Abstract
Aims
Mineralocorticoid receptor antagonists (MRAs) have been demonstrated to improve outcomes in reduced ejection fraction heart failure (HFrEF) patients. However, MRAs added to conventional treatment may lead to worsening of renal function and hyperkalaemia. We investigated, in a population‐based analysis, the long‐term effects of MRA treatment in HFrEF patients.
Methods and results
We analysed data of 6046 patients included in the Metabolic Exercise Cardiac Kidney Index score dataset. Analysis was performed in patients treated (n = 3163) and not treated (n = 2883) with MRA. The study endpoint was a composite of cardiovascular death, urgent heart transplantation, or left ventricular assist device implantation. Ten years' survival was analysed through Kaplan–Meier, compared by log‐rank test and propensity score matching.
At 10 years' follow‐up, the MRA‐untreated group had a significantly lower number of events than the MRA‐treated group (P < 0.001). MRA‐treated patients had more severe heart failure (higher New York Heart Association class and lower left ventricular ejection fraction, kidney function, and peak VO2). At a propensity‐score‐matching analysis performed on 1587 patients, MRA‐treated and MRA‐untreated patients showed similar study endpoint values.
Conclusions
In conclusion, MRA treatment does not affect the composite of cardiovascular death, urgent heart transplantation or left ventricular assist device implantation in a real‐life setting. A meticulous patient follow‐up, as performed in trials, is likely needed to match the positive MRA‐related benefits observed in clinical trials.
Keywords: Mineralocorticoid receptor antagonists, Heart failure, Worsening renal function, Hyperkalaemia
Introduction
The sustained activation of the renin–angiotensin–aldosterone system is inappropriate and pathologic in patients with chronic heart failure (HF) with reduced ejection fraction (HFrEF).1 Treatment with mineralocorticoid receptor antagonists (MRAs) has been demonstrated to improve clinical outcomes in patients with HFrEF with mild to severe symptoms and also in patients with left ventricular dysfunction after myocardial infarction.2, 3, 4 The benefit of the addition of MRA to an HF treatment regimen has been demonstrated in a few multicentre, large‐sized, placebo‐controlled randomized well‐conducted studies and specifically in the Randomized Aldactone Evaluation Study (RALES) trial,2 in the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),3 and finally, in the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF).4
In patients with HFrEF, MRA added to conventional treatment may lead to worsening of renal function (WRF) and to serious and, occasionally, life‐threatening hyperkalaemia (HK).5, 6, 7, 8 Data from the EPHESUS trial showed that early WRF with eplerenone was associated with an increased risk of adverse cardiovascular outcomes, yet the benefit of eplerenone was maintained.9 However, the prognostic significance of WRF and HK in real‐life patients with moderate to severe HF receiving MRA remains unknown, albeit some reports showed an increase in hospitalization rate due to WRF and/or HK.10
The aim of the present study was to investigate, in a population‐based analysis, the long‐term effects of MRA treatment in patients affected by HFrEF. To do so, we analysed data of the Metabolic Exercise Cardiac Kidney Index (MECKI) score research group, which consists of a sizable population of HFrEF patients.
Methods
Population and study procedures
We collected and analysed data of a cohort of 6112 patients with a history of HFrEF, enrolled and prospectively followed in 23 Italian HF centres participating in the MECKI score research group. Part of the study population (n = 2715) was derived from the MECKI score validation study11 but with an updated follow‐up. The remaining patients were derived from a recruitment extension of the MECKI score database (n = 3397). From the total population of 6112 patients, we selected those with complete treatment information in terms of MRA (n = 6110) and excluded patients with a contraindication to MRA treatment due to estimated glomerular filtration rate < 30 mL/min/1.73 m2 (n = 64),12 with a final population of 6046 subjects (Figure 1). In brief, criteria at enrolment were previous or present HF symptoms (functional New York Heart Association, NYHA, Classes I–IV, Stages B and C of American College of Cardiology/American Heart Association classification) and former documentation of a reduced left ventricular ejection fraction (LVEF <40%), unchanged HF medications for at least 3 months, ability to perform a cardiopulmonary exercise test (CPET), and no major cardiovascular treatment or intervention scheduled. As exclusion criteria, we considered history of pulmonary embolism, moderate to severe aortic and mitral stenosis, pericardial disease, severe obstructive lung disease, exercise‐induced angina and significant electrocardiography (ECG) alterations, or presence of any clinical co‐morbidity interfering with exercise performance. At enrolment, clinical history and therapy information were recorded, and then physical examination, laboratory analyses, ECG, transthoracic echocardiography, and CPET were performed, as previously described.11
Figure 1.
