Abstract
Aims
Whether or not the definition of a worsening renal function (WRF) is adequate for the evaluation of acute renal failure in patients with acute heart failure is unclear.
Methods and results
One thousand and eighty‐three patients with acute heart failure were analysed. A WRF, indicated by a change in serum creatinine ≥0.3 mg/mL during the first 5 days, occurred in 360 patients while no‐WRF, indicated by a change <0.3 mg/dL, in 723 patients. Acute kidney injury (AKI) upon admission was defined based on the ratio of the serum creatinine value recorded on admission to the baseline creatinine value and placed into groups based on the degree of AKI: no‐AKI (n = 751), Class R (risk; n = 193), Class I (injury; n = 41), or Class F (failure; n = 98). The patients were assigned to another set of four groups: no‐WRF/no‐AKI (n = 512), no‐WRF/AKI (n = 211), WRF/no‐AKI (n = 239), and WRF/AKI (n = 121). A multivariate logistic regression model found that no‐WRF/AKI and WRF/AKI were independently associated with 365 day mortality (hazard ratio: 1.916; 95% confidence interval: 1.234–2.974 and hazard ratio: 3.622; 95% confidence interval: 2.332–5.624). Kaplan–Meier survival curves showed that the rate of any‐cause death during 1 year was significantly poorer in the no‐WRF/AKI and WRF/AKI groups than in the WRF/no‐AKI and no‐WRF/no‐AKI groups and in Class I and Class F than in Class R and the no‐AKI group.
Conclusions
The presence of AKI on admission, especially Class I and Class F status, is associated with a poor prognosis despite the lack of a WRF within the first 5 days. The prognostic ability of AKI on admission may be superior to WRF within the first 5 days.
Keywords: Acute decompensated heart failure, Acute renal failure, Cardio renal syndrome, Acute kidney injury, RIFLE criteria
Introduction
A worsening renal function (WRF) is defined as an increase ≥0.3 mg/dL in the serum creatinine level compared with the value on admission,1, 2 and it has been established as the gold standard for the evaluation of acute renal failure in patients with acute heart failure (AHF).3 Renal dysfunction in AHF patients involves complex and multifactorial mechanisms by haemodynamic (renal arterial hypoperfusion and renal venous congestion) and non‐haemodynamic factors, and these mechanisms have not been completely elucidated. Recent studies have suggested that a WRF has not been associated with a poor outcome in some patients, thereby indicating the need to redefine a WRF in AHF patients.3, 4 AHF patients sometimes have acute renal failure on admission, so patients not having WRF (defined as an increase <0.3 mg/dL in the serum creatinine level vs. the value on admission) might include those who already have acute renal failure on admission. As such, the definition of a WRF might need to be reconsidered.
For the re‐evaluation of the renal dysfunction criteria in AHF patients, in some reports, the baseline creatinine value was reconsidered and defined using all measured creatinine values except for the value obtained on admission. The risk, injury, failure, loss, and end‐stage (RIFLE) criteria have been established as the standard criteria in intensive care patients.5, 6 In these reports, the component of acute kidney injury (AKI) was suggested for the evaluation of acute renal failure. We previously reported that patients with AKI, particularly those with a Class I or F status, exhibit a worse long‐term prognosis than no‐AKI patients among subjects with AHF.7, 8 Furthermore, 33.2% of AHF patients already have AKI upon admission to the intensive care unit (ICU),7 which is associated with a poor in‐hospital mortality rate and long‐term prognosis.9 The presence of AKI on admission and a Class I or F status are important factors in AHF patients. In these reports, the baseline value was defined as the lowest value recorded during admission in chronic kidney disease (CKD) patients, and the lower of either the lowest creatinine value during hospitalization or the Modification of Diet in Renal Disease (MDRD) creatinine level, which was calculated assuming a glomerular filtration rate (GFR) of 75 mL/min/1.73 m2, was used as the baseline creatinine value in patients without CKD (non‐CKD).10, 11 The definition of the baseline value was completely different with WRF.
The definitions of AKI and WRF were different criteria for the evaluation of renal dysfunction. The evaluation of the renal dysfunction based on the presence of WRF will help clarify the mechanism of renal dysfunction in patients with AHF. The aim of present study was to elucidate the prognostic impact of AKI on admission and/or WRF in patients with severely decompensated AHF.
Methods
Subjects
A total of 1083 AHF patients admitted to the ICU of Nippon Medical School Chiba Hokusoh Hospital between January 2000 and May 2016 were enrolled in this study. AHF was defined as either new‐onset HF or decompensation of chronic HF with symptoms sufficient to warrant hospitalization.12 Based on the European Society of Cardiology guidelines for the diagnosis of AHF, an abnormal electrocardiogram or the presence of pulmonary oedema on chest X‐ray and a brain natriuretic peptide (BNP) level of ≥100 pg/mL are required to diagnose AHF.13
Furthermore, all included patients were administered diuretics or vasodilators for the treatment of AHF. The treating physician in the emergency department diagnosed AHF based on these criteria within 30 min of admission. All patients had a New York Heart Association (NYHA) functional class of either III or IV. The patients who met any of the following criteria were admitted to the ICU: (i) requiring high‐flow oxygen inhalation (including mechanical support) to treat orthopnea; (ii) requiring inotrope or mechanical support due to low blood pressure; and (iii) requiring various types of diuretics to improve general or lung oedema. All of the data were retrospectively retrieved from the hospital medical records. Patients who had undergone renal replacement therapy before admission were excluded. Furthermore, only the first admission was considered for patients who were readmitted to the ICU during the 1 year period after their discharge. There were no limitations regarding the treatment of AHF, and the treatment strategy was chosen by each subject's attending physician.
