Abstract
Background
Sacubitril/valsartan significantly reduced heart failure hospitalization and mortality in PARADIGM‐HF (Prospective Comparison of Angiotensin Receptor‐Neprilysin Inhibitor With an Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). However, real‐world data from its use are lacking.
Methods and results
We retrospectively assessed all baseline and follow‐up data of consecutive heart failure patients with reduced ejection fraction receiving therapy with sacubitril/valsartan for Class I recommendation between December 2016 and July 2017. Baseline characteristics and dose titration of sacubitril/valsartan were compared between patients in clinical practice and in PARADIGM‐HF. A total of 120 patients (81% male) were switched from angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker to sacubitril/valsartan. A total of 20.1% of patients received dose uptitration. Patients were treated with an equipotential dose of renin–angiotensin system blockers before and after uptitration of sacubitril/valsartan (57 ± 29% vs. 53 ± 29% of target dose indicated by European Society of Cardiology guidelines; P = 0.286). However, they received a lower dose of sacubitril/valsartan in comparison with those in the PARADIGM‐HF (219 ± 12 vs. 375 ± 75 mg; P < 0.001). In comparison with the patients receiving sacubitril/valsartan in PARADIGM‐HF, patients in clinical practice were older and had a higher serum creatinine, higher New York Heart Association functional classification, and lower left ventricular ejection fraction (all P‐value <0.05). Even in comparison with patients who experienced dropout during the run‐in phase of PARADIGM‐HF, real‐world patients exhibited baseline characteristics indicative of more disease severity. Patients were at high absolute baseline risk for adverse outcome as illustrated by the EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) risk score of 6 (inter‐quartile range 3), in comparison with 5 (inter‐quartile range 4) in PARADIGM‐HF. After initiation of sacubitril/valsartan, New York Heart Association class significantly improved (P < 0.001), but systolic blood pressure dropped more than was reported in PARADIGM‐HF (7.1 ± 8.0 vs. 3.2 ± 0.4 mmHg; P < 0.001).
Conclusions
Patients in clinical practice exhibit baseline characteristics associated with more severe disease, which might lead to prescription of lower doses. Nevertheless, patients in clinical practice are at high risk of adverse outcome as illustrated by the EMPHASIS‐HF risk score, underscoring the large potential for sacubitril/valsartan therapy to reduce the risk of heart failure hospitalization and all‐cause mortality.
Keywords: Sacubitril/valsartan, Pharmacology, Randomized controlled trials, Real world
Introduction
In the PARADIGM‐HF (Prospective Comparison of Angiotensin Receptor‐Neprilysin Inhibitor With an Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, sacubitril/valsartan significantly reduced both heart failure hospitalization and cardiovascular mortality in comparison with guideline‐recommended doses of enalapril.1 The convincing beneficial effect of sacubitril/valsartan was demonstrated against an evidence‐based dose of enalapril.2 However, in order to attain such a high‐dosed patient population, several strategies had to be implemented. For instance, pre‐screening mandated at least toleration of an equipotential dose of 10 mg enalapril. Furthermore, a run‐in phase further assured dose optimization.1, 3 However, patients in clinical practice are often frailer and treated with lower doses of renin–angiotensin system (RAS) blockers.3, 4 Differences in the patient phenotype encountered in clinical practice might lead to hesitations of initiating or uptitrating sacubitril/valsartan (a Class I lifesaving therapy). Our analysis sought out to analyse potential hurdles associated with the use of sacubitril/valsartan in clinical practice, hereby offering a larger cardiology audience valuable information when prescribing sacubitril/valsartan.
Methods
Study population
All patients receiving therapy with sacubitril/valsartan in a single tertiary heart failure clinic (ZOL Genk, Belgium) between December 2016 and July 2017 were identified using the electronic health record. Patients receiving sacubitril/valsartan as part of an ongoing clinical were excluded. Only patients receiving sacubitril/valsartan according to the Belgian reimbursement criteria were included in this analysis, which consists of (i) symptomatic heart failure defined as New York Heart Association (NYHA) Class II–IV, (ii) left ventricular ejection fraction (LVEF) <35% measured by echocardiography, (iii) pre‐treatment with an individual optimal dose of angiotensin‐converting enzyme inhibitor (ACE‐I) or angiotensin receptor blocker (ARB). In addition, only cardiologists and not general practitioners are allowed to initiate sacubitril/valsartan. The electronic search was performed starting from December 2016, as this coincided with the timing sacubitril/valsartan became commercially available in Belgium.