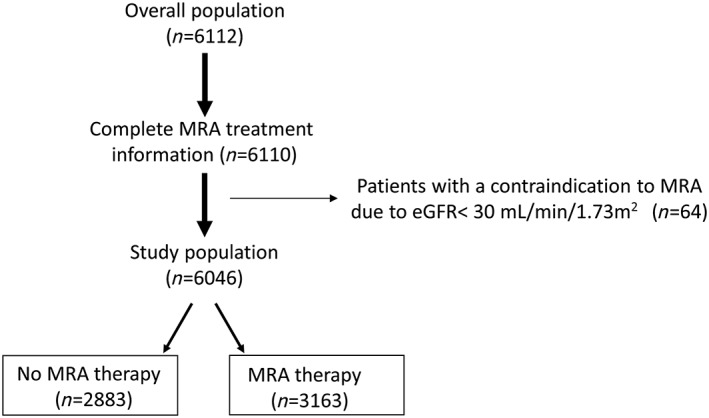
Study population and data analysis. From the whole population of 6112 patients, we selected those with complete treatment information as regards presence or not of mineralocorticoid receptor antagonist (MRA) treatment (n = 6110). Afterwards, we excluded patients with contraindication to MRA due to estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. The final analysed population was composed of 6046 subjects, including 2883 MRA‐untreated and 3163 MRA‐treated patients.
To assess the prognostic role of MRA in HF, data analysis was performed in the two groups, patients treated and untreated with MRA. The study endpoint was the composite of cardiovascular death, urgent heart transplantation defined as United Network for Organ Sharing Status 1,13 or left ventricular assist device (LVAD) implantation.
Follow‐up and data management
Patients' follow‐up and procedures of data management were performed as previously reported.11 In brief, follow‐up was carried out according to the local HF programme and ended with the last clinical evaluation or with patients' death, urgent heart transplantation, or LVAD implantation.11 If a patient died outside the hospital where he or she was followed up, medical records of the event and reported cause of death were considered.
Statistical analysis
Continuous variables are presented as mean ± standard deviation and were compared between groups using the t‐test for independent samples. Categorical variables are reported as frequency and percentage, and they were compared using the χ2 test. The association between use of MRA and endpoint (composite of cardiovascular death, urgent cardiac transplant, or LVAD implantation) was assessed by Kaplan–Meier analysis and groups compared by log‐rank test.
To reduce the treatment selection bias due to confounding variables, a propensity‐score‐matching analysis was performed. The following variables were considered: age, gender, MECKI score, year of enrolling and centre where enrolment was done, NYHA functional class, LVEF, oxygen uptake at peak exercise (peak VO2 expressed as millilitres per minute per kilogram), systolic blood pressure, serum creatinine (log), haemoglobin, serum sodium (Na+), presence of implantable cardioverter defibrillator, and drug treatments with beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, diuretic, statins, anticoagulant treatment, digitalis, and amiodarone. Each MRA user was matched to a non‐MRA user by applying a nearest‐neighbour matching analysis with 0.01 calliper. In order to validate the results obtained with the propensity score matching, we run different analyses according to the strategy proposed by Martens et al.14: (1) Cox regression on the original dataset, adjusting for all the covariates employed in the propensity score matching; (2) Cox regression on the original dataset, using propensity score as a covariate; and (3) Cox regression on the original dataset, stratified for propensity score. The hazard ratios (HRs) obtained with the different approaches were then compared with the HRs of an unadjusted Cox regression run on propensity‐score‐matched data.
In order to correct for slight imbalances in propensity score, Cox regression analysis, adjusted for propensity score, was employed. Several subgroup analyses were conducted by Cox regression on propensity‐score‐matched data. A P value <0.05 was considered statistically significant. All data were collected in an Excel database, and analyses were performed using SAS Version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient population
A total of 6046 HF patients (82% male, mean age 61.3 ± 12.7 years) fulfilled the study inclusion/exclusion criteria and were included in the present analysis. Mean LVEF of the entire population was 33 ± 10.5%; 72% were in NYHA Classes I and II and 28% in Classes III and IV; mean peak VO2 was 14.8 ± 4.8 mL/min/kg; HF aetiology was an ischaemic cardiomyopathy in 46%, an idiopathic dilated cardiomyopathy in 40%, a valvular cardiomyopathy in 5%, and other in 10%. A total of 3163 patients (52%) were on MRA while the remaining 2883 (48%) were not treated with MRA. MRA‐treated patients had more severe HF (Table 1). At study run‐in, 351 patients (185 MRA treated and 166 MRA untreated) had K+ > 5.0 meq/L.12
Table 1.
