ABSTRACT
Iron is a metal micronutrient that is essential for plant growth and development. Graminaceous and nongraminaceous plants have evolved different mechanisms to mediate Fe uptake. Generally, strategy I is used by nongraminaceous plants like Arabidopsis, while graminaceous plants, such as rice, barley, and maize, are considered to use strategy II Fe uptake. Upon the functional characterization of OsIRT1 and OsIRT2 in rice, it was suggested that rice, as an exceptional graminaceous plant, utilizes both strategy I and strategy II Fe uptake systems. Similarly, ZmIRT1 and ZmZIP3 were identified as functional zinc and iron transporters in the maize genome, along with the determination of several genes encoding Zn and Fe transporters, raising the possibility that strategy I Fe uptake also occurs in maize. This mini-review integrates previous reports and recent evidence to obtain a better understanding of the mechanisms of Fe uptake in maize.
KEYWORDS: Fe uptake, maize, mechanism, strategy I, strategy II
Iron is an essential micronutrient in plants
Iron plays an essential role in plant growth and development, as it serves as a cofactor in many physiological and biochemical processes, such as photosynthesis and respiration. Fe deficiency leads to growth arrest and reduced grain quality. On the other hand, Fe excess is toxic to plants, as a high cellular Fe level may elevate the rate of Fe3+/Fe2+ redox reactions, resulting in high oxidation-reduction potential and damage to cells.1 Therefore, a strictly regulated mechanism is required for plants to maintain Fe homeostasis, which includes the uptake, utility, and storage of Fe. As an initial process, the regulation of Fe uptake is the key step to maintain Fe homeostasis in plants.
Two mechanisms of fe uptake in plants
Graminaceous and nongraminaceous plants utilize different strategies of Fe uptake. Strategy I is used by nongraminaceous plants, such as Arabidopsis, which take up Fe(II) as their main source of iron.2,3 The whole process is divided into several steps. First, insoluble ferric compounds in soil are acidified by protons exported from the root cells via H+ATPase.4-6 Then, a membrane-bound ferric-chelate reductase, AtFRO2, reduces ferric ions into ferrous ions, which are subsequently taken up into root epidermal cells by a transmembrane transporter, AtIRT1.7,8 In contrast, graminaceous plants, such as barley, rice, and maize, use strategy II to take up Fe, which is a chelation strategy that includes a series of steps. Primarily, this involves mugineic acids (MAs) that are synthesized by a conserved pathway, which begins with the trimerization of 3 molcules of S-adenosyl-L-methionine into nicotianamine (NA) by nicotianamine synthase (NAS).9,10 Then, nicotianamine aminotransferase (NAAT) and deoxymugineic acid synthase (DMAS) further converted NA into 2′-deoxymugineic acid and MAs,11,12 which are secreted into the rhizosphere by the transporter of mugineic acid family phytosiderophores 1 (TOM1). MAs serve as phytosiderophores (PSs), which chelate insoluble Fe in soil, and the Fe(III)-MA complexes are subsequently translocated into root cells by the yellow stripe-like family transporters.13,14
Rice is thought to utilize a combined fe uptake strategy
Recently, it was proposed that rice utilizes a combined strategy to absorb Fe, as both strategy I and strategy II proteins have been functionally characterized in rice. For instance, OsNAS1, OsNAS2, OsNAAT1, and OsDMAS1 were demonstrated to be key components in the biosynthesis of MAs; TOM1 transports MAs out of cells; while OsYSL15 was reported to take part in the uptake of Fe(III)-MAs into root cells.12,15-23 It was also revealed that Fe(II) can be directly absorbed into rice roots by OsIRT1 and OsIRT2. Although there is a lack of evidence for proton extrusion and Fe(III)-chelate reductase activity,24,25 these results suggest that the strategy I mechanism is active to some extent in rice. Therefore, rice is considered to be an exceptional graminaceous plant that possesses a combined Fe uptake strategy.