Baseline characteristics and drugs dosing
The electronic health record was used to retrospectively collect all baseline data from the moment that sacubitril/valsartan was initiated. We retrospectively collected baseline demographics, physical features, aetiology of heart failure, presence of co‐morbidities, NYHA class at initiation of therapy, baseline laboratory, electrocardiogram features, transthoracic echocardiogram features, and baseline medical and heart failure therapy. To reduce heterogeneity between the different compounds of ACE‐I, ARB, and beta‐blockers, the baseline dose was expressed as per cent of target dose. For instance, if a patient takes the maximal guideline advised dose for a certain ACE‐I, this would equate to 100% of target dose. A full list of different medication with the maximal target dose can be found in Table S1 . Loop diuretic dosing were expressed as furosemide equivalents with 40 mg furosemide being equal to 1 mg bumetanide of 20 mg torsemide.5, 6 Patients were initiated on sacubitril/valsartan as outlined in the PARADIGM‐HF study. To assess the intrinsic risk for heart failure hospitalization and cardiovascular mortality, we calculated the EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) risk score.7 This score ranges between 0 and 12 and assesses the absolute baseline risk for the composite endpoint of heart failure hospitalization and cardiovascular mortality. A higher risk score indicates a higher baseline risk of adverse events. This score has been well validated and previously used to assess the baseline risk in the PARADIGM‐HF population.
Follow‐up and comparison with PARADIGM‐HF
After initiation of sacubitril/valsartan, the timing of patient follow‐up with the cardiologist was left at the discretion of the initiating cardiologist. However, patients were always seen by their general practitioner 2 weeks after initiation for ambulatory clinical control as well as check of renal function and potassium. General practitioners were instructed to uptitrate therapy according to guidelines. This had to be repeated until the maximum tolerated dose was achieved. The follow‐up consultation with the treating cardiologist was used to assess the impact of sacubitril/valsartan on the patients' clinical and biochemical status. Only follow‐up consultations >6 weeks after the initiation were used for follow‐up assessment. This was performed in order to have a substantial time frame of sacubitril/valsartan use. Furthermore, at censoring (15 September 2017), all patients without follow‐up were contacted by telephone to determine the actual dose (and degree of uptitration) of sacubitril/valsartan. Differences in baseline characteristics and follow‐up features in our clinical practice population vs. the population as included in PARADIGM‐HF were analysed. For this analysis, a distinction was made between patients that were actually enrolled in the PARADIGM‐HF study or were excluded during the run‐in phase of the trial.
Statistics
Continuous variables are expressed as mean ± standard deviation if normally distributed or median (inter‐quartile range) if not normally distributed. Normality was checked by the Shapiro–Wilk statistic. Categorical data were expressed as numbers and percentages and compared with Pearson's χ2 test or Fisher's exact, when appropriate. Continuous variables were compared with Student's t‐test, Mann–Whitney U‐test, and paired t‐test, when appropriate. Comparison of means and standard deviation from published literature was performed using the summary independent sample t‐test. For comparison of categorical variables with the literature, cross‐tabulation with Pearson's χ2 testing was performed. Statistical significance was always set at a two‐tailed probability level of <0.05. Statistics were performed using SPSS version 22 (IBM, Chicago, IL).
Results
Baseline characteristics: real world vs. PARADIGM‐HF
A total of 120 patients with heart failure with reduced ejection fraction (HFrEF) were identified according to the aforementioned criteria. An extensive overview of baseline characteristics is illustrated in Table 1. Table 2 gives a comparison of baseline characteristics of the clinical practice patients vs. the patients receiving therapy with sacubitril/valsartan in the PARADIGM‐HF study. This indicates that patients in clinical practice were older, had a higher baseline creatinine and a lower baseline LVEF, and were more often treated with a mineralocorticoid receptor antagonist, cardiac resynchronization therapy (CRT), and implantable cardioverter defibrillators (ICDs). In addition, a comparison was made between the baseline characteristics of patients in clinical practice and the patients in the PARADIGM‐HF trial that were excluded during the run‐in phase or eventually randomized. These results are reflected in Table 3. Although it is well known that patients dropping out during the run‐in phase exhibited characteristics indicative of more disease severity, patients in clinical practice exhibit features of more pronounced disease severity in comparison with both patients being enrolled and patients experiencing dropout. We noticed a higher prevalence of atrial fibrillation, lower systolic blood pressure, higher baseline serum creatinine, lower baseline LVEF, and higher use of CRT and ICDs. All patients had baseline data available to calculate the EMPHASIS‐HF risk score. The median value in our population was 6 (inter‐quartile range 3). This is in comparison with a median EMPHASIS‐HF risk score of 5 (inter‐quartile range 4) in PARADIGM‐HF. A visual description of this relationship between baseline risk predicted by the EMPHASIS‐HF risk score and relative risk reduction effect of sacubitril is depicted in Figure 1 . Patients in clinical practice were at higher baseline risk as illustrated by the higher median EMPHASIS‐HF risk score.
