Abstract
Aims
In atrial fibrillation, stroke risk is assessed by the CHA2DS2‐VASc score. Heart failure is included in CHA2DS2‐VASc, but the rationale is uncertain. Our objective was to test if heart failure is a risk factor for stroke, independent of other risk factors in CHA2DS2‐VASc.
Methods and results
We studied 300 839 patients with atrial fibrillation in the Swedish Patient Register 2005–11. Three definitions of heart failure were used in order to assess the robustness of the results. In the main analysis, heart failure was defined by a hospital discharge diagnosis of heart failure as first or second diagnosis and a filled prescription of a diuretic within 3 months before index + 30 days. The second definition counted first or second discharge diagnoses <1 year before index + 30 days and the third definition any heart failure diagnosis in open or hospital care before index + 30 days. Associations with outcomes were assessed with multivariable Cox analyses. Patients with heart failure were older (80.5 vs. 74.0 years, P < 0.001) and had higher CHA2DS2‐VASc score (4.4 vs. 2.7, P < 0.001). The 1 year incidence of ischaemic stroke without warfarin was 4.4% with heart failure and 3.1% without. Adjustment for the cofactors in CHA2DS2‐VASc eradicated the difference in stroke risk between patients with and without heart failure (hazard ratio 1.01 with 95% confidence interval 0.96–1.05). The area under the receiver operating characteristic curve for CHA2DS2‐VASc was not improved by points for heart failure.
Conclusions
A clinical diagnosis of heart failure was not an independent risk factor for stroke in patients with atrial fibrillation, which may have implications for anticoagulation management.
Keywords: Anticoagulation, Atrial fibrillation, Heart failure, Stroke, Epidemiology, Register
Introduction
The risk of stroke in atrial fibrillation (AF) varies in relation to age and comorbidity, as reflected in the CHA2DS2‐VASc stroke risk assessment scheme.1 CHA2DS2‐VASc counts one point for chronic heart failure (CHF), mainly based on an observation of increased stroke risk among patients with recent heart failure in the Stroke Prevention in Atrial Fibrillation trial2 from 1992. An early meta‐analysis of five studies in 1994 also showed a trend, although not significant, towards higher stroke risk in AF patients with heart failure.3
The inclusion of points for heart failure in CHA2DS2‐VASc was inherited from the older CHADS2 scheme where it was helpful in predicting stroke risk.4 In the more inclusive CHA2DS2‐VASc score, heart failure is accompanied by the five most important predictors for incident heart failure according to a recent long‐term follow‐up study by the Framingham Heart Study, namely, previous myocardial infarction, ischaemic heart disease, diabetes mellitus, age, and hypertension.5
In more recent studies, heart failure has not emerged as an independent risk factor or else been rated as a very weak factor compared with the other components of the stroke risk scores.6, 7, 8
Our aim was therefore to test the hypothesis that heart failure is a risk factor for stroke, independent of other risk factors in the CHA2DS2‐VASc score.
Methods
We conducted a retrospective cohort study of data from Swedish health registers maintained by the Swedish Board of Health and Welfare.9 All 325 020 patients with a diagnosis of AF in the Swedish Patient Register between 1 July 2005 and 31 December 2011 were identified. Exclusions were made of patients ≤20 years of age (n = 367), of patients with valvular AF defined as mitral stenosis or mechanical heart valve (n = 7804), and of patients who died within 30 days of the index contact (n = 16 317). After exclusions, 300 839 patients remained for the study (flow chart, Figure 1 ).
Figure 1.
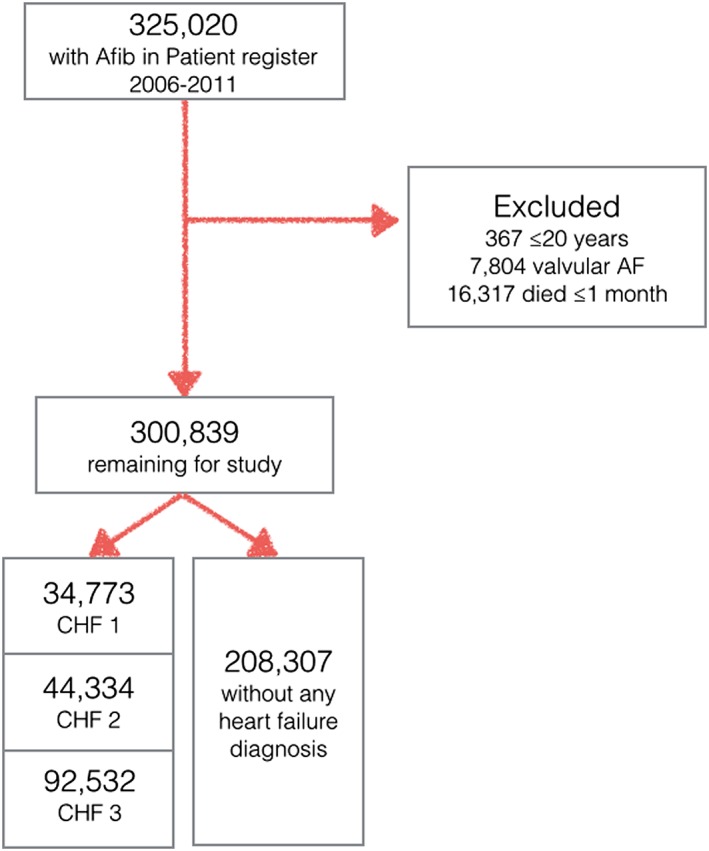
Inclusions and exclusions of study patients. AF, atrial fibrillation.
