Abstract
Aims
Patients with heart failure (HF) risk factors are described as being in Stage A of this condition (SAHF). Management is directed towards prevention of HF progression, but to date, no evidence has been described to align the intensity of this intervention to HF risk. We sought to what extent SAHF of Type 2 diabetes mellitus (T2DM) and other HF risks showed differences in subclinical left ventricular function, exercise capacity, and prognosis.
Methods and results
We recruited 551 elder asymptomatic SAHF patients (age 71 ± 5 years, 49% men, 290 T2DM) with at least one risk factor from a community‐based population with preserved ejection fraction. All underwent a comprehensive echocardiogram including global longitudinal strain (GLS) and a 6 min walk test and were followed for 2 years. The primary endpoints were new‐onset HF and all‐cause mortality. The T2DM group was associated with reduced 6 min walk test distance (451 ± 111 vs. 493 ± 87 m, P < 0.001), worse diastolic function (E/e′ 9.2 ± 2.7 vs. 8.7 ± 2.4, P = 0.028), and impaired GLS (−17.7 ± 2.6% vs. −19.0 ± 2.6%, P < 0.001). Over a median follow‐up of 1.6 years, 49 T2DM‐SAHF and 27 other‐SAHF met the primary endpoint. T2DM‐SAHF had significantly worse outcome than other‐SAHF (P = 0.021). In Cox models, obesity [hazard ratio (HR) = 2.46; P = 0.007], atrial fibrillation (HR = 2.39; P = 0.028), 6 min walk distance (HR = 0.99; P = 0.034), and GLS (HR = 1.14; P = 0.033) were independently associated with the primary endpoint in T2DM‐SAHF, independent of age and glycaemic control.
Conclusions
The T2DM‐SAHF has worse subclinical left ventricular function, exercise capacity, and prognosis than other‐SAHF. Impaired GLS, atrial fibrillation, exercise capacity, and obesity are associated with a worse prognosis in T2DM‐SAHF but not in other‐SAHF.
Keywords: Heart failure, Diabetes mellitus, 6 min walk, Global longitudinal strain
Introduction
The current American College of Cardiology/American Heart Association heart failure (HF) guidelines define Stage A HF (SAHF) as existing in subjects with risk factors for HF, in the absence of structural heart disease or symptoms.1 These risk factors include obesity, hypertension, atherosclerosis, diabetes mellitus (DM), exposure to cardiotoxins, and family history of HF, and they may be present in up to a third of subjects aged ≥45 years. The natural history of SAHF is that patients may progress to functional or structural abnormalities without symptoms [Stage B HF (SBHF)], clinical manifestations of HF (Stage C), and eventually, refractory or end‐stage HF (Stage D).2, 3 The identification of HF risk should stimulate efforts to identify SBHF, as therapeutic interventions in this setting may prevent the development of clinical HF and improve prognosis.2, 3 However, an effective screening strategy necessitates an understanding of underlying risk.4
A recent systematic review of the relative risk of a large range of HF risk factors showed that Type 2 DM (T2DM) had nearly double the risk of incident HF, while the risk with hypertension was lower.5 The frequency of HF in patients with DM is even higher among elderly subjects.6 The presence of T2DM adversely affects the prognosis of patients with HF, with contributions from coronary heart disease, diabetic cardiomyopathy, and hypertension.7, 8 Even in the absence of ischaemic heart disease, T2DM is over‐represented in HF.9 We hypothesized that non‐ischaemic SAHF due to T2DM would have more subclinical left ventricular (LV) dysfunction and worse functional capacity and clinical outcomes, compared with other SAHF. This finding might justify a more aggressive stance towards screening for SBHF in T2DM.
Methods
Patient selection
We prospectively recruited 551 asymptomatic patients aged ≥65 years, with preserved ejection fraction (EF) but at least one Stage A risk factor for HF (DM, obesity, hypertension, or known cardiac disease), from a community‐based population in Tasmania. Patients with existing HF or known ischaemic heart disease were excluded, as were patients with more than moderate valve disease, history of HF, LV EF <40%, inability to acquire adequate echocardiographic images for speckle tracking imaging analysis at baseline. All participants provided written informed consent, and the study protocol was approved by the Tasmanian Human Research Ethics Committee.
Clinical features
Type 2 DM was based on self‐report of diagnosis including medication. Obesity was defined as body mass index (BMI) ≥30 kg/m2.
Demographics, disease and family history, and medication use were obtained using a standardized questionnaire. BMI was calculated as weight in kilograms divided by height in metres squared. In addition to standardized weight and height measurements, waist circumference was measured with a tape measure to the nearest millimetre at the mid‐point between the lower costal margin and the iliac crest by a trained examiner. Supine resting blood pressure (BP) was measured twice and averaged in each patient after at least 10 min of rest in a quiet room. Uncontrolled hypertension was defined by averaged systolic BP ≥140 mmHg or a diastolic BP ≥ 90 mmHg.10 The International Federation of Clinical Chemistry standardized haemoglobin A1c (HbA1c) (cut‐off 64 mmol/mol) level, estimated glomerular filtration rate (eGFR), and creatinine were obtained from local pathology records. Missing values for HbA1c, eGFR, and creatinine were estimated by imputation using linear regression.
Six‐minute walk test
The 6 min walk test (6MWT) was performed by traversing back and forth along a marked pathway on a hard, flat surface, to test the distance an individual was able to walk over a total of 6 min. Patients were allowed to self‐pace, and the test was performed using a standardized protocol.11
Echocardiography
A comprehensive echocardiogram including standard transthoracic 2D, Doppler echocardiographic studies, and speckle tracking echocardiogram using sensitive systolic and diastolic function parameters was performed using the same ultrasound machine (Siemens ACUSON SC2000, 4V1c and 4Z1c probes, Siemens Healthcare, Mountain View, CA) in accordance with the American Society of Echocardiography guidelines.12, 13 Images were saved in raw data format and analysed offline. LV internal dimensions and wall thickness, chamber volumes, and valvular morphology were assessed. LV mass index was obtained from LV mass measurement using standard criteria and normalized for body size (body surface area or height to the power of 1.7). LV EF measurement used the modified Simpson biplane method. LV inflow was obtained using pulsed wave Doppler in the apical four‐chamber view; peak early (E) and late (A) diastolic velocities, deceleration time, and E/A ratio were assessed. Peak early diastolic medial and lateral mitral annular velocities (e′) and the ratio of mitral inflow early diastolic velocity to average e′ velocity were obtained from pulsed tissue Doppler; E/e′ >13 was used as an indicator of diastolic dysfunction. For deformation analysis, standard greyscale two‐dimensional images were acquired in conventional four‐chamber, two‐chamber, three‐chamber, parasternal short‐axis views at the mid‐level, basal level, and apical level. Global longitudinal strain (GLS) was calculated by averaging three apical views using standard software and greater than −18% was considered consistent with LV dysfunction.14, 15
Outcomes
Potential HF symptoms were assessed through regular follow‐up phone calls, followed by symptom surveillance questionnaires and clinical visits. Records of all‐cause hospitalization were obtained and collected. Suspicious HF symptoms and signs were reviewed by three independent cardiologists. The diagnosis of HF was established according to the Framingham HF criteria.16 The primary endpoint for study was new onset of HF and all‐cause mortality.
