Abstract
Aims
Sleep‐disordered breathing (SDB) is a highly prevalent co‐morbidity in patients with chronic heart failure (CHF) and can play a detrimental role in the pathophysiology course of CHF. However, the best way to manage SDB in CHF remains a matter of debate. Sacubitril–valsartan has been included in the 2016 European Society of Cardiology guidelines as an alternative to angiotensin‐converting enzyme inhibitors to further reduce the risk of progression of CHF, CHF hospitalization, and death in ambulatory patients. Sacubitril and valsartan are good candidates for correcting SDB of CHF patients because their known mechanisms of action are likely to counteract the pathophysiology of SDB in CHF.
Methods and results
The ENTRESTO‐SAS trial is a 3‐month, multicentric, prospective, open‐label real‐life cohort study. Patients eligible for sacubitril–valsartan treatment (i.e. adults with left ventricular ejection fraction ≤35%, who remain symptomatic despite optimal treatment with an angiotensin‐converting enzyme inhibitor, a beta‐blocker, and a mineralocorticoid receptor antagonist) will be evaluated before and after 3 months of treatment (nocturnal ventilatory polygraphy, echocardiography, laboratory testing, and quality‐of‐life and SDB questionnaires). The primary outcome is the change in the Apnoea–Hypopnoea Index, before and after 3 months of treatment. One hundred twenty patients are required to detect a significant 20% improvement of the Apnoea–Hypopnoea Index with a power of 90% at an alpha risk of 5%.
Conclusions
In the context of the SERVE‐HF study, physicians are waiting for new trials and alternative therapies. We sought to assess in the ENTRESTO‐SAS trial whether sacubitril–valsartan could improve the outcome of SDB in CHF patients.
Keywords: Heart failure, Sleep apnoea syndrome, Continuous positive airway pressure, Sacubitril–valsartan, Sleep‐disordered breathing
Introduction
Chronic heart failure (CHF) is a frequent pathology marked by significant morbidity and mortality.1 Approximately 1–2% of the adult population in developed countries has CHF, with a prevalence rising to ≥10% among persons 70 years of age or older.2 Despite progress in the medical management of CHF and a relative reduction in hospitalization in recent years by 30–50%,3, 4 the last European data (ESC‐HF pilot study) demonstrated that 12‐month hospitalization rates for hospitalized and stable/ambulatory CHF patients were 44% and 32%, respectively, and the 12‐month all‐cause mortality rates were 17% and 7%, respectively.5 At 4.5 years, mortality remains high in developed countries, reported in 2011 as reaching over 70% in an American cohort study.6
Sleep‐disordered breathing (SDB) is a highly prevalent co‐morbidity in CHF patients and potentially impacts the prognosis of CHF. The prevalence of SDB, either predominantly obstructive sleep apnoea (OSA) or predominantly central sleep apnoea (CSA), was reported as reaching up to 76% in CHF patients with reduced ejection fraction (HFrEF).7 While OSA has been shown to be an independent risk factor for the development of CHF8 and to increase morbidity and mortality of CHF,9 CSA appears to be more a marker of CHF severity and is thought to mirror cardiac dysfunction.10, 11 However, both OSA and CSA interfere with neurohumoral systems and thus may worsen CHF, for example, by increasing sympathetic and renin–angiotensin–aldosterone activity, both targets of CHF therapy. To date, there is a consensus to consider that the first step in the management plan for patients with SDB and CHF should be optimization of CHF treatment.12, 13, 14 Indeed, previous studies have reported that optimization of pharmacological therapy15, 16, 17, 18, 19, 20 and the use of non‐pharmacological methods21, 22 to treat CHF can lead to an improvement in SDB. However, the best way to manage SDB in CHF remains open to debate since the beneficial effect of an additional ventilatory treatment in patients with HFrEF is questioned after the results of the SERVE‐HF trial and pending the results of the ADVENT‐HF study.23