Characteristics of patients not treated and treated with MRA
| MRA− (n = 2883) | MRA+ (n = 3163) | P | |
|---|---|---|---|
| Age (years) | 61.3 ± 13.4 | 61.4 ± 12.2 | 0.6504 |
| BMI (kg/m2) | 26.7 ± 4.3 | 26.8 ± 4.4 | 0.3745 |
| LVEF (%) | 35.6 ± 10.9 | 31.0 ± 9.6 | <0.0001 |
| SBP (mmHg) | 120.4 ± 17.2 | 114.4 ± 17.2 | <0.0001 |
| HR at rest (b.p.m.) | 69.9 ± 12.0 | 71.6 ± 12.8 | <0.0001 |
| Peak VO2 (mL/min/kg) | 15.5 ± 5.2 | 14.2 ± 4.4 | <0.0001 |
| Peak VO2 (% of predicted) | 58.6 ± 17.8 | 53.7 ± 16.7 | <0.0001 |
| Peak HR (b.p.m.) | 121.6 ± 25.3 | 117.4 ± 24.2 | <0.0001 |
| VE/VCO2 slope | 31.8 ± 7.1 | 33.6 ± 8.2 | <0.0001 |
| MDRD (mL/min/1.73 m2) | 72.5 ± 24.1 | 70.5 ± 23.7 | 0.0011 |
| Hb (g/dL) | 13.5 ± 1.6 | 13.4 ± 1.6 | 0.0023 |
| Gender (n, %) | |||
| Female | 553 (19.2) | 555 (17.5) | 0.1008 |
| Male | 2330 (80.8) | 2608 (82.5) | |
| NYHA (n, %) | <0.0001 | ||
| I | 533 (18.5) | 381 (12.1) | |
| II | 1753 (60.8) | 1664 (52.6) | |
| III | 576 (20.0) | 1062 (33.6) | |
| IV | 21 (0.7) | 54 (1.7) | |
| Atrial fibrillation (n, %) | 396 (13.7) | 561 (17.8) | <0.0001 |
| ICD (n, %) | 667 (23.1) | 1216 (38.5) | <0.0001 |
| CRT (n, %) | 239 (8.4) | 503 (16.1) | <0.0001 |
| Aetiology (n, %) | 0.0005 | ||
| Idiopathic | 1109 (38.7) | 1265 (40.4) | |
| Ischaemic | 1285 (44.9) | 1476 (47.1) | |
| Valvular | 144 (5.0) | 126 (4.0) | |
| Other | 325 (11.4) | 268 (8.5) | |
| ACE inhibitors (n, %) | 2132 (73.9) | 2393 (75.7) | 0.1269 |
| ARBs (n, %) | 533 (18.5) | 585 (18.5) | 0.9941 |
| Diuretics (n, %) | 1989 (69.0) | 2857 (90.3) | <0.0001 |
| Statins (n, %) | 1197 (41.7) | 1504 (47.9) | <0.0001 |
| Allopurinol (n, %) | 603 (21.0) | 955 (30.4) | <0.0001 |
| Beta‐blockers (n, %) | 2419 (83.9) | 2833 (89.6) | <0.0001 |
| Antiplatelets (n, %) | 1543 (53.5) | 1708 (54) | 0.7092 |
| Oral anticoagulants (n, %) | 719 (24.9) | 1072 (33.9) | <0.0001 |
| Amiodarone (n, %) | 602 (20.9) | 878 (27.8) | <0.0001 |
| Digitalis (n, %) | 505 (17.5) | 710 (22.5) | <0.0001 |
| Potassium (meq/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.6483 |
| Sodium (mmol/L) | 139.7 ± 3.1 | 139 ± 3.4 | <0.0001 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blockers; BMI, body mass index; CRT, cardiac resynchronization therapy; Hb, haemoglobin; HR, heart rate; ICD, implantable cardioverter defibrillator; LVEF, ejection fraction; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; Peak VO2, oxygen uptake at peak exercise; SBP, systolic blood pressure; VE/VCO2 slope, minute ventilation/carbon dioxide production relationship.
Follow‐up
For the total population, the median follow‐up period was 3.7 years (interquartile range 1.7–6.4), while it was 3.5 (interquartile range 1.6–6.1) and 3.9 years (interquartile range 1.8–6.7) in patients receiving and not receiving MRA treatment, respectively. The events occurring at 10 year follow‐up were 1042: 46 events person/year * 1000 in MRA‐treated patients vs. 34 events person/year * 1000 (P‐value < 0.0001) in the MRA‐untreated patients. At 10 years' follow‐up, the MRA‐untreated group had significantly higher freedom from events than the MRA‐treated group (log‐rank test, P < 0.001) (Figure 2). The MRA‐untreated group had 370 cardiovascular deaths, 67 urgent heart transplants, and 1 LVAD implantation, while the MRA‐treated group had 506 cardiovascular deaths, 87 urgent heart transplants, and 11 LVAD implantations. In analogy with the RALES,2 we also analysed the study endpoint truncating the follow‐up at 2 years, but, as for the 10 years' follow‐up evaluation, MRA‐treated patients had more events than MRA‐untreated patients.