Statistical analysis
Descriptive data are presented as mean ± standard deviation, and dichotomous data as subject number and percentage. Comparisons between the groups were performed by independent‐samples t‐test; the Kruskal–Wallis test was used for comparison of non‐normally distributed variables. Univariable Cox regression was used in order to identify the predictors with the primary endpoint among clinical, demographic, and echocardiographic variables. Multivariable Cox proportional hazards model was performed to determine the independent predictors and reported as hazard ratio (HR) with 95% confidence interval, guided by univariable analyses. Analyses were performed with standard statistical computer software (SPSS 22, IBM, Chicago, IL); P < 0.05 was deemed to be statistically significant.
Results
Patient characteristics
All 551 SAHF patients (age 71 ± 5 years, 49% male) were eligible for inclusion in the final analysis. T2DM was present in 290 subjects (53%). The clinical and demographic characteristics of the SAHF patients according to T2DM status are listed in Table 1. T2DM‐SAHF was characterized by more obesity as well as central obesity, higher heart rate, and lower diastolic BP. The 6 min walk distance (6MWD) was significantly lower in T2DM‐SAHF patients compared with other‐SAHF patients (451 ± 111 vs. 493 ± 87 m, P < 0.001). Among T2DM‐SAHF patients, 24% patients were treated with insulin and 68% with metformin. The mean HbA1c level is 53.7 ± 10.3 mmol/mol with 13% having impaired HbA1c level (>64 mmol/mol). For eGFR, 14% patients had eGFR > 90 mL/min/1.73 m2, 71% had eGFR 60–90 mL/min/1.73 m2, and 15% had eGFR < 60 mL/min/1.73 m2. The mean creatinine value was 92 ± 21 μmol/L.
Table 1.
Demographic and clinical characteristics of the SAHF population according to T2DM status
| T2DM‐SAHF (n = 290) | Other‐SAHF (n = 261) | P value | |
|---|---|---|---|
| Age (years) | 71 ± 4.4 | 71 ± 5.1 | 0.877 |
| Male gender (n, %) | 163 (56.2) | 106 (40.6) | <0.001 |
| Weight (kg) | 86.2 ± 17.1 | 79.7 ± 15.4 | <0.001 |
| Height (cm) | 168.4 ± 9.9 | 166.6 ± 9.4 | 0.027 |
| BMI (kg/m2) | 30.4 ± 5.9 | 28.6 ± 4.7 | <0.001 |
| Waist circumference (cm) | 103.7 ± 13.3 | 96.8 ± 13.7 | <0.001 |
| Obesity (n, %) | 142 (49.0) | 108 (41.4) | 0.074 |
| Heart rate (n/min) | 69 ± 11 | 66 ± 11 | 0.001 |
| Systolic blood pressure (mmHg) | 139 ± 15 | 141 ± 18 | 0.143 |
| Diastolic blood pressure (mmHg) | 81 ± 10 | 83 ± 11 | 0.021 |
| Hypertension (n, %) | 222 (76.6) | 231 (88.5) | <0.001 |
| Active hypertension (n, %) | 143 (49.3) | 132 (50.6) | 0.767 |
| Past heart disease (n, %) | 20 (6.9) | 24 (9.2) | 0.321 |
| Family history of HF (n, %) | 90 (31.0) | 115 (44.1) | 0.002 |
| Past chemotherapy (n, %) | 24 (8.3) | 25 (9.6) | 0.592 |
| Atrial fibrillation (n, %) | 29 (10) | 18 (6.9) | 0.224 |
| 6MWD (m) | 451 ± 111 | 493 ± 87 | <0.001 |
| Biomarker | |||
| HbA1c (mmol/mol) | 53.7 ± 10.3 | — | — |
| Impaired HbA1c (>64 mmol/mol) | 38 (13.1) | — | — |
| eGFR | |||
| >90 mL/min/1.73 m2 | 41 (14.1) | — | — |
| 60–90 mL/min/1.73 m2 | 207 (71.4) | — | — |
| <60 mL/min/1.73 m2 | 42 (14.5) | — | — |
| Creatinine (μmol/L) | 91.8 ± 21.4 | — | — |
| Medication | |||
| Insulin | 69 (23.8) | — | — |
| Metformin | 196 (67.6) | — | — |
| ACEI/ARB (n, %) | 201 (69.3) | 190 (72.8) | 0.368 |
| Beta‐blockers (n, %) | 16 (5.5) | 13 (5.0) | 0.778 |
| Calcium antagonists (n, %) | 68 (23.4) | 68 (26.1) | 0.108 |
| Diuretics (n, %) | 33 (11.4) | 42 (16.1) | 0.479 |
| Lipid‐lowering medications (n, %) | 148 (51.0) | 149 (57.1) | 0.155 |
ARB, angiotensin II receptor blocker; ACEI, angiotensin‐converting enzyme inhibitor; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HF, heart failure; SAHF, Stage A heart failure; 6MWD, 6 min walk distance; T2DM, Type 2 diabetes mellitus. Bold P values are significant.
Echocardiographic characteristics
Table 2 shows the echocardiographic characteristics between T2DM‐SAHF and the remaining SAHF group. Overall, the mean EF was 63 ± 6% (range 40–80%), and only 2% and 1% had EF within 40–50% in T2DM‐SAHF and other‐SAHF patients, respectively. Average GLS was −18.4 ± 2.7% (range −10.4% to 26.0%). T2DM‐SAHF had higher E/e′ ratio, higher LV mass index, and worse GLS than the other‐SAHF group. The T2DM group was associated with worse diastolic function (E/e′ 9.2 ± 2.7 vs. 8.7 ± 2.4, P = 0.028) as well as impaired GLS (−17.7 ± 2.6 vs. −19.0 ± 2.6%, P < 0.001). T2DM‐SAHF also had a much higher prevalence of abnormal GLS (50% vs. 28%, P < 0.001) and abnormal E/e′ (10% vs. 5%, P = 0.011).
