddCt = dCt (Dead cells) dCt (Viable cells); dCt (Dead cells) = Ct (Dead cells with dye)–Ct (Dead cells without dye), dCt (Viable cells) = Ct (Viable cells with dye)–Ct (Viable cells without dye). Means followed by different letters are significantly different (P < 0.05)
For the heat-killed cells, the Ct value of Cmm treated with photoactivatable dye showed a significant increase compared to the control group. When the concentration of either PMA or PMAxx increased, the capacity of photoactivatable dye to eliminate the signal from dead cell DNA significantly increased. The Ct value of dead cells showed little change when the concentration of PMA and PMAxx was higher than 10 μM, and most values were greater than 34.00, except the values for dead cells treated with 30 μM PMA or 10 μM PMAxx (Table 1).
As the concentration of PMA increased, the ddCt value of PMA initially firstly increased and then decreased. The maximum value was calculated at 10 μM. After treatment with PMAxx at 40 μM, the ddCt value reached a maximum. However, this concentration of PMAxx was too high for viable cells. The second highest ddCt value was calculated when the PMAxx concentration was 20 μM (Table 1). This treatment did not affect viable cells but strictly inhibited dead cells (Ct value > 35). Based on the ddCt values calculated from viable and dead cells, the optimal concentrations of PMA and PMAxx were 10 and 20 μM. Because the ddCt value for 20 μM PMAxx treatment was higher than that for 10 μM PMA, it had no significant effect on the DNA amplification of viable Cmm cells. Thus, PMAxx was selected as the photoactivatable dye in the present study, and its optimal concentration was 20 μM.
Determination of viable Cmm cells in pure culture using PMAxx-qPCR
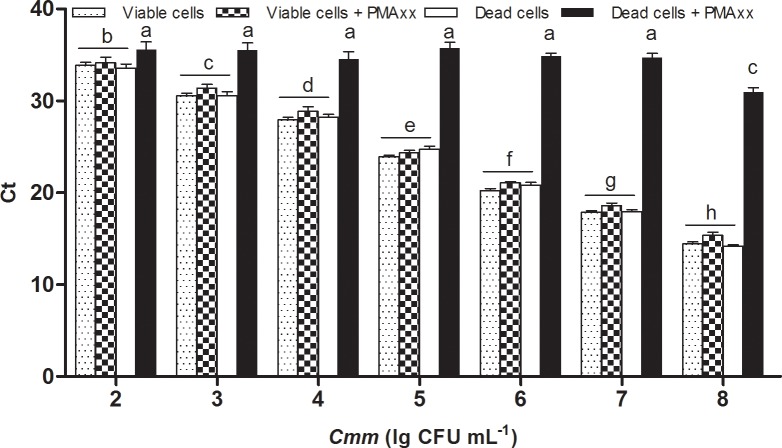
Tenfold serial dilutions of viable and dead Cmm cells treated with or without 20 μM PMAxx were used to evaluate the application range of the PMAxx-qPCR assay. The Ct values for PMAxx-treated viable cells at all concentrations were similar to those for untreated viable cells. For dead Cmm cells at 107 CFU mL-1 or lower, the 20 μM of PMAxx was sufficient to inhibit the DNA amplification. However, when the concentration of heat-killed cells reached 108 CFU mL-1, the Ct value significantly decreased compared to that at 107 CFU mL-1 and lower concentrations (Fig 1). PMAxx at 20 μM did not bind to all the Cmm DNA from dead cells when the bacterial concentration was 108 CFU mL-1.
Fig 1. Specificity and sensitivity of PMAxx-qPCR assay in detecting viable cells of Clavibacter michiganensis subsp. michiganensis (Cmm).
Viable or heat-killed Cmm cells at various concentrations were treated with 20 μM PMAxx, followed by DNA extraction and qPCR detection. Ct: threshold cycle of qPCR. CFU: colony forming unit. PMA: propidium monoazide. Columns and bars represent mean values and standard deviations. Means followed by different letters are significantly different (P < 0.05).