Figure 2.
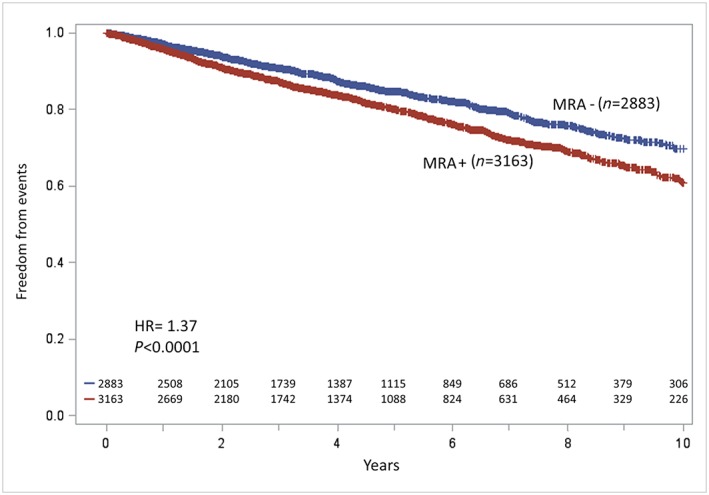
Kaplan–Meier analyses of study endpoint (cardiovascular death, urgent heart transplant, or left ventricular assist device implantation) of the mineralocorticoid receptor antagonist (MRA)‐treated (MRA+) and MRA‐untreated (MRA−) patients: at 10 years' follow‐up the MRA− group had significantly higher survival than the MRA+ group (P < 0.001). HR, hazard ratio.
Propensity score analysis
Propensity score matching allowed us to identify 1587 patients in both study groups, with a follow‐up of 3.8 (1.8–6.5) and 3.7 (1.8–6.4) years for patients not receiving MRA and those receiving MRA, respectively (Table 2). At 10 years' follow‐up, no differences as regards study endpoints were observed between the two groups (Figure 3). Similarly, no difference between groups was observed, truncating the follow‐up at 2 years. The validity of the results of the propensity score matching was tested by applying different analytical approaches (Table 3). In all analyses, the HRs for the combined endpoint comparing MRA+ vs. MRA− subjects were remarkably stable, ranging from 1.13 to 1.15.
Table 2.
Characteristics of patients not treated and treated with MRA in propensity‐score‐matching analysis
| MRA− (n = 1587) | MRA+ (n = 1587) | P | |
|---|---|---|---|
| Age (years) | 62.6 ± 13 | 62.6 ± 12.4 | 0.92 |
| BMI (kg/m2) | 26.9 ± 4.3 | 27.1 ± 4.5 | 0.36 |
| LVEF (%) | 34.2 ± 9.9 | 34.3 ± 9.8 | 0.70 |
| SBP (mmHg) | 118.1 ± 16.7 | 117.6 ± 17.1 | 0.40 |
| HR at rest (b.p.m.) | 70.3 ± 12.4 | 70.9 ± 12.7 | 0.13 |
| Peak VO2 (mL/min/kg) | 14.8 ± 4.7 | 14.8 ± 4.6 | 0.95 |
| Peak VO2 (% of predicted) | 57.1 ± 17.2 | 57 ± 16.9 | 0.92 |
| Peak HR (b.p.m.) | 118.7 ± 25.8 | 118 ± 24.4 | 0.46 |
| VE/VCO2 slope | 32.5 ± 7.3 | 32.6 ± 7.3 | 0.75 |
| MDRD (mL/min/1.73 m2) | 72.2 ± 24.7 | 71.2 ± 23.6 | 0.25 |
| Hb (g/dL) | 13.4 ± 1.6 | 13.5 ± 1.6 | 0.51 |
| Gender (n, %) | 0.32 | ||
| Female | 319 (20.1%) | 297 (18.71%) | |
| Male | 1268 (79.9%) | 1290 (81.29%) | |
| NYHA (n, %) | 0.78 | ||
| I | 231 (14.56%) | 230 (14.49%) | |
| II | 957 (60.3%) | 944 (59.48%) | |
| III | 383 (24.13%) | 401 (25.27%) | |
| IV | 16 (1.01%) | 12 (0.76%) | |
| Atrial fibrillation (n, %) | 244 (15.37%) | 254 (16.06%) | 0.60 |
| ICD (n, %) | 498 (31.38%) | 512 (32.26%) | 0.59 |
| CRT (n, %) | 188 (11.97%) | 202 (12.86%) | 0.45 |
| Aetiology (n, %) | |||
| Idiopathic | 572 (36.13%) | 603 (38.09%) | |
| Ischaemic | 759 (47.95%) | 747 (47.19%) | 0.64 |
| Valvular | 83 (5.24%) | 75 (4.74%) | |
| Other | 169 (10.68%) | 158 (9.98%) | |
| ACE inhibitors (n, %) | 1175 (74.04%) | 1179 (74.29%) | 0.87 |
| ARBs (n, %) | 312 (19.66%) | 303 (19.09%) | 0.69 |