Evaluation of a worsening renal function and acute kidney injury
A WRF was defined as an increase ≥0.3 mg/dL in the serum creatinine level during the first 5 days vs. the baseline creatinine value; meanwhile, no‐WRF was the increase <0.3 mg/dL in serum creatinine level in comparison with baseline creatinine value. The baseline creatinine value was the creatinine value upon admission in all patients. Patients who were receiving continuous renal replacement therapy during the first 5 days were defined as having a WRF even if they had an increase <0.3 mg/dL in the serum creatinine level during the first 5 days.
We evaluated the presence of AKI using only the creatinine criteria of the RIFLE classification.6 AKI upon admission was defined based on the ratio of the serum creatinine value recorded on admission to the baseline creatinine value. Patients were classified as having either no‐AKI or Class R (risk), Class I (injury), or Class F (failure) AKI. ‘No AKI’ was diagnosed as an increase in the serum creatinine level < 1.5‐fold baseline, Class R as an increase in the serum creatinine level ≥ 1.5‐fold baseline, Class I as increase in the serum creatinine level ≥ 2.0‐fold baseline, and Class F as increase in the serum creatinine level ≥ 3.0‐fold baseline. Patients who were receiving continuous renal replacement therapy within 24 h were defined as Class F. With regard to the baseline level of creatinine, in CKD patients, the baseline level was defined as the lowest value recorded during admission. In patients without CKD (non‐CKD) patients, the lower of either the lowest creatinine value during hospitalization or the MDRD creatinine level was used as the baseline creatinine value. The MDRD creatinine levels were calculated using the MDRD equation, as recommended by the Acute Dialysis Quality Initiative. The MDRD equation for serum creatinine was calculated assuming a GFR of 75 mL/min/1.73 m2.10, 11
Chronic kidney disease was diagnosed based on the creatinine value observed within 1 year. Furthermore, among patients in whom the creatinine value had not been measured within 1 year before admission, those who had been previously diagnosed with CKD in the past or at another institution were considered to have CKD. CKD was defined as a syndrome comprising a >3 month history of a low GFR (<60 mL/min/1.73 m2).14 Patients who did not have medical records at Chiba Hokusoh Hospital for the 3 months before admission were diagnosed with CKD using the previous 3 months' data from another institution. Kidney damage, as identified by abnormal findings in the urine and imaging tests, was used to diagnose CKD in some patients in the present study; therefore, CKD was diagnosed only by a >3 month history of low GFR. In the present study, 516 of 1083 patients (47.6%) were diagnosed with CKD.
Procedures
Worsening renal function occurred in 360 patients (33.2%) and did not occur in 723 patients (66.8%). The occurrence of AKI was evaluated by the RIFLE classification on admission. No AKI occurred in 751 patients (no‐AKI) on admission. AKI was therefore present upon admission in 332 patients including Class R (risk; n = 193), Class I (injury; n = 41), or Class F (failure; n = 98). The patients were assigned to four categories based on the WRF and AKI on admission: 723 patients with no WRF were divided into no AKI on admission (no‐WRF/no‐AKI group, n = 512) or AKI on admission (no‐WRF/AKI, n = 211) groups, and 360 patients with WRF were also divided into no AKI on admission (WRF/no‐AKI group, n = 239) or AKI on admission (WRF/AKI group, n = 121) groups (Figure 1 ). This study examined (i) the relationship between the WRF and/or AKI on admission and the outcomes and (ii) the relationship between the degree of AKI on admission and the outcomes.
Figure 1.
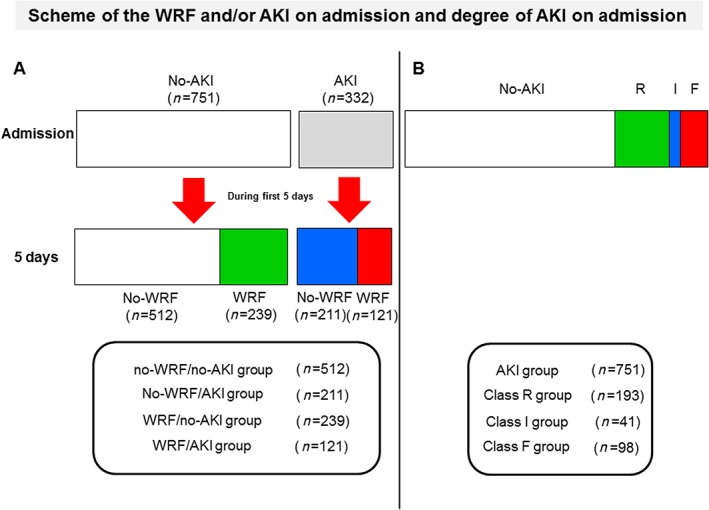
This study examined (i) the relationship between the worsening renal function (WRF) and/or acute kidney injury (AKI) on admission and the outcomes and (ii) the relationship between the degree of AKI on admission and the outcomes. This is the scheme of the group assignment. (A) The patients were assigned to four categories based on the WRF and AKI on admission: The no‐AKI patients who did not develop WRF during the first 5 days were assigned to no‐WRF/no‐AKI group (n = 512), and the no‐AKI patients who developed WRF were assigned to WRF/no‐AKI group (n = 239). The AKI patients who did not develop WRF during the first 5 days were assigned to no‐WRF/AKI (n = 211) groups, and the AKI patients who developed WRF were assigned to WRF/AKI group (n = 121) groups. (B) The patients were assigned to four categories based on the degree of AKI: No AKI was present in 751 patients, and AKI was present upon admission in 332 patients including Class R (risk; n = 193), Class I (injury; n = 41), or Class F (failure; n = 98).
