Abstract
Aims
The SHIFT trial showed that ivabradine reduced heart rate (HR) and the risk of cardiovascular outcomes. Concerns remain over the efficacy and safety of ivabradine on heart failure (HF) due to Chagas disease (ChD). We therefore conducted a post hoc analysis of the SHIFT trial to investigate the effect of ivabradine in these patients.
Methods and results
SHIFT was a randomized, double‐blind, placebo‐controlled trial in symptomatic systolic stable HF, HR ≥ 70 b.p.m., and in sinus rhythm. The ChD HF subgroup included 38 patients, 20 on ivabradine, and 18 on placebo. The ChD HF subgroup showed high prevalence of bundle branch right block and, compared with the overall SHIFT population, lower systolic blood pressure; higher use of diuretics, cardiac glycosides, and antialdosterone agents; and lower use of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker or target daily dose of beta‐blocker. ChD HF presented a poor prognosis (all‐cause mortality at 2 years was ~60%). The mean twice‐daily dose of ivabradine was 6.26 ± 1.15 mg and placebo 6.43 ± 1.55 mg. Ivabradine reduced HR from 77.9 ± 3.8 to 62.3 ± 10.1 b.p.m. (P = 0.005) and improved functional class (P = 0.02). A trend towards reduction in all‐cause death was observed in ivabradine arm vs. placebo (P = 0.07). Ivabradine was not associated with serious bradycardia, atrioventricular block, hypotension, or syncope.
Conclusions
ChD HF is an advanced form of HF with poor prognosis. Ivabradine was effective in reducing HR in these patients and improving functional class. Although our results are based on a very limited sample and should be interpreted with caution, they suggest that ivabradine may have a favourable benefit–risk profile in ChD HF patients.
Keywords: Chagas disease, Heart failure, Ivabradine, SHIFT trial, Heart rate, Chagasic cardiomyopathy
Introduction
The cardiomyopathy caused by Chagas disease (ChD) is a major cause of morbidity and mortality in Latin America.1 Also, ChD has become a global health issue mainly owing to migration. Despite the substantial burden to the healthcare system, there is uncertainty regarding the efficacy and safety of standard guideline‐oriented pharmacological interventions for treating heart failure (HF) in patients with ChD.2 The current guidelines recommend the use of non‐chagasic HF treatments for ChD HF, although no robust evidence supports this practice.3, 4, 5, 6 In addition, the specific etiological treatment for chronic ChD produces frequent side effects or it seems to be not effective.7, 8 Patients with HF due to ChD face a worse prognosis in comparison with other aetiologies.9 Many of the pathogenic and remodelling mechanisms involved in ChD HF might render chagasic HF patients unresponsive to usual treatment,10 and this may also explain the high mortality.
Thus, new evidences of novel therapeutic approaches for the treatment of ChD HF are crucial. Ivabradine, a specific inhibitor of the I f current in the sinoatrial node was associated with reduction of cardiovascular death or hospitalization for worsening HF in the SHIFT study.11 Effects of ivabradine in ChD HF were not reported. In this post hoc analysis of SHIFT, we explored the effects of ivabradine in patients with ChD HF.
Methods
Study design
The SHIFT study was described in detail previously.11 Briefly, this randomized, double‐blind, placebo‐controlled, parallel‐group, multinational clinical trial included 6505 patients with moderate to severe chronic HF and documented left ventricular (LV) systolic dysfunction (LV ejection fraction ≤ 35%). Eligible patients were in sinus rhythm with a resting heart rate (HR) of ≥70 b.p.m. on 12‐lead electrocardiography; they had been clinically stable for ≥4 weeks and had been admitted to the hospital for worsening HF within the previous 12 months. All participants were receiving guideline‐based background therapy for HF, including maximized beta‐blocker therapy, if tolerated. Other inclusion and exclusion criteria have been described previously. After a 2‐week run‐in period, patients were randomly assigned to receive either ivabradine (5 mg b.i.d.) or placebo. Ivabradine or placebo therapy was initiated with 5 mg b.i.d. At subsequent visits at 14 days, at 28 days, and every 4 months thereafter (or at any other time if necessary), the dosage of blinded study medication could be adjusted upward (7.5 mg b.i.d.) or downward (2.5 mg b.i.d.) depending on resting HR. The ethics committee approved the trial, and all subjects gave written informed consent to participate in the trial.
Objective of this analysis
A post hoc analysis of SHIFT was performed to explore the safety profile and efficacy of ivabradine in patients with systolic HF due to ChD in sinus rhythm, with resting HR ≥ 70 b.p.m. All primary and secondary endpoints of SHIFT were analysed. Main adverse events during the study also were studied.
Statistical analysis
Baseline characteristics are presented as numbers and percentages for categorical variables and means (±SD) for continuous variables. Because this study is a post hoc analysis of SHIFT data, the statistical methods employed also were selected post hoc, although they are standard for analyses of this type of data. The Graphpad Prism 5.0 was used for statistical analysis. Survival analyses were performed on a time‐to‐first‐event basis with an intention‐to‐treat principle. Time‐to‐event curves were estimated with the Kaplan–Meier method. Groups were compared with log‐rank test and Breslow (generalized Wilcoxon). The percentages of patients improving their New York Heart Association (NYHA) functional class from baseline to last evaluation were compared with a χ2 test. Intra‐group HR comparisons from baseline to last evaluation were performed by a paired t‐test.
Role of sponsor of the SHIFT trial
The first author, who was a member of the Steering Committee of the SHIFT study, requested the sponsor of SHIFT to provide the data of ChD HF patients included in the SHIFT study. The first author was the investigator of this post hoc analysis; neither the sponsor of SHIFT nor the executive committee of the trial participated in the analysis of data or in any activity related to manuscript except for data sharing.