Index date was defined as the date of the first contact with a diagnosis AF in the National Patient Register within the inclusion period. Diagnoses given from 1997 and up to the individual index date +30 days were used for characterization of the medical history. Diagnoses given after index date +30 days were used for identification of outcome events. Time at risk was counted from index date +30 days. Censoring was made at the specified event, at death or on 31 December 2012, whichever came first. Minimum follow‐up was 1 year.
Endpoints
The primary endpoint was ischaemic stroke. Secondary endpoints were other thromboembolism (unspecified stroke and pulmonary and systemic embolism), intracranial haemorrhage, any other hospital contact for a bleeding event, and a composite endpoint consisting of ischaemic or haemorrhagic stroke or death.
Data sources
The Patient Register contains detailed information about hospitalizations and visits to hospital affiliated outpatient clinics in Sweden but does not contain information about visits in primary care. More than 99% of all entries in the Patient Register are technically correct.9 A diagnosis of AF at hospital discharge is correct in 97% of cases.10 A diagnosis of heart failure made in a clinic of internal medicine or cardiology could retrospectively be verified on the basis of available documentation in 86% and 91% of the cases, respectively.11 For most other diagnoses, the positive predictive values are in the range 85–95%.12
The Patient Register (http://www.sos.se) was used to obtain information needed for calculation of individual risk scores for ischaemic stroke and bleeding according to CHA2DS2‐VASc1 and HAS‐BLED.13 In the CHA2DS2‐VASc scheme, 2 points are given for previous stroke/transient ischaemic attack/systemic embolism, and for age ≥ 75 years and 1 point each for heart failure, hypertension, diabetes, vascular disease, female sex, and age 65–74 years. HAS‐BLED gives 1 point each for hypertension, renal disease, liver disease, previous stroke, history of major bleeding, age ≥ 65 years, concomitant treatment with drugs known to increase bleeding risk, alcohol abuse, and poorly controlled anticoagulant treatment. Because we did not have access to individual international normalized ratio values, all patients received zero points for this variable, and the HAS‐BLED score may therefore have been underestimated. The specific International Classification of Diseases‐10 codes used are listed in Table S1 .
Information about oral anticoagulants and other medication use was obtained from the Drug Register, which stores details about every filled prescription in Sweden since 1 July 2005. All pharmacies are required to participate by law. It says nothing about prescriptions that were issued but not filled or about over the counter medications. The only registered oral anticoagulant in Sweden during the study period was warfarin. Medication at baseline was defined by the presence of a filled prescription within 120 days before, up to 30 days after index date.
Patient level information about socio‐economic conditions such as education, income, and immigrant status, which may have affected decisions about anticoagulation, was obtained from the LISA register maintained by the governmental agency Statistics Sweden (http://www.scb.se).
Definition of heart failure
In the absence of echocardiographic data or information about the severity of heart failure, we used three different and increasingly broad definitions as sensitivity analyses in order to assess the robustness of the results.Heart failure definition 1 (CHF 1). A hospital discharge diagnosis of heart failure as principal or first secondary diagnosis and a filled prescription of a diuretic. Both within 3 months before index + 30 days.Heart failure definition 2 (CHF 2). A hospital discharge diagnosis of heart failure as principal or first secondary diagnosis within 1 year before index + 30 days.
Heart failure definition 3 (CHF 3). Any diagnosis of heart failure in open or in hospital care before index + 30 days.
This strictly defined CHF 1 heart failure has been used for the main results, with CHF 2 and CHF 3 for sensitivity analysis purposes only. ‘No heart failure’ refers to patients without heart failure according to any of these definitions.
Statistical methods
Baseline characteristics were presented descriptively, and differences were tested with t‐test (two sided) and χ2 test. Incidences were calculated as events per 100 years at risk but expressed as percentages in the text for easier reference.
