Abstract
Introduction
This study aimed to determine the frequency of transactive response DNA binding protein 43 kDa (TDP-43) pathology in PSP, the clinical features of patients with this pathology, and genetic risk factors for it..
Methods
Hippocampal sections were screened with immunohistochemistry for TDP-43 in 945 PSP cases. A subset of 261 cases that were negative in hippocampus was screened in the amygdala. The density and disruption of this pathology, as well as regional tau burden, and clinical and genetic characteristics were analyzed.
Results
We observed TDP-43pathology in 47 cases in the hippocampus and an additional 9 cases that only affected the amygdala. Hippocampal sclerosis was the strongest risk factor, followed by Alzheimer’s disease pathology, argyrophilic grain disease, and older age at death. Five stages of TDP-43 pathology were identified in PSP: Stage A had pathology only in the amygdala (16%); stage I had pathology confined to the hippocampus and entorhinal cortex (9%); stage II included both regions of stage A and I (38%); stage III spread further to medial occipitotemporal gyrus (20%); and stage IV had pathology in the dorsolateral frontal lobe (18%). Anatomical areas vulnerable to PSP pathology had varying degrees of this pathology in stage II and later. PSP with TDP-43pathology were older at disease onset and had lower median MMSE scores; however, the latter was driven by concurrent pathologies.
Conclusions
Distribution and clinical characteristics of TDP-43pathology in PSP were influenced by concurrent pathologies. This is the first study to observe that PSP-vulnerable regions are also susceptible to this non-tau pathology.
Keywords: Progressive supranuclear palsy, TDP-43, hippocampal sclerosis
Introduction
TAR DNA binding protein of 43 kDa (TDP-43) was originally identified as a major component of ubiquitin-positive/tau-negative inclusions of the frontotemporal cortex and motor neurons in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). 1, 2 The deposition of TDP-43 in FTLD and ALS occurs in a progressive fashion, respectively. 3, 4
In addition to FTLD and ALS, TDP-43 pathology has also been reported in other neurodegenerative diseases, including Alzheimer disease (AD) 5–10, hippocampal sclerosis (HpScl),11–13 Lewy body disease (LBD),7, 14–16 argyrophilic grain disease (AGD), 17, 18 Perry syndrome,13 corticobasal degeneration (CBD),10, 19 and progressive supranuclear palsy (PSP).15 The accumulation of pathologic TDP-43 follows a stereotypical pattern in AD.5, 20 The staging scheme of TDP-43 pathology in AD correlates with clinical and neuroimaging features.5, 20 Additionally, presence and burden of TDP-43 deposition are strongly associated with memory loss.21, 22 Taken together, the regional pattern of TDP–43 pathology is a significant factor in driving clinical phenotypes of both primary (i.e. ALS and FTLD) and secondary (i.e. AD) TDP-43 proteinopathies.23
There are few studies describing TDP-43 pathology in PSP, but most are small series. Although several studies reported no TDP-43 pathology in PSP,1, 10, 24 one study showed 26% (5/19) of PSP cases had TDP-43 pathology in the limbic system and temporal lobes.15 Thus, the frequency of TDP-43 pathology in PSP remains uncertain.
Recent studies have identified genetic variants associated with TDP-43 pathology. The TMEM106B rs3173615 minor allele, which regulates progranulin (GRN) expression,25 is reported to be protective for FTLD with TDP-43 inclusions (FTLD-TDP).26, 27 The GRN rs5848 variant, whose T allele is associated with lower progranulin expression, is also reported to be a risk factor for FTLD-TDP. 28 Additionally, common variants in TMEM106B serve as distinct risk factors for TDP-43 pathology not only in FTLD patients, but also in elderly subjects without FTLD.29 No information is available on genetic associations of TDP-43 pathology in PSP.
The aims of this study were to determine the frequency and distribution of TDP-43 pathology in PSP, the clinical features of PSP patients with TDP-43 pathology, and genetic risk factors for TDP-43 pathology. To accomplish our aims, we assessed the pathologic, clinical, and genetic features of a large cohort of PSP patients.
Subjects and Methods
Case selection
Between 2000 and 2014, 982 cases in the Mayo Clinic brain bank have been given a neuropathologic diagnosis of PSP. Of the 982 PSP cases, 945 cases with available paraffin-embedded tissue and at least minimal medical documentation were included in this study. Brain autopsies were obtained after consent of the legal next-of-kin or individuals with legal power-of-attorney, and studies of autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board.
Neuropathologic assessment
One neuropathologist (D.W.D.) made all neuropathological diagnoses. The neuropathologic protocol includes assessment of Alzheimer-type pathology with assignment of Braak neurofibrillary tangle (NFT) stage and Thal amyloid phase by thioflavin S fluorescent microscopy.30–32 Neuropathological diagnosis of AD was based on the consensus criteria for the neuropathologic diagnosis of AD.33 In this study, both high and intermediate likelihood cases were diagnosed with AD. Immunohistochemistry for phospho-tau (CP13, 1:1000, from Dr. Peter Davies, Feinstein Institute, North Shore Hospital, NY) was used to establish neuropathological diagnosis of PSP.34 The severity of tau pathology, including pretangles/NFT, coiled bodies, tufted astrocytes, and tau-positive threads, was graded semi-quantitatively on a four-point scale (0 = absent, 1 = sparse, 2 = moderate, 3 = frequent).35 Select sections were processed for Gallyas silver stain and immunohistochemistry for 4-repeat tau (RD4, 1:5000; Millipore, Temecula, CA) to assist in neuropathological diagnosis of AGD.36 HpScl was assessed by H&E staining. HpScl was defined as neuronal loss and gliosis in hippocampal CA1 and subiculum in the absence of other pathologic findings that could account for neuronal loss in these regions.