Sacubitril–valsartan (Entresto™; LCZ696) is a new orally administered complex of the neprilysin inhibitor prodrug sacubitril and the angiotensin II Type 1 receptor blocker valsartan, recently approved in the United States and the European Union for the treatment of patients with HFrEF who remain symptomatic (New York Heart Association Classes II–IV) despite optimal treatment with an angiotensin‐converting enzyme (ACE) inhibitor, a beta‐blocker, and a mineralocorticoid receptor antagonist.4 Indeed, in the large randomized double‐blind PARADIGM‐HF trial,24 sacubitril–valsartan reduced the occurrence of the primary endpoint (cardiovascular death or hospitalization for worsening of CHF) by 20% when compared with the ACE inhibitor enalapril. Sacubitril–valsartan was also superior to enalapril in reducing death from any cause (16% reduction) and in limiting the progression of CHF. As a consequence, in these specified patients, sacubitril–valsartan is recommended by the 2016 European Society of Cardiology guidelines (Class I, Level B) as an alternative to ACE inhibitors.4
Two preliminary studies have reported that ACE inhibitors could improve SDB in CHF.15, 16 To the best of our knowledge, the effect of sacubitril–valsartan on SDB has never been investigated in a prospective study despite the fact that this drug combination has the potential to correct SDB in CHF patients. Indeed, the first case report to document parallel improvements in CHF and SDB after the initiation of sacubitril–valsartan was published recently.25 Their known mechanisms of action are likely to counteract key points of the pathophysiology of SDB in CHF (extracellular fluid overload, cardiac injury, and sympathetic nervous system activation).26 Thereby, sacubitril–valsartan interferes with neurohumoral systems and improves CHF by decreasing sympathetic and renin–angiotensin–aldosterone activity, both possible actors also involved in the pathophysiology of SDB.
In the context of the post‐SERVE‐HF study, where concern prevails against auto‐servoventilation, physicians are waiting for new trials and an alternative therapy. In the ENTRESTO‐SAS trial, we sought to assess in real‐life conditions whether sacubitril–valsartan could improve the outcome of SDB in CHF patients with HFrEF.
Study design
Study hypothesis
The aim of ENTRESTO‐SAS trial is to assess the impact of sacubitril‐valsartan on SDB of CHF patients. We speculate that the sacubitril–valsartan combination could provide expected beneficial effects on CHF but also modulate the severity of SDB as assessed by means of the Apnoea–Hypopnoea Index (AHI) measured before and after exposure to the drug.
Design
The ENTRESTO‐SAS trial is a 3‐month, multicentric, prospective, open‐label real‐life cohort study. Figure 1 summarizes the design of the study and the participants' timeline. Patients treated in real‐life conditions of care and eligible for sacubitril–valsartan treatment will be invited to participate in the study. After inclusion and exclusion criteria are checked (Table 1), a pre‐therapeutic evaluation will be performed [physical examination, echocardiography, laboratory testing, Minnesota Living with Heart Failure Questionnaire and EuroQol Group 5‐Dimension Self‐Report Questionnaire (EQ‐5D), Epworth Sleepiness Scale, Pichot Scale, and nocturnal ventilatory polygraphy]. Sacubitril–valsartan will be started the day after the polygraphy, and a cardiological follow‐up will then be set up to achieve the optimal treatment dose before 3 months.
Figure 1.
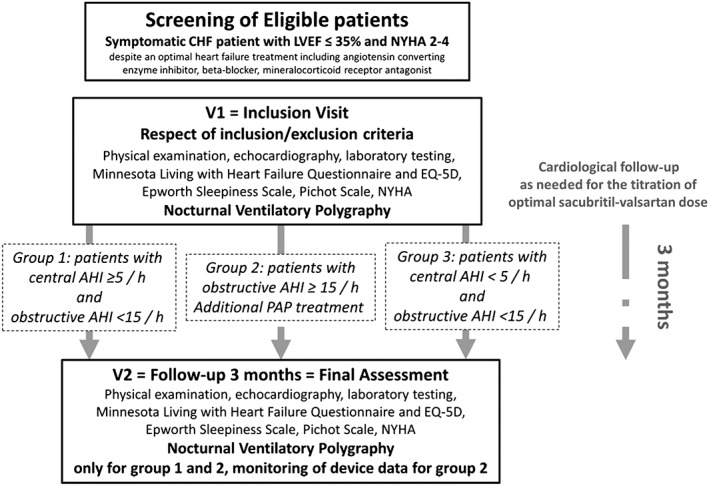
Study design. AHI, Apnoea–Hypopnoea Index; CHF, chronic heart failure; EQ‐D, EuroQol Group 5‐Dimension Self‐Report Questionnaire; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; PAP, positive airway pressure.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 18 years of age or older | Pregnancy |
| HF with LVEF ≤35% and NYHA 2–4 | Renal insufficiency (eGFR < 30 mL/min) |
| Symptomatic despite an appropriate HF treatment at the appropriate dosing per guidelines | Current positive airway pressure |
| Medically stable in terms of HF clinical status (ambulatory and receiving no IV medications) | Allergy to one compound or personal history of angioedema |
| Signed informed consent | Haemodynamic instability |
| Severe hepatopathy | |
| Current and not treated hyperkalaemia | |
| Anticipated life expectancy <6 months |
eGFR, estimated glomerular filtration rate; HF, heart failure; IV, intravenous; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction.