Table 2.
Echocardiographic characteristics of the SAHF population according to T2DM status
| T2DM‐SAHF (n = 290) | Other‐SAHF (n = 261) | P value | |
|---|---|---|---|
| LV ejection fraction (%) | 62.9 ± 6.5 | 63.9 ± 5.5 | 0.048 |
| 40–50% | 5 (1.7) | 3 (1.1) | |
| >50% | 285 (98.3) | 258 (98.9) | |
| Mitral early diastolic inflow velocity (E wave) (m/s) | 0.65 ± 0.17 | 0.63 ± 0.15 | 0.057 |
| Mitral late diastolic inflow velocity (A wave) (m/s) | 0.83 ± 0.19 | 0.78 ± 0.17 | 0.001 |
| Early‐to‐late peak diastolic transmitral flow velocity ratio (E/A) | 0.80 ± 0.21 | 0.82 ± 0.23 | 0.167 |
| Early diastolic mitral annular velocity (e′) (m/s) | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.306 |
| Mitral E/e′ septal–lateral ratio (E/e′) | 9.2 ± 2.7 | 8.7 ± 2.4 | 0.028 |
| E/e′ ratio > 13 (n, %) | 30 (10.3) | 12 (4.6) | 0.011 |
| Deceleration time (s) | 246.4 ± 52.4 | 242.8 ± 49.4 | 0.414 |
| LV mass index (g/m2) | 92.4 ± 23.8 | 92.7 ± 22.1 | 0.912 |
| LVH/LV mass index | 167 (57.6) | 179 (68.6) | 0.009 |
| GLS (%) | −17.7 ± 2.6 | −19.0 ± 2.6 | <0.001 |
| GLS > −18% (n, %) | 146 (50.3) | 74 (28.4) | <0.001 |
GLS, global longitudinal strain; LV, left ventricular; LVH, left ventricular hypertrophy; SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus. Bold P values are significant.
In order to determine the incremental impact of T2DM on subclinical systolic and diastolic functions over hypertension, Table 3 lists echocardiographic parameter comparisons between patients with isolated active hypertension (HT‐SAHF) and patients with both active hypertension and T2DM (HT + T2DM‐SAHF). HT + T2DM‐SAHF had worse GLS than the HT‐SAHF group. HT + T2DM‐SAHF also had a much higher prevalence of abnormal GLS (52% vs. 30%, P < 0.001).
Table 3.
Echocardiographic characteristics of the SAHF population according to T2DM and hypertension status
| HT‐SAHFa (n = 132) | HT + T2DM‐SAHFb (n = 143) | P value | |
|---|---|---|---|
| LV ejection fraction (%) | 63.6 ± 5.6 | 62.7 ± 6.9 | 0.247 |
| Mitral early‐diastolic inflow velocity (E wave) (m/s) | 0.61 ± 0.15 | 0.67 ± 0.17 | 0.008 |
| Mitral late diastolic inflow velocity (A wave) (m/s) | 0.80 ± 0.16 | 0.85 ± 0.20 | 0.016 |
| Early‐to‐late peak diastolic transmitral flow velocity ratio (E/A) | 0.78 ± 0.19 | 0.79 ± 0.23 | 0.513 |
| Early diastolic mitral annular velocity (e′) (m/s) | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.220 |
| Mitral E/e′ septal–lateral ratio (E/e′) | 9.0 ± 2.5 | 9.4 ± 2.8 | 0.185 |
| E/e′ ratio > 13 (n, %) | 8 (6.1) | 18 (12.6) | 0.065 |
| Deceleration time (s) | 247.5 ± 49.7 | 246.5 ± 52.1 | 0.882 |
| LV mass index (g/m2) | 93.4 ± 21.5 | 95.1 ± 25.1 | 0.547 |
| LVH/LV mass index | 41 (31.1) | 44 (30.8) | 0.989 |
| GLS (%) | −19.1 ± 2.4 | −17.7 ± 2.5 | <0.001 |
| GLS > −18% (n, %) | 39 (29.5) | 74 (51.7) | <0.001 |
GLS, global longitudinal strain; HT, hypertension; LV, left ventricular; LVH, left ventricular hypertrophy; SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus. Bold P values are significant.
HT‐SAHF: Patients had active hypertension without diabetes.
Patients had both T2DM and active hypertension.
Follow‐up
Over a median follow‐up period of 1.6 ± 0.6 years (range 0.6–3.2 years), 49 (16.9%) T2DM‐SAHF and 27 (10.3%) other‐SAHF met the primary endpoint of new onset of HF and all‐cause mortality. On examination of individual components of the primary endpoint, 46 T2DM‐SAHF and 24 other‐SAHF developed HF, and three T2DM‐SAHF and three other‐SAHF died. In the entire cohort, the annualized event rate of HF and all‐cause mortality was 8.8%—varying from 11.2% in T2DM‐SAHF to 6.4% in other‐SAHF.
Stepwise nested Cox models were constructed to determine the predictors of primary outcome in SAHF patients with and without T2DM. HRs of the primary outcome from univariable and multivariable analyses are listed in Table 4. Significant variables from univariable analyses were entered into the final age‐adjusted and sex‐adjusted model. Among T2DM‐SAHF patients, obesity [HR = 2.46 (1.28 to 4.70); P = 0.007], atrial fibrillation (AF) [HR = 2.39 (1.10 to 5.22); P = 0.028], 6MWD [HR = 0.99 (0.99 to 1.00); P = 0.034], and GLS [HR = 1.14 (1.01 to 1.30); P = 0.033] were independently associated with the primary endpoint after adjustment for age, gender, and glycaemic control. In the other‐SAHF cohort, history of heart disease [HR = 2.97 (1.19 to 7.4); P = 0.019] was predictive after adjustment for age [HR = 1.08 (1.01 to 1.15); P = 0.022] and gender.
Table 4.
