Abstract
Extracorporeal membrane oxygenation is used for acute respiratory distress syndrome, refractory cardiogenic shock, and out‐of‐hospital cardiac arrest with uncertain neurological status, and, until recently, it was the only minimally invasive option to achieve biventricular support. However, extracorporeal membrane oxygenation increases left ventricular afterload and requires systemic anticoagulation, which is a major contraindication in the context of thrombolytic therapy following an ischaemic stroke. Conversely, the Impella heart pumps by design unload the ventricle and require minimal anticoagulation. We report the first case of mechanical circulatory supported with Impella CP on the left and Impella RP on the right (Abiomed Inc., Danvers, MA) for acute biventricular failure due to suspected acute myocarditis in the context of thrombolytic therapy for ischaemic stroke.
Keywords: Cardiogenic shock, Unloading, Myocardial recovery
Case report
A 37‐year‐old man, with no past medical history, presented at the emergency department complaining of dyspnoea and tachycardia. Initial assessment showed de novo atrial fibrillation and a left ventricular ejection fraction of 10% on echocardiogram. Subsequently, the patient developed aphasia and right side upper and lower limbs paresis while receiving intravenous infusion of antiarrhythmic medications. A brain computed tomography (CT) scan confirmed the diagnosis of ischaemic stroke. Despite thrombolytic therapy with recombinant tissue plasminogen activator, neurological symptoms persisted. A second CT scan revealed a thrombus in the mid‐cerebral artery. The patient was then transferred to a tertiary hospital for endovascular cerebral thrombectomy. The patency of left mid‐cerebral artery was obtained; however, the (CT) scan was positive for ischaemic brain damage at the level of anterior left temporal lobe, insula, and the left lenticular nucleus. Thereafter, the patient developed acute heart failure refractory to inotropic therapy and was transferred to the intensive care unit. Within 10 h of admission, the patient had a total inotrope dose of 40 (calculated according to inotropic score)1 and was in overt cardiogenic shock (systolic blood pressure 90 mmHg, heart rate 140 b.p.m., and oliguria) complicated by pulmonary congestion (central venous pressure 19 mmHg) and bilateral pleural effusion. Following pleural drainage, the central venous pressure fell to 13 mmHg; however, the pulmonary artery pressure remained high at 39/29/33 mmHg. The patient remained in cardiogenic shock with severe reduction of right and left ventricular contractility on transthoracic echocardiogram. Evaluation for mechanical circulatory support (MCS) raised a major risk of severe bleeding, due to recent thrombolytic therapy. Therefore, we elected to proceed with simultaneous MCS in the catheterization laboratory with Impella CP to support the left ventricle and Impella RP the right ventricle (Figures 1 and 2 ). The patient underwent, in the same setting, coronary angiography and myocardial biopsy that was complicated by pericardial effusion and drainage. The Impella RP was implanted via 23Fr sheath in right femoral vein and achieved 3.6 L/min of flow at performance level 7 (performance level ranges from P0 to P9). Impella CP was inserted via 14Fr sheath in left femoral artery and achieved 3.4 L/min of flow activated at P8 (Figure 1 ). The anticoagulation therapy targeted an activated partial thromboplastin time of 50–55″ with bivalirudin. Intravascular haemolysis was observed [lactate dehydrogenase (LDH) max value 1240 U/L] initially when Impella pumps were at high rotational speed. Both ventricles were unloaded, and progressively, the ventricles regained contractility, filling pressure decreased to a normal range, and the rotational speed was reduced gradually. Weaning off mechanical haemodynamic support included stepwise reduction of support level to assess the recovery of each ventricle. Inotropes were tapered down, haemodynamic parameters improved, and end‐organ function recovered within 24 h of initiation of biventricular support. Furthermore, the patient recovered from right side hemiplegia within 48 h of the thrombolytic therapy, but the dysarthria persisted. Seven days post‐initiation of support, the right ventricle recovered, and Impella RP was then removed. Two days later, Impella CP was explanted as well. However, following removal of the arterial sheath, left leg ischaemia occurred and was treated surgically. On Hospital Day 14, the patient was discharged from the intensive care unit. Myocardial biopsy was not conclusive; nevertheless, a cardiac magnetic resonance imaging on the 20th day revealed areas of residual inflammation and necrosis compatible with acute myocarditis, involving also the right ventricle at the level of inferior wall. Later, the patient was discharged home on Day 22 with a right ventricular ejection fraction and left ventricular ejection fraction of 49% and 35%, respectively, and minimal residual neurologic deficits.