There was a negative correlation between the number bacterial cells and Ct value of PMAxx-qPCR for culturable Cmm cells (Fig 2). Plotting the Ct value versus the log-concentration of Cmm yielded a straight-line regression, and the R2 was 0.996. Based on the standard curve, when the bacterial concentration ranged from 103 to 107 CFU mL-1, the number of viable Cmm cells could be calculated by using the Ct values obtained from PMAxx-qPCR.
Fig 2. Correlation between bacterial population on agar plates and threshold cycle (Ct) of PMAxx-qPCR for culturable Clavibacter michiganensis subsp. michiganensis (Cmm).
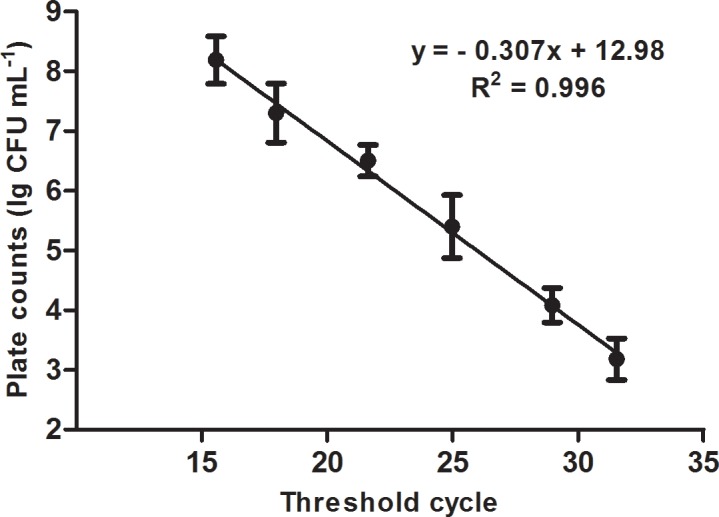
The final concentration of PMAxx used in bacterial cells treatment was 20 μM. The X-axis indicates the Ct value of PMAxx-qPCR and the Y-axis indicates the lg CFU mL-1 of bacterial cells.
Comparison of flow cytometry and PMAxx-qPCR for the detection of VBNC cells
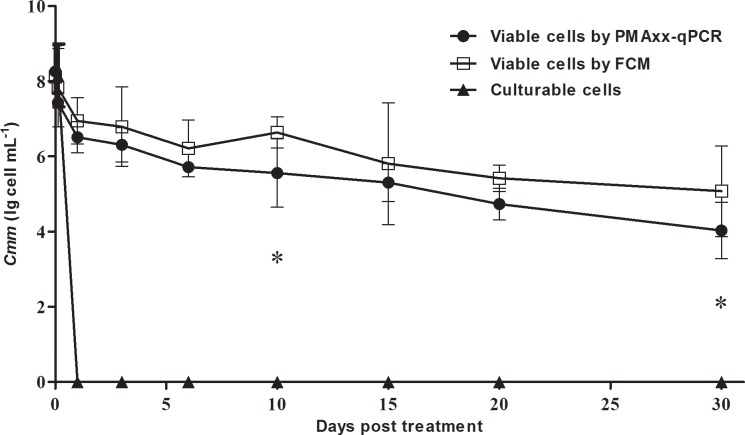
Both PMAxx-qPCR and flow cytometry methods exported a similar tendency of viable Cmm cells at each time point by detecting all viable cells, whereas the dilution plating method only showed the number of culturable cells. After 1-day incubation, no culturable cells were found on LB agar plates. In contrast, the viable cells detected by both PMAxx-qPCR and flow cytometry were above 104 CFU mL-1 during 30 days of incubation, although this number decreased with time (Fig 3).
Fig 3. Detection of VBNC cells of Clavibacter michiganensis subsp. michiganensis (Cmm) by PMAxx-qPCR and flow cytometry (FCM).