Prognostic value of baseline clinical and echocardiographic characteristics over time
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| T2DM‐SAHF | Other‐SAHF | T2DM‐SAHF (χ2 = 54.7) | Other‐SAHF (χ2 = 25.8) | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.06 (0.99, 1.12) | 0.070 | 1.10 (1.04, 1.17) | 0.002 | 1.02 (0.96, 1.09) | 0.455 | 1.08 (1.01, 1.15) | 0.022 |
| Male gender | 1.58 (0.87, 2.86) | 0.136 | 1.47 (0.69, 3.13) | 0.319 | 1.02 (0.51, 2.04) | 0.946 | 1.51 (0.65, 3.51) | 0.654 |
| BMI | 1.10 (1.06, 1.14) | <0.001 | 0.98 (0.90, 1.07) | 0.651 | ||||
| Waist | 1.05 (1.03, 1.08) | <0.001 | 1.01 (0.98, 1.04) | 0.548 | ||||
| Heart rate | 1.00 (0.97, 1.03) | 0.856 | 0.98 (0.95, 1.02) | 0.412 | ||||
| Systolic blood pressure | 1.00 (0.98, 1.02) | 0.861 | 1.00 (0.98, 1.02) | 0.776 | ||||
| Diastolic blood pressure | 1.00 (0.99, 1.03) | 0.811 | 1.01 (0.97, 1.04) | 0.732 | ||||
| History of heart disease | 1.93 (0.82, 4.56) | 0.131 | 3.95 (1.73, 9.03) | 0.001 | 2.97 (1.19, 7.4) | 0.019 | ||
| Obesity | 2.77 (1.51, 5.10) | 0.001 | 1.12 (0.52, 2.42) | 0.771 | 2.46 (1.28, 4.70) | 0.007 | ||
| Active hypertension | 1.05 (0.60, 1.84) | 0.866 | 0.87 (0.41, 1.85) | 0.712 | ||||
| Past chemotherapy | 1.34 (0.53, 3.39) | 0.537 | 1.01 (0.24, 4.28) | 0.987 | ||||
| Family history of HF | 0.62 (0.32, 1.21) | 0.164 | 0.78 (0.36, 1.70) | 0.525 | ||||
| Atrial fibrillation | 3.96 (2.06, 7.63) | <0.001 | 2.22 (0.77, 6.44) | 0.141 | 2.39 (1.10, 5.22) | 0.028 | ||
| Dyslipidaemia | 1.48 (0.83, 2.65) | 0.187 | 1.14 (0.54, 2.45) | 0.728 | ||||
| Beta‐blocker | 2.89 (1.23, 6.81) | 0.015 | 1.39 (0.33, 5.87) | 0.654 | ||||
| ACEI/ARB | 1.37 (0.71, 2.63) | 0.343 | 1.00 (0.42, 2.37) | 0.998 | ||||
| Insulin | 1.57 (0.86, 2.86) | 0.140 | — | — | ||||
| Metformin | 0.89 (0.50, 1.59) | 0.688 | — | — | ||||
| 6MWD | 0.996 (0.994, 0.999) | 0.003 | 0.995 (0.990, 0.999) | 0.010 | 0.997 (0.995, 1.000) | 0.034 | 0.99 (0.99, 1.00) | 0.237 |
| Biomarker | ||||||||
| HbA1c | 1.03 (1.01, 1.05) | 0.008 | — | — | 1.01 (0.99, 1.04) | 0.283 | ||
| HbA1c > 64 mmol/mol | 2.83 (1.52, 5.27) | 0.001 | — | — | ||||
| eGFR | 0.67 (0.40, 1.12) | 0.666 | — | — | ||||
| Creatinine (μmol/L) | 1.01 (1.01, 1.02) | 0.065 | — | — | ||||
| Echocardiography | ||||||||
| GLS | 1.27 (1.15, 1.41) | <0.001 | 1.17 (1.03, 1.32) | 0.016 | 1.14 (1.01, 1.30) | 0.033 | 1.04 (0.90, 1.20) | 0.586 |
| GLS > −18% | 2.13 (1.18, 3.84) | 0.012 | 1.87 (0.87, 4.04) | 0.109 | ||||
| E/e′ | 1.06 (0.96, 1.17) | 0.251 | 1.08 (0.93, 1.25) | 0.295 | ||||
| E/e′ ratio > 13 | 1.35 (0.58, 3.18) | 0.489 | 0.84 (0.11, 6.16) | 0.860 | ||||
| LV mass index | 1.03 (1.01, 1.04) | <0.001 | 1.02 (1.00, 1.04) | 0.052 | 1.01 (0.99, 1.02) | 0.087 | ||
| LVH/ LV mass index | 3.52 (2.00, 6.18) | <0.001 | 1.02 (0.45, 2.32) | 0.969 | ||||
ARB, angiotensin II receptor blocker; ACEI, angiotensin‐converting enzyme inhibitor; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HF, heart failure; SAHF, Stage A heart failure; 6MWD, 6 min walk distance; T2DM, Type 2 diabetes mellitus. Bold P values are significant.
Kaplan–Meier survival curves were constructed for the primary endpoint, with log‐rank testing for significance between strata. Among the whole cohort, T2DM‐SAHF had significantly worse outcome than other‐SAHF (χ2 = 5.36; P = 0.021; Figure 1 ). Subclinical LV systolic dysfunction (LVSD) (defined as GLS > −18%) was predictive of primary outcome (HF and all‐cause mortality) in T2DM‐SAHF in Kaplan–Meier analysis (χ2 = 6.75; P = 0.009; Figure 2 A). However, there was no statistically significant difference in survival when comparing other‐SAHF with or without LVSD (χ2 = 2.68; P = 0.101; Figure 2 B). There were significantly more events in T2DM‐SAHF with obesity compared with those without (χ2 = 11.86; P = 0.001; Figure 3 A), but there were no differences when other‐SAHF was divided by obesity (χ2 = 0.09; P = 0.770; Figure 3 B). In T2DM‐SAHF patients, the most serious prognosis pertained to those with impaired GLS or obesity. However, as shown in Figure 4 , in other‐SAHF individuals, the most serious prognosis was seen in those with a history of heart disease (χ2 = 12.49; P < 0.001).
Figure 1.