Considering the results of the polygraphy, patients will be divided into three groups:
Group 1: patients characterized by a central AHI ≥5/h and an obstructive AHI <15/h.
Group 2: patients characterized by an obstructive AHI ≥15/h whatever the central component. In this group of patients, a ventilator treatment with positive airway pressure [PAP, i.e. constant PAP (CPAP) or bilevel pressure or auto‐servoventilation] can be started. In the case of predominantly central events and reduced left ventricular ejection fraction (LVEF), the use of auto‐servoventilation treatment was not allowed.
Group 3: patients characterized by a central AHI <5/h and an obstructive AHI <15/h.
After 3 months of treatment, the final evaluation will include physical examination, echocardiography, laboratory testing, Minnesota Living with Heart Failure Questionnaire and EQ‐5D, Epworth Sleepiness Scale, and Pichot Scale, for all patients included in the study. Nocturnal polygraphy will be repeated only in Groups 1 and 2. For PAP‐treated patients from Group 2, observance, residual AHI, and level of PAP measured by the device equipped with a built‐in software will be recorded.
Ethics approval
The ENTRESTO‐SAS trial is conducted in keeping with Good Clinical Practice Guidelines, the principles outlined in the Declaration of Helsinki, and applicable local laws and regulations. The trial has been approved by the Ethics Committee for the Protection of Persons (CPP Sud Méditerranée IV), the French Drug Safety Agency (Agence Nationale de Sécurité du Médicament), the French Advisory Committee on health research information processing (Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé), and the French National Commission for Data Protection (Commission Nationale de l'Informatique et des Libertés). Informed consent is required. The trial is registered at ClinicalTrials.gov under the identification number NCT02916160.
Study endpoints
The primary endpoint of the ENTRESTO‐SAS trial is the AHI change determined by nocturnal polygraphy, before and after 3 months of treatment with sacubitril–valsartan. The 2012 American Academy of Sleep Medicine recommendations are used to characterize apnoea and hypopnoea events, the phenotype, whether central, obstructive, or mixed.
Secondary endpoints include the following:
The proportion of patients with a 20% decrease in their AHI, before and after sacubitril–valsartan.
The proportion of patients with a 50% decrease in their AHI, before and after sacubitril–valsartan.
The proportion of patients with AHI < 5/h, 5 ≤ AHI < 15, 15 ≤ AHI < 30, and 30 ≤ AHI, before and after sacubitril–valsartan, and the proportion of central events before and after sacubitril–valsartan.
A comparison of AHI results assessed by JAWAC® technology.27
A Global Subject Assessment (heart rhythm, systolic and diastolic blood pressure, and New York Heart Association functional class), before and after sacubitril–valsartan.
Echocardiographic measures of structure and function before and after sacubitril–valsartan [ejection fractions, left ventricular diameters, atrial surface, diastolic function (strain), and filling pressures].
Renal function as compared with baseline (estimated glomerular filtration rate will be calculated using the Chronic Kidney Disease Epidemiology Collaboration formula).
Serum BNP and pro‐BNP concentrations before and after sacubitril–valsartan.
Laboratory testing also included creatinine, potassium, sodium, haemoglobin, and alanine and aspartate transaminase.
Concomitant medications will be assessed before and after sacubitril–valsartan.
The proportion of patients without an optimal dose of sacubitril–valsartan after 3 months.
Quality of life as measured by the Minnesota Living with Heart Failure Questionnaire and EQ‐5D‐3L Questionnaire, before and after sacubitril–valsartan.