| Diuretics (n, %) | 1352 (85.19%) | 1349 (85%) | 0.88 |
| Statins (n, %) | 820 (51.67%) | 822 (51.8%) | 0.94 |
| Allopurinol (n, %) | 450 (28.36%) | 482 (30.37%) | 0.21 |
| Beta‐blockers (n, %) | 1416 (89.22%) | 1417 (89.29%) | 0.95 |
| Antiplatelets (n, %) | 907 (57.15%) | 931 (58.66%) | 0.39 |
| Oral anticoagulants (n, %) | 425 (26.78%) | 431 (27.16%) | 0.81 |
| Digitalis (n, %) | 227 (14.3%) | 220 (13.86%) | 0.72 |
| Amiodarone (n, %) | 360 (22.68%) | 401 (25.27%) | 0.09 |
| Potassium (meq/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.64 |
| Sodium (mmol/L) | 139.5 ± 3 | 139.5 ± 3.1 | 0.99 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blockers; BMI, body mass index; CRT, cardiac resynchronization therapy; Hb, haemoglobin; HR, heart rate; ICD, implantable cardioverter defibrillator; LVEF, ejection fraction; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; Peak VO2, oxygen uptake at peak exercise; SBP, systolic blood pressure; VE/VCO2 slope, minute ventilation/carbon dioxide production relationship.
Figure 3.
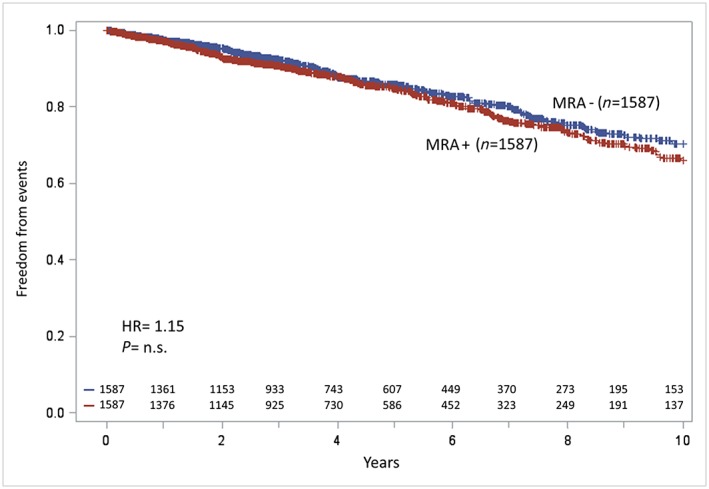
Kaplan–Meier analyses of study endpoint (cardiovascular death, urgent heart transplant, or left ventricular assist device implantation)of the mineralocorticoid receptor antagonist (MRA)‐treated (MRA+) and MRA‐untreated (MRA−) patients after propensity‐score‐matching analysis: at 10 years' follow‐up, survival differences between MRA+ and MRA− patients reduced and statistical significance was lost (P = 0.12). HR, hazard ratio.
Table 3.
Hazard ratios for mineralocorticoid receptor antagonists obtained with different analytical approaches
| Analysis | Hazard ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Multivariable Cox regression analysis (original dataset) | 1.15 | 0.98 | 1.34 | 0.09 |
| Cox regression using PS as a covariate (original dataset) | 1.13 | 0.97 | 1.32 | 0.13 |
| Cox regression stratified for PS (original dataset) | 1.14 | 0.97 | 1.34 | 0.12 |
| Unadjusted Cox regression (on PS matched data) | 1.15 | 0.96 | 1.38 | 0.12 |
PS, propensity score.
Further analysis
All the previous analyses have been performed also excluding subjects with K+ > 5.0 meq/L but without any significant difference. Furthermore, supplementary analysis were performed to evaluate MRA treatment effects in different subgroups of HFrEF patients and specifically defined according to gender, LVEF, NYHA class, HF aetiology, peak VO2, serum K+, drug treatment, kidney function and systolic blood pressure. MRA‐treated patients never showed a better outcome, as analysed through the study endpoint, compared with MRA‐untreated patients.