Results
From 6505 patients randomized in SHIFT, 20 patients with HF due to ChD HF were randomized to receive ivabradine and 18 patients to placebo. Sixteen patients (42%) were randomized in Argentina, 21 (55%) in Brazil, and 1 (3%) in Chile. The mean studied follow‐up for ChD HF patients was 13 ± 7 months and concluded on 31 March 2010. Baseline characteristics of ChD HF are given in Table 1. The characteristics of this substudy population were similar to those of the population of the main study (Table 1) except for lower percentages of men, NYHA functional class III patients, hypertension, diabetes, history of atrial arrhythmias, and device indication; longer duration of symptoms; and lower systemic blood pressure. The ChD HF subgroup showed high prevalence of bundle branch right block. In contrast to other SHIFT patients, ChD HF patients more frequently received antialdosterone agents and lower doses of beta‐blockers. Hypotension was the most frequent reason for failing to reach target dose of beta‐blocker: 68% vs. 45% in main study (Table 2). The mean ivabradine dose prescribed after the titration phase for ivabradine was 6.26 ± 1.15 mg and for placebo 6.43 ± 1.55 mg twice daily.
Table 1.
Baseline characteristics of patients with Chagas disease heart failure enrolled in the SHIFT trial
|
SHIFT trial (n = 6505) |
ChD HF
n = 38 |
ChD HF
Iva n = 20 |
ChD HF
Pla n = 18 |
|
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 60.9 ± 11.6 | 60 ± 12 | 62 ± 11 | 58 ± 13 |
| Sex (male), n (%) | 4970 (76.4%) | 26 (68.4%) | 13 (65%) | 13 (72%) |
| Ethnic origin, n (%) | ||||
| White (Caucasian) | 5771 (88.7%) | 31 (81.5%) | 17 (85%) | 14 (78%) |
| Black | NR | 3 (7.8%) | 0 | 3 (17%) |
| Other | 202 (3.1%) | 4 (10.5%) | 3 (15%) | 1 (6%) |
| Current smoking | NR | 3 (7.8%) | 1 (5%) | 2 (11%) |
| BMI (kg/m2) | 25.8 ± 2.4 | 25.5 ± 5.2 | 26 ± 6.5 | 25 ± 4 |
| Cardiac parameters | ||||
| Heart rate (b.p.m.) | 79.9 ± 9.6 | 78.3 ± 7.2 | 77.9 ± 3.8 | 78.8 ± 10.6 |
| SBP (mmHg) | 121.7 ± 16 | 111 ± 15.5 | 112 ± 16 | 110 ± 15 |
| DBP (mmHg) | 75.7 ± 9.5 | 70 ± 8.5 | 71 ± 9 | 69 ± 8 |
| LVEF (%) | 29 ± 5.2 | 27.5 ± 5.5 | 28 ± 5 | 27 ± 6 |
| Bundle branch block right | NR | 9 (23.6%) | 4 (20%) | 5 (28%) |
| Bundle branch block left | 912 (14%) | 6 (15.7%) | 2 (10%) | 4 (22%) |
| NYHA, n (%) | ||||
| Class II | 3169 (48.7%) | 30 (78.9%) | 16 (80%) | 14 (78%) |
| Class III | 3223 (49.5%) | 8 (21.1%) | 4 (20%) | 4 (22%) |
| Class IV | 111 (1.7%) | 0 | 0 | 0 |
| Medical history | ||||
| Duration HF (years) | 4.19 ± 5.04 | 3.9 ± 10.79 | 4.62 ± 8.22 | 3.19 ± 5.14 |
| Hypertension, n (%) | 4314 (66.3%) | 15 (39.4%) | 8 (40%) | 7 (39%) |
| Diabetes | 1979 (30.4%) | 3 (7.9%) | 2 (10%) | 1 (5.6%) |
| Previous stroke, n (%) | 523 (8%) | 2 (5.2%) | 0 | 2 (11%) |
| Atrial fibrillation or flutter | 522 (8%) | 1 (2.6%) | 1 (5%) | 0 |
| COPD | 730 (11.2%) | 4 (10.5%) | 1 (5%) | 3 (16.7%) |
| Hypothyroidism | NR | 4 (10.5%) | 2 (10%) | 2 (11%) |
| Depression | NR | 2 (5.2%) | 1 (5%) | 1 (5.6%) |
| Anaemia | NR | 1 (2.6%) | 0 | 1 (5.6%) |
| eGFR (mL/min/1.73 m2) | 74.6 | 72 ± 32.5 | 61 ± 23 | 83 ± 42 |
| Treatment at randomization | ||||
| Βeta‐blocker | 5820 (89.5%) | 33 (86.8%) | 18 (90%) | 15 (83.3%) |
| ACE inhibitor | 5116 (78.6%) | 25 (65.7%) | 15 (75%) | 10 (56%) |
| Enalapril (mg) | NR | 8 ± 4.95 | 7.3 ± 5 | 8.8 ± 4.9 |
| ARB | 927 (14.3%) | 7 (18.4%) | 3 (15%) | 4 (22%) |
| Losartan (mg) | NR | 44.5 ± 23 | 33 ± 14 | 56 ± 32 |
| Diuretic | 5414 (83.2%) | 35 (92%) | 18 (90%) | 17 (94%) |
| ACEI and /or ARB | NR | 32 (84.2%) | 18 (90%) | 14 (78% |
| Antialdosterone agents | 3922 (60.2%) | 35 (92%) | 20 (100%) | 15 (83%) |
| Spironolactone (mg) | NR | 26.5 ± 4 | 28 ± 8 | 25 ± 0 |
| Cardiac glycosides | 1416 (21.7%) | 17 (44.7%) | 9 (45%) | 8 (44%) |
| Devices | 279 (4.2%) | 0 | 0 | 0 |
| Amiodarone | NR | 6 (15.7%) | 2 (10%) | 4 (22.13%) |
| Nitrates | NR | 1 (2.6%) | 0 | 1 (6%) |
| Antithrombotic agents | NR | 18 (47.3%) | 9 (45%) | 9 (50%) |
| Acetylsalicylic acid | NR | 16 (42.1%) | 9 (45%) | 7 (39%) |
| Clopidogrel | NR | 1 (2.6%) | 1 (5%) | 0 |
| Vitamin K antagonists | 1082 (16.6%) | 2 (5.2%) | 0 | 2 (11.2%) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; ChD HF, Chagas disease heart failure; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Iva, ivabradine; LVEF, left ventricular ejection fraction; NR, not reported; Pla, placebo; SBP, systolic blood pressure.