Epworth Sleepiness Scale before and after sacubitril–valsartan
Pichot Scale before and after sacubitril–valsartan
Device‐related measures in Group 2 patients: PAP observance (number of hours per day and number of days with ≥3 h in the past 2 months), type of device used (CPAP, bilevel pressure, or auto‐servoventilation), settings of the device, type of mask used and historical use of mask, and AHI reported by the device.
Patient population
The ENTRESTO‐SAS study will enrol adult (≥18 years old), clinically stable patients with heart failure (HF; defined by an LVEF ≤35% at transthoracic echo examination for 1 month at least, without exacerbation of HF and receiving appropriate HF treatment at the appropriate dosing as per guidelines). Table 1 summarizes the inclusion and exclusion criteria of the study.
Study drug
Patients will receive sacubitril–valsartan orally twice daily with adjustment for renal function and haemodynamic tolerance, as per the European‐Union‐approved dose. Subjects will be advised to take the study drug at the same time every day according to approved instructions.
Sample size and statistical methods
Data will be collected and recorded on case report forms by trained local research coordinators or residents. Data will be handled in compliance with French law. All original records will be archived at trial sites for 15 years. The clean database file will be anonymized and kept for 15 years.
The primary outcome is the change in the AHI determined by nocturnal polygraphy, before and after 3 months of treatment. For this study, 68 evaluable patients are needed to detect a 20% difference after 3 months, with a standard deviation of 5 points (standardized effect size of 0.4), at a two‐sided α level of 0.05 and a statistical power of 90%. After potential withdrawn consents after inclusion, inclusions not fulfilling inclusion criteria, or patients with a central AHI <5/h and an obstructive AHI <15/h are taken into account, 120 patients will be included in this study.
Statistical analysis will be performed on an intention‐to‐treat population, encompassing all included patients except those who have withdrawn their consent or who did not meet the inclusion criteria. Baseline features of the overall population and of each group will be described, using n (%) for categorical variables and the minimum, maximum, mean, standard deviation, and quartiles for quantitative variables.
Paired Student's t test or Wilcoxon's test when appropriate will be used for primary outcome analysis.
The χ2 test (or Fisher's exact test as appropriate) will be used for secondary binary outcomes. Continuous variables will be compared with the use of parametric or non‐parametric ANOVA.
All analyses will be conducted by the medical statistical department of the Montpellier University Hospital using statistical software (SAS, V.9.3; SAS Institute; Cary, NC, USA, and R V.3.2.3). A two‐sided P value of <0.05 will be considered to indicate statistical significance.
Safety
Safety endpoints include death, onset of acute HF episodes, frequency and type of serious adverse event (SAEs), and adverse drug reaction (ADRs) as defined by Good Clinical Practices. Investigators and site staff are responsible for detecting, documenting, and reporting SAEs and ADRs on electronic case report forms, which are continuously monitored.
Participating sites
To preserve the real‐life nature of the study, university hospitals and non‐university hospitals/clinics participate in the study. University hospitals include the Nîmes, Montpellier, Bordeaux, Marseille, and Toulouse hospitals, and non‐university hospitals/clinics include Pasteur clinic (Toulouse) and the Béziers hospital and Saint‐Privat clinic of Boujan‐sur‐Libron.
Discussion
To date, the best way to manage SDB in CHF patients remains a point of debate. The SERVE‐HF study23 has raised serious concerns about the safety of treating patients with CHF and CSA with adaptative servoventilation, so that physicians are waiting for new trials and alternative therapies. Pharmacological and non‐pharmacological approaches are being developed and evaluated to correct SDB associated with CHF. Table 2 depicts a summary of the studies registered on the ClinicalTrials.gov website using a non‐ventilatory therapeutic approach targeting SDB in patients with CHF.
Table 2.
