ABSTRACT
Previous study reported a novel type of self-discrimination in the tendrils of the vine Cayratia japonica (Vitaceae). However, whether self-discrimination in tendrils is common in vine plant species has not been elucidated. Here, we investigated whether tendrils of Momordica charantia var. pavel (Cucurbitaceae), Cucumis sativus (Cucurbitaceae) and Passiflora caerulea (Passifloraceae) can discriminate self and non-self plants. We also investigated whether the tendrils of M. charantia and C. sativus can discriminate differences in cultivars to determine the discrimination ability for genetic similarity. We found that tendrils of the M. charantia and P. caerulea were more likely to coil around non-self plant than self plants, but not in C. sativus. Our findings support the common occurrence of self-discrimination in tendrils in different plant taxa, although some species lacked it. Furthermore, tendrils of M. charantia more rapidly coil around different cultivars than around same cultivars. The tendrils of M. charantia may can discriminate differences in cultivars.
KEYWORDS: Climbing plant, competition, conspecific interactions, mcultivar, self-discrimination
Introduction
Many organisms can discriminate self from non-self. Self-discrimination has roles in diverse biological functions such as self-incompatibility in flowering plants1,2 and ascidians3 to prevent self-fertilization, and in the vertebrate immune system, where it plays a central role in the identification and destruction of non-self antigens.4 The mechanisms of self-discrimination are most often based on genotype-specific differences (allogeneic recognition), such as the S-locus in plants and the major histocompatibility complex in vertebrates.1,2,4
Different mechanisms of self-discrimination in plant roots have been reported.5 Self-discrimination in roots allows plants to alter their root allocation, morphology and distribution in response to neighbours.6-12 Unlike in self-incompatibility and immune systems, root discrimination is thought to be mediated by environmentally mediated physiological coordination rather than by genotype-specific differences.7-9, but see 10 For example, clones derived from the same plant treat each other as genetic aliens after temporal separation.13
Recently, a study reported a novel type of self-discrimination in the tendrils of the perennial vine Cayratia japonica (Vitaceae).14 Tendrils were more likely to coil around neighbouring non-self plants than around neighbouring self plants, and around a physiologically severed self plant than around a physiologically connected self plant. The latter result suggests that self-discrimination is mediated by physiological coordination between the tendril and the touched plant, as in roots.13 However, whether self-discrimination in tendrils is common in vine plant species has not been elucidated. Furthermore, recent research shows that plants can discriminate the genetic similarity of conspecific neighbours,15,16 possibly mediated via root exudates or leaf volatiles.17-19 Here, we investigated whether tendrils of Momordica charantia var. pavel (Cucurbitaceae), Cucumis sativus (Cucurbitaceae) and Passiflora caerulea (Passifloraceae) can discriminate self and non-self or differences in cultivars. These plant species have many tendrils that enable it to grasp and climb host plants. Because these plants grow rapidly in both vertical and horizontal directions, tendrils are likely to contact their own plants' stems and leaves. Whereas coiling around a non-self neighbour would be an adaptive response, providing an advantage in competition for light and space, coiling around itself would be harmful.
To detect self-discrimination in vine tendrils, we conducted a coiling experiment using M. charantia, C. sativus and P. caerulea. We compared coiling responses to self plants, other plants of the same cultivar, and plants of different cultivars in M. charantia and C. sativus. In P. caerulea, we compared coiling responses to the following neighbours: self plants, other individuals. If self-discrimination in tendrils were present, we would expect the tendrils to be more likely to coil around non-self plants than around self plants. If M. charantia and C. sativus could discriminate differences in cultivars, we would expect the tendrils to be more likely to coil around plants of different cultivars than around plants of the same cultivar.