Data are number of patients (%) or means ± 1 standard deviation.
Table 2.
Distribution of beta‐blocker use at baseline in patients with Chagas disease heart failure enrolled in the SHIFT trial
|
Ivabradine group n = 20 |
Placebo group n = 18 |
|
|---|---|---|
| Patients receiving beta‐blocker | 18 (90%) | 15 (83.3%) |
| Βeta‐blocker | ||
| Carvedilol | 18 (90%) | 12 (67%) |
| Mean daily dose, mg | 17 ± 16 | 15 ± 13 |
| Bisoprolol | 0 | 1 |
| Mean daily dose, mg | — | 1.25 |
| Metoprolol succinate | 0 | 1 |
| Mean daily dose, mg | — | 100 |
| Optimum tolerated dose | 18 (100%) | 15 (100%) |
| At least half of the target daily dose | 5 (28%) | 4 (29%) |
| Target daily dose | 3 (17%) | 1 (7%) |
| Reasons for no target daily dose | ||
| Bradycardia | 0 | 1 (8%) |
| Fatigue | 4 (27%) | 1 (8%) |
| Hypotension | 9 (60%) | 10 (77%) |
| Cardiac decompensation | 0 | 2 (15%) |
| Pulmonary dyspnoea | 1 (7%) | 0 |
| Other reason | 2 (13%) | 1 (8%) |
Heart rate effects of ivabradine
Figure 1 A shows changes in HR. In comparison with baseline HR at 28 days and 12 months after randomization, the HR reduced in the ivabradine group (−12 ± 7 and −11 ± 10 b.p.m.) and in the placebo group (−8 ± 11 and −6 ± 16 b.p.m.). From the baseline to post‐randomization last evaluation, the HR reduced from 77.9 ± 3.8 to 62.3 ± 10.1 b.p.m. (−16 ± 9 b.p.m.) in the ivabradine group (P = 0.005), while in the placebo group, the HR was 78.8 ± 10.6 b.p.m. at baseline and 79.6 ± 13.7 b.p.m. in the last evaluation (1 ± 16 b.p.m. in comparison with baseline) (Figure 1 B).
Figure 1.
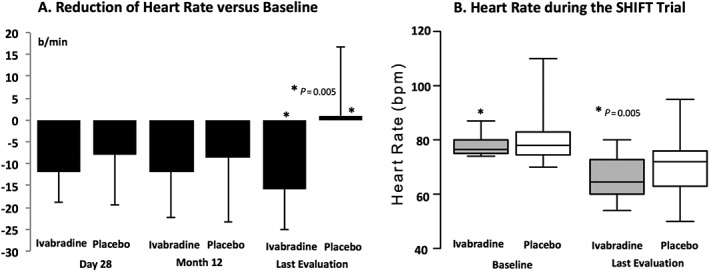
Heart rate at baseline and during the follow‐up in patients with Chagas disease heart failure treated with ivabradine and placebo (with intra‐group paired t‐test P value).
Functional class
Figure 2 shows the NYHA functional class from baseline to 12 months and at last post‐randomization evaluation. The functional class improved over this period in the ivabradine group in 30% of patients, while it remained stable in 60%, and worsened in 10%. In the placebo group, functional class improved in 22%, remained stable in 44%, and worsened in 33% (comparison X on χ2 test: P = 0.02).
Figure 2.
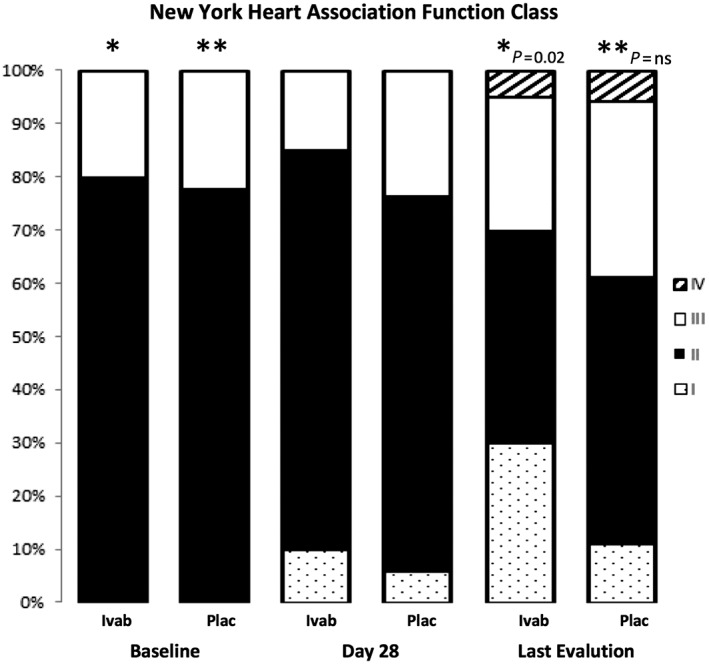
New York Heart Association functional class at baseline and during the follow‐up in patients with Chagas disease heart failure treated with ivabradine and placebo (χ2 test).