Discussion
The present study provides important insights into the long‐term effects of MRA treatment in real‐life HFrEF patients. When compared with MRA‐untreated patients, MRA‐treated patients have a more severe HF, likely because of previous guidelines indications and suggestions, which were guided by RALES entry criteria and results. Indeed, the MECKI score dataset includes patients enrolled during several years starting from 1993. Notably, after performing propensity score match so as to overwhelm HF severity differences, we found that at 10 years' follow‐up MRA use does not emerge as an independent predictor of long‐term better or worse survival assessed as the composite of cardiovascular death, urgent heart transplantation or LVAD implantation.
These results were unexpected because the RALES,2 EPHESUS,3 and EMPHASIS‐HF4 trials all had demonstrated relevant benefits of the addition of MRA to an HF treatment regimen.
In the RALES trial, the MRA spironolactone, added to recommended medical therapy, reduced the rates of death from any cause and hospitalization for cardiovascular reasons among patients with HFrEF (in NYHA Class III or IV).2 The EPHESUS trial showed that adding the selective MRA eplerenone to recommended therapy in patients with acute myocardial infarction complicated by left ventricular systolic dysfunction and HF decreased the rates of death from any cause and hospitalization for cardiovascular reasons.3 Finally, in the EMPHASIS‐HF, eplerenone reduced the primary endpoint of cardiovascular death or HF hospitalization and the secondary endpoint of all‐cause mortality in comparison with placebo when added to evidence‐based therapy among patients with HFrEF and mild symptoms.4 According to these clinical trials, the usefulness of MRA in HFrEF treatment is particularly relevant. In this regard, it should be underlined that the RALES and EPHESUS were both stopped ahead of time because an interim analysis determined the efficacy of MRA; consequently, the median follow‐up of the RALES, EPHESUS, and EMPHASIS‐HF was 24, 16, and 21 months, respectively.2, 3, 4 In contrast, we showed in a sizable population of HFrEF patients, followed for an average duration longer than that of research trials, that MRA did not improve the present study endpoint. Notably truncating the follow‐up at 2 years as in the RALES2or excluding patients with K+ > 5.0 meq/L, we showed no endpoint differences between the MRA‐treated and MRA‐untreated groups. Moreover, in none of the subgroup analysis MRA treatment was associated with a better outcome.
In the real world, HF patients may be prone to serious HK when taking the combination of MRA and angiotensin‐converting enzyme inhibitors in the presence of co‐morbidities like diarrhoea, pre‐renal failure, renovascular disease and diabetes mellitus.8 Indeed, an increase of in‐hospital death among HF patients was described also by Juurlink's group in a Canadian population study. In that population, the publication of RALES was associated with an increase in spironolactone use and in the rates of hospital admission for HK and subsequent in‐hospital death among HF patients.10 Similarly, another population study showed that spironolactone use was not associated with improved long‐term survival in the general HF population.15 However, the above‐reported observations do not mean that conclusions gained by the RALES trial are wrong; it is likely, indeed, that physicians may have prescribed inappropriately high doses of spironolactone without monitoring potassium levels closely or that they may have extended the RALES findings to patients who were not affected by left ventricular systolic dysfunction or with other co‐morbidities, which might have led to a reduced efficacy of the drug or even to worsening of patients' clinical condition.
It needs to be underlined that the MECKI score database is continuously updated and new patients are added. However, the dataset used for the present analysis included patients enrolled from January 1993 to December 2015.Accordingly, for a long time, MRA treatment was reserved to NYHA Class III and Class IV patients. Indeed, it was only since 2011, with the publication of EMPHASIS‐HF,4 that the use of MRA was also admitted in HF patients in NYHA Class II. It might represent an ‘indication bias’; however, also patients more recently enrolled showed a similar HF severity distribution and prognostic effects. Indeed, albeit guidelines' changes, clinical habits have a slow modification rate. Notably, in the MECKI score, treatment choice and drug dosage were independently decided by local doctors. In clinical trials, eplerenone and spironolactone retained their clinical benefit, despite being associated with more frequent WRF and HK. In the EMPHASIS‐HF and in the EPHESUS, eplerenone induced a modest but statistically significant decline in estimated glomerular filtration rate and a rise in serum potassium.9, 16 WRF and HK were also described in patients treated with spironolactone in the RALES trial. In all clinical trials, WRF and HK were not associated with an increased risk for death.17
There are several possible reasons for our study's failure to detect the positive effect of MRA use on HFrEF survival observed in randomized trial. First of all, this is a retrospective study, and we recognize that it cannot be compared with the statistical power of the randomized controlled trials mentioned above. Then, HK is likely a more common occurrence in clinical practice than it is in the carefully controlled setting of RALES, EPHESUS, and EMPHASIS for several reasons. Indeed, in the real world, a less compelling patient follow‐up compared with what happens in research trial is frequently the case so that initial laboratory signs of body organ deterioration may not be appreciated. Specifically, outside the world of trials, physicians may not monitor potassium levels closely in patients receiving MRA, alone or in combination with other medications that contribute to HK.18 In patients with severe HF enrolled in RALES, the greatest change in potassium occurred within the first 4 weeks after initiation of spironolactone.19 Therapeutic optimization with MRA is often conducted during ambulatory visits or during hospitalization in referral HF hospitals far from patients' area. In these conditions, a direct close monitoring is very difficult. In addition, physician may neglect baseline attributes that predispose patients to HK (e.g. diabetes mellitus or renal diseases)20 and may overlook conditions that develop during therapy (e.g. renal dysfunction). Besides, some patients may purposefully increase their dietary potassium intake, as is often recommended during treatment with diuretics such as furosemide.