Table 1.
Baseline characteristics
| Variable | Total population (n = 120) |
|---|---|
| Demographics | |
| Age, years | 66.0 ± 10.5 |
| Male | 98 (81%) |
| Active smoker | 29 (24%) |
| Duration of heart failure, years | 2.7 (0.6–7.0) |
| Heart failure aetiology | |
| Ischaemic | 80 (66%) |
| Non‐ischaemic | 40 (33%) |
| Physical features | |
| Systolic blood pressure, mmHg | 120 ± 20 |
| Diastolic blood pressure, mmHg | 65 ± 11 |
| Weight, kg | 82.5 ± 14.0 |
| BMI, kg/m2 | 28.9 ± 9.0 |
| Heart rate, b.p.m. | 69 ± 15 |
| Peripheral oedema (>Grade 1) | 16 (13%) |
| Co‐morbidities | |
| Atrial fibrillation | 51 (42%) |
| Anaemia | 28 (23%) |
| COPD | 12 (10%) |
| Hypertension | 56 (46%) |
| Dyslipidaemia | 56 (46%) |
| Diabetes | 29 (24%) |
| History valve surgery | 37 (31%) |
| Laboratory analysis | |
| Sodium, mmol/L | 138.6 ± 3.4 |
| Potassium, mmol/L | 4.5 ± 0.5 |
| Haemoglobin, g/dL | 13.9 ± 1.6 |
| Serum creatinine, mg/dL | 1.28 ± 0.4 |
| NYHA class | |
| Class II | 76 (62.5%) |
| Class III | 43 (35.5%) |
| Class IV | 1 (0.8%) |
| Electrocardiogram feature | |
| QRS duration, ms | 129 ± 33 |
| PR duration, ms | 172 ± 44 |
| Echocardiography | |
| LVEF, % | 26 ± 6 |
| LVESV, mL | 161 ± 53 |
| LVEDV, mL | 218 ± 69 |
| Guideline directed heart failure therapy | |
| ACE‐I | 98 (82%) |
| ARB | 22 (18%) |
| Beta‐blocker | 115 (95%) |
| Aldosterone antagonist | 99 (82%) |
| Loop diuretic | 72 (60%) |
| CRT | 52 (43%) |
| ICD | 67 (55%) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association.
Table 2.
Comparison of baseline characteristics with PARADIGM‐HF sacubitril/valsartan arm
| Variable | Real world (n = 120) | Sacubitril/valsartan arm (n = 4187) | P‐value |
|---|---|---|---|
| Demographics | |||
| Age, years | 66.0 ± 10.5 | 63.8 ± 11.5 | 0.038 |
| Male | 98 (81%) | 3308 (79%) | 0.480 |
| Heart failure aetiology | 0.159 | ||
| Ischaemic | 80 (66.6%) | 2506 (59.9%) | |
| Non‐ischaemic | 40 (33.3%) | 1681 (40.1%) | |
| Physical features | |||
| Systolic blood pressure, mmHg | 120 ± 20 | 122 ± 15 | 0.154 |
| BMI, kg/m2 | 28.9 ± 9.0 | 28.1 ± 5.5 | 0.125 |
| Heart rate, b.p.m. | 69 ± 15 | 72 ± 12 | 0.007 |
| Co‐morbidities | |||
| Atrial fibrillation | 51 (42%) | 1517 (36%) | 0.159 |
| Hypertension | 56 (46%) | 2969 (71%) | <0.001 |
| Diabetes | 29 (24%) | 1451 (35%) | 0.017 |
| Laboratory analysis | |||
| Serum creatinine, mg/dL | 1.28 ± 0.4 | 1.13 ± 0.3 | <0.001 |
| NYHA class | 0.013 | ||
| Class I | 0 (0%) | 180 (4.3%) | |
| Class II | 76 (63.5%) | 2998 (71.6%) | |
| Class III | 43 (35.5%) | 969 (23.1%) | |
| Class IV | 1 (1%) | 33 (0.8%) | |
| Echocardiography | |||
| LVEF, % | 26 ± 6 | 29 ± 6 | <0.001 |
| Heart failure therapies | |||
| ACE‐I | 98 (82%) | 3266 (78%) | 0.480 |
| ARB | 22 (18%) | 929 (22%) | 0.316 |
| Beta‐blocker | 115 (95%) | 3899 (93%) | 0.520 |
| Aldosterone antagonist | 99 (82%) | 2271 (54%) | <0.001 |
| Loop diuretic | 72 (60%) | 3363 (80%) | <0.001 |
| CRT | 52 (43%) | 292 (7%) | <0.001 |
| ICD | 67 (55%) | 623 (15%) | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Table 3.