Endpoints were analysed using univariable and multivariable Cox regressions with stepwise inclusion of cofactors and with the Efron method for handling ties. A list of the cofactors used is presented in the footnote to Table 1. The original CHA2DS2‐VASc score was calculated for each definition of heart failure (CHF 1–3) and compared with a ‘HA2DS2‐VASc’ score, that is without counting points for heart failure. Non‐parametric receiver operating characteristic analysis (receiver operator curves) and net reclassification improvement were calculated for the primary endpoint to assess concordance between model predictions and observed outcomes. P values <0.05 were considered significant. Analyses were performed in Stata 14.0 (Stata Corp, College Station, TX 77845, USA).
Table 1.
Heart failure as predictor of outcomes
| CHF 1 | CHF 2 | CHF 3 | |||||
|---|---|---|---|---|---|---|---|
| Endpoint | Analysis | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Ischaemic stroke | HF univariate | 1.41 | 1.35–1.47 | 1.37 | 1.32–1.43 | 1.33 | 1.30–1.37 |
| + age and sex | 0.98 | 0.94–1.03 | 1.00 | 0.96–1.04 | 1.01 | 0.98–1.04 | |
| + CHA2DS2‐VASc components | 0.98 | 0.93–1.02 | 0.98 | 0.94–1.02 | 0.97 | 0.94–1.00 | |
| + comorbidity and socio‐economya | 0.97 | 0.95–1.04 | 1.00 | 0.96–1.04 | 0.97 | 0.94–1.00 | |
| + medicationb | 1.01 | 0.96–1.06 | 1.02 | 0.97–1.07 | 1.01 | 0.97–1.04 | |
| Other thromboembolism (unspecified stroke and peripheral and pulmonary embolism) | HF univariate | 1.93 | 1.81–2.07 | 1.88 | 1.77–2.00 | 1.72 | 1.64–1.81 |
| + age and sex | 1.41 | 1.31–1.51 | 1.42 | 1.33–1.51 | 1.34 | 1.28–1.41 | |
| + CHA2DS2‐VASc components | 1.36 | 1.27–1.46 | 1.37 | 1.28–1.46 | 1.28 | 1.21–1.34 | |
| + comorbidity and socio‐economya | 1.28 | 1.19–1.38 | 1.28 | 1.20–1.37 | 1.20 | 1.14–1.26 | |
| + medicationb | 1.15 | 1.06–1.25 | 1.18 | 1.10–1.28 | 1.14 | 1.07–1.20 | |
| Intracranial haemorrhage | HF univariate | 1.31 | 1.20–1.42 | 1.26 | 1.17–1.36 | 1.24 | 1.18–1.31 |
| + age and sex | 1.00 | 0.92–1.09 | 0.99 | 0.92–1‐07 | 1.01 | 0.95–1.06 | |
| + HAS‐BLED components | 1.00 | 0.92–1.09 | 0.99 | 0.92–1.07 | 0.98 | 0.92–1.03 | |
| + comorbidity and socio‐economya | 1.00 | 0.91–1.09 | 1.01 | 0.90–1.07 | 0.98 | 0.93–1.04 | |
| + medicationb | 0.99 | 0.89–1.09 | 0.98 | 0.93–1.09 | 0.97 | 0.90–1.03 | |
| Other bleeding | HF univariate | 2.02 | 1.96–2.09 | 1.96 | 1.90–2.02 | 1.87 | 1.83–1.92 |
| + age and sex | 1.53 | 1.48–1.58 | 1.53 | 1.48–1.57 | 1.51 | 1.47–1.54 | |
| + HAS‐BLED components | 1.41 | 1.37–1.46 | 1.41 | 1.37–1.45 | 1.38 | 1.35–1.42 | |
| + comorbidity and socio‐economya | 1.32 | 1.27–1.36 | 1.30 | 1.26–1.34 | 1.29 | 1.26–1.32 | |
| + medicationb | 1.14 | 1.10–1.19 | 1.15 | 1.11–1.20 | 1.17 | 1.14–1.21 | |
| Death from any cause | HF univariate | 3.21 | 3.16–3.26 | 3.03 | 2.98–3.07 | 2.60 | 2.57–2.63 |
| + age and sex | 2.00 | 1.97–2.03 | 2.00 | 1.97–2.03 | 1.80 | 1.78–1.83 | |
| + comorbidity and socio‐economya | 1.73 | 1.70–1.76 | 1.70 | 1.67–1.73 | 1.54 | 1.52–1.56 | |
| + medicationb | 1.45 | 1.42–1.48 | 1.47 | 1.45–1.50 | 1.36 | 1.34–1.37 | |
| Composite of ischaemic stroke, intracranial bleed, and death | HF univariate | 2.86 | 2.82–2.90 | 2.71 | 2.67–2.75 | 2.36 | 2.33–2.38 |
| + age and sex | 1.83 | 1.80–1.86 | 1.83 | 1.80–1.86 | 1.66 | 1.64–1.68 | |
| + comorbidity and socio‐economya | 1.61 | 1.59–1.64 | 1.59 | 1.56–1.61 | 1.44 | 1.42–1.46 | |
| + medicationb | 1.40 | 1.37–1.42 | 1.41 | 1.39–1.44 | 1.30 | 1.28–1.32 | |
CI, confidence interval; CHF, congestive heart failure; HF, heart failure; HR, hazard ratio.