Summary of the studies registered on the clinical trial website using a non‐ventilatory therapeutic approach targeting sleep‐disordered breathing in patients with chronic heart failure
| Country | Drug/intervention | Type of SDB | CHF category | Number of patients | Primary endpoint | Time frame | Design | Putative end of study | NCT |
|---|---|---|---|---|---|---|---|---|---|
| USA | Oxygen vs. CPAP | OSA and CSA | LVEF < 45% | 161 | LVEF | 3 months | Randomized; single blind | December 2017 | NCT01807897 |
| Brazil | Inspiratory muscle training | OSA | LVEF < 51% for man and LVEF < 53% for woman | 30 | AHI | 3 months | Randomized; double blind | December 2017 | NCT02794935 |
| USA | Acetazolamide, 4 mg/kg, once daily before b.i.d., for 7 days | OSA and CSA | LVEF <50% and HFpEF | 85 | AHI | 1 week | Randomized; double blind | 2016 | NCT01377987 |
| UK | Carbon dioxide | CSA | LVEF < 40% | 24 | Safety | Night | Single blind | Unknown | NCT01041924 |
| UK | CRT | CSA | LVEF < 40% | 40 | The change in gradient of minute ventilation vs. end tidal CO2 before and after CRT | 6 weeks; 6 months | Observational | Unknown | NCT02203383 |
| Austria | CRT vs. conventional right ventricular stimulation | CSA | LVEF < 50% | 80 | Improvement of central sleep apnoea | 3–5 months | Randomized; crossover assignment | January 2018 | NCT01970423 |
AHI, Apnoea–Hypopnoea Index; CHF, chronic heart failure; CPAP, constant positive airway pressure; CRT, cardiac resynchronization therapy; CSA, central sleep apnoea; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnoea; SDB, sleep‐disordered breathing.
Previous studies have reported that optimization of pharmacological therapy can lead to an improvement in SDB.15, 16, 17, 18, 19, 20 Table 3 summarizes the published data on the effect of medical treatment of CHF in patients presenting SDB. Paradoxically, the evidence‐based beneficial effect of medical treatment on SDB in patients with HFrEF is weak. Only six studies encompassing a total of 67 patients are available, and only one is a randomized controlled trial with a crossover design. The ENTRESTO‐SAS trial is a pharmacological trial whose main purpose is to evaluate the new sacubitril–valsartan combination effect on the AHI of CHF patients presenting SDB.
Table 3.
Summary of studies evaluating the impact of medical treatment on the Apnoea–Hypopnoea Index of chronic heart failure patients
| Study | Type of study | Clinical characteristics | Treatment | AHI (number of events per hour) | AI (number of events per hour) | |||
|---|---|---|---|---|---|---|---|---|
| No. of patients | LVEF (%) | Before | After | Before | After | |||
| Walsh et al.15 | OO | 9 | <30 | Captopril 75 mg/day | 35 ± 7 | 20 ± 5 | ||
| 4 weeks | ||||||||
| Solin et al.16 | OO | 7 | 18.9 ± 1.3 | Diuretics: 6 patients | 38.5 ± 7.7 (central) | 18.1 ± 5.8 (central) | ||
| ACE inhibitor: 4 patients | ||||||||
| Carvedilol: 2 patients | ||||||||
| Nitrate: 2 patients | ||||||||
| 1 to 6 months | ||||||||
| Javaheri et al.17 | CO and DB | 12 | 19 ± 6 | Acetazolamide 3.5 mg/kg/day | 55 ± 24 | 34 ± 20 | 44 ± 23 (central) | 23 ± 21 (central) |
| 6 days | ||||||||
| Tamura et al.18 | OO | 5 | 36 ± 8.6 | Carvedilol 2.5 to 20 mg/day | 28.8 ± 7.5 | 12.4 ± 9.1 | 9.5 ± 4.9 (central) | 1.3 ± 2.4 (central) |
| 6 months | 1.8 ± 1.4 (obstructive) | 1.4 ± 1.5 (obstructive) | ||||||
| Bucca et al.19 | OO | 15 | 65.85 ± 1.7 | Furosemide 40 mg/day | 74.89 ± 6.95 | 57.17 ± 5.4 | ||
| Spironolactone 200 mg/day | ||||||||
| 3 days | ||||||||
| Tamura et al.20 | OO | 19 | 32 ± 7.4 | Carvedilol 2.5 to 20 mg/day | 34 ± 13 | 14 ± 13 | 13 ± 11 (central) | 1.9 ± 4.3 (central) |
| 6 months | 1.1 ± 1.5 (obstructive) | 3.1 ± 3.4 (obstructive) | ||||||
For the apnoea index, the type of event (central/obstructive) is mentioned if data are available. ACE, angiotensin‐converting enzyme; AHI, Apnoea–Hypopnoea Index; AI, Apnoea Index; CO, crossover study; DB, double‐blind study; LVEF, left ventricular ejection fraction; OO, open and observational study.