Materials and methods
Cultivation of plants
We bought seeds of four cultivars of M. charantia: ‘Nigauri Goya’ (Aritaya Nouen, Hiroshima, Japan), ‘Shironigakun’ (Tokita Shyubyou, Saitama, Japan), ‘Suzume Goya’ (Hutaba Syubyou, Okinawa, Japan), and ‘Nigauri Goya’ (Sakatanotane, Yokohama, Japan). We also bought seeds of three cultivars of C. sativus: ‘Kyoukenn-Tasyuu Kyuri’ (Aritaya Nouen, Hiroshima, Japan), ‘Kyoukenn-Kasyuu Kyuri’ (Aritaya Nouen, Hiroshima, Japan) and ‘Tuyoi-Kyuri’ (Aritaya Nouen, Hiroshima, Japan). In September 2016, we filled a plastic container (45 cm × 35 cm × 5 cm deep) with wet vermiculite and sowed 10 seeds per cultivar on the surface. The container was maintained at 25 °C under a 12-L: 12-D photoperiod in a growth chamber for 30 days. We selected 5 to 10 healthy plants per cultivar for the experiment in October 2016, when their leaves had not yet begun to flush. The plants were transplanted individually into plastic pots (20 cm × 20 cm × 25 cm) containing garden soil (Sun & Hope Co., Tokyo, Japan). They were maintained at 25 °C under a 12-L: 12-D photoperiod in the growth chamber for another 30 days. Plants at least 30 cm tall with multiple tendrils were used for experiment.
The 9 P. caerulea plants were bought from a gardener in Hirosaki City in May 2017. These plants had about 60 leaves and 30cm-40cm long. These plants were maintained at 25°C under a 12L12D photoperiod condition in the growth chamber for 30 days. Plants of at least 40 cm in height with multiple tendrils were used for experiment.
Touch experiments
We performed touch experiments (commonly used to evaluate tendril coiling14) from 3 to 21 November 2016 in M. charantia and C. sativus. In P. caerulea plants, the touch experiment was conducted from 23 July to 30August 2017. Every morning at 08:00, the subject plants with fully expanded tendrils were moved from the growing shelves to a table in the growing room. Subject plant tendrils were placed in contact with the petiole of self or a neighbouring (object) plant by tying the petiole of the object plant to an anchored bamboo stick. The 1st to 3rd petioles from the shoot tip were used for experiment. Neighbouring plants consisted of either the same cultivar or a different cultivar. We also touch tendrils with bamboo sticks (0.6 cm in diameter) as control. We recorded the degree of coiling as uncoiled, slightly coiled (tendrils turned partially around the petiole but not to 360°), just coiled (at least 360° but not tight), or completely coiled (at least 720° and tight) at 2, 12, and 24 h after contact. Each tendril was used only once, and measurements were conducted on 1 to 3 tendrils per subject plant. Plants were randomly assigned, and plant ID was considered as a random effect in the statistical analysis.
Statistical analysis
All data were analysed in R v. 3.3. software. Ordinal logistic regression with a random effect was used to examine the effects of the one or two types of neighbouring plants and a bamboo stick on tendril coiling around the petiole because the levels of the response variable (the degree of coiling response) are defined on an ordinal scale. The models were fitted by using the clmm function in the ‘ordinal’ package in R. We used the level of coiling at 2, 12 and 24 h after contact as a response variable, the type of the neighbouring plant as an explanatory variable and the target plant ID and cultivars as a random effect. We analyzed the model for each species and time point separately. To examine the effect of self or non-self on coiling, we tested the differences between self and same cultivar plants. To examine the effect of genetic differences among the types of non-self plants, we tested the differences between the same and different cultivar plants in M. charantia and C. sativus. Multiple comparisons were performed using the false discovery rate.
Results
Tendril coiling responses in C. sativus did not differed among targets (Fig. 1d-f), it in M. charantia and P. caerulea differed among targets. Tendrils of M. charantia were significantly more likely to coil around petioles of other individuals of the same cultivar or a different cultivar than around self petioles at 24 h after the start of the experiment (Fig. 1c), and around a bamboo stick than around self petioles and same cultivar petioles at 2 and 12 h (Fig. 1a, b). Tendrils of M. charantia more likely to coil around petioles of different cultivar than around self petioles at 12h (Fig. 1b). Tendrils of P. caerulea were significantly more likely to coil around petioles of other individuals than around self petioles at 24 h after the start of the experiment (Fig. 1i).