Real‐world vs. dropout vs. randomized patients
| Variable | Real world (n = 120) | Dropout during run‐in phase (n = 2079) | P‐value | Randomized in PARADIGM‐HF (n = 8442) | P‐value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 66.0 ± 10.5 | 64.8 ± 11.8 | 0.276 | 63.8 ± 11.4 | 0.025 |
| Male | 98 (81%) | 1580 (76%) | 6595 (78%) | ||
| Heart failure aetiology | 0.515 | 0.134 | |||
| Ischaemic | 80 (66.6%) | 1325 (63.7%) | 5058 (59.9%) | ||
| Non‐ischaemic | 40 (33.3%) | 754 (36.3%) | 3384 (40.1%) | ||
| Physical features | |||||
| Systolic BP, mmHg | 120 ± 20 | 125 ± 17 | 0.008 | 128 ± 15 | <0.001 |
| BMI, kg/m2 | 28.9 ± 9.0 | 27.5 ± 5.7 | 0.095 | 28.1 ± 5.5 | 0.118 |
| Co‐morbidities | |||||
| Atrial fibrillation | 51 (42%) | 697 (34%) | 0.044 | 3111 (37%) | 0.203 |
| Hypertension | 56 (46%) | 1371 (66%) | <0.001 | 5970 (71%) | <0.001 |
| Diabetes | 29 (24%) | 753 (36%) | 0.007 | 2916 (35%) | 0.018 |
| Laboratory analysis | |||||
| Serum creatinine, mg/dL | 1.28 ± 0.40 | 1.20 ± 0.35 | 0.034 | 1.11 ± 0.28 | <0.001 |
| NYHA class | 0.845 | 0.558 | |||
| Class I/II | 73 (62.5%) | 1274 (61.5%) | 5481 (65.0%) | ||
| Class III/IV | 44 (36.3%) | 798 (38.5%) | 2952 (35.0%) | ||
| Echocardiography | |||||
| LVEF, % | 26.0 ± 6 | 28.5 ± 6.5 | <0.001 | 29.5 ± 6.2 | <0.001 |
| Heart failure therapies | |||||
| ACE‐I | 98 (82%) | 1578 (76%) | 0.149 | 6560 (78%) | 0.300 |
| ARB | 22 (18%) | 499 (24%) | 0.156 | 1907 (23%) | 0.268 |
| CRT | 52 (43%) | 199 (10%) | <0.001 | 575 (7%) | <0.001 |
| ICD | 67 (55%) | 417 (20%) | <0.001 | 1246 (15%) | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Figure 1.