Mortality and hospitalization
For the overall ChD HF patients, Figure 3 A (Kaplan–Meier curve) shows 40% probability of survival at 750 day follow‐up, and Figure 3 B (Kaplan–Meier curve) shows ~30% probability to be free of death or hospitalization due to worsening HF at the same time of follow‐up. Table 3 shows the effects of ivabradine in comparison with placebo in components of primary endpoints, and secondary endpoints. The data show a trend towards reduction in any cause of death in the ivabradine group (P < 0.07) (Figure 4 A) (Kaplan–Meier curve) during the follow‐up. Figure 4 B shows the effect of ivabradine vs. placebo on the primary composite endpoint (cardiovascular death or hospitalization for worsening HF) during the study follow‐up.
Figure 3.
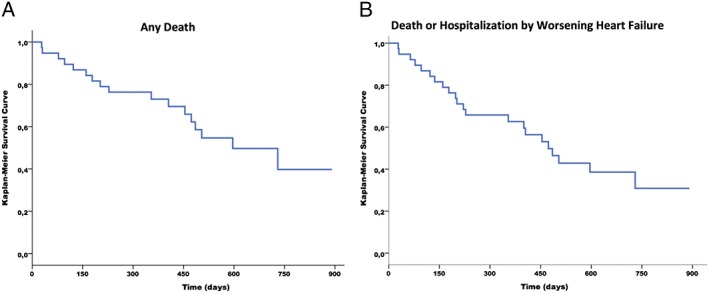
Death and hospitalization events in overall patients with Chagas disease heart failure. (A) Kaplan–Meier survival curves free of any cause of death. (B) Kaplan–Meier survival curves free of death or hospitalization due to heart failure.
Table 3.
Number and % of patients with at least one event in ivabradine/placebo groups for primary and secondary endpoints
|
Ivabradine group n = 20 |
Placebo group n = 18 |
|
|---|---|---|
| Primary endpoint components | ||
| Cardiovascular death or HF hospitalization | 10 (50%) | 10 (56%) |
| Cardiovascular death | 6 (30%) | 8 (44%) |
| Hospital admission for worsening HF | 8 (40%) | 8 (44%) |
| Secondary endpoints | ||
| Death of any cause | 7 (35%) | 9 (50%) |
| Hospitalization for any cause | 13 (65%) | 10 (56%) |
| Death from heart failure | 4 (20%) | 5 (28%) |
| Other | ||
| Non‐cardiovascular hospitalization | 6 (30%) | 2 (11%) |
| AMI hospitalization or death | 0 | 0 |
| Other cardiovascular hospitalization | 3 (30%) | 5 (28%) |
| Other cardiovascular death | 0 | 2 (11%) |
| Non‐cardiovascular death | 1 (5%) | 1 (6%) |
| Sudden cardiac death | 2 (10%) | 1 (6%) |
AMI; acute myocardial infarction; HF, heart failure.
Figure 4.
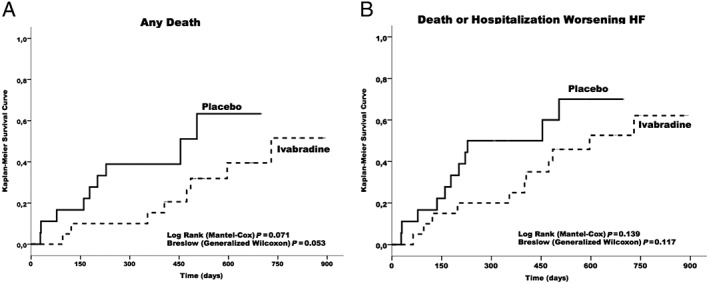
Kaplan–Meier cumulative event curves for patients with Chagas disease heart failure. (A) Kaplan–Meier cumulative event curves for death of any cause (with P values). (B) Kaplan–Meier cumulative event curves for SHIFT primary endpoint (cardiovascular death or hospitalization due to worsening heart failure).
Adverse effects
Table 4 presents the adverse effects reported in the ivabradine group in comparison with placebo. No differences were observed comparing both groups concerning total clinical events. Ivabradine was not associated with serious bradycardia, atrioventricular block, hypotension, or syncope.
Table 4.
Main adverse events during the study in patients with Chagas disease heart failure enrolled in the SHIFT trial
|
Ivabradine group n = 20 |
Placebo group n = 18 |
|
|---|---|---|
| Atrial fibrillation | 1 (5%) | 3 (17%) |
| Supraventricular arrhythmia | 2 (10%) | 0 |
| Atrial flutter | 1 (5%) | 0 |
| Complete atrioventricular block | 0 | 2 (11%) |
| First–second degree atrioventricular block | 2 (10%) | 0 |
| Serious bradycardia | 0 | 1 (6%) |
| Infections | 6 (30%) | 7 (39%) |
| Gastrointestinal disorders | 4 (20%) | 5 (28%) |
| Metabolism and nutrition disorders | 5 (25%) | 2 (11%) |
| Hypokalaemia | 2 (10%) | 0 |
| Renal failure or impairment | 4 (20%) | 2 (11%) |
| Nervous system disorders | 2 (10%) | 3 (17%) |
| Syncope | 0 | 2 (11%) |
| Hypotension | 0 | 2 (11%) |
| Heart transplant | 0 | 1 (6%) |
| Implantable defibrillator insertion | 0 | 1 (6%) |
| Neoplasms | 1 (5%) | 0 |
| All clinical events | 17 (85%) | 15 (83%) |
Discussion
This investigation is, to the best of our knowledge, the first to report ivabradine effects in ChD HF patients and confirmed that ChD HF is an advanced form of HF with very poor prognosis. Ivabradine reduced HR, improved NYHA functional class, and was associated with a trend to reduction in mortality. Ivabradine was not associated with an increment in adverse events in comparison with placebo. Also, optimized, guideline‐oriented HF treatment was not well tolerated in ChD HF.