Our study has some important limitations. Firstly, patients were not randomized to MRA active treatment or placebo, but it is an analysis of a large dataset of HFrEF patients followed in several HF units. Consequently, the reasons beyond MRA treatment or non‐treatment are unknown. Secondly, the analysis is based on HFrEF patients able to perform a CPET, and this may result in selection of a population not closely representative of a general population, including subjects with worse HFrEF stages. Thirdly, it should be considered that this analysis was performed considering a static picture of the population at baseline without taking into account the possible changes in treatments during follow‐up, carrying a possible prognostic association. Indeed, during the long follow‐up, MRA treatment might have been added to treatment as recommended by guidelines.12 However, also when analysis was truncated at 2 years, MRA‐treated and MRA‐untreated patients showed similar results. Moreover, the dosage of MRA was not recorded, and we cannot analyse any dose‐related effect on prognosis, albeit a high MRA dose is rarely prescribed. It is also recognized that very few patients received eplerenone owing to the only recent commercial availability of this drug in Italy so that our results should not be extended to this specific MRA. Moreover, we have not evaluated rehospitalization rates, a study endpoint that was reported significantly reduced in a few MRA trials.21, 22 Lastly, the results of this study can only be applicable to HFrEF patients, as the role of MRA in HF patients with preserved systolic function has not been addressed.
In conclusion, this study shows that in the real world MRA treatment is not associated with HFrEF patients' survival improvement and suggests that a strict adherence to the inclusion criteria, dosing, and monitoring regimens used in RALES, EPHESUS, and EMPHASIS‐HF should be applied also in clinical practice to realize the positive benefit/risk ratio achieved by MRA in the clinical trials. Further prospective studies exploring long‐term mortality may be interesting and useful in the scope of therapy with MRA in HF.
Conflict of interest
None declared.
Funding
Not provided.
The other members of the MECKI score research group are as follows: Stefania Farina, Emanuele Spadafora from the Centro Cardiologico Monzino, IRCCS, Milano; Alessandro Ferraironi from the Cardiology University Department, Heart Failure Unit and Cardiopulmonary Laboratory Santo Spirito Hospital, Roma; Francesca Pietrucci from Cardiologia Riabilitativa, Azienda Ospedali Riuniti, Ancona; Gabriella Malfatto, Sergio Caravita, and Elena Viganò from Istituto Auxologico Italiano; Fabio Valente, Rossella Vastarella, Rita Gravino, Teo Roselli, and Andrea Buono from Cardiologia SUN, Ospedale Monaldi Napoli; Renata De Maria from CNR‐Milano; Andrea Passantino, Daniela Santoro, Saba Campanale, and Domenica Caputo from Istituti Clinici Scientifici Maugeri, Cassano delle Murge; Donatella Bertipaglia from Istituti Clinici Scientifici Maugeri, Tradate; Marco Confalonieri, Emanuela Berton, Elena Zambon, Marco Morosin from Ospedali Riuniti and University of Trieste; Armando Ferraretti from the Department of Cardiology, University of Foggia, Foggia; Chiara Minà from the Department for the Treatment and Study of Cardiothoracic Diseases and Cardiothoracic Transplantation IRCCS‐ISMETT, Palermo; Elisa Battaia from the Department of Cardiology, S. Chiara Hospital, Trento; Giovanni Marchese from the Cardiac Rehabilitation Unit, Istituti Clinici Scientifici Maugeri, Milan; Annamaria Iorio from Ospedale Papa Giovanni XXIII, Bergamo; and Luigi Pastormerlo from Fondazione Gabriele Monasterio, CNR‐Regione Toscana, Pisa.