Pathophysiology of sleep‐disordered breathing and medical treatment of chronic heart failure
Chronic HF patients may have an increased risk for OSA due to extracellular fluid overload. Achieving fluid homeostasis is a potential point of care since pharyngeal oedema and narrowing may develop during supine sleep with redistribution of fluid from the legs and subsequent pharyngeal collapsibility and airway obstruction in CHF patients with OSA.28 Indeed, it has been reported that the aldosterone antagonist spironolactone improves postural fluid shifts and AHI in patients with OSA.29
Central sleep apnoea in CHF is likely caused by the instability of ventilatory control systems. Patients with CSA have increased chemoresponsiveness that promotes hyperventilation and hypocapnia.10, 12, 13 A factor contributing to hyperventilation is pulmonary vagal irritant receptor stimulation by pulmonary venous congestion. Induced hyperventilation often drives the arterial CO2 pressure below the apnoeic threshold, leading to decreased central respiratory drive.10, 12, 13
Thereby, optimal CHF therapy could counteract some mechanisms of the OSA/CSA pathophysiology since it includes diuretics aiming to reduce the total lung water content, beta‐blockers to blunt the effects of sympathetic nervous system activation, and ACE inhibitors to reduce ventricular afterload and improve cardiac output by blocking the effects of the renin–angiotensin–aldosterone system.3, 4
Why sacubitril–valsartan is a candidate for correcting sleep‐disordered breathing in chronic heart failure patients
To date, the only study evaluating valsartan did not find any benefit on AHI, but the patients included in the study did not represent CHF30 and the effect of sacubitril has never been evaluated on AHI.
Sacubitril and valsartan are good candidates for correcting SDB of CHF patients because their known mechanisms of action are likely to counteract the pathophysiology of both OSA and CSA in HF patients. Indeed, inhibition of neprilysin by sacubitril increases the level of numerous endogenous peptides (atrial natriuretic peptide, BNP, C‐type natriuretic peptide, bradykinin, substance P, adrenomedullin, and angiotensin II) with favourable natriuretic and vasodilatory properties and a favourable decrease of sympathetic tone.26 The antagonism of angiotensin II Type 1 receptors by valsartan decreases sodium retention, endothelin and aldosterone secretion, and vasopressin release and increases renin secretion, all these actions leading to natriuretic and vasodilatory beneficial effects.31 Valsartan also decreases cardiac injury by decreasing angiotensin II Type‐1‐receptor‐mediated hypertrophy, inflammation, fibrosis, and sympathetic activity.31 Thereby, sacubitril–valsartan interferes with neurohumoral systems and improves CHF by decreasing sympathetic and renin–angiotensin–aldosterone activity, both possible factors also involved in the pathophysiology of SDB in CHF patients.
Study design and limitations
Testing a recently licensed drug strongly recommended in the population of interest, in real‐life conditions, has raised many new challenges regarding study design. First, it appeared unethical to impose a placebo‐controlled randomization: even though the study will not exceed 3 months, beneficial effects of sacubitril–valsartan on mortality and hospitalization rates were noticed very early in the PARADIGM trial, and a placebo arm would have been considered as a potential loss of chance for patients.
Physicians in charge of patients with CHF and an obstructive AHI ≥15/h (irrespective of the central AHI) are facing a complex dilemma. They can initiate additional PAP treatment because CPAP has previously demonstrated a decrease in mortality in this population of CHF patients with OSA.32 However, as underlined recently by Bradley et al.,33 there is currently no published randomized double‐blind study demonstrating the benefit of CPAP vs. sham CPAP in these patients with HFrEF.