TDP-43 immunohistochemistry
The screening for TDP-43 pathology consists of two phases. As the phase 1, 5-μm thick paraffin sections of the anterior hippocampus at the level of subthalamic nucleus were screened with TDP-43 immunohistochemistry (MC2085, 1:3,000, from Dr. Leonard Petrucelli, Mayo Clinic, FL), which is phosphorylation-independent rabbit polyclonal antibody with high specificity for pathological TDP-43, in all cases using a DAKO Autostainer (Universal Staining System, Carpinteria, CA).37 The following regions were further screened in cases with positive hippocampal TDP-43 pathology: entorhinal cortex, amygdala at the level of the anterior commissure, medial occipitotemporal gyrus, middle frontal gyrus, putamen, globus pallidus, subthalamic nucleus, substantia nigra, internal capsule, pontine tegmentum including locus coeruleus, and cerebellar dentate nucleus. All slides were reviewed simultaneously by two observers (D.W.D., S.K.) who agreed on the presence of TDP-43 immunoreactivity, defined as neuronal cytoplasmic inclusion (NCI), dystrophic neurites, fine neurites, neuronal intranuclear inclusion (NII), perivascular inclusions or coiled body-like inclusion in any region. The severity of TDP-43 deposition was graded semi-quantitatively on a five-point scale (0 = absent, 1 = scant, 2 = sparse, 3 = moderate, 4 = frequent) as shown in Supplementary Figure 1.
To avoid overlooking cases with earlier stage of TDP-43 pathology, we screened amygdala sections in a subset of cases, because the amygdala is the most frequently affected site in AD, LBD and cognitively normal elderly individuals (phase 2).5, 7, 18, 20, 38 Sections from amygdala in 261 cases that were negative in screens of the hippocampus were processed for TDP-43 immunohistochemistry. All 63 cases with concurrent AD, a case with concurrent HpScl, 17 cases having Braak NFT stage 4 or greater, and remaining 180 cases with available amygdala sections were included in these 261 cases.
Clinical Assessment
We assessed clinical information in all TDP-43 positive PSP (PSP-TDP) cases and a subset of TDP-43 negative PSP cases (N = 88). The latter group was described as the Mayo Clinic patient series in our previous reports.35 Ante-mortem clinical information for age at onset, clinical diagnosis, cognitive impairment, and Mini Mental State Examination (MMSE) scores were gathered from clinical reports and/or a brain bank questionnaire filled out by a close family member. Patients were considered to have cognitive impairment if at least short-term memory loss, disorientation, or executive dysfunction were diagnosed by a physician, or there were recorded complaints of these symptoms by the patient or their family members.39
Genetic Assessment
We performed genetic assessment in PSP cases with available frozen brain tissues: all 56 PSP-TDP cases and 844 PSP cases without TDP-43 pathology. For genotyping, genomic DNA was extracted from cerebellum of frozen brain tissue using standard procedures. Genotyping for GRN (SNP rs5848 C/T SNPs, T minor allele) and TMEM106B (rs3173615 C/G SNPs, G minor allele), and MAPT H1/H2 (SNP rs1052553 A/G, A = H1, G = H2) was assessed with TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA) as previously reported 26, 40–42. Genotype calls were obtained with SDS v2.2.2 software (Applied Biosystems).
Statistical Analysis
All statistical analyses were performed using SigmaPlot 12.3 (Systat, San Jose, CA, USA). A chi-square test or Fisher’s exact test was performed for group comparisons of categorical data, as appropriate. The t-test or Mann-Whitney rank sum test was used for analyses of continuous variables, as appropriate. P-values <0.05 were considered statistically significant. Multivariable logistic regression models were built for each combination of the pathologic groups using the significant pathological and clinical variables from univariate analyses.
Results
Of the 945 PSP cases, 47 (5%) had TDP-43 pathology in the hippocampal CA1 and subiculum (phase 1). Of 898 TDP-43 negative cases, 261 were processed for further screening on amygdala sections (phase 2). This led to identification of an additional 9 TDP-43 positive cases [5 in AD (5/61, 8.2%), 4 in the remaining cases (4/180, 2.2%)]. In total, 56 cases had TDP-43 pathology in hippocampus and/or amygdala.
Demographic and pathologic features for all subjects are shown in Table 1. PSP-TDP cases had an older age at death than PSP cases without TDP-43 pathology (81 vs. 74, P <0.001). The median Braak NFT stage and Thal amyloid phase were higher in PSP-TDP compared to PSP (III vs. II, P < 0.001; 3 vs. 1, P < 0.001, respectively). The frequency of concurrent AD, HpScl, and AGD was higher in PSP-TDP than in PSP (39% vs. 7%, P <0.001; 18% vs. 0.1%, P < 0.001; 46% vs. 25%, P < 0.001, respectively). Associations of TDP-43 pathology with the presence of concurrent AD, HpScl and AGD, as well as age at death were assessed by a multiple logistic regression model. PSP-TDP cases had older age at death compared to PSP cases without TDP-43 pathology (OR 1.10, 95% CI 1.05–1.15). The presence of concurrent AD, HpScl, and AGD were also independently associated with the TDP–43 pathology (AD: OR 6.07, 95% CI 2.99–12.3; HpScl: OR 156 95% CI 16.3–1490; AGD: OR 2.00, 95% CI 1.04–3.85).
Table 1.