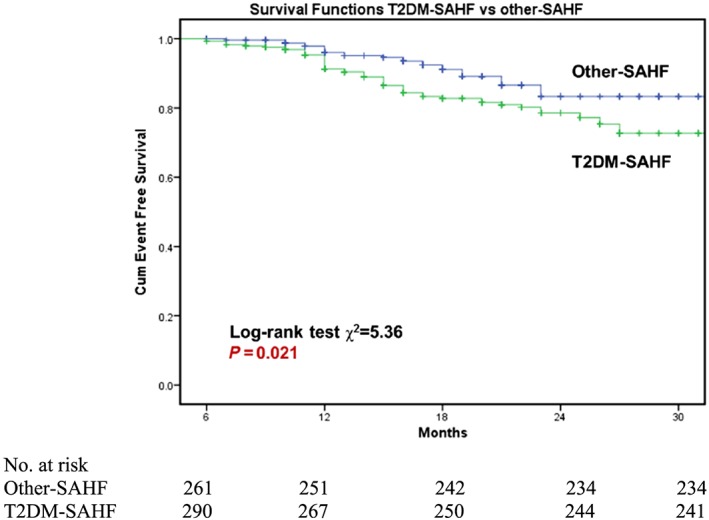
Comparison of primary outcomes between patients with T2DM‐SAHF and other‐SAHF. SAHF patients with T2DM had significantly worse outcome than those without T2DM. SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus.
Figure 2.
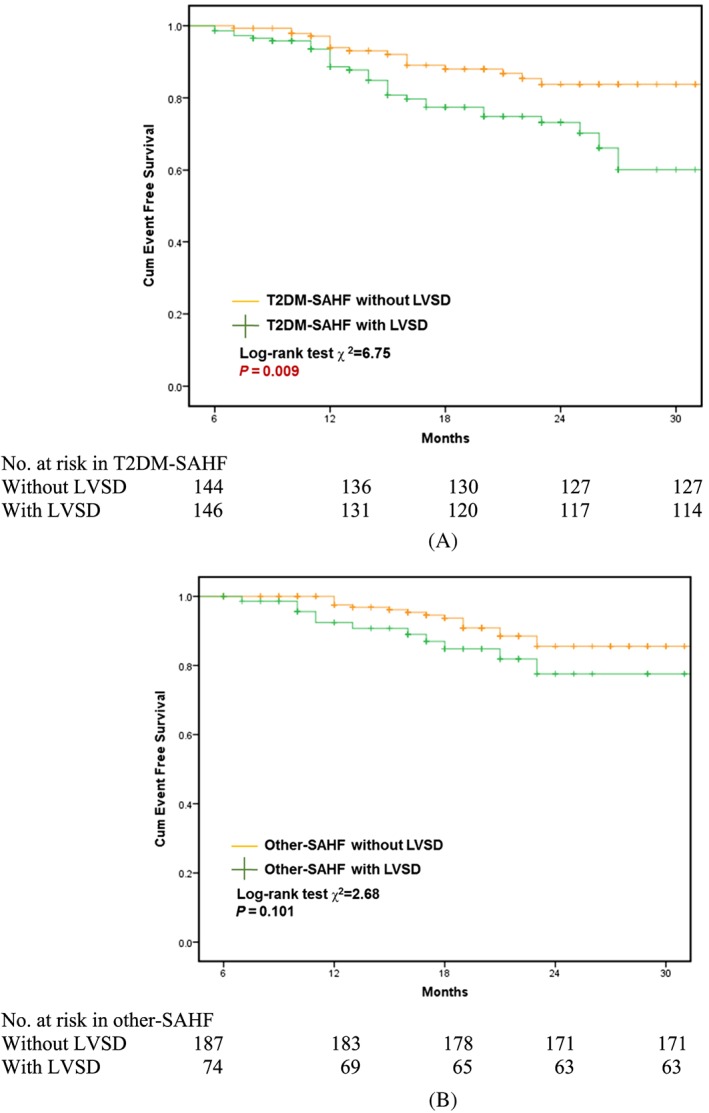
Prognostic implications of T2MD‐SAHF and other‐SAHF and the role of left ventricular systolic dysfunction. SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus.
Figure 3.
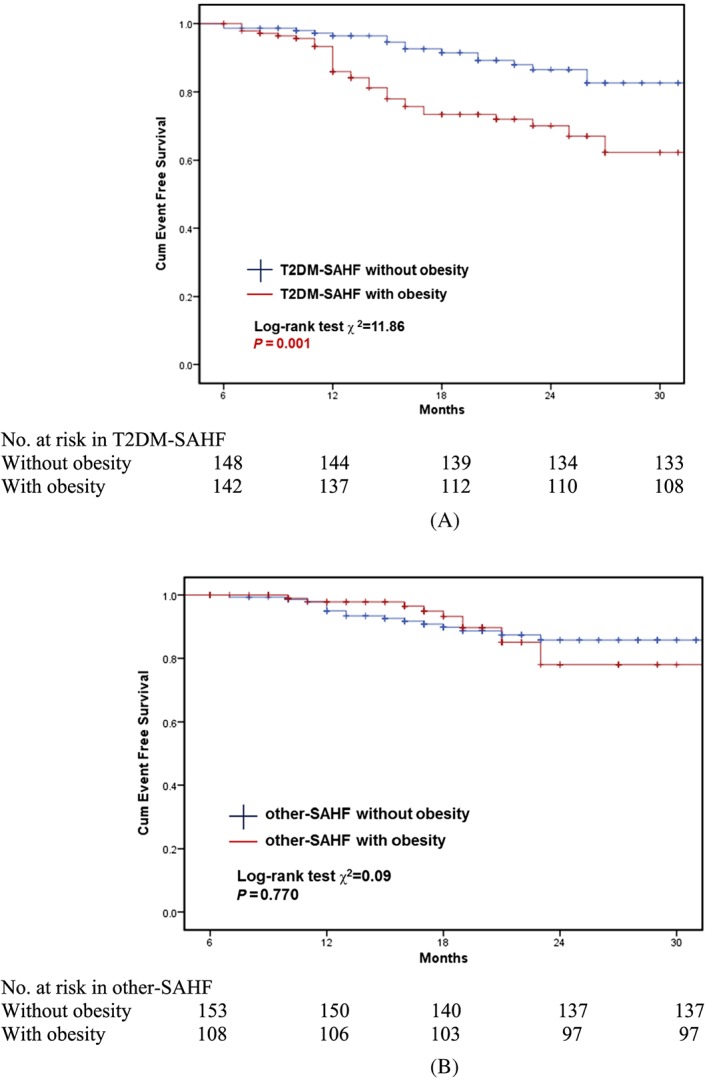
Prognostic implications of T2MD‐SAHF and other‐SAHF and the role of obesity. SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus.
Figure 4.