The finding of ivabradine‐dependent HR reduction with acceptable safety profile suggests that the I f current in the sinoatrial node is not importantly affected by ChD HF pathogenic mechanisms in patients with HR heart rate ≥ 70 b.p.m. Therefore, use of ivabradine may be feasible in ChD HF patients fulfilling the SHIFT inclusion criterion. The reduction of HR at 28 days compared with pre‐treatment observed in ChD HF was close to that described in the SHIFT study.11 Our findings disagree with the potential concern about use of ivabradine in ChD HF. Chronotropic incompetence and sick sinus node syndrome may be found in ChD cardiomyopathy (ChHD), even in the latent phase when the heart may be apparently normal.12 The inflammatory and fibrotic processes may damage sinus cells, destroy the innervation, and cause significant barriers to sinus node impulse progression.12 However, autoantibodies reported in ChHD may exert sometimes effects in favour of increment or decrease in heart chronotropism, depending on the type of antibody or patient characteristics.13, 14, 15
Beyond the proposed effects of ivabradine in overall aetiologies, some mechanisms should be taken in account to explain the tendency to reduction of mortality and improvement in functional class ChD HF. ChD HF in comparison with other HF aetiologies is characterized by a remodelling process with frequent right and LV dysfunction besides persistent myocarditis associated with inflammatory infiltrate oedema, contraction band necrosis, myocytolysis, focal and diffuse areas of myocellular hypertrophy, fibrosis, damage to the parasympathetic system causing sympathetic over activity, and microvascular abnormalities.16, 17 The reported improvement in right ventricular function with ivabradine might be clinically significant in ChD HF because right ventricular dysfunction is a challenge in the management of ChD HF.18 Concerning the myocarditis, beneficial pleiotropic effects of ivabradine beyond HR were reported in experimental coxsackievirus B3 myocarditis partially mediated by the inhibition of both the production of proinflammatory cytokines and the synthesis of NO by iNOS.19, 20 Also, ivabradine in chronic viral myocarditis in mice significantly increased the survival rate; attenuated the myocardial lesions and fibrosis; improved the impairment of the LV function; diminished the heart dimension; decreased the production of collagen I and collagen III; reduced the expression of the proinflammatory cytokines tumour necrosis factor alpha and interleukins 1β and 6; and lowered the production of phospho‐p38 MAPK.21 In HF patients, ivabradine increased LV ejection fraction and led to a significant reduction of tumour necrosis factor alpha serum levels and a reconstitution of circulating dendritic cells.22 Furthermore, ivabradine reduced microvascular derangements in experimental sepsis and improved endothelial function in patients undergoing percutaneous coronary intervention,23, 24
The mortality observed in our study (60%) in ChD HF is much higher in comparison with that of the SHIFT overall population (~17.5%) and of the recent PARADIGM‐HF trial (~15%) at 750 day follow‐up.11, 25 This finding is in agreement with previous publication.9, 26 Multiple mechanisms can contribute such as the remodelling process in ChD HF, the low dosage of medications for HF observed in our results, and the lower systemic blood pressure of patients in this post hoc analysis in comparison with that of the overall SHIFT population.
Limitation of the study
The present study is based on a post hoc analysis of a prospective randomized controlled trial in chronic stable HF patients (SHIFT trial), and this trial was not designed to investigate the effect of ivabradine treatment in ChD HF patients. Thus, the data have to be interpreted with caution given the rather small sample size, the low statistical power, and the retrospective analysis. However, a high percentage of primary and secondary endpoints were observed. Some differences between the groups ChD HF ivabradine and ChD HF placebo were observed concerning baseline frequency and dose of medication under use for HF. However, the impact of these differences on outcome is controversial because there are no prospective trials proving that these medications improve outcome in ChD HF. Despite the safety of ivabradine use in ChD HF reported in this study, the risk of bradycardia should be carefully assessed in ChD HF because patients were selected to be enrolled based on HR ≥ 70 b.p.m.
Clinical implications
There are doubts if the beneficial effects of standard HF treatment reported in other aetiologies can be translatable to ChD HF. In addition, medication tolerance in ChD HF may differ from other aetiologies. Sometimes, the heart transplantation is the unique alternative.27, 28 The challenge in clinical practice is when to consider new drugs, such as ivabradine, in the scenario of neglected disease such as ChD. The finding of favourable benefit–risk profile of ivabradine in ChD HF patients can improve the decision‐making process in the treatment of ChD HF.
Conclusions
Our analysis demonstrates that the overall outcome in ChD HF patients included in SHIFT was poor. ChD HF seems to be an advanced form of HF. The need for new medications in the treatment of ChD HF is clear. Although our results are based on a very limited sample and should be interpreted with caution, they suggest that ivabradine may have a favourable benefit–risk profile in CH HF patients. These findings encourage for further investigations of HR as a potential treatment target in ChD HF and provide the rationale for a randomized controlled trial to evaluate the benefit of ivabradine treatment in patients with ChD HF.
Conflict of interest
Edimar Alcides Bocchi reports fees for board membership for AstraZeneca and Novartis; consultancy fees from Servier; speaker's bureau for Novartis; and grants and personal fees from Merck Sharp & Dohme, Janssen Research & Development—Bayer, and Servier while conducting studies according to contract of the companies with the Heart Institute (InCor). Salvador Rassi reports fees for board membership for Novartis; speaker's bureau for Novartis; and fees from Servier while conducting studies according to contract of the companies with the Heart Institute (InCor). Guilherme Guimarães does not have anything to disclose.
Funding
The SHIFT trial was sponsored by Servier, France. No other funding was provided to the authors for the current analysis.
Acknowledgements
The authors would like to thank all participating investigators for their contributions to the SHIFT study specially Latin America by inclusion of ChD HF patients. We thank also Fabienne Dominjon (France), who provided the data on patients with ChD HF of the SHIFT trial on behalf of the Sponsor (Servier, France), and subsequent data sharing; and Dr Abraham Epelman (Servier, Brazil), who was enthusiastic for the development of this analysis.