Figure 1.
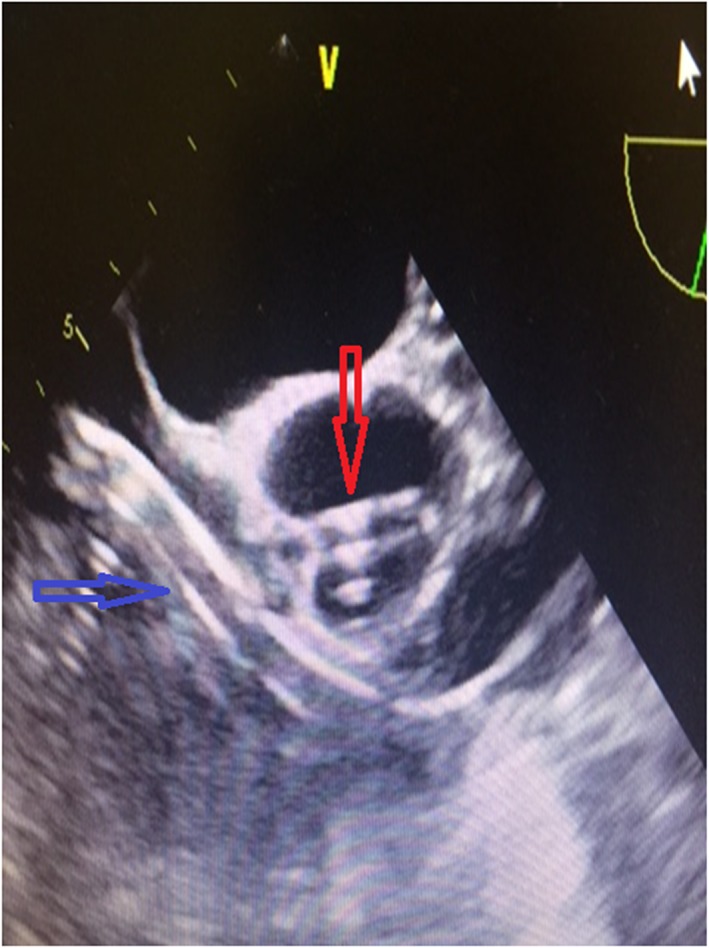
Echocardiographic view of the two pumps in place. Red arrow refers to left ventricular Impella pump. Blue arrow shows right ventricular Impella pump.
Figure 2.
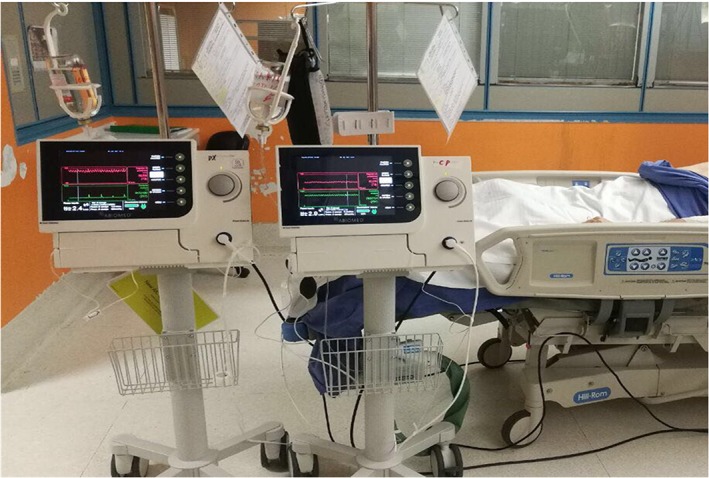
In situ Bi‐Pella: consoles for right and left ventricle.
Discussion
Full biventricular support is a major challenge in the current era of MCS. Venoarterial extracorporeal membrane oxygenation (VA ECMO) at present is increasingly used to support patients with cardiogenic shock refractory to standard medical therapy and especially in case of unknown neurological status. However, VA ECMO increases left ventricular after load, thus hindering the chances of myocardial recovery, especially in the ischaemic setting. VA ECMO requires also systemic anticoagulation because of blood–surface interactions; it is therefore contraindicated following ischaemic stroke thrombolytic therapy due to concrete risk of uncontrollable bleeding. Conversely, Impella pumps require a minimal degree of systemic anticoagulation enough to maintain an activated clotting time between 160 and 180 s during support. The pump does not trigger activation of coagulation system that is typical of ECMO with unbalanced phenomenon of clotting and consumption of coagulation factors ending up with bleeding. We took advantage of the positive profile of Impella systems about coagulation: do require anticoagulation but are not associated with coagulopathy. The Bi‐Pella approach mitigates both shortcomings of VA ECMO while providing percutaneous biventricular unloading and haemodynamic support. This approach fulfils the requirements of the acute MCS contemporary concept allowing for (i) ease of access, (ii) rapid deployment, (iii) non‐surgical percutaneous insertion, (iv) potent biventricular support, and (v) stepwise weaning of univentricular or biventricular support2 (Table 1). The Bi‐Pella approach was initiated mainly to address the systemic anticoagulation contraindication of ECMO in the present case of biventricular failure but also because simultaneous initiation of support with Impella CP and Impella RP has been associated with improved survival outcomes as compared with staged initiation of support.3 Acute biventricular failure in the context of cardiogenic shock is clinically catastrophic and remains accompanied with excessive mortality mainly because of unrecognized right ventricular failure and limited treatment options.4, 5 Indeed, in most of the cases, concomitant right ventricular failure, requiring MCS, is uncovered following initiation of left ventricular MCS or following initial assessment of a patient presenting with overt biventricular failure. This results in different haemodynamic pictures, needs of MCS, and patterns of recovery; from this aspect, manipulation of two different pumps might offer significant advantages. Of course, currently available Impella hardware and console require more active care and could be more complex to manage compared with other devices (Figure 2). Actually, Impella is technically a more difficult device compared with ECMO: managing low cardiac output syndrome is not only a matter of pump flow but also the efficacy of treatment is mainly a consequence of appropriate unloading. This strictly depends on rotor positioning and on adaptation of the heart to the percutaneous ventricular assist device. Impella physiology is indeed proximal to durable or surgical implanted ventricular assist devices. This case is an ideal scenario for dual percutaneous support with the Impella technology due to limited applicability of other approaches,6 for whom exist major contraindications, but it is a clear example of the potential of this strategy in the chance of myocardial recovery.
Table 1.
Technical features of Impella and ECMO systems
| Bi‐Pella | ECMO | |
|---|---|---|
| Implantation time |