Viable and non-culturable (VBNC) cells of Cmm was induced by 50 μM CuSO4 in 0.85% NaCl. The bacterial cells were collected at 0 h, 3 h, 1 d, 3 d, 6 d, 10 d, 15 d, 20 d and 30 d, and used for viability detection by PMAxx-qPCR and flow cytometry method, while the culturability was determined on LB plates. Each data point represents the mean of two biological replicates. * indicates that the means were significantly different (P < 0.05) at the corresponding time point.
Detection of VBNC Cmm from tomato seed using PMAxx-qPCR
Tomato (Solanum lycopersicum) seed ‘908’ was confirmed as Cmm negative by agar plating and Bio-PCR (data not shown). The Ct values for conventional qPCR showed no difference for the seed samples inoculated with culturable, VBNC and dead Cmm cells, but the Ct values of PMAxx-qPCR were significantly different (Fig 4). For seed samples containing viable cells, there was no significant difference of the Ct values for PMAxx-qPCR and conventional qPCR; for seed samples containing dead cells, Ct value of PMAxx-qPCR was higher than conventional qPCR (Fig 4). The total number of Cmm cells in 10 tomato seeds, which inoculated with VBNC cells, was 2.53 × 105 CFU mL-1 (Ct value of conventional qPCR = 24.68), while the number of viable Cmm cells was 4.45 × 104 CFU mL-1 (Ct value of PMAxx-qPCR = 27.14). VBNC cells in the microcosm were calculated as approximately 17.57% of the total cells. Therefore, PMAxx-qPCR detected VBNC Cmm cells in a tomato seed sample.
Fig 4. Detection of culturable, viable but non-culturable (VBNC) and dead cells of Clavibacter michiganensis subsp. michiganensis (Cmm) by PMAxx-qPCR from artificially inoculated tomato seed.
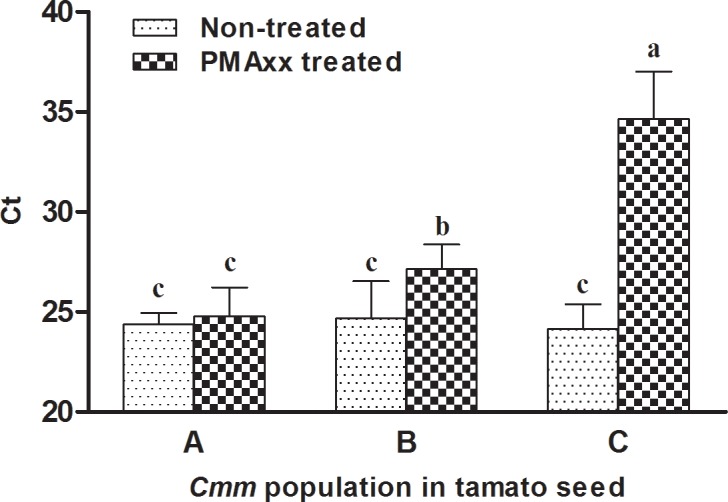
Ten tomato seeds were soaked in log phase (A), copper-induced VBNC (B) and heat-killed (C) Cmm cells suspension (108 CFU mL-1) by vacuum infiltration. After inoculation, the seed was broken by a ball mill and diluted 20-fold with 0.85% NaCl solution, followed by treatment with or without PMAxx at a final concentration of 20 μM. DNA was extracted after PMAxx treatment and used for qPCR assay. Cycle threshold (Ct) of qPCR was separated using multiple range test, and means labeled with different letters were significantly different (P < 0.05).
Discussion
We have demonstrated an improved and effective strategy to detect Cmm in the VBNC state, which cannot be otherwise detected by using conventional bio-PCR, and confirmed that the new photoactivatable dye PMAxx had higher sensitivity to differentiate viable and dead cells and less false binding to the DNA of viable cells compared to PMA. This result is supported by studies on the detection of hepatitis A virus (HAV) and Norovirus [35,39].