Pathologic and genetic findings of PSP-TDP compared to PSP
| PSP-TDP N = 56 |
PSP N = 889 |
P value | ||
|---|---|---|---|---|
| Male, No. (%) | 26 (46%) | 497 (56%) | 0.213 | |
| Age at death, years | 81 (78, 86) | 74 (69, 80) | <0.001 | |
| Pathologic characteristics | ||||
| Brain weight, g | 1120 (1020, 1180) | 1160 (1060, 1260) | 0.031 | |
| Braak neurofibrillary tangles stage | III (II, IV) | II (II, III) | <0.001 | |
| Thal amyloid phase | 3 (1, 4) | 1 (0, 3) | <0.001 | |
| Concurrent pathologic diagnosis | ||||
| Alzheimer disease | 22 (39%) | 58 (7%) | <0.001 | |
| Hippocampal sclerosis | 10 (18%) | 1 (0.1%) | <0.001 | |
| Argyrophilic grains | 26 (46%) | 225 (25%) | <0.001 | |
| Genetic findings* | ||||
| TMEM106B, | G allele | 42 (38%) | 689 (41%) | 0.499 |
| G/G | 5 (9%) | 145 (17%) | 0.198 | |
| G/C | 32 (57%) | 399 (47%) | ||
| C/C | 19 (34%) | 299 (36%) | ||
| GRN, | T allele | 36 (32%) | 493 (29%) | 0.580 |
| T/T | 4 (7%) | 65 (8%) | 0.590 | |
| T/C | 28 (50%) | 363 (43%) | ||
| C/C | 24 (43%) | 416 (49%) | ||
| MAPT, | H1 allele | 97 (87%) | 1407 (83%) | 0.442 |
| H1/H1 | 41 (73%) | 589 (70%) | 0.403 | |
| H1/H2 | 15 (27%) | 229 (27%) | ||
| H2/H2 | 0 (0%) | 26 (3%) | ||
Data are displayed as median (25th, 75th range).
Genetic analysis is performed for PSP-TDP (N = 56) and PSP (N = 844), respectively. One case is not available for TMEM106B. Abbreviations: PSP, progressive supranuclear palsy; PSP-TDP, PSP with TDP-43 pathology.
Genetic Assessment
To determine if reported genetic risk variants for FTLD-TDP could contribute to TDP-43 pathology in PSP-TDP, we performed genetic analyses in 56 PSP-TDP and 844 PSP cases. As shown in Table 1, there was a decreased frequency of TMEM106b homozygous minor allele in PSP-TDP compared to PSP (9% vs 17%, P = 0.198). The frequency of homozygous minor allele of GRN was similar between the two groups. The frequency of MAPT H1/H1 genotype and H1 haplotype was similar between the two groups.
Clinical Assessment
Table 2 summarizes clinical characteristics and frequency of concurrent pathology of PSP-TDP and a subset of PSP cases. Age at onset was older in PSP-TDP cases than PSP cases (75 vs. 66, P <0.001), but disease duration was not different between the two groups. The frequency of cognitive impairment was not different between the two groups, but the median score of MMSE was lower in PSP-TDP than PSP cases (20 vs. 25, P = 0.003). Same as the parent population of TDP-negative PSP (Table 1), these 88 PSP cases had less concurrent pathology as assessed by concurrent AD, HpScl or AGD. The hypothesis that differences in clinical characteristics were driven by concurrent pathology was assessed by a multiple logistic regression model. PSP-TDP cases had older age at onset compared to PSP cases without TDP-43 pathology (OR 1.18, 95% CI 1.09–1.27). The presence of concurrent AD, HpScl, and AGD were also independently associated with the TDP-43 pathology (AD: OR 5.64, 95% CI 1.83–17.4; HpScl: OR 24.7, 95% CI 1.94–314; AGD: OR 2.60, 95% CI 1.02–6.65). In contrast, the association of the presence of cognitive impairment was not independently associated with TDP-43 pathology (P = 0.988).
Table 2.
Clinical characteristics and total tau burden in PSP-TDP compared to PSP
| PSP-TDP N = 56 |
PSP N = 88 |
P value | |
|---|---|---|---|
| Male, No. (%) | 26 (46%) | 55 (62%) | 0.085 |
| Age at death, years | 81 (78, 86) | 74 (68, 80) | <0.001 |
| Clinical characteristics | |||
| Age at onset, years | 75 (70, 80) | 66 (61, 71) | <0.001 |
| Disease duration, years | 7 (4, 9) | 7 (5, 9) | 0.295 |
| Clinical diagnosis of PSP | 38/56 (68%) | 73/88 (83%) | 0.058 |
| Cognitive impairment* | 40/47 (85%) | 65/88 (74%) | 0.201 |
| Mini-Mental State Examination score** | 20 (10, 23) | 25 (21, 27) | 0.003 |
| Concurrent pathologic diagnosis | |||
| Alzheimer disease | 22 (39%) | 8 (10%) | <0.001 |
| Hippocampal sclerosis | 10 (18%) | 1 (1%) | <0.001 |
| Argyrophilic grain disease | 26 (46%) | 23 (26%) | 0.020 |
| Total tau burden | |||
| Dentate gyrus | 2 (1, 3) | 1 (1, 2) | <0.001 |
| CA4 | 2 (1, 3) | 1 (1, 2) | 0.001 |
| CA2/3 | 3 (2, 3) | 2 (1, 3) | <0.001 |
| CA1/subiculum | 3 (2, 3) | 2 (1, 3) | 0.003 |
| Entorhinal cortex | 3 (2, 3) | 2 (1, 3) | <0.001 |
| Occipitotemporal gyrus | 2 (1, 3) | 1 (1, 2) | <0.001 |
| Amygdala | 3 (2, 3) | 2 (1, 3) | 0.031 |
| Substantia nigra | 2 (1, 2) | 2 (1, 2) | 0.122 |
| Globus pallidus | 2 (1, 2) | 2 (1, 3) | <0.001 |
| Putamen | 1 (1, 2) | 2 (1, 2) | 0.176 |
| Subthalamic nucleus | 2 (1, 3) | 2 (2, 3) | 0.410 |
| Pontine tegmentum | 2 (1, 3) | 2 (1, 3) | 0.058 |
| Cerebellar dentate nucleus | 1 (0, 2) | 1 (0, 2) | 0.895 |
Data are displayed as median (25th, 75th range).
Descriptions of cognitive impairment are available for PSP-TDP (n = 33) and PSP (N = 88).
Mini-Mental State Examination scores are available for PSP-TDP (N = 19) and PSP (N = 42).