Bocchi, E. A. , Rassi, S. , Guimarães, G. V. , and Argentina, Chile, and Brazil SHIFT Investigators (2018) Safety profile and efficacy of ivabradine in heart failure due to Chagas heart disease: a post hoc analysis of the SHIFT trial. ESC Heart Failure, 5: 249–256. doi: 10.1002/ehf2.12240.
Argentina, Chile, and Brazil SHIFT Investigators:
Argentina: https://www.ncbi.nlm.nih.gov/pubmed/?term=Amuchastegui%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Barrios%20AR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Bendersky%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Bene%C3%ADtez%20CF, https://www.ncbi.nlm.nih.gov/pubmed/?term=Buscema%20JJ, https://www.ncbi.nlm.nih.gov/pubmed/?term=Labarta%20GB, https://www.ncbi.nlm.nih.gov/pubmed/?term=Cacharr%C3%B3n%20JL, https://www.ncbi.nlm.nih.gov/pubmed/?term=Caime%20JD, https://www.ncbi.nlm.nih.gov/pubmed/?term=Colombo%20H, https://www.ncbi.nlm.nih.gov/pubmed/?term=Colque%20RM, https://www.ncbi.nlm.nih.gov/pubmed/?term=Costello%20AR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Diez%20F, https://www.ncbi.nlm.nih.gov/pubmed/?term=Fern%C3%A1ndez%20A, https://www.ncbi.nlm.nih.gov/pubmed/?term=Ferrari%20GM, https://www.ncbi.nlm.nih.gov/pubmed/?term=Ferreyra%20R, https://www.ncbi.nlm.nih.gov/pubmed/?term=Fuselli%20J, https://www.ncbi.nlm.nih.gov/pubmed/?term=Gambarte%20AJ, https://www.ncbi.nlm.nih.gov/pubmed/?term=Garrido%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Gorini%20NU, https://www.ncbi.nlm.nih.gov/pubmed/?term=Carrillo%20NC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Guzm%C3%A1n%20L, https://www.ncbi.nlm.nih.gov/pubmed/?term=Hasbani%20E, https://www.ncbi.nlm.nih.gov/pubmed/?term=Jure%20HO, https://www.ncbi.nlm.nih.gov/pubmed/?term=Kuschnir%20E, https://www.ncbi.nlm.nih.gov/pubmed/?term=Lastiri%20H, https://www.ncbi.nlm.nih.gov/pubmed/?term=Marquez%20LL, https://www.ncbi.nlm.nih.gov/pubmed/?term=Luciardi%20HL, https://www.ncbi.nlm.nih.gov/pubmed/?term=Luquez%20H, https://www.ncbi.nlm.nih.gov/pubmed/?term=Majul%20CR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Marino%20J, https://www.ncbi.nlm.nih.gov/pubmed/?term=Martingano%20R, https://www.ncbi.nlm.nih.gov/pubmed/?term=Miriuka%20SG, https://www.ncbi.nlm.nih.gov/pubmed/?term=Muntaner%20J, https://www.ncbi.nlm.nih.gov/pubmed/?term=Perna%20E, https://www.ncbi.nlm.nih.gov/pubmed/?term=Piasentin%20JA, https://www.ncbi.nlm.nih.gov/pubmed/?term=Piskorz%20D, https://www.ncbi.nlm.nih.gov/pubmed/?term=Pomposiello%20JC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Porcile%20R, https://www.ncbi.nlm.nih.gov/pubmed/?term=Poy%20CA, https://www.ncbi.nlm.nih.gov/pubmed/?term=Ramos%20HR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Acu%C3%B1a%20AA, https://www.ncbi.nlm.nih.gov/pubmed/?term=Rusculleda%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Nisi%20JS, https://www.ncbi.nlm.nih.gov/pubmed/?term=Sanchez%20JM, https://www.ncbi.nlm.nih.gov/pubmed/?term=Sarjanovich%20R, https://www.ncbi.nlm.nih.gov/pubmed/?term=Scaro%20G, https://www.ncbi.nlm.nih.gov/pubmed/?term=Secchi%20JM, https://www.ncbi.nlm.nih.gov/pubmed/?term=Severino%20P, https://www.ncbi.nlm.nih.gov/pubmed/?term=Snitman%20MJ, https://www.ncbi.nlm.nih.gov/pubmed/?term=Liprandi%20MI, https://www.ncbi.nlm.nih.gov/pubmed/?term=Sultan%20MG, https://www.ncbi.nlm.nih.gov/pubmed/?term=V%C3%A1zquez%20RJ, https://www.ncbi.nlm.nih.gov/pubmed/?term=Vigo%20CF, https://www.ncbi.nlm.nih.gov/pubmed/?term=Vulcano%20N
Brazil: https://www.ncbi.nlm.nih.gov/pubmed/?term=Albuquerque%20DC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Almeida%20DR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Aziz%20JL, https://www.ncbi.nlm.nih.gov/pubmed/?term=Boas%20FV, https://www.ncbi.nlm.nih.gov/pubmed/?term=Bocchi%20EA, https://www.ncbi.nlm.nih.gov/pubmed/?term=Bodanese%20LC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Braga%20JC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Chaves%20R, https://www.ncbi.nlm.nih.gov/pubmed/?term=Figueiredo%20JC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Greco%20OT, https://www.ncbi.nlm.nih.gov/pubmed/?term=Kohler%20I, https://www.ncbi.nlm.nih.gov/pubmed/?term=Leaes%20PE, https://www.ncbi.nlm.nih.gov/pubmed/?term=Mady%20C, https://www.ncbi.nlm.nih.gov/pubmed/?term=Maia%20LN, https://www.ncbi.nlm.nih.gov/pubmed/?term=Manenti%20E, https://www.ncbi.nlm.nih.gov/pubmed/?term=Montera%20MW, https://www.ncbi.nlm.nih.gov/pubmed/?term=Motta%20MS, https://www.ncbi.nlm.nih.gov/pubmed/?term=Moura%20LZ, https://www.ncbi.nlm.nih.gov/pubmed/?term=Precoma%20DB, https://www.ncbi.nlm.nih.gov/pubmed/?term=Rassi%20S, https://www.ncbi.nlm.nih.gov/pubmed/?term=Saraiva%20JF, https://www.ncbi.nlm.nih.gov/pubmed/?term=Silveira%20JR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Filho%20DS, https://www.ncbi.nlm.nih.gov/pubmed/?term=Teixeira%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Zimmermann%20SL