It has been reported that the sensitivity of PMA-qPCR varies depending on bacterial species [40]. In the present study, in the range of 103 to 107 CFU mL-1 bacteria, PMAxx worked properly, and no false amplification occurred on dead bacteria, which was an appropriate range for a precise detection. When the Cmm concentration increased to or was more than 108 CFU mL-1, dead cells could be partially picked up by PMAxx-qPCR. However, it was difficult to distinguish the difference of Ct values between viable and dead Cmm cells at a low bacterial concentration (< 103 CFU mL-1). Additionally, we found that a proper concentration of PMAxx improved the accuracy of detection. This result was consistent with that of Luo et al., who used a TaqMan probe and EMA-qPCR to detect viable Cmm cells [27]. Thus, Cmm cells at a concentration higher than 108 CFU mL-1 or lower than 103 CFU mL-1 should be avoided for detecting viable cells. In other words, PMA-qPCR and PMAxx-qPCR can be used when the bacterial concentration is in an appropriate range.
The consistency and reliability of PMAxx-qPCR results were confirmed by flow cytometry and culture-based methods. Because the primers are highly specific, PMAxx-qPCR could only detect Cmm without showing other bacteria, thus this method can be used for the precise detection of viable Cmm cells in tomato plants and seed. Moreover, PMAxx-qPCR requires a minimal population of Cmm as low as 103 CFU mL-1, which was much lower than that (105 CFU mL-1) by flow cytometry [14]. A similar result was obtained in assessing the viability status of Listeria monocytogenes in the food industry [29].
Cmm can survive on (externally) or in (internally) tomato seed [38], suggesting that whole seed tissues should be examined for bacterial detection. In the present study, Cmm was successfully detected in artificially inoculated tomato seeds, which required only 10 seeds for reliable results, although the inoculum concentration was as low as 105 CFU mL-1 (S1 Fig). In PMAxx treatment, the turbidity of the seed extracts could influence the exposure and photolysis, as the original thick seed extracts affected the transmission of the light from halogen bulbs [31]. To ensure the PMAxx photolysis, the seed extracts must be diluted prior to light exposure. In the present study, we found that 20 times or higher dilution of seed extract generated reliable PMAxx-qPCR results (S2 Fig). However, dilution of the sample also reduces bacterial concentration, which may affect the PCR result. Apparently, the sample preparation can be further improved. Ideally, we would expect to remove plant materials from the sample without significantly reduce the bacterial concentration. Compared to log phase cells from seed extract, the Ct value of copper-induced Cmm cells significantly increased in the PMAxx-qPCR detection, suggesting that most of the Cmm cells were dead and lost their intact membrane during VBNC induction; this result was consistent with the detection of VBNC Escherichia coli O157:H7 cells by PMA-qPCR [26].
In conclusion, the PMAxx-qPCR method was the most effective in the detection and quantification of VBNC Cmm from both pure culture and tomato seeds. This method will provide a precise detection of bacterial pathogen and evaluation for risks of VBNC cells in seed lots and other plant samples.
Supporting information
Each group of 10 tomato seeds was treated with Cmm suspension at concentrations from 108 to 104 CFU mL-1 by using vacuum infiltration. The treated seeds were ground by a ball mill in 1 mL 0.85% (w/v) NaCl solution to produce a bacterial extract and DNA extraction.
(TIF)
Ten tomato seeds were treated with Cmm cells suspension at a concentration of 108 CFU mL-1 for inoculation with vacuum infiltration. These seeds were ground by a ball mill in 1 mL 0.85% (w/v) NaCl solution to produce a bacterial extract. The seed extract was diluted to different concentrations (X axis) prior to treatment with PMAxx (final concentration 20 μM). The DNA was extracted after PMAxx treatment and homogeneous exposure and subsequently used for qPCR assay.