Regional tau burden
To clarify the association of TDP-43 and regional tau burden, we performed immunohistochemistry for phospho-tau in all PSP-TDP cases (N = 56) and a subset of PSP cases (N = 88). The comparison of the regional tau burden and other pathology findings are shown in Table 2. The regional tau burden in medial temporal lobe (i.e. hippocampus and amygdala) was greater in PSP-TDP than in PSP. In contrast, the total tau burden in PSP-vulnerable regions was not different between the two groups.
Influence of concomitant pathology
To exclude the influence of concomitant pathology, we defined “pure” PSP-TDP as PSP-TDP cases without AD, HpScl and AGD. Fifteen cases were identified as “pure” PSP-TDP. Compared to pure PSP TDP-43 negative cases (N = 57), age at onset and death were both greater in pure PSP-TDP than in pure PSP; however, other clinical characteristics, including the frequency of cognitive impairment, MMSE scores, and correct clinical diagnoses of PSP, were not significantly different between the two groups (Supplementary Table 1). This result is consistent with the results of multiple logistic regression models performed above and suggest that these clinical characteristics of are driven by concomitant pathology. Regarding total tau burden, the median scores of tau burden were not different in medial temporal lobe and in PSP-vulnerable regions.
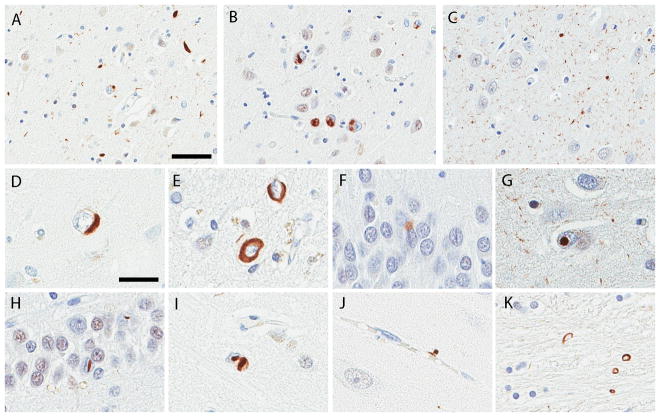
Morphology of TDP-43 pathology
A range of morphologic lesions were TDP-43 immunopositive (Figure 1), with lesions in both neurons and glia. NCIs were most frequent and observed in all 56 TDP-43 positive cases. Most cases had a mixture of NCIs and short dystrophic neurites in the hippocampus or adjacent cortices (Fig. 1A). Ten cases had numerous NCIs with minimal DNs (Fig. 1B). TDP-43 immunoreactive fine neurites were observed in CA1 and subiculum of 25 cases (Fig. 1C). The NCIs were morphologically heterogeneous, including round, crescent (Fig. 1D) r ring shaped NCI (Fig. 1E), as well as Pick body-like NCI (Fig. 1F), NFT-like inclusions. Only a few had skein-like morphology. Thirteen cases had NIIs in CA2 (Fig. 1G), CA1, subiculum, dentate gyrus (Fig. 1H), amygdala (Fig. 1I), putamen, globus pallidus, substantia nigra, or medial occipitotemporal gyrus. Two cases had perivascular TDP-43 inclusions characterized by small, globular, dense structures in close proximity to small vessels in CA1 and subiculum (Fig. 1J). Coiled body-like glial inclusions were observed in internal capsule (Fig. 1K).
Figure 1.
Immunohistochemistry for TDP-43. (A) A of neuronal cytoplasmic inclusions (NCIs) and dystrophic neurites in the hippocampal CA1. (B) Moderate NCIs and minimal dystrophic neurites in hippocampal CA1. (C) TDP-43 immunoreactive fine neurites in hippocampal CA1. Various NCI morphologies: crescent-shape in the hippocampal CA1 (D), ring-shape in the entorhinal cortex (E), and Pick body-like inclusions in dentate gyrus (F). Neuronal intranuclear inclusions (NII): round NII in hippocampal CA2 (G) and lentiform NII in the dentate gyrus (H). A neuron with both NCI and NII in the amygdala (I). Perivascular inclusions in CA1 (J). Coiled body-like glial inclusions in internal capsule (K). Bars = 50 μm in (A), 25 μm in (D). Images (A) to (C) and (D) to (K) are the same magnification, respectively.
Distribution and severity of TDP-43 pathology
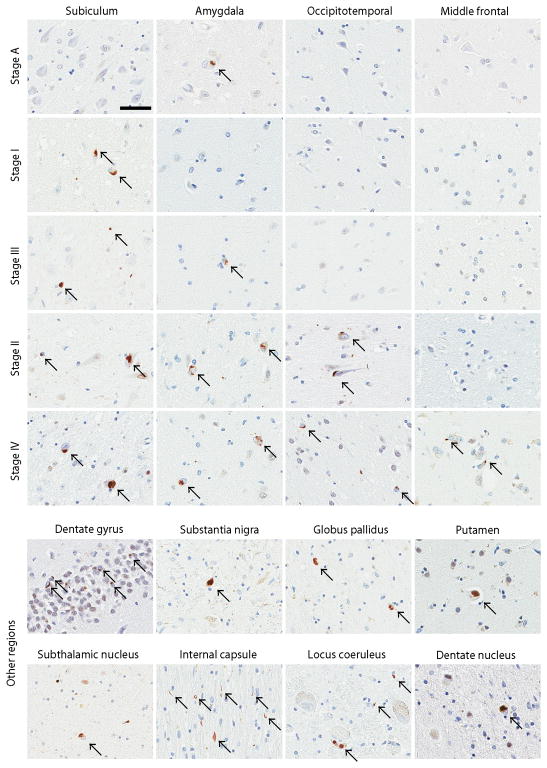
The distribution of TDP-43 pathology and a summary of clinicopathologic features of all 56 cases are shown in Table 3. TDP-43 pathology was most frequent in the amygdala (49/56), followed by the hippocampus (47/56), entorhinal cortex (40/56), dentate gyrus (26/56), substantia nigra (25/55), medial occipitotemporal gyrus (20/56), subthalamic nucleus (19/55), internal capsule (18/56), globus pallidus (17/56), pontine tegmentum (12/51), putamen (11/56), middle frontal gyrus (10/56), and cerebellar dentate nucleus (8/55). The severity of TDP-43 pathology varied among the regions. The hippocampus, entorhinal cortex, and amygdala were most severe, while there were scant NCIs in cerebellar dentate nucleus. Four stages were identified based on sequential anatomical progression of TDP-43 pathology (Table 3 and Fig. 2).