Adjustment for heart failure, age (continuous), gender, previous stroke, venous thromboembolism, any previous diagnosis of bleeding, platelet or coagulation defect, anaemia, previous myocardial infarction, ischaemic heart disease without infarction, peripheral artery disease, valvular disease (not qualifying as exclusion criteria), pacemaker/implantable cardioverter defibrillator, hypertension, diabetes, renal failure, liver disease, hypothyroidism, thyrotoxicosis, chronic pulmonary disease, alcohol index, dementia, cancer within 3 years, income, education, living alone, and working.
Adjustment for baseline use of warfarin, aspirin, clopidogrel, noac, beta‐blockers, class I antiarrhythmic agents, class III antiarrhythmic agents, digoxin, verapamil or diltiazem, dihydropyridine calcium blockers, diuretics, aldosterone receptor blockers, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, statins and non steroidal anti‐inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors.
The study was approved by the regional ethics committee and conformed to the Declaration of Helsinki. Individual patient consent was not required or obtained.
Results
During the 7.5 year study period, 300 839 patients with AF met the inclusion criteria. Of these, 34 773 (11.6%) had received a hospital discharge diagnosis of heart failure and also filled a prescription for a diuretic within the preceding 3 months (CHF 1), 44 334 (14.7%) had received a discharge diagnosis within the preceding year (CHF 2), and 92 532 (30.8%) had in one way or the other received a diagnosis of heart failure at any time before index date (CHF 3). There were 2462 men (4.9% of all), who had received their one and only point on the CHA2DS2‐VASc scale due to heart failure. Irrespective of definition, AF patients with heart failure were older, for example patients with CHF 1 were more than 7.5 years older than patients without (80.5 vs. 72.8 years, P < 0.001), and had higher risk scores both for ischaemic stroke (CHA2DS2‐VASc 4.4 vs. 2.6, P < 0.001) and bleeding (HAS‐BLED 2.6 vs. 2.1, P < 0.001) than other patients (Table 2). As expected, the use of diuretics, beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists was more common among patients with than without heart failure ( Table S2 ). Less than half of the patients used warfarin: crude rates 44.5% of those with, and 43.1% of those without, CHF 1 (P < 0.001).
Table 2.
Baseline characteristics in relation to definition of heart failure
| CHF 1 | No CHFa | P | CHF 2 | CHF3 | ||
|---|---|---|---|---|---|---|
| n = 34 773 | n = 208 307 | CHF1 vs. no CHF | n = 44 334 | n = 92 532 | ||
| Age, mean (median) | 80.5 (82) | 72.8 (75) | <0.001 | 79.8 (82) | 79.2 (81) | |
| Women | 47.9% | 44.0% | <0.001 | 46.4% | 45.8% | |
| Living alone | 68.7% | 51.1% | <0.001 | 67.3% | 64.2% | |
| Annual income and/or pension after taxes, 1000 SEK mean (median) | 162 (130) | 189 (144) | <0.001 | 164 (130) | 165 (131) | |
| University/college studies | 29.4% | 26.0% | <0.001 | 29.3% | 27.2% | |
| Immigrant | 10.6% | 10.4% | 0.268 | 10.9% | 10.8% | |
| CHA2DS2‐VASc score, mean (median) | 4.4 (4) | 2.6 (3) | <0.001 | 4.4 (4) | 4.4 (4) | |
| HAS‐BLED score, mean (median) | 2.6 (3) | 2.1 (2) | <0.001 | 2.6 (3) | 2.6 (3) | |
| Previous thromboembolism | Ischaemic stroke | 14.0% | 14.2% | 0.394 | 14.5% | 16.4% |
| Unspecified stroke and systemic or pulmonary embolism | 6.1% | 5.1% | <0.001 | 6.2% | 6.7% | |
| Previous bleeding | Intracranial | 2.4% | 2.4% | 0.774 | 2.4% | 2.7% |
| Other | 14.9% | 9.2% | <0.001 | 15.2% | 15.6% | |
| Anaemia | 10.6% | 4.4% | <0.001 | 10.8% | 9.4% | |
| Platelet or coagulation defect | 1.0% | 0.8% | <0.001 | 1.1% | 1.1% | |
| Ischaemic heart disease | Myocardial infarction | 30.4% | 12.5% | <0.001 | 31.3% | 31.3% |
| w/o myocardial infarction | 15.2% | 11.4% | <0.001 | 15.5% | 17.1% | |
| Peripheral arterial disease | 9.7% | 5.4% | <0.001 | 9.9% | 10.2% | |
| Valvular disease (other than exclusion criteria) | 15.5% | 6.2% | <0.001 | 15.4% | 15.0% | |
| Pacemaker or ICD | 10.3% | 6.5% | <0.001 | 11.3% | 12.0% | |
| Hypertension | 51.8% | 46.4% | <0.001 | 51.6% | 53.5% | |
| Diabetes | 23.5% | 13.7% | <0.001 | 23.5% | 23.8% | |
| Renal failure | 11.2% | 2.6% | <0.001 | 11.2% | 9.7% | |
| Liver disease | 1.3% | 0.9% | <0.001 | 1.4% | 1.4% | |
| Thyroid disease | Hypo | 5.0% | 3.3% | <0.001 | 5.0% | 4.6% |
| Hyper | 0.7% | 0.8% | 0.012 | 0.7% | 0.8% | |
| Chronic obstructive pulmonary disease | 12.8% | 4.9% | <0.001 | 13.2% | 12.7% | |
| Alcohol indexb | 2.2% | 2.4% | 0.016 | 2.4% | 2.5% | |
| Dementia | 4.7% | 3.3% | <0.001 | 4.7% | 5.2% | |
| Cancer within 3 years | 14.2% | 13.9% | 0.257 | 14.5% | 15.4% | |
CHF, congestive heart failure; ICD, implantable cardioverter defibrillator.