(TIF)
Acknowledgments
This work was financially supported through a grant from the National Natural Science Foundation of China (Grant No. 31571972) and the National Key Research and Development Program of China (2017YFD0201601).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31571972; the website address is http://www.nsfc.gov.cn/) and National Key Research and Development Program of China grant 2017YFD0201601 to LL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith EF. A new tomato disease of economic importance. Science. 1910; 31: 794–796. [Google Scholar]
- 2.Davis MJ, Gillaspie AGJ, Vidaver AK, Harris RW. Clavibacter: a new genus containing some phytopathogenic Coryneform bacteria, including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and bermudagrass stunting disease. Int J Syst Bacteriol. 1984; 34: 107–117. doi: 10.1099/00207713-34-2-107 [Google Scholar]
- 3.Gartemann KH, Kirchner O, Engemann J, Gräfen I, Eichenlaub R, Burger A. Clavibacter michiganensis subsp. michiganensis: first steps in the understanding of virulence of a gram-positive phytopathogenic bacterium. J Biotechnol. 2003; 106: 179–191. doi: 10.1016/j.jbiotec.2003.07.011 [DOI] [PubMed] [Google Scholar]
- 4.de León L, Siverio F, López MM, Rodríguez A. Clavibacter michiganesis subsp. michiganensis, a seedborne tomato pathogen: healthy seeds are still the goal. Plant Dis. 2011; 95: 1328–1338. doi: 10.1094/PDIS-02-11-0091 [DOI] [PubMed] [Google Scholar]
- 5.Hausbeck MK, Bell J, Medina-Mora C, Podolsky R, Fulbright DW. Effect of bactericides on population sizes and spread of Clavibacter michiganensis subsp. michiganensis on tomatoes in the greenhouse and on disease development and crop yield in the field. Phytopathology. 2000; 90: 38–44. doi: 10.1094/PHYTO.2000.90.1.38 [DOI] [PubMed] [Google Scholar]
- 6.Frenkel O, Bornestein M, Shulhani R, Sharabani G, Sofer M, Abo-Moch F, et al. Secondary spread of Clavibacter michiganensis subsp. michiganensis in nurseries and the conditions leading to infection of tomato seedlings. Eur J Plant Pathol. 2016; 144: 569–579. doi: 10.1007/s10658-015-0795-4 [Google Scholar]
- 7.Eichenlaub R, Gartemann KH. The Clavibacter michiganensis subspecies: molecular investigation of gram-positive bacterial plant pathogens. Annu Rev Phytopathol. 2011; 49: 445–464. doi: 10.1146/annurev-phyto-072910-095258 [DOI] [PubMed] [Google Scholar]
- 8.Sen Y, van der Wolf J, Visser RGF, van Heusden S. Bacterial canker of tomato: Current knowledge of detection, management, resistance, and interactions. Plant Dis. 2015; 99: 4–13. doi: 10.1094/PDIS-05-14-0499-FE [DOI] [PubMed] [Google Scholar]
- 9.Gitaitis RD, Beaver RW, Voloudakis AE. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis. 1991; 75: 834–838. doi: 10.1094/PD-75-0834 [Google Scholar]
- 10.Franken AAJM, Kamminga GC, Snijders W, van der Zouwen PS, Birnbaum YE. Detection of Clavibacter michiganensis ssp. michiganensis in tomato seeds by immunofluorescence microscopy and dilution plating. Neth J Plant Pathol. 1993; 99: 125–137. doi: 10.1007/BF01974265 [Google Scholar]
- 11.de León L, Rodríguez A, López MM, Siverio F. Evaluation of the efficacy of immunomagnetic separation for the detection of Clavibacter michiganensis subsp michiganensis in tomato seeds. J Appl Microbiol. 2008; 104: 776–786. doi: 10.1111/j.1365-2672.2007.03595.x [DOI] [PubMed] [Google Scholar]
- 12.Yasuhara-Bell J, Kubota R, Jenkins DM, Alvarez AM. Loop-Mediated amplification of the Clavibacter michiganensis subsp. michiganensis micA gene is highly specific. Phytopathology. 2013; 103: 1220–1226. doi: 10.1094/PHYTO-03-13-0078-R [DOI] [PubMed] [Google Scholar]