Table 3.
Summary of 56 PSP-TDP cases
| Stage | # | AAO | AAD | Sex | Clin Dx | Braak | Thal | AD | HS | AGD | NII | FN | TDP-43 pathology
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | Hp | EC | DG | OT | MF | SN | STN | GP | Pu | IC | PT | DN | |||||||||||||
| I | 1 | ND | 66 | F | PSP | I | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 2 | 73 | 86 | M | PSP | III | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 3 | 78 | 80 | M | PSP | III | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 4 | 75 | 80 | M | PSP | III | 2 | + | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 5 | 60 | 85 | F | PSP | V | 4 | ++ | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 6 | 68 | 78 | F | PSP | V | 4 | ++ | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 7 | 80 | 84 | F | CBD | IV | 5 | ++ | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 8 | 77 | 86 | F | PSP | IV | 3 | ++ | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 9 | 70 | 78 | F | PSP | IV | 4 | ++ | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
|
| |||||||||||||||||||||||||
| II | 10 | 70 | 74 | F | PSP | III | 0 | + | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 11 | 85 | 90 | M | PSP | III | 1 | + | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 12 | 67 | 80 | M | PSP | IV | 0 | + | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 13 | 73 | 77 | F | PSP | II | 3 | + | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 14 | 74 | 82 | M | PDD | IV | 3 | + | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 15 | ND | 64 | M | PSP | III | 3 | + | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 16 | 65 | 72 | M | VaD | II | 4 | + | + | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 17 | 78 | 80 | M | AD | IV | 3 | + | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||||
| 18 | 69 | 77 | F | PSP | 0 | 0 | + | 1 | 3 | 0 | 0 | 0 | 0 | 3 | 3 | 2 | 0 | 3 | 0 | 0 | |||||
| 19 | 84 | 93 | F | AD | VI | 3 | ++ | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 20 | 69 | 78 | F | CBD | VI | 4 | ++ | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 21 | 79 | 86 | F | PSP | II | 3 | + | 2 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 22 | 72 | 82 | M | PSP | IV | 5 | + | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 23 | 64 | 82 | F | MSA | IV | 5 | + | + | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | ||||
| 24 | 84 | 91 | M | PD | IV | 3 | + | + | + | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | |||
| 25 | 75 | 83 | M | PSP | II | 3 | + | 2 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | |||||
| 26 | 78 | 81 | M | CBD | I | 1 | + | 0 | 2 | 3 | 2 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 0 | |||||
| 27 | 77 | 84 | F | AD | IV | 3 | + | + | 1 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 28 | ND | 83 | F | PSP | IV | 1 | + | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 29 | 79 | 81 | F | PSP | III | 2 | + | + | + | 4 | 3 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 30 | 78 | 83 | M | PSP | III | 1 | + | + | 2 | 3 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | ||||
| 31 | 70 | 80 | M | PSP | III | 1 | + | + | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | ||||
| 32 | 84 | 92 | M | CBD | IV | 3 | + | + | 3 | 3 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||
| 33 | 59 | 68 | F | PSP | IV | 4 | + | + | + | 4 | 4 | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | |||
| 34 | 70 | 76 | M | PSP | IV | 3 | + | + | + | 4 | 3 | 2 | 3 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | |||
| 35 | 76 | 79 | F | FTD | II | 3 | + | + | + | 3 | 2 | 4 | 2 | 0 | 0 | 3 | 3 | 0 | 0 | 2 | 2 | 0 | |||
|
| |||||||||||||||||||||||||
| III | 36 | 69 | 78 | F | PSP | VI | 4 | ++ | 4 | 3 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 37 | 68 | 76 | M | PSP | II | 2 | + | + | + | 2 | 3 | 4 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| 38 | 89 | 89 | F | PSP | III | 0 | + | + | + | + | 3 | 4 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 39 | 80 | 85 | M | PSP | III | 3 | + | + | + | 3 | 2 | 3 | 4 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | |||
| 40 | 83 | 88 | F | PDD | III | 0 | + | 4 | 2 | 4 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | |||||
| 41 | 74 | 81 | F | PSP | IV | 5 | + | 4 | 2 | 2 | 2 | 3 | 0 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | |||||
| 42 | 75 | 79 | M | PSP | IV | 3 | + | + | + | + | + | 3 | 3 | 4 | 3 | 3 | 0 | 3 | 2 | 0 | 0 | 2 | 3 | 0 | |
| 43 | 73 | 79 | F | PSP | II | 3 | + | + | 1 | 4 | 2 | 2 | 2 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 0 | ||||
| 44 | 71 | 77 | M | PSP | III | 0 | + | + | 2 | 2 | 2 | 2 | 3 | 0 | 3 | 2 | 2 | 2 | 3 | 2 | 0 | ||||
| 45 | 78 | 81 | M | PSP | IV | 4 | + | + | + | 3 | 3 | 3 | 2 | 2 | 0 | 4 | 4 | 2 | 0 | 2 | 2 | 1 | |||
| 46 | 87 | 98 | F | AD | V | 4 | ++ | + | + | 4 | 3 | 3 | 4 | 2 | 0 | 3 | 2 | 0 | 1 | 2 | 2 | 1 | |||
|
| |||||||||||||||||||||||||