Chile: https://www.ncbi.nlm.nih.gov/pubmed/?term=Bus%20AA, https://www.ncbi.nlm.nih.gov/pubmed/?term=Bugue%C3%B1o%20C, https://www.ncbi.nlm.nih.gov/pubmed/?term=Castro%20P, https://www.ncbi.nlm.nih.gov/pubmed/?term=Cobos%20L, https://www.ncbi.nlm.nih.gov/pubmed/?term=Escobar%20E, https://www.ncbi.nlm.nih.gov/pubmed/?term=Moreno%20M, https://www.ncbi.nlm.nih.gov/pubmed/?term=Castro%20CR, https://www.ncbi.nlm.nih.gov/pubmed/?term=Schnettler%20MC, https://www.ncbi.nlm.nih.gov/pubmed/?term=Sep%C3%BAlveda%20P, https://www.ncbi.nlm.nih.gov/pubmed/?term=Stockins%20B
References
- 1. Bocchi EA, Guimarães G, Tarasoutshi F, Spina G, Mangini S, Bacal F. Cardiomyopathy, adult valve disease and heart failure in South America. Heart 2009; 95: 181–189. [DOI] [PubMed] [Google Scholar]
- 2. Martí‐Carvajal AJ, Kwong JS. Pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy. Cochrane Database Syst Rev 2016; 7: CD009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bocchi EA, Marcondes‐Braga FG, Bacal F, Ferraz AS, Albuquerque D, Rodrigues Dde A, Mesquita ET, Vilas‐Boas F, Cruz F, Ramires F, Villacorta H Jr, Souza Neto JD, Rossi Neto JM, Moura LZ, Beck‐da‐Silva L, Moreira LF, Rohde LE, Montera MW, Simões MV, Moreira Mda C, Clausell N, Bestetti R, Mourilhe‐Rocha R, Mangini S, Rassi S, Ayub‐Ferreira SM, Martins SM, Bordignon S, Issa VS. Updating of the Brazilian guideline for chronic heart failure—2012. Arq Bras Cardiol 2012; 98: 1–33. [DOI] [PubMed] [Google Scholar]
- 4. Andrade JP, Marin‐Neto JA, Paola AA, Vilas‐Boas F, Oliveira GM, Bacal F, Bocchi EA, Almeida DR, Fragata Filho AA, Moreira Mda C, Xavier SS, Oliveira Junior WA, Dias JC, Sociedade Brasileira de Cardiologia . I Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy. Arq Bras Cardiol 2011; 97: 1–48. [PubMed] [Google Scholar]
- 5. Bocchi EA, Braga FG, Ferreira SM, Rohde LE, Oliveira WA, Almeida DR, Moreira Mda C, Bestetti RB, Bordignon S, Azevedo C, Tinoco EM, Rocha RM, Issa VS, Ferraz A, Fd C, Guimarães GV, Montera V d S, Albuquerque DC, Bacal F, Souza GE, Rossi Neto JM, Clausell NO, Martins SM, Siciliano A, Souza Neto JD, Moreira LF, Teixeira RA, Moura LZ, Beck‐da‐Silva L, Rassi S, Azeka E, Horowitz E, Ramires F, Simões MV, Castro RB, Salemi VM, Villacorta Junior H, Vila JH, Simões R, Albanesi F, Montera MW, Sociedasde Brasileira de Cardiologia . III Brazilian guidelines on chronic heart failure. Arq Bras Cardiol 2009; 93: 3–70. [PubMed] [Google Scholar]
- 6. Bocchi EA, Vilas‐Boas F, Perrone S, Caamaño AG, Clausell N, Moreira Mda C, Thierer J, Grancelli HO, Serrano Junior CV, Albuquerque D, Almeida D, Bacal F, Moreira LF, Mendonza A, Magaña A, Tejeda A, Chafes D, Gomez E, Bogantes E, Azeka E, Mesquita ET, Reis FJ, Mora H, Vilacorta H, Sanches J, Dd SN, Vuksovic JL, Moreno JP, Aspe y Rosas J, Moura LZ, Campos LA, Rohde LE, Javier MP, Garrido Garduño M, Tavares M, Castro Gálvez P, Spinoza R, Castro de Miranda R, Rocha RM, Paganini R, Castano Guerra R, Rassi S, Lagudis S, Bordignon S, Navarette S, Fernandes W, Pereira Barretto AC, Issa V, Guimarães JI, Grupo de Estudos de Insuficiência Cardíaca , Brazilian Society of Cardiology , Argentine Federation of Cardiology , Argentine Society of Cardiology , Chilean Society of Cardiology , Costa Rican Association of Cardiology , Colombian Society of Cardiology , Equatorian Society of Cardiology , Guatemalan Association of Cardiology , Peruvian Society of Cardiology , Uruguayan Society of Cardiology , Venezuelan Society of Cardiology , Mexican Society of Cardiology , Mexican Society of Heart Failure , Interamerican Society of Heart Failure . I Latin American guidelines for the assessment and management of decompensated heart failure. Arq Bras Cardiol 2005; 85: 1–48. [PubMed] [Google Scholar]
- 7. Morillo CA, Marin‐Neto JA, Avezum A, Sosa‐Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao‐Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investigators . RANDOMIZED TRIAL OF BENZNIDAZOLE FOR CHRONIC CHAGAS' CARDIOMYOPATHY. N Engl J Med 2015; 373: 1295–1306. [DOI] [PubMed] [Google Scholar]
- 8. Issa VS, Bocchi EA. Antitrypanosomal agents: treatment or threat? Lancet 2010; 376: 768. [DOI] [PubMed] [Google Scholar]