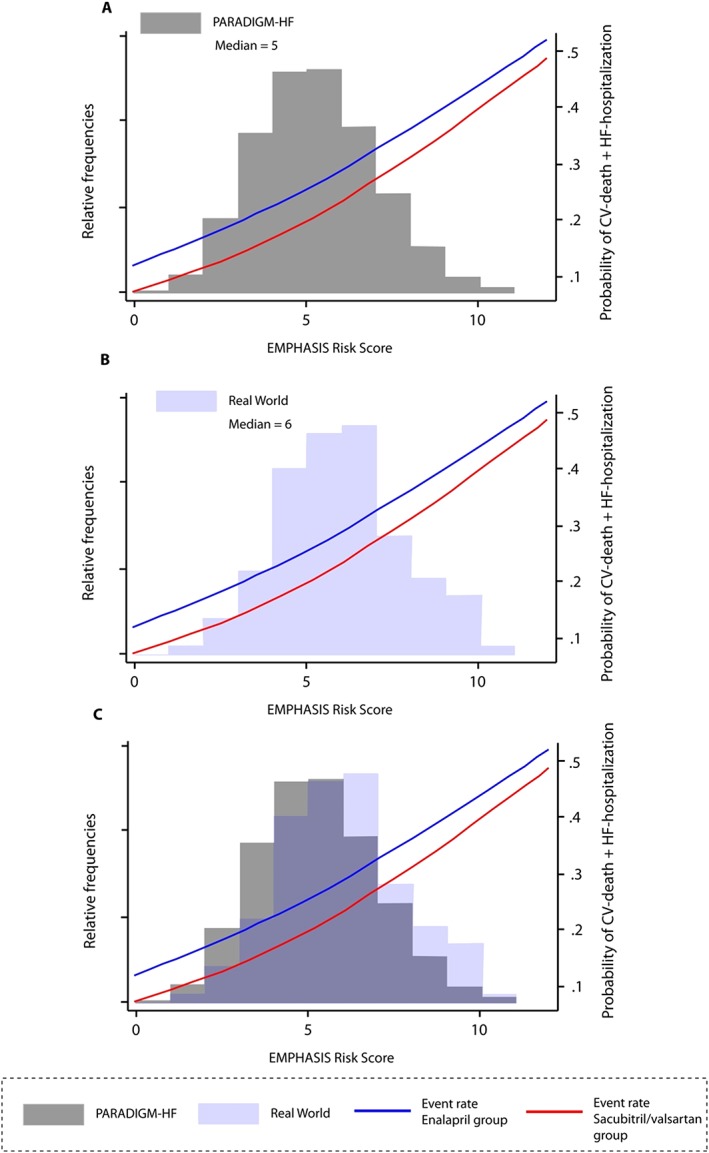
Distribution of EMPHASIS‐HF risk score in real‐world vs. PARADIGM‐HF patients. CV denotes cardiovascular; HF denotes heart failure. Gray and light blue boxes indicate relative frequencies of a certain EMPHASIS‐HF risk score in PARADIGM‐HF (A) vs. real world (B). (C) The overlap of risk‐score distribution. Adapted from Simpson et al. with permission.12
Clinical and biochemical status at follow‐up
During a mean follow‐up time of 90 ± 49 days, a total of 67 patients were seen during a cardiology follow‐up consultation. One patient died 8 weeks after the initiation of sacubitril/valsartan. In all the 119 remaining patients, vital status was checked using the electronic health care record. Figure 2 illustrates the longitudinal impact of sacubitril/valsartan on clinical and biochemical features. Only a significant drop in systolic blood pressure was noticed but no significant change in renal function or potassium levels. On average, patients in clinical practice experienced a drop of 7.1 ± 8.0 mmHg in systolic blood pressure. This drop in systolic blood pressure was significantly more pronounced in comparison with the 3.2 ± 0.4 mmHg drop seen in PARADIGM‐HF (P < 0.001). Figure 3 illustrates the impact on NYHA class before and after initiation of sacubitril/valsartan.
Figure 2.
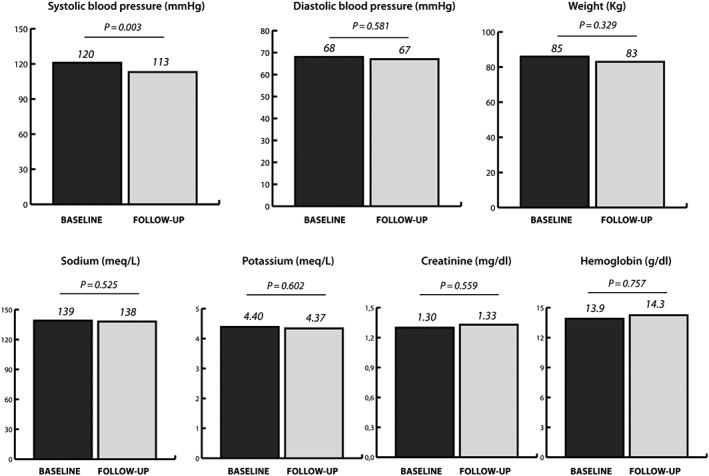
Longitudinal changes in clinical and biochemical features.
Figure 3.
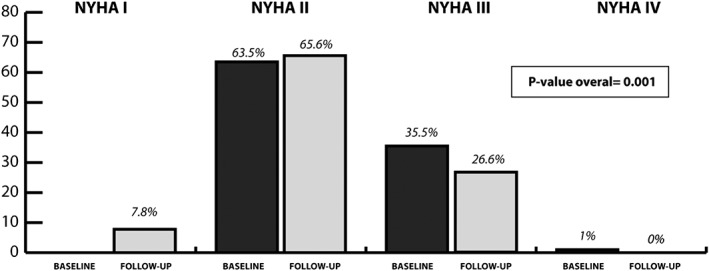
Longitudinal change in NYHA class. NYHA, New York Heart Association.