Bruno, N. , Sinagra, G. , Paolillo, S. , Bonomi, A. , Corrà, U. , Piepoli, M. , Veglia, F. , Salvioni, E. , Lagioia, R. , Metra, M. , Limongelli, G. , Cattadori, G. , Scardovi, A. B. , Carubelli, V. , Scrutino, D. , Badagliacca, R. , Guazzi, M. , Raimondo, R. , Gentile, P. , Magrì, D. , Correale, M. , Parati, G. , Re, F. , Cicoira, M. , Frigerio, M. , Bussotti, M. , Vignati, C. , Oliva, F. , Mezzani, A. , Vergaro, G. , Di Lenarda, A. , Passino, C. , Sciomer, S. , Pacileo, G. , Ricci, R. , Contini, M. , Apostolo, A. , Palermo, P. , Mapelli, M. , Carriere, C. , Clemenza, F. , Binno, S. , Belardinelli, R. , Lombardi, C. , Perrone Filardi, P. , Emdin, M. , and Agostoni, P. (2018) Mineralocorticoid receptor antagonists for heart failure: a real‐life observational study. ESC Heart Failure, 5: 267–274. doi: 10.1002/ehf2.12244.
The study complies with the Declaration of Helsinki and was approved by the local ethics committee on human research (protocol number CE no. R116/14‐CCM127).
References
- 1. Weber KT. Aldosterone and spironolactone in heart failure. N Engl J Med 1999; 341: 753–755. [DOI] [PubMed] [Google Scholar]
- 2. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 4. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 5. Berry C, McMurray JJ. Serious adverse events experienced by patients with chronic heart failure taking spironolactone. Heart 2001; 85: E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life‐threatening hyperkalemia during combined therapy with angiotensin‐converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med 2001; 110: 438–441. [DOI] [PubMed] [Google Scholar]
- 7. Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. Hyperkalaemia and impaired renal function in patients taking spironolactone for congestive heart failure: retrospective study. BMJ 2003; 327: 1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarman PR, Kehely AM, Mather HM. Hyperkalaemia in diabetes: prevalence and associations. Postgrad Med J 1995; 71: 551–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, Lamiral Z, Dobre D, Pitt B, Zannad F. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation 2012; 125: 271–279. [DOI] [PubMed] [Google Scholar]
- 10. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551. [DOI] [PubMed] [Google Scholar]
- 11. Agostoni P, Corra U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magri D, Fiorentini C, Mezzani A, Salvioni E, Scrutinio D, Ricci R, Bettari L, Di Lenarda A, Pastormerlo LE, Pacileo G, Vaninetti R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Confalonieri M, Giannuzzi P, Passantino A, Cas LD, Piepoli MF, Passino C. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710–2718. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. U.S. Department of Health & Human Services . Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_06 (14 Nov 2017).
- 14. Martens EP, de Boer A, Pestman WR, Belitser SV, Stricker BH, Klungel OH. Comparing treatment effects after adjustment with multivariable Cox proportional hazards regression and propensity score methods. Pharmacoepidemiol Drug Saf 2008; 17: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Ouzounian M, Hassan A, Cox JL, Johnstone DE, Howlett J. The effect of spironolactone use on heart failure mortality: a population‐based study. J Card Fail 2007; 13: 165–169. [DOI] [PubMed] [Google Scholar]
- 16. Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Circ Heart Fail 2014; 7: 51–58. [DOI] [PubMed] [Google Scholar]
- 17. Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012; 60: 2082–2089. [DOI] [PubMed] [Google Scholar]
- 18. Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol 2003; 41: 211–214. [DOI] [PubMed] [Google Scholar]
- 19. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD. Incidence, predictors, and outcomes related to hypo‐ and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail 2014; 7: 573–579. [DOI] [PubMed] [Google Scholar]
- 20. Jarman PR, Mather HM. Diabetes may be independent risk factor for hyperkalaemia. BMJ 2003; 327: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maisel A, Xue Y, van Veldhuisen DJ, Voors AA, Jaarsma T, Pang PS, Butler J, Pitt B, Clopton P, de Boer RA. Effect of spironolactone on 30‐day death and heart failure rehospitalization (from the COACH study). Am J Cardiol 2014; 114: 737–742. [DOI] [PubMed] [Google Scholar]
- 22. Koifman E, Kopel E, Maor E, Fefer P, Matezky S, Tofler G, Hamdan A, Grossman E, Goldenberg I, Klempfner R. Mineralocorticoid receptor antagonist use in eligible patients following acute myocardial infarction: real world data from the Acute Coronary Syndrome Israeli Surveys: 2004–2010. Int J Cardiol 2013; 168: 3971–3976. [DOI] [PubMed] [Google Scholar]