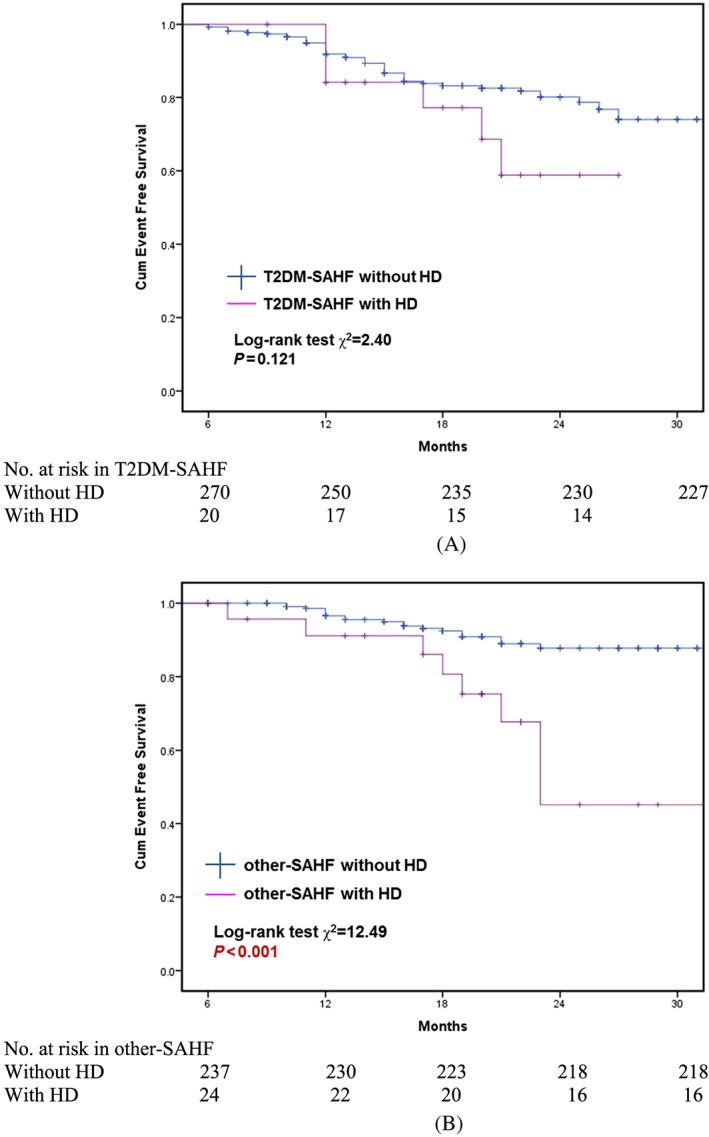
Prognostic implications of T2MD‐SAHF and other‐SAHF and the role of heart disease history. SAHF, Stage A heart failure; T2DM, Type 2 diabetes mellitus.
Discussion
In these data from a prospectively enrolled cohort, we examined the prevalence of subclinical LV dysfunction, impaired exercise capacity, and prognosis in a community‐based population with HF risk factors. The results suggest that SAHF due to T2DM has a worse echocardiographic manifestation, functional capacity, and clinical outcome than other‐SAHF. This work builds on previous reports of an association between increasing HF stage and worse functional status and prognosis,17 by adding evidence about clinical features and functional capacity within the early asymptomatic phases of HF.
Left ventricular mechanics in Stage A heart failure
Previous studies have shown that changes of subclinical LV longitudinal systolic and diastolic function begin before structural LV changes in asymptomatic patients with HF risk factors.18, 19, 20, 21 However, comparisons of the degree of LV myocardial dysfunction in early diabetic cardiomyopathy and in other‐SAHF have not been documented previously. Our results show that abnormalities of diastolic function are more common in T2DM than in other‐SAHF groups, as are disturbances of LV longitudinal systolic function (GLS: −17.7 ± 2.6% vs. −19.0 ± 2.6%, P < 0.001). In the current study, we report that the prevalence of systolic longitudinal dysfunction is 50% among community population‐based T2DM‐SAHF and 28% among other‐SAHF. Alterations of systolic strain may exist despite normal diastolic function.22 Importantly, impaired GLS as a marker of SBHF is associated with an increased risk of further transition to symptomatic Stage C HF with preserved EF in diabetes.2 However, in real life, few asymptomatic SAHF patients undergo tests of cardiac morphology and function, and perhaps because of this, many of them develop into Stage C/D directly without experiencing SBHF. Effectively, many SBHF ‘hide’ in SAHF, and our results highlight the importance of echocardiography for identifying SBHF and initiating cardioprotection.
These findings highlight the fact that the severity of the early LV function impairment is not necessarily analogous within different aetiologies of SAHF. Despite rigorous adjustment for covariables, each unit decrement of GLS in subjects with T2DM showed a 1.14‐fold higher risk of HF and mortality, whereas in subjects with other risks, the predictive value of GLS was not found. The worse LV function in T2DM‐SAHF may be attributable to diabetic complications and associated co‐morbidities as well as the presence of a distinct diabetic cardiomyopathy.4, 18 The pathogenesis of the latter is complex and likely multifactorial, involving cardiac autonomic neuropathy, altered myocardial metabolism, small‐vessel and large‐vessel damage, insulin resistance, and myocardial fibrosis.18 Additionally, in our study, patients with uncontrolled hypertension showed relatively preserved GLS, but a combined group with active hypertension and T2DM had more abnormal GLS (−19.1 ± 2.4% vs. −17.7 ± 2.5%, P < 0.001; Table 3), as well as a higher prevalence of abnormal GLS than in T2DM without hypertension (52% vs. 30%). Although hypertension and T2DM are the two leading aetiologies of early HF, impaired GLS is more likely due to diabetic cardiomyopathy than hypertensive heart disease.23
Functional capacity in Stage A heart failure
In this study, functional capacity at submaximal stress was assessed using 6MWT. This is a simple, safe test of functional capacity that is a well‐established diagnostic procedure in cardiovascular and pulmonary disease, which has prognostic value.24 A systematic review25 to investigate the reliability between 6MWT and other methods to assess functional capacity showed that 6MWT has moderate to good ability to predict VO2 in chronic HF, especially in those patients who are unable to walk >490 m. Compared with healthy controls, patients with T2DM have lower 6MWD.26 The results of this study show that, compared with that in other‐SAHF, functional capacity in T2DM‐SAHF was more impaired.