| 47 | 83 | 89 | M | CBD | V | 5 | ++ | + | + | 4 | 4 | 2 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 48 | 86 | 91 | M | AD | IV | 1 | + | 0 | 4 | 4 | 3 | 2 | 2 | NA | NA | 0 | 0 | 0 | NA | 1 | |||||
| 49 | 73 | 81 | F | PSP | I | 1 | + | + | + | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 0 | 3 | 0 | 2 | 2 | 0 | |||
| 50 | 61 | 69 | F | PSP | I | 1 | + | 2 | 3 | 2 | 0 | 0 | 2 | 4 | 3 | 0 | 0 | 4 | 2 | 1 | |||||
| 51 | 72 | 76 | F | CBD | II | 3 | + | + | + | 3 | 2 | 0 | 2 | 2 | 2 | 4 | 3 | 3 | 3 | 3 | NA | 1 | |||
| IV | 52 | 73 | 80 | M | PSP | II | 3 | + | + | 4 | 2 | 2 | 0 | 2 | 2 | 4 | 4 | 2 | 3 | 4 | NA | 1 | |||
| 53 | 77 | 83 | M | PSP | I | 0 | + | 2 | 4 | 4 | 0 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | NA | NA | |||||
| 54 | 82 | 88 | F | PSP | IV | 3 | + | + | + | 3 | 2 | 4 | 4 | 2 | 2 | 4 | 3 | 2 | 2 | 3 | NA | 0 | |||
| 55 | 81 | 88 | F | PSP | II | 0 | + | 2 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 0 | |||||
| 56 | 65 | 77 | F | PD | II | 2 | + | + | + | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 4 | 3 | 1 | |||
Abbreviations: TDP-43 pathology in scores 0 = absent, 1 =scant, score 2 = sparse, score 3 = moderate, and 4 = frequent. + = presence of pathology. ++ in AD = high likelihood, + in AD = intermediate likelihood. Abbreviations: PSP, progressive supranuclear palsy; #, case number; AAO, age at onset; AAD, age at death; Clin Dx, clinical diagnosis; Braak, Braak neurofibrillary tangles stage; Thal, Thal amyloid phase; NII, Neuronal intranuclear inclusions ; FN, Fine neurites ; AD, Alzheimer disease ; HS, Hippocampal sclerosis ; AGD, Argyrophilic grain disease; Hp, hippocampal CA1/subiculum; EC, entorhinal cortex; AM, amygdala; DG, dentate gyrus; OT, occipitotemporal gyrus; MF, middle frontal gyrus; SN, substantia nigra; GP, globus pallidus; Pu, putamen ; STN, subthalamic nucleus; IC, internal capsule; PT, pontine tegmentum; DN, cerebellar dentate nucleus; PDD, Parkinson disease with dementia; VaD, vascular dementia; CBD, corticobasal degeneration; MSA, multiple system atrophy; FTD, frontotemporal dementia; PD, Parkinson disease; ND, not described; NA, not available.
Figure 2.
Pathological findings from representative cases for each of the four stages. Arrows point to neuronal NCI in all panels except for coiled body-like glial inclusions in internal capsule. Bars = 50 μm. All images are the same magnification. Supplementary Figure 1: Semi-quantitative score on a five-point scale (0 = absent, 1 = scant, 2 = sparse, 3 = moderate, 4 = frequent) of TDP-43 pathology. Sections of amygdala (left) and the hippocampal CA1 (right) are shown. Arrows point to NCI. Bars = 50 μm. All images are the same magnification.
Nine cases (16%) had Stage I, defined as TDP-43 pathology only in the amygdala. These cases were identified in phase 2 screening and all had no TDP-43 pathology in the hippocampus.
Twenty six cases (46%) had Stage II, which included involvement of amygdala, hippocampus, entorhinal cortex, and dentate gyrus. Six cases did not have TDP-43 pathology in amygdala; five were affected only in hippocampus or entorhinal cortex (Case 10–14). Twelve of Stage II cases also had TDP-43 pathology in regions vulnerable to tau pathology and neuronal loss in PSP, including the substantia nigra, globus pallidus, putamen and subthalamic nucleus, although these regions were not included in the staging scheme.
Eleven cases (20%) were considered to have Stage III, which was defined by extensive TDP-43 pathology in medial occipitotemporal gyrus. All Stage III cases had involvement in almost all of regions affected in earlier stages. Ten Stage III cases (91%) had involvement of the substantia nigra, but the frequency in other PSP-vulnerable regions was variable: 36% in globus pallidus and putamen, 55% in subthalamic nucleus and pontine tegmentum, and 18% in cerebellar dentate nucleus.
Ten cases (18%) had Stage IV, defined as extensive TDP-43 pathology in middle frontal gyrus. The majority of Stage IV cases had involvement of hippocampus, entorhinal cortex, amygdala, substantia nigra, globus pallidus, putamen, and medial occipitotemporal gyrus. The frequency of TDP-43 pathology was 77% in subthalamic nucleus, 80% in pontine tegmentum, and 56% in cerebellar dentate nucleus.
Comparison of the clinico-pathologic findings between Stage I/II and Stage III/IV is shown in Supplementary Table 2. The frequency of HpScl and having NIIs in Stage III/IV was significantly higher than that seen in Stage I/II. The severity of TDP-43 pathology in Stage III/IV were higher than that in Stage I/II in substantia nigra, globus pallidus, putamen, subthalamic nucleus, internal capsule, pontine tegmentum, and cerebellar dentate nucleus. The median MMSE score was lower in Stage III/IV than that in Stage I/II. It is noteworthy that the severity of AD pathology in Stage IV was less than Stage I-III; only three of ten (30%) stage IV cases had Braak stage greater than II, although 36 of 46 (78%) stage I-III cases had.