Figure 1.
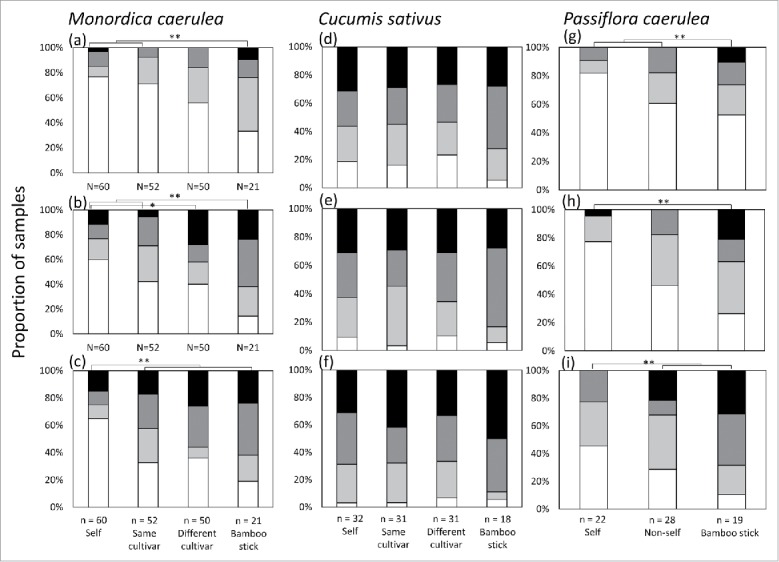
Degree of tendril coiling in Momordica charantia, Cucumis sativus and Passiflora caerulea seedlings that touched self plants, non-self plants of the same cultivar or a different cultivar, or a bamboo stick at (a, d, g) 2, (b, e, h) 12, and (c, f, i) 24 h from the start of the experiment. Proportions of completely coiled (black), just coiled (dark grey), slightly coiled (light grey), and uncoiled (white) tendrils are shown. Asterisks indicate significant differences: *P < 0.05, **P < 0.01.
Discussion
Tendrils of M. charantia and P. caerulea (Passifloraceae) were significantly more likely to coil around petioles of other individuals of the same or different cultivar than around self petioles by 24 h. Following the previous report of self-discrimination in tendrils of C. japonica,14 our findings suggested that the self-discrimination in tendrils were evolved multi-time independently in the various plant taxa.
Self-discrimination in C. japonica tendrils depends on physiological coordination,14 as in roots of other species.13 Greater coiling around a physiologically severed self-plant than around a physiologically connected self-plant suggests that self-discrimination is mediated by physiological coordination between the tendril and the touched plant, as in roots. The effect of physiological coordination in self-discrimination by M. charantia and P. caerulea, needs to be investigated to reveal common mechanisms of self-discrimination in plants.
Our results suggest that the tendrils of M. charantia can discriminate differences in cultivars. The tendrils tended to coil faster around petioles of different cultivars than around those of other individuals of the same cultivar at 2 h and 12 h (Fig. 1a, b), although not by 24 h (Fig. 1c). This result indicates that the efficacy of self-discrimination depends on genetic similarity, but the final result does not. Future studies need to investigate the effects of genetic similarity among cultivars on self-discrimination to reveal discrimination accuracy in tendrils.
On the other hand, C. sativus plants did not expressed the self-discrimination in tendrils (Fig. 1d-f), despite it is same taxa in M. charantia. Crops in monoculture may have reduced the ability to discriminate their kin during the domestication process because there is little chance to meet with the non-self and little benefit for avoiding the self.20 Also, artificial selection may favour the individual that has an ability of fast coiling without any discrimination. The potential differences in the domestication history and condition between C. sativus and M. charantia may be associated with the differences in the self-discrimination ability between them.