Considering the real‐life pattern of the study and the evolution of the recommendations,34 no strict protocol of treatment has been made in our study protocol. It is noteworthy that sacubitril–valsartan should be initiated cautiously following a progressive incremental‐dose scheme. Because the time to obtain a safe and efficient dose of sacubitril–valsartan may differ from patient to patient, the ideal duration of the study may exceed 3 months. In the first version of the protocol submitted to an independent ethics committee, the final evaluation was scheduled at 4 months. The committee's response (taking into account the literature at the time) was to reduce the study time to 3 months. They considered that that one extra month could represent increased risk for patients. The literature at that time indicated that the absence of specific treatment for SDBs could be associated with an increased morbimortality, in particular in patients with OSA. Indeed, for patients with a potential indication of additional PAP treatment, a longer duration of the study could be considered as a loss of chance.32 A 3‐month study appeared to be a good compromise and was recommended by the ethics committee. In any case, the study evaluates the number of patients for whom the cardiologist considers that the optimal dose of sacubitril–valsartan is not reached at 3‐months.
Of course, we are fully aware that polygraphy is not the gold standard diagnostic test of SDB, but in real‐life conditions, it is the only easily and rapidly available examination. Furthermore, it has nonetheless been shown as relevant. Current guidelines acknowledge polygraphy as an important contributor to SDB assessment. For ethical and financial considerations, Group 3 patients (i.e. patients with a central AHI <5/h and an obstructive AHI <15/h) will not benefit from a second polygraphy.
Conclusions
In the context of the SERVE‐HF study,23 a trial that has raised serious concerns about the effect of adaptative servoventilation in patients with HFrEF and CSA, physicians are waiting for new trials and alternative therapies. Because of its reported beneficial effects and its mechanism of action, we believe that sacubitril–valsartan needs to be evaluated.25 The ENTRESTO‐SAS study will be the first prospective real‐life study evaluating the potential interest of sacubitril–valsartan in patients with SDB and CHF with HFrEF.
Conflict of interest
J.D. reports advisory board membership and participation as an investigator in clinical trials sponsored by Loewenstein Medical and ResMed and Philips. N.M. has declared that no competing interest exists in relation with the present trial. P.B. reports advisory board membership and participation as an investigator in clinical trials sponsored by Novartis. A.P. reports advisory board membership and participation as an investigator in clinical trials sponsored by Novartis. M.G. reports advisory board membership and participation as an investigator in clinical trials sponsored by Novartis and ResMed. M.D. have declared that no competing interest exists in relation with the present trial. J.P.M. reports advisory board membership and participation as an investigator in clinical trials sponsored by Loewenstein Medical and ResMed and Philips. A.B. reports advisory board membership and participation as an investigator in clinical trials sponsored by GlaxoSmithKline, Chiesi, Actelion, Teva, Regeneron, Boehringer Ingelheim, Novartis, and AstraZeneca. F.R. reports advisory board membership and participation as an investigator in clinical trials sponsored by Novartis.
Acknowledgements
The authors are grateful to Ms Anne Verchere and Ms Erika Nogue for their administrative and organizational support. The authors would like to thank Dr Nicolas Combes (Cardiology Unit, Pasteur Clinic, Toulouse, France), Dr Kamila Solecki (Department of Cardiology, Regional University Hospital, Hôpital Arnaud de Villeneuve, Montpellier, France), and Dr Carey M. Suehs (Department of Medical Information, Regional University Hospital, Hôpital La Colombière, Montpellier, France) for their review of the present manuscript, as well as Ms Mireille Renaud‐Mallet (translator–interpreter, member of EMWA‐AMWA) for editing this paper.
Jaffuel, D. , Molinari, N. , Berdague, P. , Pathak, A. , Galinier, M. , Dupuis, M. , Ricci, J.‐E. , Mallet, J.‐P. , Bourdin, A. , and Roubille, F. (2018) Impact of sacubitril–valsartan combination in patients with chronic heart failure and sleep apnoea syndrome: the ENTRESTO‐SAS study design. ESC Heart Failure, 5: 222–230. doi: 10.1002/ehf2.12270.