- 13.Hadas R, Kritzman G, Klietman F, Gefen T, Manulis S. Comparison of extraction procedures and determination of the detection threshold for Clavibacter michiganensis ssp. michiganensis in tomato seeds. Plant Pathol. 2005; 54: 643–649. doi: 10.1111/j.1365-3059.2005.01230.x [Google Scholar]
- 14.Jiang N, Lv QY, Xu X, Cao YS, Walcott RR, Li JQ, et al. Induction of the viable but nonculturable state in Clavibacter michiganensis subsp. michiganensis and in planta resuscitation of the cells on tomato seedlings. Plant Pathol. 2016; 65: 826–836. doi: 10.1111/ppa.12454 [Google Scholar]
- 15.Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005; 43: 93–100. [PubMed] [Google Scholar]
- 16.Pinto D, Santos MA, Chambel L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms. Crit Rev Microbiol. 2015; 41: 61–76. doi: 10.3109/1040841X.2013.794127 [DOI] [PubMed] [Google Scholar]
- 17.Ordax M, Marco-Noales E, López MM, Biosca EG. Survival strategy of Erwinia amylovora against copper: induction of the viable-but-nonculturable state. Appl Environ Microbiol. 2006; 72: 3482–3488. doi: 10.1128/AEM.72.5.3482-3488.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducret A, Chabalier M, Dukan S. Characterization and resuscitation of ‘non-culturable’ cells of Legionella pneumophila. BMC Microbiol. 2014; 14: 3 doi: 10.1186/1471-2180-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014; 5: 258 doi: 10.3389/fmicb.2014.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullrich S, Karrasch B, Hoppe HG, Jeskulke K, Mehrens M. Toxic effects on bacterial metabolism of the redox dye 5-cyano-2,3-ditolyl tetrazolium chloride. Appl Environ Microbiol. 1996; 62: 4587–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riedy MC, Muirhead KA, Jensen CP, Stewart CC. Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell populations. Cytometry. 1991; 12: 133–139. doi: 10.1002/cyto.990120206 [DOI] [PubMed] [Google Scholar]
- 22.Nocker A, Cheung CY, Camper AK. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Meth. 2006; 67: 310–320. doi: 10.1016/j.mimet.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Rudi K, Naterstad K, Drømtorp SM, Holo H. Detection of viable and dead Listeria monocytogenes on gouda-like cheeses by real-time PCR. Lett Appl Microbiol. 2005; 40: 301–306. doi: 10.1111/j.1472-765X.2005.01672.x [DOI] [PubMed] [Google Scholar]
- 24.Josefsen MH, Löfström C, Hansen TB, Christensen LS, Olsen JE, Hoorfar J. Rapid quantification of viable Campylobacter Bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl Environ Microbiol. 2010; 76: 5097–5104. doi: 10.1128/AEM.00411-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawthorn DM, Witthuhn RC. Selective PCR detection of viable Enterobacter sakazakii cells utilizing propidium monoazide or ethidium bromide monoazide. J Appl Microbiol. 2008; 105: 1178–1185. doi: 10.1111/j.1365-2672.2008.03851.x [DOI] [PubMed] [Google Scholar]
- 26.Xiao XL, Tian C, Yu YG, Wu H. Detection of viable but nonculturable Escherichia coli O157:H7 using propidium monoazide treatments and qPCR. Can J Microbiol. 2013; 59: 157–163. doi: 10.1139/cjm-2012-0577 [DOI] [PubMed] [Google Scholar]
- 27.Luo LX, Walters C, Bolkan H, Liu XL, Li JQ. Quantification of viable cells of Clavibacter michiganensis subsp. michiganensis using a DNA binding dye and a real-time PCR assay. Plant Pathol. 2008; 57: 332–337. doi: 10.1111/j.1365-3059.2007.01736.x [Google Scholar]
- 28.Dinu LD, Bach S. Detection of viable but non-culturable Escherichia coli O157:H7 from vegetable samples using quantitative PCR with propidium monoazide and immunological assays. Food Control. 2013; 31: 268–273. doi: 10.1016/j.foodcont.2012.10.020 [Google Scholar]
- 29.Ferrentino G, Tamburini S, Bath K, Foladori P, Spilimbergo S, Jousson O. Application of culture-independent methods for monitoring Listeria monocytogenes inactivation on food products. Process Biochem. 2015; 50: 188–193. doi: 10.1016/j.procbio.2014.12.014 [Google Scholar]