In the no CHF group, patients with heart failure according to all three definitions were excluded.
‘Alcohol index’ is a set of diagnostic codes used by the Swedish Board of Health and Welfare for annual reporting of alcohol‐related mortality (International Classification of Diseases‐10 codes).
The primary endpoint: ischaemic stroke
The 1 year incidences of ischaemic stroke among AF patients without warfarin were 4.4%, 4.3%, and 4.1% with CHF 1–3, respectively, and 3.1% among patients without any previous diagnosis of heart failure. Among patients with warfarin at baseline, the corresponding incidences were 1.7%, 1.7%, and 1.8% with CHF 1–3 and 1.5% among patients without heart failure.
Adjustment for the cofactors in CHA2DS2‐VASc eradicated the difference in ischaemic stroke risk between patients with and without heart failure [hazard ratio (HR) 0.98 with 95% confidence interval (CI) 0.93–1.02] (Figure 2 ). The results were similar with all three definitions of heart failure (Table 1).
Figure 2.
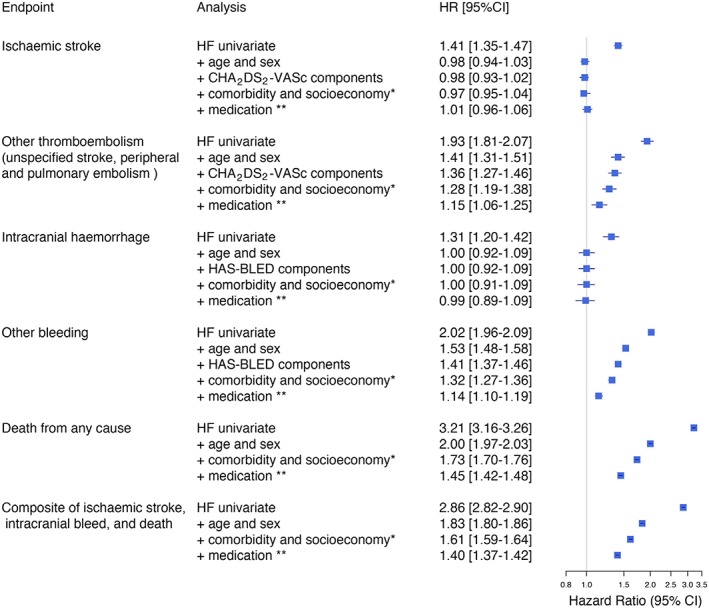
Forest plot showing the association between congestive heart failure (CHF 1) and outcome among 300 839 patients with atrial fibrillation. Multivariable adjustments to the hazard ratio (HR) and corresponding 95% confidence interval (CI) with stepwise inclusion of cofactors.
Secondary endpoints
Our secondary endpoint ‘other thromboembolism’, which did not count ischaemic stroke but counted unspecified stroke (which also may be haemorrhagic) and pulmonary and systemic embolism, occurred more often in patients with heart failure. The 1 year incidences among AF patients without warfarin were 1.9%, 1.9%, and 1.7% with CHF 1–3 and 1.0% among patients without heart failure.
The unadjusted HR for such other thromboembolism with heart failure was 1.93 (95% CI 1.81–2.07) compared with no heart failure (Figures 2 and 3 ). Adjustment for CHA2DS2‐VASc did not neutralize this excess risk. After adjustment for 45 cofactors including comorbidity, medication, and socio‐economic conditions, patients with heart failure still were at higher risk than patients without heart failure (HR 1.15, 95% CI 1.06–1.25). The results were similar with our more inclusive definitions of heart failure (Table 3).