Acute kidney injury and the prognosis
The short‐term prognosis was evaluated as the length of the ICU stay and the length of total hospitalization. Furthermore, the long‐term prognosis was also evaluated as any‐cause death and heart failure (HF) events, defined as including death and readmission for HF within 1 year. The patients were clinically followed up at a routine outpatient clinic. In the patients followed up at another institute, their prognoses were determined by telephoning the other institutes. HF events were defined as death or readmission to the hospital for HF within 1 year. All variables on admission, including age, type of HF (new onset or worsening), aetiology of HF (ischaemic or non‐ischaemic), gender, NYHA class (III or IV), blood urea nitrogen, total bilirubin, sodium, potassium, haemoglobin, C‐reactive protein, systolic blood pressure (SBP), heart rate, and WRF/AKI, that were retrieved from all 1083 cases were selected for inclusion in the multivariate logistic regression model. The continuous variables were evaluated by every 1‐ or 10‐unit increase based on the meaning of each category. The prognostic value for the 365 day mortality and HF event was evaluated using the Cox regression hazard model and Kaplan–Meier curve.
Statistical analyses
All data were statistically analysed using the SPSS 22.0 J software program (SPSS Japan Institute, Tokyo, Japan). All numerical data were expressed as the medians (25–75% interquartile range). The Mann–Whitney U‐test was used to compare two groups (AKI group vs. no‐AKI group). Comparisons of all proportions were performed with a chi‐squared analysis. A P value < 0.05 was considered to be statistically significant.
The prognostic value of the presence of WRF or AKI was assessed using a Cox regression hazard model. A Cox regression analysis was performed to determine the hazard ratio (HR) for 365 day mortality and HF events. All clinically relevant factors affecting the prognosis, including age (per 1 year increase), SBP (per 10 mmHg increase), LVEF (per 1% increase), sodium (per 1.0 mmol/L increase), haemoglobin level (per 1.0 mg/dL increase), and BNP (per 10 pg/mL increase) on Day 1, were selected for inclusion in the multivariate Cox regression hazard model associated with 365 days all‐cause death and HF event. Multivariate Cox regression hazard model was performed by the backward stepwise selection. The cumulative survival rates and HF events in each of the four groups (presence of WRF or AKI and the degree of AKI) were analysed using Kaplan–Meier curves, and the log‐rank test was used to calculate the statistical significance of the differences.
Ethical concerns
The research ethics committee of Nippon Medical School Chiba Hokusoh Hospital approved the study protocol.
Results
Patients' characteristics
The patient cohort comprised 65.4% male with a median age of 74 years. A total of 776 (70.7%) patients had new‐onset HF, 449 (41.5%) had ischaemic heart disease, and 634 (58.5%) had non‐ischaemic heart disease, including cardiomyopathy (n = 121), hypertensive heart disease (n = 178), and valvular disease (n = 252). Most patients were NYHA class IV (79.4%). The median LVEF on admission was 36.0%.
The relationships between the patient characteristics, including the baseline values on admission and medications prescribed during the ICU hospitalization, and the WRF and the AKI are shown in Table 1. The age was significantly older, the male gender significantly less likely, and the SBP significantly lower in the no‐WRF/AKI group than in the no‐WRF/no‐AKI group. The serum levels of total bilirubin, uric acid, blood urea nitrogen, creatinine, and C‐reactive protein were significantly higher and the serum levels of sodium and haemoglobin significantly lower in the no‐WRF/AKI group than in the no‐WRF/no‐AKI group. The SBP was also significantly lower in the WRF/AKI group than in the WRF/no‐AKI group. The serum levels of total bilirubin, blood urea nitrogen, creatinine, C‐reactive protein, and BNP were significantly higher and the serum levels of sodium and haemoglobin significantly lower in the WRF/AKI group than in the WRF/no‐AKI group (Table 1).
Table 1.