Dose titration in clinical practice
In all 120 patients, dose of heart failure medical therapy could be verified at the time of initiation of sacubitril/valsartan and is reflected in Table 4. Of the 120 patients starting sacubitril/valsartan, the starting dose was 24/26 mg b.i.d. in 61 patients (51%), 49/51 mg b.i.d. in 46 patients (38%), and 97/103 mg b.i.d. in 14 patients (11%). In 14 patients, the treating physician decided to initiate the 97/103 mg dose, and all 14 patients were treated with a maximal dose of ACE‐I/ARB, beta‐blocker, and mineralocorticoid receptor antagonist, with a systolic blood pressure >135 mmHg and glomerular filtration rate > 60 mL/min. In all but one patient, the dose of sacubitril/valsartan could be verified at the time of censoring (in 67 patients by classic clinical follow‐up and in 53 patients by telephone). Of them, 24 patients had dose uptitration, three patients had a dose down‐titration, and 92 had no change in the dose of sacubitril/valsartan. Reasons for absence of uptitration were only determined in the patients with clinical follow‐up (n = 67), as telephone follow‐up might be less reliable. Of the patients with in‐hospital follow‐up (n = 67), uptitration was performed in 14 (21%) and down‐titration in one (1.5%), and the dose remained unchanged in 53 (77.5%). Of the patients who did not undergo dose uptitration, 14 patients were already treated with the maximal dose at baseline, 20 patients had a specific reason mentioned why further uptitration was not possible, and in 18 patients, dose uptitration was not yet performed for unknown reasons and this was at a mean follow‐up period of 90 ± 49 days. Reasons for no further uptitration of sacubitril/valsartan was symptomatic hypotension (n = 10, 50%), worsening renal function (n = 6, 30%), an increase in potassium >5.5 mmol/L (n = 1, 5%), itching (n = 1, 5%), diarrhoea (n = 1, 5%), and blurred vision (n = 1, 5%). The mean achieved total daily dose of sacubitril/valsartan was 207 ± 117 mg in the entire cohort, which was significantly lower as reported in the PARADIGM‐HF trial 375 ± 75 mg (P < 0.001). However, when calculating the valsartan dose in sacubitril/valsartan as per cent of target dose and comparing this with pre‐initiation ACE‐I/ARB dose, there was no difference in the dose of RAS blocker prescribed (per cent target dose ACE‐I/ARB before 57 ± 29% vs. 53 ± 29% on sacubitril/valsartan; P = 0.286).
Table 4.
Dosing of heart failure therapy before initiation of sacubitril/valsartan
| Variable | Total population (n = 120) |
|---|---|
| ACE‐I, % of target dose | 59 ± 29 |
| ARB, % of target dose | 49 ± 29 |
| Beta‐blocker, % of target dose | 54 ± 25 |
| Spironolactone, mg | 25 (25–25) |
| Loop diuretic, mg furosemide equivalents | 40 (20–40) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Discussion
To the best of our knowledge, this article is the first to describe the use of sacubitril/valsartan in clinical practice. The main findings of this analysis are that patients in clinical practice exhibit baseline characteristics of more pronounced disease severity in comparison with patients being randomized or experiencing dropout in PARADIGM‐HF. This might have resulted in a more pronounced drop in systolic blood pressure and lower drug dose usage in comparison with those in the PARADIGM‐HF trial. Nevertheless, protocol‐driven uptitration assured dosing with at least an equipotential dose of RAS blocker. Finally, the high intrinsic risk of adverse events in real‐world patients suggests a large benefit of sacubitril/valsartan in absolute terms.
The use of sacubitril/valsartan has convincingly proven its benefit in reducing heart failure hospitalization and cardiovascular mortality in selected patients with HFrEF.1, 8, 9 Despite the firm evidence of benefit, several factors could interfere with drug prescription in clinical practice. First, the underappreciating of the intrinsic risk for heart failure‐related morbidity and mortality in ostensibly stable HFrEF patients might result in low rates of drug prescriptions.8, 10 Second, differences in the patient phenotype encountered in clinical practice might further lead to hesitations of initiating or uptitrating sacubitril/valsartan. Third, practical difficulties with regard to reimbursement requests and immediate increased cost of the drug in comparison with ACE‐I/ARB might also contribute towards hesitation. Our analysis reports on the initial 6 month experience in a tertiary referral centre with the use of sacubitril/valsartan, which might be insightful for the general cardiologist to prescribe sacubitril/valsartan in Class I recommended indications.