Figure 3.
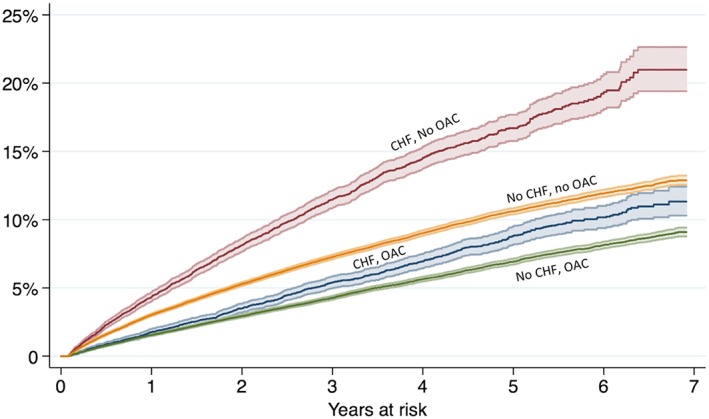
Incidence of ischaemic stroke among 300 839 patients with atrial fibrillation in relation to heart failure and warfarin use at baseline; CHF definition 1. CHF, congestive heart failure; OAC, oral anticoagulation.
Table 3.
Predictive efficacy of CHA2DS2‐VASc with and without points for heart failure. Only patients not on warfarin
| Ischaemic stroke within 1 year | ||||||
|---|---|---|---|---|---|---|
| Cut‐off for high risk | Scheme | c‐statistic | 95% CI | Positive predictive value | Negative predictive value | Net reclassification improvement |
| ≥1 point | CHF 1 CHA2DS2‐VASc | 0.542 | 0.540–0.543 | 0.031 | 0.996 | 0.001 |
| CHF 2 CHA2DS2‐VASc | 0.541 | 0.539–0.543 | 0.031 | 0.996 | 0.001 | |
| CHF 3 CHA2DS2‐VASc | 0.535 | 0.533–0.536 | 0.032 | 0.996 | −0.002 | |
| HA2DS2‐VASc | 0.536 | 0.533–0.536 | 0.032 | 0.995 | Reference | |
| ≥2 points | CHF 1 CHA2DS2‐VASc | 0.587 | 0.583–0.590 | 0.035 | 0.993 | 0.023 |
| CHF 2 CHA2DS2‐VASc | 0.584 | 0.580–0.587 | 0.035 | 0.993 | 0.017 | |
| CHF 3 CHA2DS2‐VASc | 0.572 | 0.569–0.575 | 0.035 | 0.993 | −0.016 | |
| HA2DS2‐VASc | 0.575 | 0.572–0.578 | 0.035 | 0.993 | Reference | |
| Continuous | CHF 1 CHA2DS2‐VASc | 0.638 | 0.631–0.646 | n/a | ||
| CHF 2 CHA2DS2‐VASc | 0.633 | 0.626–0.641 | ||||
| CHF 3 CHA2DS2‐VASc | 0.620 | 0.613–0.627 | ||||
| HA2DS2‐VASc | 0.628 | 0.622–0.635 | ||||
CI, confidence interval; n/a, not applicable.
CHF 1. A hospital discharge diagnosis of heart failure as principal or first secondary diagnosis and a filled prescription of a diuretic. Both within 3 months before index + 30 days.
CHF 2. A hospital discharge diagnosis of heart failure as principal or first secondary diagnosis within 1 year before index + 30 days.
CHF 3. Any diagnosis of heart failure in open or in hospital care before index + 30 days.
The 1 year incidence extracranial bleedings were more common among heart failure patients than among patients without heart failure (6.5% vs. 3.8%, P < 0.001). The excess bleeding risk associated with heart failure remained after adjustment for cofactors (HR 1.14, 95% CI 1.10–1.19). Regarding intracranial haemorrhages, no difference was seen in relation to heart failure (HR 0.99, 95% CI 0.89–1.09).
Heart failure patients more often met the composite endpoint of stroke (regardless of cause) and death than other patients. This was mostly driven by mortality component, which was three times higher (unadjusted) among heart failure patients. The annualized rates among patients with and without heart failure were 25.0% and 7.5%, respectively (P < 0.001). This difference between patients with and without heart failure remained after multivariable adjustment (HR 1.40, 95% CI 1.37–1.42) (Table 1). Among patients with heart failure, those who used warfarin at baseline more seldom met the composite endpoint than heart failure patients without warfarin (HR 0.72, 95% CI 0.70–74). The apparent benefit of warfarin treatment was similar in the control population of patients without heart failure (HR 0.68, 95% CI 0.66–0.69).