Patients' characteristics and the presence of WRF or AKI
| No‐WRF | P value | WRF | P value | |||
|---|---|---|---|---|---|---|
| Characteristic | No‐AKI | AKI | No‐AKI | AKI | ||
| (n = 512) | (n = 211) | (n = 239) | (n = 121) | |||
| Age (years old) | 74 (64–81) | 76 (68–82) | 0.023 | 74 (65–82) | 74 (65–80) | 0.655 |
| Type (new onset, %) | 368 (71.9%) | 153 (72.5%) | 0.855 | 163 (68.2%) | 82 (67.8%) | 0.905 |
| Aetiology (ischaemia, %) | 190 (37.1%) | 85 (40.3%) | 0.500 | 123 (51.5%) | 51 (42.1%) | 0.120 |
| Gender (male, %) | 337 (65.8%) | 115 (54.5%) | 0.004 | 173 (72.4%) | 83 (68.6%) | 0.539 |
| Past medical history | ||||||
| Hypertension (yes, %) | 371 (72.5%) | 148 (70.1%) | 0.525 | 201 (84.1%) | 74 (61.2%) | <0.001 |
| Diabetes mellitus (yes, %) | 218 (42.6%) | 78 (37.0%) | 0.184 | 112 (46.9%) | 56 (46.3%) | 0.911 |
| Dyslipidemia (yes, %) | 232 (45.3%) | 95 (45.0%) | 0.137 | 145 (60.7%) | 54 (44.6%) | 0.824 |
| Vital signs and status | ||||||
| Systolic blood pressure (mmHg) | 162 (140–188) | 140 (112–170) | <0.001 | 170 (148–194) | 132 (98–166) | <0.001 |
| Pulse (beats/min) | 114 (96–132) | 112 (92–134) | 0.302 | 110 (96–126) | 104 (83–121) | 0.008 |
| LVEF (%) | 36 (26–49) | 35 (25–51) | 0.826 | 36 (27–48) | 32 (23–49) | 0.251 |
| NYHA (IV, %) | 398 (77.7%) | 169 (80.1%) | 0.551 | 190 (79.5%) | 103 (85.2%) | 0.200 |
| Arterial blood gas | ||||||
| pH | 7.34 (7.23–7.42) | 7.34 (7.21–7.44) | 0.983 | 7.32 (7.19–7.42) | 7.34 (7.19–7.42) | 0.673 |
| PCO2 (mmHg) | 43 (35–55) | 40 (32–54) | 0.054 | 42 (34–60) | 38 (29–52) | 0.003 |
| PO2 (mmHg) | 93 (69–137) | 85 (63–130) | 0.086 | 84 (66–130) | 85 (66–126) | 0.829 |
| HCO3 − (mmol/L) | 22.4 (20.2–24.7) | 21.4 (18.1–24.1) | <0.001 | 21.5 (19.5–23.6) | 19.8 (15.7–23.7) | 0.003 |
| SaO2 (%) | 96 (92–98) | 96 (89–98) | 0.257 | 95 (91–98) | 95 (90–98) | 0.773 |
| Lactate (mmol/L) | 1.5 (1.1–3.4) | 2.5 (1.5–5.9) | <0.001 | 1.4 (1.0–2.5) | 2.2 (1.2–5.4) | 0.001 |
| Laboratory data | ||||||
| Total bilirubin (mg/dL) | 0.6 (0.4–0.8) | 0.7 (0.5–1.0) | 0.003 | 0.5 (0.4–0.7) | 0.6 (0.4–1.2) | 0.002 |
| Uric acid (mg/dL) | 6.6 (5.3–7.7) | 7.3 (5.7–8.7) | <0.001 | 6.8 (5.3–8.1) | 7.1 (5.6–9.7) | 0.062 |
| BUN (mg/dL) | 20.2 (16.6–26.9) | 26.0 (19.0–36.2) | <0.001 | 25.2 (18.5–35.2) | 39.9 (27.4–62.8) | <0.001 |
| Creatinine (mg/dL) | 1.01 (0.78–1.31) | 1.14 (0.97–1.52) | <0.001 | 1.38 (0.97–1.92) | 1.84 (1.35–2.90) | <0.001 |
| Sodium (mmol/L) | 140 (138–142) | 139 (136–141) | <0.001 | 140 (138–142) | 138 (133–142) | <0.001 |
| Potassium (mmol/L) | 4.2 (3.8–4.5) | 4.4 (3.9–4.9) | <0.001 | 4.1 (3.8–4.7) | 4.6 (4.0–5.3) | <0.001 |
| Haemoglobin (g/dL) | 12.9 (11.2–14.6) | 12.5 (10.6–14.1) | 0.041 | 12.1 (10.7–14.1) | 11.3 (9.6–13.5) | 0.006 |
| C‐reactive protein (mg/dL) | 0.52 (0.16–1.61) | 0.77 (0.25–2.53) | 0.004 | 0.54 (0.21–1.80) | 2.45 (0.44–7.77) | <0.001 |
| BNP (pg/mL) | 674 (365–1214) | 757 (400–1357) | 0.170 | 801 (432–1285) | 1188 (650–2000) | <0.001 |
| Medication (cases) during ICU hospitalization | ||||||
| Furosemide (yes, %) | 482 (94.1%) | 197 (93.4%) | 0.732 | 232 (97.1%) | 112 (92.6%) | 0.061 |
| Nitroglycerin (yes, %) | 348 (68.0%) | 113 (53.6%) | <0.001 | 179 (74.9%) | 48 (39.7%) | <0.001 |
| Nicorandil (yes, %) | 77 (15.0%) | 28 (13.3%) | 0.485 | 39 (16.3%) | 21 (17.4%) | 0.767 |
| Carperitide (yes, %) | 267 (52.1%) | 84 (39.8%) | 0.002 | 140 (58.6%) | 56 (46.3%) | 0.034 |
| Dopamine (yes, %) | 79 (15.4%) | 65 (30.8%) | <0.001 | 46 (19.2%) | 52 (43.0%) | <0.001 |
| Dobutamine (yes, %) | 66 (12.9%) | 65 (30.8%) | <0.001 | 41 (17.1%) | 52 (43.0%) | <0.001 |
| ACE‐I/ARB (yes, %) | 248 (48.4%) | 73 (34.6%) | 0.001 | 87 (36.4%) | 13 (10.7%) | <0.001 |
| Beta‐blocker (yes, %) | 130 (25.4%) | 44 (20.9%) | 0.214 | 70 (29.3%) | 22 (18.2%) | 0.022 |
| Spironolactone (yes, %) | 227 (44.3%) | 80 (37.9%) | 0.115 | 81 (33.9%) | 22 (18.2%) | 0.003 |
| During hospitalization | ||||||
| ICU hospitalization (days) | 4 (3–5) | 5 (3–8) | <0.001 | 5 (3–7) | 8 (4–17) | <0.001 |