Although the PARADIGM‐HF trial is lauded for its impressive ability to compare evidence‐based doses of enalapril with sacubitril/valsartan, the way of achieving such doses might induce misconceptions in clinical practice. First, in order to be eligible for screening in PARADIGM‐HF, patients had to be on a stable dose of ACE‐I or ARB of at least 50% of the target dose (≥10 mg enalapril equivalents). Afterwards, patients entered a sequential single‐blind run‐in period to both assure tolerability of the study medication and achieve a guideline‐recommended dose of enalapril or the maximal dose of sacubitril/valsartan. Of the 10 521 patients initially screened, a total of 2079 were not able to complete the run‐in phase.11 In roughly two‐thirds of the patients, this was due to adverse events or an abnormal laboratory result (e.g. estimated glomerular filtration rate decrease of >25% during uptitration of study medication). Baseline characteristics of the patients that we encountered in clinical indicated higher disease severity in comparison with those of patients being randomized, and also compared with those of patients experiencing dropout in the run‐in phase.11 Therefore, our data indicate that in real‐world patients, it might be more challenging to achieve high doses of sacubitril/valsartan. For instance, the drop in systolic blood pressure was more pronounced in our patient population in comparison with that seen in PARADIGM‐HF.1, 3 Nevertheless, it is of upmost importance to emphasize that this does not mean that these patients benefit less from a switch of their ACE‐I or ARB to sacubitril/valsartan. Using an elegant inverse probability weighting analysis, Desai et al. attributed more weight to the patients enrolled in PARADIGM‐HF with baseline characteristics similar to the ones experiencing dropout in the run‐in phase.11 When subsequently calculating the Cox proportional hazards ratio on the primary endpoint, this inverse probability weighting did not influence the magnitude of benefit gained from therapy with sacubitril/valsartan in patients with characteristics favouring run‐in dropout.
The median EMPHASIS‐HF risk score of 6 in our population exceeds the median score of 5 seen in PARADIGM‐HF. This score assesses the intrinsic baseline risk for the composite endpoint of heart failure hospitalization and cardiovascular mortality.7 A previous analysis of the PARADIGM‐HF dataset indicates that throughout the entire EMPHASIS‐HF risk‐score spectrum (0 to 12), therapy with sacubitril/valsartan exhibits a similar relative risk‐reducing effect (not favouring one end of the risk‐score spectrum more or less).12 Therefore, although patients in clinical practice exhibit baseline characteristics of more pronounced frailty, the impact in absolute terms is not reduced.10
The ultimate dose achieved in our cohort was significantly lower than that achieved in PARADIGM‐HF. However, this should not come as a surprise, as the screening faze, run‐in faze, and the trial itself resulted in selection of tolerant patients. For instance, the reported dose of 375 ± 75 mg sacubitril/valsartan leaves out the 19.8% of patients that discontinued the study drug during the trial and the 2079 patients excluded during the run‐in faze.1, 11 It is unclear if these patients could potentially undergo even more uptitration; however, we employed a clinical care pathway together with the general practitioner to assure maximal drug uptitration. At least some of the patients were already on a maximal dose or had specific adverse events hampering further uptitration. Importantly, even a lower dose of sacubitril/valsartan compared with that in PARADIGM is reflected at reducing heart failure‐related mortality and morbidity in comparison with a similar dose of ACE‐I/ARB.13 It is therefore reassuring to see that the amount of RAS inhibition before and after the initiation of sacubitril/valsartan was not different in our population (P = 0.286). This indicates that the patients that were switched to sacubitril/valsartan received an equipotential dose of RAS blocker as previously. Not only do sicker real‐world patients tolerate this switch to an equipotential dose of sacubitril/valsartan well, it is also well tolerated illustrated with the absence of any drug discontinuations and absence of biochemical complications (hyperkalaemia or worsening of renal function).