Discussion
In a large cohort of 945 PSP cases, we identified only 47 (5%) with TDP-43 immunoreactivity in hippocampus. We also screened 261 amygdala sections for cases with no hippocampal TDP-43 and identified 9 additional cases with isolated amygdala pathology [5/61 in AD (8.2%) and 4/180 in the remaining cases (2.2%)]. Based on these results, the expected number of cases with isolated amygdala pathology in the remaining 637 cases is estimated to be 14 (2.2% of 637 cases) because no cases with concurrent AD or HpScl were included in these 637 cases. We estimate, therefore, that the frequency of TDP-43 pathology in PSP is at least 7% in this cohort because if different sections such as substantia nigra, and subthalamic nucleus are screened, more TDP-43 positive cases might also be identified. The frequency of TDP-43 in PSP is lower than that previously reported.15 The discrepancy between the two studies might be due to the different profile of cases with concurrent pathology. Although the frequency of AD was not described, HpScl was seen in 16% of all PSP cases in Yokota’s study in comparison to 1% in our study. Different sample sizes, with the Yokota’s study based on only 19 PSP cases, may also contribute differences.
The four stages of TDP-43 progression in PSP fit better with staging proposed for AD staging than for proposed staging of ALS or FTLD. Our staging of PSP-TDP builds upon AD staging proposed by Josephs et al.20 with some modifications. The similarities of distribution of TDP-43 pathology in AD and PSP may be explained by concurrent AD pathology in PSP-TDP. A major difference in staging of TDP-43 pathology between AD and PSP-TDP is involvement of basal ganglia and PSP-vulnerable regions. In contrast to AD, in which basal ganglia are rarely affected, PSP cases more often had TDP-43 pathology in the basal ganglia, as well as in other PSP-vulnerable regions. It is worth noting that even a subset of early stage cases (46%) had TDP-43 pathology in PSP-vulnerable regions. This discrepancy suggests the existence of a disease-specific vulnerability to TDP-43 pathology.
Our study disclosed several risk factors for TDP-43 pathology in PSP: older age at onset and death, and the presence of AD, HpScl or AGD, as previously reported.6–8, 14, 15, 18 Concurrent AD pathology also influenced the distribution pattern of TDP-43 pathology in PSP as we discussed above. The high frequency of cognitive impairment and low frequency of having clinical diagnosis of PSP were driven by the presence of AD, HpScl, and AGD. To exclude the influence of concurrent pathologies, we defined “pure PSP-TDP” (N = 15) as PSP-TDP without AD, HpScl or AGD. Clinical and pathologic findings were not different between the two groups, so differences seen in PSP-TDP are likely driven by concomitant pathology. The age at onset and death were older in “pure” PSP-TDP than “pure” PSP. This result is consistent with a recent study in which TDP-43 pathology was proposed to be a consequence of aging.43 Intriguingly, nine of fifteen “pure” PSP-TDP had extension of TDP-43 pathology to PSP-vulnerable regions, which are not affected by aging. It suggests that not only aging, but also PSP-related factors influence distribution of TDP-43 pathology in PSP.
We assessed the relationship between TDP-43 pathology and tau pathology, and found that regional tau burden in medial temporal lobe was greater in PSP-TDP than PSP. This result is consistent with the study by Yokota et al. that showed that tau burden in PSP-TDP tended to be greater than in TDP-negative PSP in amygdala, entorhinal cortex, hippocampal formation, and occipitotemporal gyrus.15 It is likely that the high frequency of AD (as well as AGD) in PSP–TDP contributes to greater tau burden in medial temporal lobe, which is supported by studies of pure PSP-TDP (Supplementary Table 1). Specifically, when PSP with concurrent AD, AGD or HpScl are excluded, regional tau burdens in medial temporal lobes are no longer greater in PSP-TDP. We further assessed the tau burden in PSP-vulnerable regions, but the tau burden was not significantly different between the two groups. Therefore, we conclude that no interaction between the regional tau burden and TDP-43 pathology in PSP. This fits with observations of TDP-43 in FTLD and ALS.44
Genetic analyses in our study were at variance with previous studies of TDP-43 in other neurodegenerative diseases. No statistically significant differences were found between PSP–TDP and PSP for TMEM106B rs3173615, GRN, and MAPT. The small sample size of the PSP-TDP group may contribute to this difference.
There are some limitations of our study. First, we screened sections of anterior hippocampus for TDP-43 pathology in all cases, but the result of the phase 2 screening, which was based on only a subset of 261 cases, suggested that the amygdala was the region affected first. Because not all sections of amygdala were screened in phase 2, the frequency of the TDP–43 pathology in PSP may be an underestimate. We suspect, however, that the discrepancy between the estimated frequency and the actual frequency of TDP-43 in PSP might be small, if present at all, because our validation cohort included high-risk cases such as those with concurrent pathology of AD and HpScl. Another limitation is that the staging scheme is biased by the screening sites. We observed that PSP-vulnerable regions, such as substantia nigra or internal capsule, may be affected and it is theoretically possible that had these regions been screened in all cases the frequency of PSP-TDP would differ.
Given the retrospective nature of this study, clinical information was limited. To evaluate possible clinical correlates of TDP-43 pathology and to validate the TDP-43 staging scheme, it will be important to evaluated PSP patients who come to autopsy form prospective clinical studies. To extract pertinent clinical information, we selected 88 PSP cases without TDP-43 pathology from the Mayo Clinic patient series. This might cause a selection bias because complicated cases tend to be referred to specialty clinics; however, demographic features and the frequency of concurrent pathology were not different between these 88 cases and the rest of the PSP cases.