| Total hospitalization (days) | 24 (16–37) | 31 (20–55) | <0.001 | 28 (18–46) | 43 (25–70) | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; ICU; intensive care unit; LVEF, left ventricular ejection fraction measured by echocardiography; NYHA, New York Heart Association; WRF, worsening renal function.
P value, between the no‐AKI group and the AKI group in each no‐WRF or WRF group, determined by the Mann–Whitney U‐test or the χ2 test.
All numerical data were expressed as the medians (25–75% interquartile range)
Outcomes
The length of ICU hospitalization and total hospitalization were both significantly longer in the no‐WRF/AKI group than in the no‐WRF/no‐AKI group, as well as in the WRF/AKI group than in the WRF/no‐AKI group (Table 1).
One hundred and seventy‐six of the 1083 patients died within 365 days' follow‐up, including 50 of the 512 (9.8%) no‐WRF/no‐AKI patients, 42 of the 211 (19.9%) no‐WRF/AKI patients, 32 of 239 (13.4%) WRF/no‐AKI patients, and 52 of 121 (43.0%) WRF/AKI patients. The multivariate Cox regression model indicated that the no‐WRF/AKI group and WRF/AKI group were independently associated with the 365 day mortality [HR: 1.916; 95% confidence interval (CI): 1.234–2.974 and HR: 3.622; 95% CI: 2.332–5.624], and WRF/AKI group were independent predictors of an HF event within 365 day (HR: 3.288; 95% CI: 2.431–4.446) (Table 2). The Kaplan–Meier survival curves showed that the prognosis, including all‐cause death, was significantly poorer in the WRF/AKI group than in the no‐WRF/AKI, WRF/no‐AKI, and no‐WRF/no‐AKI groups and in the no‐WRF/AKI group than in the WRF/no‐AKI and no‐WRF/no‐AKI groups ( Figure 2A ). The prognosis, including likelihood of HF events, was significantly poorer in the WRF/AKI group than in the no‐WRF/AKI, WRF/no‐AKI, and no‐WRF/no‐AKI groups (Figure 2B ). The Kaplan–Meier survival curves showed the prognosis, including likelihood of all‐cause death and HF event, to be significantly poorer in the Class I than in the no‐AKI and Class R groups and to be significantly poorer in Class F patients than in the patients in the no‐AKI and Class R groups (Figure 3A,B ).
Table 2.
A Cox regression analysis of the associations between 365 day mortality, cumulative heart failure events
| 365 day mortality | Heart failure event | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Anaemia status on Day 3 | ||||||||||||
| No‐AKI/no‐WRF | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||
| No‐AKI/WRF | 1.395 | 0.895−2.174 | 0.141 | 1.248 | 0.768–2.028 | 0.370 | 1.210 | 0.896–1.635 | 0.214 | 1.110 | 0.803–1.534 | 0.527 |
| AKI/no‐WRF | 2.234 | 1.482–3.368 | <0.001 | 1.916 | 1.234–2.974 | 0.004 | 1.214 | 0.883–1.667 | 0.232 | 1.060 | 0.748–1.502 | 0.742 |
| AKI/WRF | 5.942 | 4.025–8.771 | <0.001 | 3.622 | 2.332–5.624 | <0.001 | 3.288 | 2.431–4.446 | <0.001 | 2.424 | 1.718–3.419 | <0.001 |
| Adjusted factors (data of Day 1) | ||||||||||||
| Age (per 1 year increase) | 1.034 | 1.019–1.049 | <0.001 | 1.030 | 1.013–1.047 | <0.001 | 1.026 | 1.015–1.036 | <0.001 | 1.023 | 1.011–1.035 | <0.001 |
| SBP (per 10 mmHg increase) | 0.857 | 0.827–0.889 | <0.001 | 0.889 | 0.852–0.928 | <0.001 | 0.931 | 0.906–0.957 | <0.001 | 0.960 | 0.930–0.992 | 0.014 |
| LVEF (per 1% increase) | 0.999 | 0.991–1.008 | 0.895 | 0.993 | 0.987–1.000 | 0.063 | 0.989 | 0.981–0.996 | 0.003 | |||
| Sodium (per 1.0 mmol/L increase) | 0.937 | 0.913–0.961 | <0.001 | 0.953 | 0.933–0.973 | <0.001 | 0.974 | 0.952–0.997 | 0.030 | |||
| Haemoglobin (per 1.0 mg/dL increase) | 0.843 | 0.797–0.891 | <0.001 | 0.934 | 0.874–0.999 | 0.046 | 0.892 | 0.855–0.930 | <0.001 | 0.936 | 0.888–0.986 | 0.012 |
| BNP (per 10 pg/mL increase) | 1.002 | 1.002–1.003 | <0.001 | 1.001 | 1.001–1.002 | 0.001 | 1.002 | 1.001–1.002 | <0.001 | |||
AKI; acute kidney injury; BNP, brain natriuretic peptide; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction measured on echocardiography; SBP, systolic blood pressure; WRF; worsening renal function.