- 9. Issa VS, Amaral AF, Cruz FD, Ferreira SM, Guimarães GV, Chizzola PR, Souza GE, Bacal F, Bocchi EA. Beta‐blocker therapy and mortality of patients with Chagas cardiomyopathy: a subanalysis of the REMADHE prospective trial. Circ Heart Fail 2010; 3: 82–88. [DOI] [PubMed] [Google Scholar]
- 10. Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P, Interamerican Society of Cardiology . The reality of heart failure in Latin America. J Am Coll Cardiol 2013; 62: 949–958. [DOI] [PubMed] [Google Scholar]
- 11. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 12. Pachón JC. Chronotropic incompetence in Chagas disease: usefulness of dual sensor pacemaker based on volume minute and accelerometer. Rev Bras Cir Cardiovasc 2015; 30: III–IIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farias de Oliveira S, Pedrosa RC, Nascimento JHMS, Campos de Carvalho AC, Masuda MO. Sera from chronic chagasic patients with complex cardiac arrhythmias depress electrogenesis and conduction in isolated rabbit hearts. Circulation 1997; 96: 2031–2037. [DOI] [PubMed] [Google Scholar]
- 14. Chiale PA, Rosenbaum MB, Elizari MV, Hjalmarson A, Magnusson Y, Wallukat G et al. High prevalence of antibodies against beta 1‐ and beta 2‐adrenoceptors in patients with primary electrical cardiac abnormalities. J Am Coll Cardiol 1995; 26: 864–869. [DOI] [PubMed] [Google Scholar]
- 15. Rosenbaum MB, Chiale P, Schejtman D, Levin M, Elizari M. Antibodies to β1‐adrenergic receptors disclosing agonist‐like properties in idiopathic dilated cardiomyopathy and Chagas' heart disease. J Cardiovasc Electrophysiol 1994; 5: 367–375. [DOI] [PubMed] [Google Scholar]
- 16. Bocchi EA. Exercise training in Chagas' cardiomyopathy: trials are welcome for this neglected heart disease. Eur J Heart Fail 2010; 12: 782–784. [DOI] [PubMed] [Google Scholar]
- 17. Bellotti G, Bocchi EA, de Moraes AV, Higuchi ML, Barbero‐Marcial M, Sosa E, Esteves‐Filho A, Kalil R, Weiss R, Jatene A, Pileggi F. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas' heart disease. Am Heart J 1996; 131: 301–307. [DOI] [PubMed] [Google Scholar]
- 18. Lofrano‐Alves MS, Issa VS, Biselli B, Chizzola P, Ayub‐Ferreira SM, Bocchi EA. Control of sinus tachycardia as an additional therapy in patients with decompensated heart failure (CONSTATHE‐DHF): a randomized, double‐blind, placebo‐controlled trial. J Heart Lung Transplant 2016; 35: 1260–1264. [DOI] [PubMed] [Google Scholar]
- 19. Li YC, Luo Q, Ge LS, Chen YH, Zhou ND, Zhang T, Guan XQ, Lin JF. Ivabradine inhibits the production of proinflammatory cytokines and inducible nitric oxide synthase in acute coxsackievirus B3‐induced myocarditis. Biochem Biophys Res Commun 2013; 431: 450–455. [DOI] [PubMed] [Google Scholar]
- 20. Yue‐Chun L, Teng Z, Na‐Dan Z, Li‐Sha G, Qin L, Xue‐Qiang G, Jia‐Feng L. Comparison of effects of ivabradine versus carvedilol in murine model with the Coxsackievirus B3‐induced viral myocarditis. PLoS One 2012; 7: e39394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yue‐Chun L, Guang‐Yi C, Li‐Sha G, Chao X, Xinqiao T, Cong L, Xiao‐Ya D, Xiangjun Y. The protective effects of ivabradine in preventing progression from viral myocarditis to dilated cardiomyopathy. Front Pharmacol 2016; 7: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohm I, Kretzschmar D, Pistulli R, Franz M, Schulze PC, Stumpf C, Yilmaz A. Impact of ivabradine on inflammatory markers in chronic heart failure. J Immunol Res 2016; 2016: 6949320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miranda ML, Balarini MM, Balthazar DS, Paes LS, Santos MS, Bouskela E. Ivabradine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology 2017; 126: 140–149. [DOI] [PubMed] [Google Scholar]
- 24. Mangiacapra F, Colaiori I, Ricottini E, Balducci F, Creta A, Demartini C, Minotti G, Di Sciascio G. Heart Rate reduction by IVabradine for improvement of ENDothELial function in patients with coronary artery disease: the RIVENDEL study. Clin Res Cardiol 2017; 106: 69–75. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 26. Bocchi EA. Update on indications and results of the surgical treatment of heart failure. Arq Bras Cardiol 1994; 63: 523–530. [PubMed] [Google Scholar]
- 27. Bocchi EA, Fiorelli A, First Guideline Group for Heart Transplantation of the Brazilian Society of Cardiology . The Brazilian experience with heart transplantation: a multicenter report. J Heart Lung Transplant 2001; 20: 637–645. [DOI] [PubMed] [Google Scholar]
- 28. Beck‐da‐Silva L, Piardi D, Soder S, Rohde LE, Pereira‐Barretto AC, de Albuquerque D, Bocchi E, Vilas‐Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 2013; 168: 3439–3442. [DOI] [PubMed] [Google Scholar]