A notable strength of our study is the number of patients with PSP screened for TDP-43 pathology, which is many fold greater than any previous study. The estimated frequency of PSP-TDP was only 7%, and we have identified four stages of TDP-43 pathology in PSP. The progression pattern of TDP-43 in PSP closely resembles that seen in AD more than that of ALS or FTLD. The distribution of TDP-43 pathology and clinical characteristics were influenced by concurrent pathologies including AD, HpScl, and AGD; however, we observed for the first time that PSP-vulnerable regions were also susceptible to TDP-43 pathology.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families who donated brains to help further our knowledge of neurodegeneration. The authors would also like to acknowledge Dr. Peter Davies (Feinstein Institute, LIJ-North Shore Health System) for the monoclonal anti-tau antibodies; Linda Rousseau (Mayo Clinic) and Virginia Phillips (Mayo Clinic) for histologic support;, and Monica Castanedes-Casey (Mayo Clinic) for immunohistochemistry support.
Footnotes
Author Contributions
SK: Acquisition, analysis and interpretation of data; drafting of manuscript; execution of the statistical analysis; writing of the first draft
MSC: Acquisition, analysis and interpretation of data; review and critique
KAJ: Review and critique; contribution of patients
RJU: Review and critique; contribution of patients
NGR: Review and critique; contribution of patients
JAG: Review and critique; contribution of patients
WPC: Review and critique; contribution of patients
ZKW: Review and critique; contribution of patients
RR: Review and critique; Interpretation of data
DWD: Study concept and design; interpretation of data; review and critique
Financial Disclosures of all authors
Dr. Koga reports no disclosures.
Dr. Sanchez-Contreras reports no disclosures.
Dr. Josephs receives research support from the NIH (R01-DC010367, R01-DC012519 & R01-AG037491) and the Alzheimer’s Association. Dr. Josephs is an editorial board member of Acta Neuropathologica, Journal of Neurology and Parkinsonism and Related Disorders.
Dr. Uitti receives research support by the NIH (P50-NS072187 and R01-NS057567), from Advanced Neuromodulation Systems, Inc./St. Jude Medical, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. Dr. Uitti is an editorial board member of Neurology.
Dr. Graff-Radford reports no disclosures.
Dr. van Gerpen receives research funds from the Mayo Clinic CR program and NIH (P50-NS072187). This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Dr. Cheshire is consultant for American Academy of Neurology, Neuro SAE examination writer, 2013; and receives support from NIH, Autonomic Rare Diseases Clinical Research Consortium. He is editorial board member of Autonomic Neuroscience.
Dr. Wszolek serves as Co-Editor-in-Chief of Parkinsonism and Related Disorders, Associate Editor of the European Journal of Neurology, and on the editorial boards of Neurologia i Neurochirurgia Polska, the Medical Journal of the Rzeszow University, and Clinical and Experimental Medical Letters; holds and has contractual rights for receipt of future royalty payments from patents re: A novel polynucleotide involved in heritable Parkinson’s disease; receives royalties from editing Parkinsonism and Related Disorders (Elsevier, 2015, 2016) and the European Journal of Neurology (Wiley-Blackwell, 2015, 2016).
Dr. Rademakers receives research support from the NIH (P50-NS072187, R01-NS076471 and R01-NS080882).
Dr. Dickson receives support from the NIH (P50-AG016574; P50-NS072187; P01-AG003949) and CurePSP: Foundation for PSP | CBD and Related Disorders. Dr. Dickson is an editorial board member of Acta Neuropathologica, Annals of Neurology, Brain, Brain Pathology, and Neuropathology, and he is editor in chief of American Journal of Neurodegenerative Disease, and International Journal of Clinical and Experimental Pathology.
References
- 1.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brettschneider J, Del Tredici K, Irwin DJ, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127(3):423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127(3):441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117(2):125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 8.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120(1):43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122(6):703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 10.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67(6):555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(Pt 5):1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wider C, Dickson DW, Stoessl AJ, et al. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord. 2009;15(4):281–286. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114(3):221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 15.Yokota O, Davidson Y, Bigio EH, et al. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120(1):55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki N, Murray ME, Ogaki K, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol. 2015;129(1):53–64. doi: 10.1007/s00401-014-1358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujishiro H, Uchikado H, Arai T, et al. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009;117(2):151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126(1):51–57. doi: 10.1007/s00401-013-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouri N, Oshima K, Takahashi M, et al. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol. 2013;125(5):741–752. doi: 10.1007/s00401-013-1087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131(4):571–585. doi: 10.1007/s00401-016-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70(11):1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127(6):811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan RH, Kril JJ, Fatima M, et al. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138(Pt 10):3110–3122. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- 24.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 25.Finch N, Carrasquillo MM, Baker M, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76(5):467–474. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Deerlin VM, Sleiman PM, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42(3):234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Blitterswijk M, Mullen B, Nicholson AM, et al. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol. 2014;127(3):397–406. doi: 10.1007/s00401-013-1240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis. 2010;7(1–3):170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015;84(9):927–934. doi: 10.1212/WNL.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 31.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 32.Wider C, Ross OA, Nishioka K, et al. An evaluation of the impact of MAPT, SNCA and APOE on the burden of Alzheimer’s and Lewy body pathology. J Neurol Neurosurg Psychiatry. 2012;83(4):424–429. doi: 10.1136/jnnp-2011-301413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44(11):2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 35.Koga S, Josephs KA, Ogaki K, et al. Cerebellar ataxia in progressive supranuclear palsy: An autopsy study of PSP-C. Mov Disord. 2016;31(5):653–662. doi: 10.1002/mds.26499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131(Pt 6):1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YJ, Xu YF, Cook C, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106(18):7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota O, Davidson Y, Arai T, et al. Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol. 2010;120(6):789–801. doi: 10.1007/s00401-010-0731-9. [DOI] [PubMed] [Google Scholar]
- 39.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85(5):404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17(23):3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutherford NJ, Carrasquillo MM, Li M, et al. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012;79(7):717–718. doi: 10.1212/WNL.0b013e318264e3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128(3):411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchino A, Takao M, Hatsuta H, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson AC, Thompson JC, Weedon L, et al. No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease. Neuropathol Appl Neurobiol. 2014;40(7):844–854. doi: 10.1111/nan.12155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.