Figure 2.
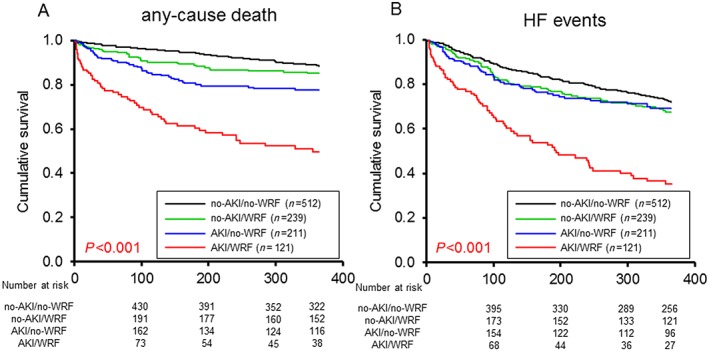
Kaplan–Meier curves based on the presence of a worsening renal function (WRF) or acute kidney injury (AKI). (A) The all‐cause death rate was significantly poorer in the WRF/AKI group than in the no‐WRF/AKI, WRF/no‐AKI, and no‐WRF/no‐AKI groups and in the no‐WRF/AKI group than in the WRF/no‐AKI and no‐WRF/no‐AKI groups. (B) The prognosis, including the likelihood of a heart failure (HF) event, was significantly poorer in the WRF/AKI group than in the no‐WRF/AKI, WRF/no‐AKI, and no‐WRF/no‐AKI groups.
Figure 3.
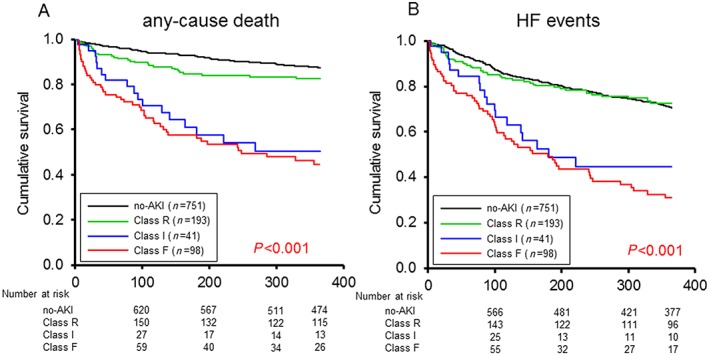
Kaplan–Meier curves regarding the degree of acute kidney injury (AKI). (A and B) The Kaplan–Meier survival curves showed the prognosis, including likelihood of all‐cause death and heart failure (HF) events, to be significantly poorer in the Class I than in the no‐AKI and Class R groups and to be significantly poorer in the Class F patients than in the patients in the no‐AKI and Class R groups.
Discussion
In the present study, the absence of AKI on admission was not associated with a worse outcome in AHF patients, even if they developed WRF within the first 5 days. Furthermore, the presence of AKI on admission, especially Class I and Class F status, was associated with a poor prognosis in AHF patients, even if they did not develop WRF within the first 5 days. These results suggest that the prognostic ability of acute renal failure might be superior to the definition of AKI upon admission compared with that of WRF within first 5 days. Using the AKI criteria in addition to the definition of WRF is therefore advised for the evaluation of acute renal failure in patients with severely decompensated AHF.