- 30.Santiago P, Moreno Y, Ferrús MA. Identification of viable Helicobacter pylori in drinking water supplies by cultural and molecular techniques. Helicobacter. 2015; 20: 252–259. doi: 10.1111/hel.12205 [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Liang WL, Kan B. Enumeration of viable non-culturable Vibrio cholerae using propidium monoazide combined with quantitative PCR. J Microbiol Meth. 2015; 115: 147–152. doi: 10.1016/j.mimet.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 32.Kibbee RJ, Örmeci B. Development of a sensitive and false-positive free PMA-qPCR viability assay to quantify VBNC Escherichia coli and evaluate disinfection performance in wastewater effluent. J Microbiol Meth. 2017; 132: 139–147. doi: 10.1016/j.mimet.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 33.Temple TN, du Toit LJ, Derie ML, Johnson KB. Quantitative molecular detection of Xanthomonas hortorum pv. carotae in carrot seed before and after hot-water treatment. Plant Dis. 2013; 97: 1585–1592. doi: 10.1094/PDIS-03-13-0262-RE [DOI] [PubMed] [Google Scholar]
- 34.Tian Q, Feng JJ, Hu J, Zhao WJ. Selective detection of viable seed-borne Acidovorax citrulli by real-time PCR with propidium monoazide. Sci Rep. 2016; 6: 354–357. doi: 10.1038/srep35457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randazzo W, López-Gálvez F, Allende A, Aznar R, Sánchez G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int J Food Microbiol. 2016; 229: 1–6. doi: 10.1016/j.ijfoodmicro.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 36.Waters CM, Bolkan HA. An improved semi selective medium and method of extraction for detecting Clavibacter michiganensis ssp. michiganensis in tomato seeds (Abstract). Phytopathology. 1992; 82: 1072. [Google Scholar]
- 37.Dikin A, Sijam K, Zainal-Abidin MA, Seman IA. Biological control of seedborne pathogen of oil palm, Schizopyllum commune Fr. with antagonistic bacteria. Int J Agric Biol. 2003; 5: 507–512. [Google Scholar]
- 38.Ribeiro DH, Souza RM, van der Wolf JM. Generation of tomato seeds artificially infected with a GFP-tagged strain of Clavibacter michiganensis subsp. michiganensis. Seed Sci Technol. 2016; 44: 486–499. doi: 10.15258/sst.2016.44.3.11 [Google Scholar]
- 39.Randazzo W, Piqueras J, Rodríguez-Díaz J, Aznar R, Sánchez G. Improving efficiency of viability-qPCR for selective detection of infectious HAV in food and water samples. J Appl Microbiol. 2017; Epub ahead of print. doi: 10.1111/jam.13519 [DOI] [PubMed] [Google Scholar]
- 40.Li F, Xie G, Zhou BQ, Yu P, Yu S, Aguilar ZP, et al. Rapid and simultaneous detection of viable Cronobacter sakazakii, Staphylococcus aureus, and Bacillus cereus in infant food products by PMA-mPCR assay with internal amplification control. LWT-Food Sci Technol. 2016; 74: 176–182. doi: 10.1016/j.lwt.2016.07.044 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each group of 10 tomato seeds was treated with Cmm suspension at concentrations from 108 to 104 CFU mL-1 by using vacuum infiltration. The treated seeds were ground by a ball mill in 1 mL 0.85% (w/v) NaCl solution to produce a bacterial extract and DNA extraction.
(TIF)
Ten tomato seeds were treated with Cmm cells suspension at a concentration of 108 CFU mL-1 for inoculation with vacuum infiltration. These seeds were ground by a ball mill in 1 mL 0.85% (w/v) NaCl solution to produce a bacterial extract. The seed extract was diluted to different concentrations (X axis) prior to treatment with PMAxx (final concentration 20 μM). The DNA was extracted after PMAxx treatment and homogeneous exposure and subsequently used for qPCR assay.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.