The determinants of functional capacity are complex and depend on both psychological and physical factors. Although it might be considered that the association of T2DM with functional capacity could be driven by obesity—and indeed, advanced age and obesity had strong associations with functional capacity—our results suggest that the association of T2DM with functional capacity was independent of these features. Subclinical cardiac dysfunction, which is often present in both T2DM‐SAHF and other‐SAHF, could influence functional capacity but cannot fully explain the difference of exercise capacity between the two SAHF subgroups. Previous work has shown that exercise intolerance in T2DM is independent of obesity and even presents with good glycaemic control and without clinically apparent cardiovascular disease.27 The presence of endothelial dysfunction, decreased myocardial perfusion, decreased muscle mitochondrial function, abnormal tissue haemoglobin oxygen saturation, and insulin resistance may be mediators in the relationship between diabetic oxidative dysfunction and defects in functional capacity.27 In addition, there is evidence of involvement of both cardiac sympathetic and cardiac parasympathetic nervous system dysfunctions.28 Parasympathetic control may be weakened by persistent hyperglycaemia, with relative enhancement of sympathetic activity.29 The association of heart rate recovery with vagal tone leads to T2DM being associated with poor heart rate recovery and chronotropic incompetence.30 Cardiac autonomic dysfunction is associated with reduced cardiac output response to functional capacity in diabetes, which is probably due to haemodynamic instability during exercise.31 Left atrial (LA) dysfunction is also common in patients with HF with preserved EF and asymptomatic T2DM and contributes to exercise intolerance. However, while LA enlargement in diabetes is an independent predictor of LA dysfunction, probably owing to a combination of diastolic dysfunction and diabetic atrial myopathy,32 the specific influence of diabetes on LA dysfunction is more controversial.
Determinants of prognosis in Stage A heart failure
Despite current epidemiological evidence of the greater risk of development of HF in patients with diabetes, the natural history of asymptomatic subjects at risk of HF remains poorly identified.33, 34 This study extends present knowledge about the prognosis of the entire SAHF cohort in 2 years, based on a community (as opposed to a clinic‐based) population. In the entire SAHF cohort, the annualized event rate of incident HF and all‐cause mortality was 8.8%. The rate in T2DM‐SAHF (11.2%) was almost twice that of other‐SAHF (6.4%).
Type 2 DM was associated with more serious outcome, irrespective of whether or not other risk factors were present. A recent systematic review of 15 observational studies indicated that diabetes (HR = 2.0) showed the strongest predictive value for incident HF among the non‐ischaemic SAHF risks, followed by hypertension (HR = 1.61) and BMI (per 5 kg/m2) (HR = 1.15).5 In the present study, the prognosis was associated with obesity, exercise capacity, and abnormal GLS independent of glycaemic control and renal function. However, while hyperglycaemia may be associated with the development of HF, the association between intensive glycaemic control and the reduction of cardiovascular complications remains controversial.35 Although a large cohort study reported an HbA1c ≥10% was associated with 1.6‐fold risk of incident HF relative to an HbA1c <7%, several large clinical trials have reported no significant benefit for primary cardiovascular events with intensive glycaemic control.35 Our findings do not show that poor glycaemic control (HbA1c > 64 mmol/mol) is a marker of the composite endpoint (HF and all‐cause death) in this elderly asymptomatic cohort.
Previous research showed that reduced eGFR is a risk factor of cardiovascular events and death in diabetes patients without advanced renal disease, but the risks are small when considering other risk factors.36 Although we found no association between renal impairment and outcomes in T2DM‐SAHF, such an association might become apparent with longer follow‐up.
A recent systematic review and meta‐analysis of 15 cohort studies confirmed the association of AF with myocardial infarction, HF, and all‐cause mortality.37 In our study, AF showed an independent association with increased risk of new‐onset HF and all‐cause mortality in T2DM‐SAHF but not in other‐SAHF. This inconsistent result in other‐SHAF may be due to the relatively low prevalence of AF (7%) and the exclusion of ischaemic heart disease at baseline.
The staging system serves as a reminder to clinicians of the importance of early detection and prevention of patients at risks of transitioning to higher stages of HF. In the absence of screening, many SAHF patients (i.e. patients with HF risk factors) have structural or functional abnormalities that that are unrecognized. However, echo screening in SAHF is currently not supported by guidelines or payers, and if these patients had not been recruited to this trial, they would have been indistinguishable from those without any echo abnormalities (i.e. ‘pure’ SAHF). Nonetheless, these results highlight the notion that SAHF associated with diabetes is clinically different from other causes of SAHF and perhaps should have different targets for prevention. These findings have important implications for awareness and identification of high‐risk individuals and optimal management to prevent or delay progression to adverse outcome in SAHF.
Limitation
Several limitations are pertinent to this study. First, the population for the present study was a selected group, based on the presence of at least one known non‐ischaemic HF risk factor and excluding patients with known HF or established asymptomatic LVSD. In addition, the prevalence of T2DM is relatively high in this SAHF population, as we preferentially recruited patients with diabetes by specific advertising to this population. Second, patients with Type 1 DM were excluded from our study, as the frequency and mechanism of cardiac involvement may not be the same as in T2DM. Third, we chose longitudinal strain as the most robust of the myocardial deformation parameters and did not record circumferential strain, which could have provided additional details about myocardial mechanics. Finally, we do not have data on biomarkers, which may also be used as a potential predictor of HF and adverse outcome.
Conclusion
In this community cohort of patients with HF risks, T2DM‐SAHF had worse subclinical LV function, functional capacity, and adverse outcome than other causes of SAHF. Impaired GLS, worse exercise capacity, and the presence of obesity or AF were independently associated with prognosis in T2MD‐SAHF, whereas a history of heart disease was the driver of subsequent HF and mortality in other causes of SAHF. The clinical application of this study provides an important caveat that not all types of SAHF are the same. Better targeting of interventions at the most vulnerable SAHF group—those with T2DM—seems appropriate.
Conflict of interest
None declared.
Funding
This study was partially supported by Tasmanian Community Fund and Diabetes Australia Research Trust. Neither of these agencies had any role in the design, analysis, or interpretation of this study.
Ethics
The study was approved by the Tasmanian Human Research Ethics Committee.
Acknowledgements
The authors gratefully acknowledge the contribution of our tireless volunteer coordinators, Diane Binns, Jasmine Prichard, and Jane Mitchell.
Wang, Y. , Yang, H. , Nolan, M. , Pathan, F. , Negishi, K. , and Marwick, T. H. (2018) Variations in subclinical left ventricular dysfunction, functional capacity, and clinical outcomes in different heart failure aetiologies. ESC Heart Failure, 5: 343–354. doi: 10.1002/ehf2.12257.