Points for heart failure in CHA2DS2‐VASc
We calculated CHA2DS2‐VASc scores without giving points for heart failure, that is ‘HA2DS2‐VASc’ scores, and compared its ability to predict ischaemic stroke with CHA2DS2‐VASc scores calculated with the three different definitions of heart failure (Table 3). With a cut‐off limit for high risk at ≥2 points, both the old and the new scheme had the same negative predictive value of 0.993, meaning that a patient with a low score of 0–1 points have an annual risk of ischaemic stroke of 0.7% irrespective of whether points are counted for heart failure or not. The positive predictive values were low for both schemes (0.035) indicating that the schemes are extremely poor in predicting who will actually suffer an ischaemic stroke. The area under the receiver operating characteristic curve was marginally better with CHA2DS2‐VASc (0.587, 95% CI 0.583–0.590) than with HA2DS2‐VASc (0.575, 95% CI 0.572–0.578) if the restrictive definition of heart failure was used, that is only counting heart failure diagnoses with recent hospitalizations as primary or first secondary diagnoses and with baseline use of diuretics (Table 3). If any previous diagnosis of heart failure in the medical history was considered when counting points for CHA2DS2‐VASc, it performed no better than HA2DS2‐VASc (c‐statistic 0.572, 95% CI 0.569–0.575). With this inclusive definition of heart failure (CHF 3), the net reclassification improvement of using CHA2DS2‐VASc over HA2DS2‐VASc even tended to be negative (Table 3).
Discussion
To date, this is the largest and most comprehensively adjusted analysis of stroke risk in AF patients with heart failure. The results suggest that a clinical diagnosis of heart failure is not a valid independent risk factor for stroke among these patients. This finding may be highly relevant for those 5% of the male AF population who have received their only CHA2DS2‐VASc point due to heart failure (Figure 3 ).
Although we performed adjustment for extensive covariates, heart failure lost its association with stroke already after adjustment for age, gender, and the CHA2DS2‐VASc components. The most common causes for heart failure are hypertension, ischaemic heart disease, diabetes, and age, which already are included in the CHA2DS2‐VASc scheme. Adding heart failure to the score therefore adjusts for what has already been adjusted for. It is true that heart failure patients are at higher risk of stroke than patients without heart failure, but this can thus be accounted for by age comorbidity.
It may be questioned if it is meaningful to count points for heart failure in the CHA2DS2‐VASc scheme. However, the CHA2DS2‐VASc score is ingrained in clinical practice and likely facilitates appropriate use of oral anticoagulants. Tampering with new acronyms in order to maximize the predictive power of the risk score schemes may cause confusion and abandonment of structured risk assessment all together.
We however propose that physicians recognize that ‘heart failure’ is a weak point in in the CHA2DS2‐VASc scheme, which only should be used for patients with recent and severe incompensation. Just any mentioning of heart failure in the medical records is not enough. This proposal however requires further studies, preferably with echocardiographic data.
Our results confirm the findings in some studies3, 6, 14 and contradict the results of others,15, 16, 17 as in a study of 6438 patients in Tours in France where the risk of thromboembolism was doubled in heart failure (HR 2.16, 95% CI (1.20–3.90).15 It is, however, difficult to make comparisons between these studies because differences in patient selection and design. For example, the Tours study classified 51.4% of the patients as heart failure compared with 11.6% in our study. Furthermore, while heart failure did not appear to independently increase the risk of stroke, oral anticoagulation was nevertheless effective in reducing stroke in patients with heart failure, as expected. Many patients with heart failure will have other risk factors and clear indications for anticoagulation regardless of presence of heart failure.
Limitations
A limitation of our study is that we were dependent on the diagnostic codes given by doctors all over the country, without possibility to validate data by scrutiny of random samples of medical records and examinations because we only had access to anonymized data. However, previous validation studies have shown that it is possible to verify a heart failure diagnosis retrospectively in approximately 90% of the cases.11, 12 An even higher proportion of diagnoses may be correct, although the documentation is no longer available.
We had no access to echocardiographic data. In previous studies, heart failure has been defined in many different ways, both with and without use of echocardiographic data.7, 8 The results of studies using echocardiographic studies have not differed markedly from studies not doing so. We did not have access to severity of heart failure. Heart failure is a clinical diagnosis, which includes both patients with reduced and preserved ejection fraction, often called diastolic heart failure. Previous studies suggest that 1/3 to 1/2 of all heart failure patients have preserved ejection fraction.18, 19 Hypothetically, if heart failure with reduced ejection confers increased stroke risk, and heart failure with preserved ejection does not (or vice versa for that matter), our inability to differentiate between these conditions could have obscured the results.