Mechanisms of acute renal failure
The mechanisms underlying renal dysfunction in patients with AHF have been reported to be complex and multifactorial, involving such factors as low cardiac output–renal hypoperfusion, fluid overload–renal venous congestion, neurohormonal activation and sympathetic activity, inflammatory response, intrinsic tubular damage, and therapeutic interventions.3 Traditionally, AKI has been attributed to hypoperfusion of the kidney because of progressive impairment of the cardiac output.15 However, attention has shifted from this cardiac output (‘forward failure’) to venous congestion (‘backward failure’) as the most important haemodynamic determinant.1 Current evidences did not support low cardiac output as the main determinant of renal dysfunction in patients with AHF.1, 16 The development of ‘congestive kidney failure’ induced by the increased renal venous pressure arising from venous congestion (increased renal afterload) and increased renal interstitial pressure (intrinsic renal compromise) might be important mechanisms underlying the development of renal dysfunction in AHF patients.1
The neurohormonal activation and sympathetic activity has also been reported to be a mechanism. Persistent stimulation of the renin–angiotensin–aldosterone system induces kidney damage through cell hypertrophy, oxidative stress, and activation of an inflammatory condition.17 Angiotensin II decreases the renal blood flow and stimulates the sympathetic nervous system, which increases the systemic vascular tone and has direct, untoward effects on the heart and kidney by promoting apoptosis and fibrosis.17 The development of intrinsic tubular damage has also been proposed as another mechanism. The levels of neutrophil gelatinase‐associated lipocalin, which reflect the presence of renal tubular injury, have been investigated to detect AKI in patients with AHF.18, 19
A number of studies have also shown that potent diuretics are associated with renal dysfunction.20, 21, 22 It is clear that patients with pre‐existing renal dysfunction are vulnerable to developing renal dysfunction on diuretic administration.21, 22 Furthermore, bolus infusions do not promote gradual diuresis and therefore do not allow time for the fluid in the periphery to move from the extravascular to the intravascular space, which leads to intravascular volume depletion and significant drops in renal perfusion, subsequently leading to renal dysfunction.23 Aggressive decongestion therefore leads to transit renal dysfunction during the acute phase of AHF; however, current research suggested that transient renal dysfunction with the use of high‐dose diuretics was associated with early clinical improvement and not a poor outcome.23, 24 It was reported recently that some mechanisms of the renal dysfunction were not associated with an adverse outcome in patients with AHF. The prognosis might be different depending on the mechanism of renal dysfunction in AHF.
As mentioned earlier, the mechanism of renal dysfunction is likely multifactorial. It might therefore be impossible to define acute renal dysfunction using a single definition. The definitions of WRF and AKI may represent different mechanisms; therefore, an evaluation based on the combination of WRF and AKI would be more effective for the precise prediction of adverse outcomes in patients with AHF.
Difference in the definition of acute renal failure—worsening renal function or acute kidney injury
There is no consensus definition in international HF management guidelines for acute renal dysfunction. WRF has been defined as a change in serum creatinine ≥0.3 mg/mL in most reports, a definition that has been established as the gold standard and shown to be associated with an increased risk for long‐term all‐cause/cardiovascular mortality and morbidity in AHF patients for a long time.25, 26, 27 In fact, in a retrospective study of 200 063 hospitalized AHF patients, Kociol et al. found that 17.8% developed WRF, with 64.5% of these patients being readmitted and 35.4% dying within 1 year.25 Cowie et al. defined WRF as an increase in serum creatinine of 0.3 mg/dL from baseline and reported that the mortality rate was higher in the WRF group than in the no renal dysfunction group during admission (12% vs. 2%), rising to 15% by 30 days and 28% in total by 6 months, compared with 5% and 18%, respectively, in patients without renal dysfunction.28 However, the epidemiology and definition of a WRF are not well defined in the literature, and there is no universally accepted definition.
During the previous decades, the component of AKI had been proposed instead of the WRF, and AKI has also been reported to affect the outcome of patients admitted to ICUs.29, 30, 31 The RIFLE criteria have been established as the standard method for evaluating AKI in intensive care patients.5, 6 Based on the wealth of evidence in the intensive care field, we suggested the prognostic efficacy of the AKI criteria in patients with AHF.7, 8, 9 The AKI criteria have been adequately established in intensive care patients; therefore, it would be reasonable to use these criteria for the evaluation of acute renal failure for AHF patients. Roy et al. compared the outcome predictive ability of the definitions for AKI (RIFLE, Acute Kidney Injury Network, and Kidney Disease Improving Global Outcomes) and the often used WRF definition in hospitalized patients.32 They found that the Acute Kidney Injury Network, RIFLE, and Kidney Disease Improving Global Outcomes AKI classification systems have an advantage in their predictive ability over the commonly used WRF in HF definition, as those systems include a severity scale in their definition criteria.32
The most important factor for defining renal dysfunction is the baseline creatinine level, which most studies have determined to be the level on admission in the definition of WRF. The routinely monitored creatinine value would be the best to use as the baseline creatinine value; however, it might be impossible to determine this value in all patients. It would be particularly difficult to estimate the AKI or CKD in an emergency setting in patients with a high creatinine value on admission. This might be a major limitation in the definition of WRF. The prognostic ability would likely be improved by dividing patients into another group based on the presence of AKI in patients with or without a WRF. The traditionally used WRF criteria for AHF filed included the patients who were not associated with their prognosis. It might therefore be necessary to redefine the reasonable criteria to include AKI on admission.
Study limitations
Several limitations associated with the present study warrant mention. First, the present study was a single‐centre study, and a small number of patients were included in each of the groups used to evaluate the prognosis, so patient bias might exist. Second, no urine volume criteria were used for the definition of AKI in the present study. The definition of AKI used in the present study included the presence of AKI on admission; therefore, it was impossible to include urinary criteria. Finally, this is the retrospective study. We were unable to determine the baseline creatinine value precisely in clinical situations. The RIFLE classification could not be evaluated quickly in our study; therefore, a further study will be needed to detect the occurrence of AKI at an earlier stage of hospitalization.