Strategy I fe uptake mechanism may also be present in maize
Although the Fe uptake mechanism has not been as well characterized in maize as in rice, it was generally believed that maize, as a graminaceous plant, utilizes strategy II to take up Fe. The evidence supporting this includes the following: ZmYS1 was reported to transport Fe-PS from the soil into root epidermis.13,26 In addition, 2 classes of ZmNAS genes were identified in the maize genome and the complementary expression pattern of class I and II ZmNAS suggests that they may play different roles in the uptake and transport of iron.27 As observed in rice, several genes involved in strategy I were also identified in maize. Nine ZmZIP genes were cloned by genome mining and their functions were preliminarily confirmed by a yeast complementation test.28 Moreover, the divalent cation transporting activity of ZmIRT1 and ZmZIP3 was confirmed in plants, as the levels of Zn and Fe were increased in ZmIRT1- and ZmZIP3-overexpressing Arabidopsis.29 Although there is a lack of evidence regarding whether the strategy I system is intact in maize, several genes encoding transcription factors, plasma membrane H+ATPase, and ferric reductases that participate in strategy I were identified in the maize genome. As shown in Table 1, the putative maize orthologs of FIT, H+ATPase (MHA), and FRO were identified by BLAST searches using Arabidopsis genes as queries. It was reported that the transcript factor AtFIT regulates the expression of FRO and IRT1 in Arabidopsis,30-32 while AtAHA and AtFRO are responsible for increasing Fe(III) solubility and reducing ferric ions into ferrous ions, respectively.2,3 The existence of FRO and MHA genes in maize, which are absent from the rice genome, indicates that maize may have a regulatory mechanism similar to that in Arabidopsis, and the strategy I Fe uptake mechanisms retained in maize and rice may differ from each other.
Table 1.
The predicted genes participated in the strategy I mechanism of Fe uptake in maize.
| Gene name | Gene ID | Chromosome no. | CDS length | Protein length |
|---|---|---|---|---|
| ZmFIT (FER like) | 103641578 | 10 | 1038 | 345 |
| H+ATPase 2 (MHA2) | 542048 | 2 | 2856 | 951 |
| ZmFRO2 | 100281526 | 2 | 2280 | 759 |
Conclusions and perspectives
An understanding of the Fe uptake mechanism in maize may help to explain the regulation of Fe assimilation in graminaceous plants, and provide genetic resources for the biofortification of maize varieties that are valuable due to their levels of essential metal micronutrients. In this mini-review, we have summarized the functionally characterized transporters and pathways involved in Fe uptake in maize and identified putative strategy I genes in maize, such as ZmFIT, ZmMHA, and ZmFRO by homolog searching. From our results, we hypothesize that strategy I may act as a complementary mechanism for Fe uptake in maize (Fig. 1). Further studies regarding the physiological functions of putative ZmFIT, ZmFRO, and ZmMHA should be undertaken to clarify their roles in mediating Fe uptake, and to confirm whether the strategy I mechanism is active in maize or whether the findings simply represent a group of functionally degenerate genes that are an evolutionary artifact.
Figure 1.
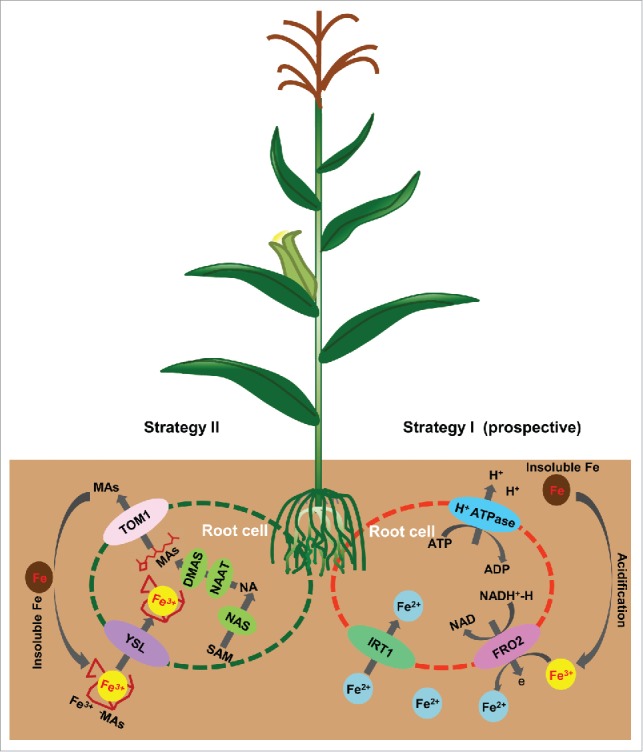
A hypothetical scheme showing the co-existence of strategy I and strategy II Fe uptake system in maize. In strategy II, MAs are synthesized in root cells by NAS, NAAT and DMAS, and MAs are secreted into rhizosphere by TOM1. Then, Fe(III)-MAs are transported in to cells by YSLs. Strategy I may serve as a complementary mechanism in maize, which involves the direct acquisition of Fe(II) by ZmIRT1 on root surface. The genes corresponding for acidification and ferric reduction are still uncovered in maize. MAs, mugineic acids; NAS, nicotianamine synthase; NAAT, nicotianamine aminotransferase; DMAS, deoxymugineic acid synthase; YSL, yellow strip like; TOM1, transporter of mugineic acid family phytosiderophores 1; IRT1, iron regulated transporter 1.