We cannot rule out the possibility that severe heart failure with recent decompensation or conversely, longer duration heart failure with more remodelling, may increase thromboembolic risk. Nor were we able to account for new‐onset heart failure during follow‐up in the control group, which may have biased the results towards the null. Our sensitivity analyses did however not show any important differences in the results whether only hospitalizations within the preceding 3 months, with the diagnosis as principal or first secondary position, and concomitant treatment with diuretics or if previous diagnosis of heart failure at any time was used.
Register studies are in general prone to underestimate risk factors that have not been diagnosed, and despite large and unselective population and extensive covariate adjustment, any risk factors or treatment effects remain associations only.
Under‐diagnosis is much more common than overdiagnosis. Thus, assessment of individual risk scores from register data is likely to give lower scores than the true situation. The extent of under‐diagnosis is largely unknown because assessment requires screening of the general population and in addition is likely to change over time.
Any registry is subject to bias and confounding, which limits interpretation of treatment effects in particular. With regard to warfarin use, confounding by indication is often a problem. Patients with poor compliance or short life expectancy may have been less likely to receive warfarin. Such circumstances are often not reflected by codes or data in registers.
Our information about exposure to warfarin assumes that all patients who purchased warfarin also used it, which rarely is the case. The treated share of patients is therefore most likely even lower than our estimates. However, we analysed warfarin use at baseline only, in analogy to intention to treat analysis, and any crossover during follow‐up would dilute the benefits that we observed.
Finally, in patients with AF, all ischaemic strokes are not cardioembolic, which limits the ability to detect specifically AF‐related cardioembolism.
Conclusions
A clinical diagnosis of heart failure was not an independent risk factor for stroke in patients with AF. The role of heart failure in CHA2DS2‐VASc requires further investigation.
Conflict of interest
L.F. has no conflicts related to the present work. Other conflicts include consulting and lecture fees from Boehringer Ingelheim, Bayer, Pfizer, Bristol‐Myers Squibb, and sanofi‐aventis.
L.H.L. has no conflicts related to the present work. Other conflicts include research grants from AstraZeneca, Novartis, and Boston Scientific and consulting and lecture fees from AstraZeneca, Novartis, Vifor Pharma, Relypsa Inc., Bayer, Fresenius, and St. Jude Medical.
Author contributions
Both authors participated equally in the conception and design of the study. L.F. prepared the data file and performed the statistical analyses. Both authors contributed equally to the first draft and to the subsequent revisions of the manuscript.
Funding
This work was funded by grants to L.H.L.'s institution from the Swedish Research Council [grant 2013‐23897‐104604‐23], the Swedish Heart‐Lung Foundation [grants 20100419 and 20120321], the Stockholm County Council [grants 20110120 and 20140220] and the Swedish Society of Medicine [grants 174111 and 504881].
Supporting information
Table S1. Definition of diagnoses by ICD‐10 codes and Swedish surgical procedural codes.
Table S2. Baseline medication in relation to definition of heart failure.
Friberg, L. , and Lund, L. H. (2018) Heart failure: a weak link in CHA2DS2‐VASc. ESC Heart Failure, 5: 231–239. doi: 10.1002/ehf2.12262.
References
- 1. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 2. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med 1992; 116: 1–5. [DOI] [PubMed] [Google Scholar]
- 3. AFI‐Investigators . Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154: 1449–1457. [PubMed] [Google Scholar]
- 4. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 5. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013; 6: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012; 33: 1500–1510. [DOI] [PubMed] [Google Scholar]
- 7. Hughes M, Lip GY. Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost 2008; 99: 295–304. [DOI] [PubMed] [Google Scholar]
- 8. Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology 2007; 69: 546–554. [DOI] [PubMed] [Google Scholar]
- 9. The Swedish Board of Health and Welfare . Quality of coding in the national patient registry, in‐hospital care 2008. Only avaliable in Swedish [Socialstyrelsen. Kodningskvalitet i patientsregistret, slutenvård 2008] http://www.socialstyrelsen.se/publikationer2010/2010-6-27 (3 June 2015).
- 10. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmö Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010; 25: 95–102. [DOI] [PubMed] [Google Scholar]
- 11. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005; 7: 787–791. [DOI] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 14. Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. Stroke Prevention in Atrial Fibrillation Investigators. JAMA 1998; 279: 1273–1277. [PubMed] [Google Scholar]
- 15. Olesen JB, Fauchier L, Lane DA, Taillandier S, Lip GY. Risk factors for stroke and thromboembolism in relation to age among patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest 2012; 141: 147–153. [DOI] [PubMed] [Google Scholar]
- 16. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA 1994; 271: 840–844. [PubMed] [Google Scholar]
- 17. Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013; 2: e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jonsson A, Edner M, Alehagen U, Dahlstrom U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail 2010; 12: 25–31. [DOI] [PubMed] [Google Scholar]
- 19. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of diagnoses by ICD‐10 codes and Swedish surgical procedural codes.
Table S2. Baseline medication in relation to definition of heart failure.