References
- 1. McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart 2000; 83: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 6. Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes 2011; 4: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep disordered breathing in patients with symptomatic heart failure. Eur J Heart Fail 2007; 9: 251–257. [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, Pleister A, Abraham WT. Sleep disordered breathing and post‐discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naughton MT. Heart failure and sleep‐disordered breathing. The chicken or the egg? Am J Respir Crit Care Med 2016; 193: 482–483. [DOI] [PubMed] [Google Scholar]
- 11. Grimm W, Sosnovskaya A, Timmesfeld N, Hildebrandt O, Koehler U. Prognostic impact of central sleep apnea in patients with heart failure. J Card Fail 2015; 21: 126–133. [DOI] [PubMed] [Google Scholar]
- 12. Costanzo MR, Khayat R, Ponikowski P, Augostini R, Stellbrink C, Mianulli M, Abraham WT. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol 2015; 65: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khayat R, Small R, Rathman L, Krueger S, Gocke B, Clark L, Yamokoski L, Abraham WT. Sleep‐disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Card Fail 2013; 19: 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence‐based literature review and meta‐analyses. Sleep 2012; 35: 17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh JT, Andrews R, Starling R, Cowley AJ, Johnston ID, Kinnear WJ. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive cardiac failure. B Heart J 1995; 73: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation 1999; 99: 1574–1579. [DOI] [PubMed] [Google Scholar]
- 17. Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double‐blind, prospective study. Am J Respir Crit Care Med 2006; 173: 234–237. [DOI] [PubMed] [Google Scholar]
- 18. Tamura A, Kawano Y, Naono S, Kotoku M, Kadota J. Relationship between beta‐blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest 2007; 131: 130–135. [DOI] [PubMed] [Google Scholar]
- 19. Bucca CB, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, Cicolin A. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest 2007; 132: 440–446. [DOI] [PubMed] [Google Scholar]
- 20. Tamura A, Kawano Y, Kadota J. Carvedilol reduces the severity of central sleep apnea in chronic heart failure. Circ J 2009; 73: 295–298. [DOI] [PubMed] [Google Scholar]
- 21. Kara T, Novak M, Nykodym J, Bybee KA, Meluzin J, Orban M, Novakova Z, Lipoldova J, Hayes DL, Soucek M, Vitovec J, Somers VK. Short‐term effects of cardiac resynchronization therapy on sleep‐disordered breathing in patients with systolic heart failure. Chest 2008; 134: 87–93. [DOI] [PubMed] [Google Scholar]
- 22. Mansfield DR, Solin P, Roebuck T, Bergin P, Kaye DM, Naughton MT. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest 2003; 124: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 23. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE‐HF: more questions than answers. Chest 2016; 149: 900–904. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 25. Fox H, Bitter T, Horstkotte D, Oldenburg O. Resolution of Cheyne–Stokes respiration after treatment of heart failure with sacubitril/valsartan: a first case report. Cardiology 2017; 137: 96–99. [DOI] [PubMed] [Google Scholar]
- 26. Chen CH. Critical questions about PARADIGM‐HF and the future. Acta Cardiol Sin 2016; 32: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maury G, Cambron L, Jamart J, Marchand E, Senny F, Poirrier R. Added value of a mandible movement automated analysis in the screening of obstructive sleep apnea. J Sleep Res 2013; 22: 96–103. [DOI] [PubMed] [Google Scholar]
- 28. Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, Bradley TD. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med 2009; 179: 241–246. [DOI] [PubMed] [Google Scholar]
- 29. Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens 2010; 24: 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heitmann J, Greulich T, Reinke C, Koehler U, Vogelmeier C, Becker HF, Schmidt AC, Canisius S. Comparison of the effects of nebivolol and valsartan on BP reduction and sleep apnoea activity in patients with essential hypertension and OSA. Curr Med Res Opin 2010; 26: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 31. Burnier M. Angiotensin II Type 1 receptor blockers. Circulation 2001; 103: 904–912. [DOI] [PubMed] [Google Scholar]
- 32. Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M, Yamaguchi T, Momomura S. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008; 133: 690–696. [DOI] [PubMed] [Google Scholar]
- 33. Bradley TD, Floras JS. Adaptive servo‐ventilation and the treatment of central sleep apnea in heart failure. Let's not throw the baby out with the bathwater. Am J Respir Crit Care Med 2016; 193: 357–359. [DOI] [PubMed] [Google Scholar]
- 34. Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, Buyse B, De Backer W, Eckert DJ, Grote L, Hagmeyer L, Hedner J, Jennum P, La Rovere MT, Miltz C, McNicholas WT, Montserrat J, Naughton M, Pepin JL, Pevernagie D, Sanner B, Testelmans D, Tonia T, Vrijsen B, Wijkstra P, Levy P. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017; 49 pii: 1600959. [DOI] [PubMed] [Google Scholar]