References
- 1. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119: e391–e479. [DOI] [PubMed] [Google Scholar]
- 2. Lam CS. Diabetic cardiomyopathy: an expression of Stage B heart failure with preserved ejection fraction. Diab Vasc Dis Res 2015; 12: 234–238. [DOI] [PubMed] [Google Scholar]
- 3. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 4. Murtagh G, O'Connell JO, O'Connell EO, Tallon E, Watson C, Gallagher J, Baugh J, Patle A, O'Connell L, Griffin J, O'Hanlon R, Voon V, Ledwidge M, O'Shea D, McDonald K. Importance of risk factor management in diabetic patients and reduction in Stage B heart failure. QJM 2015; 108: 307–314. [DOI] [PubMed] [Google Scholar]
- 5. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open Heart 2015; 2: e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 7. Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J 2000; 139: 72–77. [DOI] [PubMed] [Google Scholar]
- 8. Zarich SW, Nesto RW. Diabetic cardiomyopathy. Am Heart J 1989; 118: 1000–1012. [DOI] [PubMed] [Google Scholar]
- 9. Johansson I, Dahlstrom U, Edner M, Nasman P, Ryden L, Norhammar A. Prognostic implications of Type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol 2016; 68: 1404–1416. [DOI] [PubMed] [Google Scholar]
- 10. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357. [DOI] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 13. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur Heart J Cardiovasc Imaging 2009; 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 14. Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta‐analysis. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 2013; 26: 185–191. [DOI] [PubMed] [Google Scholar]
- 15. Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2‐dimensional speckle‐tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2009; 2: 80–84. [DOI] [PubMed] [Google Scholar]
- 16. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 17. Azevedo A, Bettencourt P, Alvelos M, Martins E, Abreu‐Lima C, Hense H‐W, Barros H. Health‐related quality of life and stages of heart failure. Int J Cardiol; 129: 238–244. [DOI] [PubMed] [Google Scholar]
- 18. Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with Type 2 diabetes mellitus. Am J Cardiol 2009; 104: 1398–1401. [DOI] [PubMed] [Google Scholar]
- 19. Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusma‐Piccione M, Di Bella G, Saitta C, Saitta A. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography (Mount Kisco, NY) 2011; 28: 649–657. [DOI] [PubMed] [Google Scholar]
- 20. Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, Murphy RT. Subclinical anthracycline‐ and trastuzumab‐induced cardiotoxicity in the long‐term follow‐up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart (British Cardiac Society) 2010; 96: 701–707. [DOI] [PubMed] [Google Scholar]
- 21. Kusunose K, Yamada H, Nishio S, Mizuguchi Y, Choraku M, Maeda Y, Hosokawa S, Yamazaki N, Tomita N, Niki T, Yamaguchi K, Koshiba K, Soeki T, Wakatsuki T, Akaike M, Sata M. Validation of longitudinal peak systolic strain by speckle tracking echocardiography with visual assessment and myocardial perfusion SPECT in patients with regional asynergy. Circulation Journal: Official Journal of the Japanese Circulation Society 2011; 75: 141–147. [DOI] [PubMed] [Google Scholar]
- 22. Ernande L, Bergerot C, Rietzschel ER, De Buyzere ML, Thibault H, Pignonblanc PG, Croisille P, Ovize M, Groisne L, Moulin P, Gillebert TC, Derumeaux G. Diastolic dysfunction in patients with Type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 2011; 24: 1268, e1261–1275. [DOI] [PubMed] [Google Scholar]
- 23. Yang H, Wang Y, Negishi K, Nolan M, Marwick TH. Pathophysiological effects of different risk factors for heart failure. Open Heart 2016; 3: e000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucas C, Stevenson LW, Johnson W, Hartley H, Hamilton MA, Walden J, Lem V, Eagen‐Bengsten E. The 6‐min walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J 1999; 138: 618–624. [DOI] [PubMed] [Google Scholar]
- 25. Pollentier B, Irons SL, Benedetto CM, Dibenedetto AM, Loton D, Seyler RD, Tych M, Newton RA. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulmonary Physical Therapy Journal 2010; 21: 13–21. [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart T, Caffrey DG, Gilman RH, Mathai SC, Lerner A, Hernandez A, Pinto ME, Huaylinos Y, Cabrera L, Wise RA, Miranda JJ, Checkley W. Can a simple test of functional capacity add to the clinical assessment of diabetes? Diabetic Medicine: A Journal of the British Diabetic Association 2016; 33: 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord 2013; 14: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sucharita S, Bantwal G, Idiculla J, Ayyar V, Vaz M. Autonomic nervous system function in Type 2 diabetes using conventional clinical autonomic tests, heart rate and blood pressure variability measures. Indian Journal of Endocrinology and Metabolism 2011; 15: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanninen E, Uusitupa M, Lansimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed Type 2 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association 1993; 10: 66–73. [DOI] [PubMed] [Google Scholar]
- 30. Loimaala A, Huikuri HV, Koobi T, Rinne M, Nenonen A, Vuori I. Exercise training improves baroreflex sensitivity in Type 2 diabetes. Diabetes 2003; 52: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 31. Roy TM, Peterson HR, Snider HL, Cyrus J, Broadstone VL, Fell RD, Rothchild AH, Samols E, Pfeifer MA. Autonomic influence on cardiovascular performance in diabetic subjects. Am J Med 1989; 87: 382–388. [DOI] [PubMed] [Google Scholar]
- 32. Kadappu KK, Boyd A, Eshoo S, Haluska B, Yeo AE, Marwick TH, Thomas L. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging 2012; 13: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 33. Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, Chen HH. The prevalence of diabetic cardiomyopathy: a population‐based study in Olmsted County, Minnesota. J Card Fail 2014; 20: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, Levy D, Aragam J, Benjamin EJ, Cheng S, Wang TJ, Mitchell GF, Vasan RS. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart failure 2016; 4: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in Type 2 diabetes: now that the dust is settling from large clinical trials. Ann N Y Acad Sci 2013; 1281: 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, Fassett R, Ansquer J‐C, Dixon P, Davis TME, Pardy C, Colman P, Keech A. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in Type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011; 54: 32–43. [DOI] [PubMed] [Google Scholar]
- 37. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all‐cause mortality and heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol 2017; 24: 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]