Conclusions
The absence of AKI on admission was associated with a good prognosis, even if a WRF developed within the first 5 days. Furthermore, the presence of AKI on admission, especially Class I and Class F status, was associated with a poor prognosis despite the lack of a WRF within the first 5 days. The traditionally used WRF criteria for AHF filed included the patients who were not associated with their prognosis. The prognostic ability of AKI might be superior to the definition of WRF within the first 5 days. Using the AKI criteria in addition to the definition of WRF is therefore advised for the evaluation of acute renal failure in patients with severely decompensated AHF.
Conflict of interest
None declared.
Funding
This research received no grants from any funding agency in the public, commercial, or not‐for‐profit sectors.
Acknowledgements
We are grateful to the staff of the ICU and the medical records office at Chiba Hokusoh Hospital, Nippon Medical School for collecting the medical data.
Shirakabe, A. , Hata, N. , Kobayashi, N. , Okazaki, H. , Matsushita, M. , Shibata, Y. , Nishigoori, S. , Uchiyama, S. , Asai, K. , and Shimizu, W. (2018) Worsening renal function definition is insufficient for evaluating acute renal failure in acute heart failure. ESC Heart Failure, 5: 322–331. doi: 10.1002/ehf2.12264.
References
- 1. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 2004; 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 3. Núñez J, Miñana G, Santas E, Bertomeu‐González V. Cardiorenal syndrome in acute heart failure: revisiting paradigms. Rev Esp Cardiol 2015; 68: 426–435. [DOI] [PubMed] [Google Scholar]
- 4. Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med 2007; 33: 409–413. [DOI] [PubMed] [Google Scholar]
- 5. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup . Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hata N, Yokoyama S, Shinada T, Kobayashi N, Shirakabe A, Tomita K, Kitamura M, Kurihara O, Takahashi Y. Acute kidney injury and outcomes in acute decompensated heart failure: evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010; 12: 32–37. [DOI] [PubMed] [Google Scholar]
- 7. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Long‐term prognostic impact after acute kidney injury in patients with acute heart failure: evaluation of the RIFLE criteria. Int Heart J 2012; 53: 313–319. [DOI] [PubMed] [Google Scholar]
- 8. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J 2013; 77: 687–696. [DOI] [PubMed] [Google Scholar]
- 9. Mathew TH, Johnson DW, Jones GR. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: revised recommendations. Med J Aust 2007; 187: 459–463. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 11. Sokolski M, Zymliński R, Biegus J, Siwołowski P, Nawrocka‐Millward S, Todd J, Yerramilli MR, Estis J, Jankowska EA, Banasiak W, Ponikowski P. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur J Heart Fail 2017; 19: 760–767. [DOI] [PubMed] [Google Scholar]
- 12. Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L, International Working Group on Acute Heart Failure S . Acute heart failure syndromes: current state and framework for future research. Circulation 2005; 112: 3958–3968. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891‐975 [DOI] [PubMed] [Google Scholar]
- 14. Iseki K. Chronic kidney disease in Japan. Intern Med 2008; 47: 681–689. [DOI] [PubMed] [Google Scholar]
- 15. Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990; 39: 10–24. [DOI] [PubMed] [Google Scholar]
- 16. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 17. Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J 2012; 33: 2135–2142. [DOI] [PubMed] [Google Scholar]
- 18. Shrestha K, Shao Z, Singh D, Dupont M, Tang WH. Relation of systemic and urinary neutrophil gelatinase‐associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol 2012; 110: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF, Roll SN, Sperling MI, Sawyer DB, Storrow AB. Elevated urinary neutrophil gelatinase‐associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail 2012; 14: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation 2004; 110: 1514–1517. [DOI] [PubMed] [Google Scholar]
- 21. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 2004; 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 22. Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, Horwitz RI. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol 2000; 85: 1110–1113. [DOI] [PubMed] [Google Scholar]
- 23. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM, Heart Failure Clinical Research Network NHLBI. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kociol RD, Greiner MA, Hammill BG, Phatak H, Fonarow GC, Curtis LH, Hernandez AF. Long‐term outcomes of Medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol 2010; 105: 1786–1793. [DOI] [PubMed] [Google Scholar]
- 26. Patel UD, Greiner MA, Fonarow GC, Phatak H, Hernandez AF, Curtis LH. Associations between worsening renal function and 30‐day outcomes among Medicare beneficiaries hospitalized with heart failure. Am Heart J 2010; 160: 132–138.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei CL. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 2008; 10: 188–195. [DOI] [PubMed] [Google Scholar]
- 28. Cowie MR, Komajda M, Murray‐Thomas T, Underwood J, Ticho B, POSH Investigators . Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the Prospective Outcomes Study in Heart Failure (POSH). Eur Heart J 2006; 27: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 29. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators . Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818. [DOI] [PubMed] [Google Scholar]
- 30. de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 2000; 26: 915–921. [DOI] [PubMed] [Google Scholar]
- 31. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34: 1913–1917. [DOI] [PubMed] [Google Scholar]
- 32. Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, Murray PT. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013; 3: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