Limitations
Several limitations should be addressed when interpreting the results. First, this is a single‐centre experience and therefore might reflect local practice. The higher use of CRT and ICD might, for instance, be such a reflection of local practice. Nevertheless, patients were on at least as robust heart failure therapies as in PARADIGM‐HF, indicating selection of Class I indication patients. Second, all patients were aware of the treatment assigned to them; therefore, the longitudinal change in NYHA class needs to be interpreted with care, as they are liable to a placebo effect. Nevertheless, the results corroborate the findings of PARADIGM‐HF. Third, we only had clinical and biochemical follow‐up in 67 patients, and it is not possible to exclude that these patients are different than the ones without clinical follow‐up. However, for the analysis of dose uptitration, all remaining patients with missing clinical follow‐up were contacted to determine the actual dose of sacubitril/valsartan at the time of censoring.
Conclusions
Patients in clinical practice exhibit baseline characteristics associated with more severe disease, which might lead to prescription of lower doses and a more pronounced drop in blood pressure. Nevertheless, patients in clinical practice are at high absolute risk of adverse outcome as illustrated by the EMPHASIS‐HF risk score. Importantly, in the PARADIGM‐HF trial, patients at high baseline risk had an equal relative risk reduction with sacubitril/valsartan in comparison with patients with a lower baseline risk. Given the high baseline risk, the absolute risk reduction effect of therapy with sacubitril/valsartan therapy in clinical practice is expected to be high.
Conflict of interest
None declared.
Supporting information
Table S1. Conversion table RAS‐ and beta‐blockers.
Acknowledgements
P.M. is supported by a doctoral fellowship by the Research Foundation ‐ Flanders (FWO; grant number 1127917N). P.M., Petra Nijst, and W.M. are researchers for the Limburg Clinical Research Program (LCRP) UHasselt‐ZOL‐Jessa, supported by the foundation Limburg Sterk Merk (LSM), Hasselt University, Ziekenhuis Oost‐Limburg, and Jessa Hospital.
Martens, P. , Beliën, H. , Dupont, M. , and Mullens, W. (2018) Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Failure, 5: 275–283. doi: 10.1002/ehf2.12258.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. SOLVD Investigators , Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pellicori P, Urbinati A, Shah P, MacNamara A, Kazmi S, Dierckx R, Zhang J, Cleland JGF, Clark AL. What proportion of patients with chronic heart failure are eligible for sacubitril–valsartan? Eur J Heart Fail 2017; 19: 768–778. [DOI] [PubMed] [Google Scholar]
- 5. Martens P, Verbrugge FH, Boonen L, Nijst P, Dupont M, Mullens W. Value of routine investigations to predict loop diuretic down‐titration success in stable heart failure. Int J Cardiol 2018; 250: 171–175. [DOI] [PubMed] [Google Scholar]
- 6. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Changes in loop diuretic dose and outcome after cardiac resynchronization therapy in patients with heart failure and reduced left ventricular ejection fractions. Am J Cardiol 2017; 120: 267–273. [DOI] [PubMed] [Google Scholar]
- 7. Collier TJ, Pocock SJ, McMurray JJ, Zannad F, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS‐HF trial. Eur Heart J 2013; 34: 2823–2829. [DOI] [PubMed] [Google Scholar]
- 8. Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, Jhund P, Lefkowitz M, Shi V, Solomon SD. Estimating the long‐term treatment benefits of sacubitril–valsartan. N Engl J Med 2015; 373: 2289–2290. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez‐Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS, Jr. , Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 10. Packer M, Armstrong WM, Rothstein JM, Emmett M. Sacubitril–valsartan in heart failure: why are more physicians not prescribing it? Ann Intern Med 2016; 165: 735–736. [DOI] [PubMed] [Google Scholar]
- 11. Desai AS, Solomon S, Claggett B, McMurray JJ, Rouleau J, Swedberg K, Zile M, Lefkowitz M, Shi V, Packer M. Factors associated with noncompletion during the run‐in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM‐HF trial. Circ Heart Fail 2016; 9: pii: e002735. [DOI] [PubMed] [Google Scholar]
- 12. Simpson J, Jhund PS, Silva CJ, Martinez F, Mosterd A, Ramires F, Rizkala AR, Senni M, Squire I, Gong J, Lefkowitz MP, Shi VC, Desai AS, Rouleau JL, Swedberg K, Zile MR, McMurray JJ, Packer M, Solomon SD. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS‐HF risk scores: an analysis of mortality and morbidity in PARADIGM‐HF. J Am Coll Cardiol 2015; 66: 2059–2071. [DOI] [PubMed] [Google Scholar]
- 13. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2016; 18: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Conversion table RAS‐ and beta‐blockers.