Abbreviations
- Zm,
Zea mays;
- YSL,
yellow stripe like;
- FRO,
ferric reductase oxidase;
- IRT1,
iron regulated transporter 1;
- FIT,
FER-LIKE Fe-deficiency-induced transcription factor;
- NAS,
nicotianamine synthase;
- MHA,
plasma-membrane H+ATPase;
- PS,
phytosiderophores;
- MAs,
muginetic acids;
- NAS,
nicotianamine synthase;
- NAAT,
nicotianamine aminotransferase;
- DMAS,
deoxymugineic acid synthase;
- TOM1,
transporter of mugineic acid family phytosiderophores 1.
Funding Statement
This work was supported by the National Special Program for GMO Development of China (grant number 2014ZX08003-002), the National Natural Science Foundation of China (grant number 31101095) and the Graduate Student Innovation Foundation of Hebei Province (grant number 1099009).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Briat JF, Lebrun M. Plant responses to metal toxicity. CR Acad Sci III 1999; 322:43-54; PMID:10047953; https://doi.org/22483849 10.1016/S0764-4469(99)80016-X [DOI] [PubMed] [Google Scholar]
- 2.Hindt MN, Guerinot ML. Getting a sense for signals: regulation of the plant iron deficiency response. Biochim Biophys Acta 2012; 1823:1521-30; PMID:22483849; https://doi.org/ 10.1016/j.bbamcr.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivanov R, Brumbarova T, Bauer P. Fitting into the harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Mol Plant 2012; 5:27-42; PMID:21873619; https://doi.org/ 10.1093/mp/ssr065 [DOI] [PubMed] [Google Scholar]
- 4.Santi S, Cesco S, Varanini Z, Pinton R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol Biochem 2005; 43:287-92; PMID:15854837; https://doi.org/ 10.1016/j.plaphy.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Santi S, Schmidt W: Laser microdissection-assisted analysis of the functional fate of iron deficiency-induced root hairs in cucumber. J Exp Bot 2008; 59:697-704; PMID:18316319; https://doi.org/ 10.1093/jxb/erm351 [DOI] [PubMed] [Google Scholar]
- 6.Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 2009; 183:1072-84; PMID:19549134; https://doi.org/ 10.1111/j.1469-8137.2009.02908.x [DOI] [PubMed] [Google Scholar]
- 7.Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002; 14:1223-33; PMID:12084823; http//dx.doi.org/21742768 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T. AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol 2011; 52:1433-42; PMID:21742768; https://doi.org/ 10.1093/pcp/pcr089 [DOI] [PubMed] [Google Scholar]
- 9.Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S. Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 1999; 119:471-80; PMID:9952442; https://doi.org/ 10.1104/pp.119.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S. Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 1999; 121:947-56; PMID:10557244; https://doi.org/ 10.1104/pp.121.3.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 2003; 15:1263-80; PMID:12782722; https://doi.org/ 10.1105/tpc.010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 2006; 281:32395-402; PMID:16926158; https://doi.org/ 10.1074/jbc.M60-4133200 [DOI] [PubMed] [Google Scholar]
- 13.Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL: Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001; 409:346-9; PMID:11201743; https://doi.org/ 10.1038/35053080 [DOI] [PubMed] [Google Scholar]
- 14.Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 2009; 103:1-11; PMID:18977764; https://doi.org/ 10.1093/aob/mcn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi K, Watanabe S, Takahashi M, Kawasaki S, Nakanishi H, Nishizawa NK, Mori S. Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J 2001; 25:159-167; PMID:11169192; https://doi.org/ 10.1046/j.1365-313x.2001.00951.x [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 2003; 36:366-81; PMID:14617093; https://doi.org/ 10.1046/j.1365-313X.2003.01878.x [DOI] [PubMed] [Google Scholar]