Funding Statement
This work was supported by JSPS Grant-in-Aid for Young Scientists (B) (No. 15K18611) to A.Y.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nasrallah JB. Recognition and rejection of self in plant reproduction. Science. 2002;296:305–8. doi: 10.1126/science.296.5566.305. [DOI] [PubMed] [Google Scholar]
- 2.Nasrallah JB. Recognition and rejection of self in plant self-incompatibility: comparisons to animal histocompatibility. Trends Immunol. 2005;26:412–8. doi: 10.1016/j.it.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Harada Y, Sawada H. Allorecognition mechanisms during ascidian fertilization. Int J Dev Biol. 2008;52:637–45. doi: 10.1387/ijdb.072544yh. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway CA. Decoding the patterns of self and non self by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 5.Chen BJW, During HJ, Anten NPR. Detect thy neighbor: identity recognition at the root level in plants. Plant Sci. 2012;195:157–67. doi: 10.1016/j.plantsci.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Mahall B, Callaway R. Root communication mechanisms and intracommunity distributions of two Mojave Desert shrubs. Ecology. 1992;73:2145–51. doi: 10.2307/1941462. [DOI] [Google Scholar]
- 7.Falik O, Reides P, Gersani M, Novoplansky A. Self/non-self discrimination in roots. J Ecol. 2003;91:525–31. doi: 10.1046/j.1365-2745.2003.00795.x. [DOI] [Google Scholar]
- 8.Holzapfel C, Alpert P. Root cooperation in a clonal plant: Connected strawberries segregate roots. Oecologia. 2003;134:72–77. doi: 10.1007/s00442-002-1062-x. [DOI] [PubMed] [Google Scholar]
- 9.Gruntman M, Novoplansky A. Physiologically mediated self/non-self discrimination in roots. Proc Natl Acad Sci USA. 2004;101:3863–7. doi: 10.1073/pnas.0306604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang S, Clark RT, Zheng Y, Iyer-Pascuzzi AS, Weitz JS, Kochian LV, Edelsbrunner H, Liao H, Benfey PN. Genotypic recognition and spatial responses by rice roots. Proc Natl Acad Sci USA. 2013;110:2670–5. doi: 10.1073/pnas.1222821110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamawo A, Sato M, Mukai H. Experimental evidence for benefit of self discrimination in roots of a clonal plant. AoB PLANTS. 2017;9:Plx049. doi: 10.1093/aobpla/plx049. [DOI] [Google Scholar]
- 12.Mahall BE, Callaway RM. Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am J Bot. 1996;83:93–98. doi: 10.1002/j.1537-2197.1996.tb13879.x. [DOI] [Google Scholar]
- 13.Falik O, Reides P, Gersani M, Novoplansky A. Self/non‐self discrimination in roots. J Ecol. 2003;91:525–31. doi: 10.1046/j.1365-2745.2003.00795.x. [DOI] [Google Scholar]
- 14.Fukano Y, Yamawo A. Self-discrimination in the tendrils of the vine Cayratia japonica is mediated by physiological connection. Proce. R Soc Lond B Biol Sci. 2015;282:20151379. doi: 10.1098/rspb.2015.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley SA, File AL. Kin recognition in an annual plant. Biol Let. 2007;3:435–8. doi: 10.1098/rsbl.2007.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biernaskie JM. Evidence for competition and cooperation among climbing plants. Proce R Soc Lond B Biol Sci. 2011;278:1989–96. doi: 10.1098/rspb.2010.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biedrzycki ML, Jilany TA, Dudley SA, Bais HP. Root exudates mediate kin recognition in plants. Communicative & Integrative Biol. 2010;3:28–35. doi: 10.4161/cib.3.1.10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karban R, Shiojiri K. Self‐recognition affects plant communication and defense. Ecology Letters. 2009;12:502–6. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Semchenko M, Saar S, Lepik A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytologist. 2014;204:631–7. doi: 10.1111/nph.12930. [DOI] [PubMed] [Google Scholar]
- 20.Fang S, Gao X, Deng Y, Chen X, Liao H. Crop root behavior coordinates phosphorus status and neighbors: From field studies to three-dimensional in situ reconstruction of root system architecture. Plant physiol. 2011;155:1277–85. doi: 10.1104/pp.110.167304. [DOI] [PMC free article] [PubMed] [Google Scholar]