|

|
| Bedside |

|

|
| Primary haemodynamic effect | RV | RV |
| Volume unloading |

|

|
| Pressure unloading |

|

|
| Primary haemodynamic effect | LV | LV |
| Volume unloading |

|

|
| Pressure unloading |

|

|
| Limb ischaemia |

|

|
| Lung support |

|

|
| Haemolysis |

|

|
| Anticoagulation |

|

|
| Bleeding diathesis |

|

|
| Transportability |

|

|
| Flexibililty |

|

|
| Post‐implantation management complexity |

|

|
ECMO, extracorporeal membrane oxygenation; LV, left ventricle; RV, right ventricle.
Conflict of interest
None declared.
Pappalardo, F. , Scandroglio, A. M. , and Latib, A. (2018) Full percutaneous biventricular support with two Impella pumps: the Bi‐Pella approach. ESC Heart Failure, 5: 368–371. doi: 10.1002/ehf2.12274.
References
- 1. Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castañeda AR, Newburger JW, Wessel DL. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low‐flow cardiopulmonary bypass and circulatory arrest. Circulation 1995; 92: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 2. Aghili N, Bader Y, Vest AR, Kiernam MS, Kimmelstiel C, DeNofrio D, Kapur NK. Biventricular circulatory Support using 2 axial flow catheters for cardiogenic shock without the need for surgical vascular access. Circ Cardiovasc Interv 2016; 9. [DOI] [PubMed] [Google Scholar]
- 3. Kapur NK, Bretón C, O'kelly RJ, Esposito ML, Rheude T, Mullin A, Annamalai SK, Grise M, Kiernan MP, Pham DT, Anderson M, Morris D. Simultaneous, not staged, deployment of biventricular micro‐axial flow Impella catheters (BiPella) is associated with improved survival for cardiogenic shock involving biventricular failure. J Am Coll Cardiol 2016; 68. [Google Scholar]
- 4. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG, Henriques JP. Percutaneous mechanical circulatory support versus intra‐aortic ballon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287. [DOI] [PubMed] [Google Scholar]
- 5. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott‐Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra‐aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008; 52: 1584–1588. [DOI] [PubMed] [Google Scholar]
- 6. Kapur NK, Jumean M, Ghuloom A, Aghili N, Vassallo C, Kiernan MS, DeNofrio D, Pham DT. First successful use of 2 axial flow catheters for percutaneous biventricular circulatory support as a bridge to a durable left ventricular assist device. Circ Heart Fail 2015; 8: 1006–1008. [DOI] [PubMed] [Google Scholar]