- 17.Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, Nishizawa NK. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol 2008; 66:193-203; PMID:18034312; https://doi.org/ 10.1007/s11103-007-9262-8 [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Wang F, Shou H, Huang F, Zheng L, He F, Li J, Zhao FJ, Ueno D, Ma JF et al.. Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol 2007; 145:1647-57; PMID:17951455; https://doi.org/ 10.1104/pp.107.107912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozoye T, Itai RN, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Diurnal changes in the expression of genes that participate im phytosiderophore synthesis in rice. Soil Sci Plant Nutr 2004; 50:1125-31; https://doi.org/ 10.1080/00380768.2004.10408585 [DOI] [Google Scholar]
- 20.Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 2011; 286:5446-54; PMID:21156806; https://doi.org/ 10.1074/jbc.M110.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 2009; 284:3470-9; PMID:19049971; https://doi.org/ 10.1074/jbc.M806042200 [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol 2009; 150:786-800; PMID:19376836; https://doi.org/ 10.1104/pp.109.135418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricachenevsky FK, Sperotto RA. There and back again, or always there? The evolution of rice combined strategy for Fe uptake. Front Plant Sci 2014; 5:189; PMID:24860581; https://doi.org/ 10.3389/fpls.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M et al.. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 2006; 45:335-46; PMID:16412081; https://doi.org/ 10.1111/j.1365-313X.2005.02624.x [DOI] [PubMed] [Google Scholar]
- 25.Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol 2008; 11:530-5; PMID:18722804; https://doi.org/ 10.1016/j.pbi.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 26.von Wiren N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC. Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol 1999; 119:1107-14; PMID:10069850; https://doi.org/ 10.1104/pp.119.3.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Li S, Zhao Q, Liu X, Zhang S, Sun C, Fan Y, Zhang C, Chen R. Genome-wide identification, classification and expression profiling of nicotianamine synthase (NAS) gene family in maize. BMC Genom 2013; 14:238; PMID:23575343; https://doi.org/26317616 10.1186/1471-2164-14-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Zhou X, Huang Y, Zhu L, Zhang S, Zhao Y, Guo J, Chen J, Chen R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol 2013; 13:114; PMID:23924433; https://doi.org/26317616 10.1186/1471-2229-13-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Zhou X, Li H, Liu Y, Zhu L, Guo J, Liu X, Fan Y, Chen J, Chen R. Overexpression of ZmIRT1 and ZmZIP3 enhances iron and zinc accumulation in transgenic Arabidopsis. PLoS ONE 2015; 10:e0136647; PMID:26317616; https://doi.org/ 10.1371/journal.pone.0136647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colangelo EP, Guerinot ML. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004; 16:3400-12; PMID:15539473; https://doi.org/ 10.1105/tpc.104.024-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Y, Wu H, Wang N, Li J, Zhao W, Du J, Wang D, Ling HQ. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res 2008; 18:385-97; PMID:18268542; https://doi.org/ 10.1038/cr.2008.26 [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Cui Y, Liu Y, Fan H, Du J, Huang Z, Yuan Y, Wu H, Ling HQ. Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol Plant 2013; 6:503-13; PMID:22983953; https://doi.org/ 10.1093/mp/sss089 [DOI] [PubMed] [Google Scholar]


