Abstract
Background
In the era of effective antiretroviral treatment (ART) CD4:CD8 ratio is proposed as a potential marker for HIV-positive (HIV+) patients at increased risk for non-AIDS comorbidities. The current study aims to compare CD4:CD8 ratio between Asian and Caucasian HIV+ patients.
Methods
HIV+ patients from the Australian HIV Observational Database (AHOD) and the TREAT Asia HIV Observational Database (TAHOD) meeting specific criteria were included. In these analyses Asian and Caucasian status were defined by cohort. Factors associated with a low CD4:CD8 ratio (cut-off <0.2) prior to ART commencement, and with achieving a normal CD4:CD8 ratio (>1) at 12 and 24 months post ART commencement were assessed using logistic regression.
Results
There were 591 patients from AHOD and 2620 patients from TAHOD who met the inclusion criteria. TAHOD patients had a significantly (p<0.001) lower odds of having a baseline (prior to ART initiation) CD4:CD8 ratio greater than 0.2. After 12 months of ART, AHOD patients were more than twice as likely to achieve a normal CD4:CD8 ratio compared to TAHOD patients (15% vs 6%). However, after adjustment for confounding factors there was no significant difference between cohorts in the odds of achieving a CD4:CD8 ratio > 1 (p=0.475).
Discussion
We found a significantly lower CD4:CD8 ratio prior to commencing ART in TAHOD compared to AHOD even after adjusting for confounders. However, after adjustment, there was no significant difference between the cohorts in odds of achieving normal ratio. Baseline CD4+ and CD8+ counts seem to be the main driver for this difference between these two populations.
Keywords: Asians, Caucasians, race HIV-positive, CD4:CD8 ratio, cohorts
Introduction
HIV positive (HIV+) patients have significantly improved survival in the current era of effective antiretroviral therapy (ART). Yet mortality rates still remain elevated compared to HIV-negative people and HIV+ patients are increasingly experiencing many age related comorbidities. In addition to the natural ageing process, and lifestyle factors, HIV infection itself is commonly implicated due to the ongoing immune activation and inflammation [1].
CD4 lymphocyte (CD4+) count has been the single most important surrogate marker of mortality risk in HIV+ patients. The availability of effective ART means the majority of patients now achieve and maintain high CD4+ counts. For patients with CD4+ counts >500 cells/mm3 other surrogate markers of immune function, such as CD4:CD8 ratio, are being investigated for their capacity to differentiate patients who are at increased risk of non-infectious comorbidities.
In HIV-negative people an inverted CD4:CD8 ratio is an indicator of immune-senescence and an independent predictor of all-cause mortality [2, 3]. Prior to the availability of ART, HIV infection lead to a dramatic decrease in CD4+count along with increasing CD8+ counts resulting in an inverted CD4:CD8 ratio (<1). In the current ART era, although most patients begin ART with an inverted CD4:CD8 ratio, studies report that overtime ratios reach normal levels (ratio >1) for some patients. However, these studies also report that the rate of normalisation is quite slow and in some cases as many as 70% of patients have been reported to not having achieved a normal ratio after 5 years of ART[4, 5], suggesting ongoing immune dysregulation. Studies have also shown that an inverted CD4:CD8 ratio is independently associated with predicting morbidity and mortality over many years among HIV positive patients [5-7]. These studies also suggest a loss in the predictive value of CD4+ count.
It is known that Asian and Caucasian populations have different absolute CD4+ counts. Previous work comparing an Asian HIV+ cohort (TAHOD) with predominately Caucasian HIV+ cohorts (Aquitaine and AHOD) showed that for any given CD4 percentage [CD4% strata as indicator of disease stage] Asians had a lower CD4+ count pre-treatment [8], and have slightly lower CD4+ count responses after starting ART, even after adjusting for baseline CD4+ counts[9] compared to Caucasians. Further follow-up work from these investigators reported that these differences were not clinically significant [10]. It is not known whether this variability in CD4+ count between Caucasian and Asian populations is also evident when investigating the CD4:CD8 ratio. The aim of this study is to compare the CD4:CD8 ratio between Asian and Caucasian HIV+ patients, prior to initiation of antiretroviral therapy, and at 12 and 24 months post commencement of ART.
Methods
Study population
The Australian HIV Observational Database (AHOD) and the TREAT Asia HIV Observational Database (TAHOD) are observational cohort studies of HIV positive patients in Australia and Asia. AHOD and TAHOD have similar methodologies which have previously been explained in detail [11, 12]. Briefly AHOD and TAHOD commenced in 1999 and 2003 and include 30 clinical sites throughout Australia (AHOD) and 20 clinical sites throughout 12 countries in the Asia Pacific region (TAHOD). In 2014 AHOD was expanded to include two sites in New Zealand. Retrospective and prospective HIV related data are recorded for patients recruited to these cohorts. These data include basic demographics, antiretroviral treatment uptake as well as immunological (CD4+ and CD8+ count) and virological markers of HIV disease, AIDS diagnoses and coinfections with Hepatitis B (HBV) and Hepatitis C (HCV). Date and cause of death are also reported. These cohorts are also currently part of the International Epidemiology Databases to Evaluate AIDS initiative (IeDEA) global cohort consortium [13]. Ethics approval for both AHOD and TAHOD are obtained from the UNSW Australia Human Research Ethics Committee and from institutional review boards at all sites and the coordinating centre at TREAT Asia. All AHOD and TAHOD study procedures are in accordance with the 1975 Helsinki declaration.
Inclusion criteria
For this analysis, patients in AHOD and TAHOD were included if they met the following criteria: previously treatment naïve patients who commenced combination antiretroviral therapy (ART) on or after 1 January 2000 and remained on treatment for at least 14 days. All included patients had at least one CD4:CD8 ratio measurement within 12 months prior to commencing ART, and at least one CD4:CD8 ratio measurement at least 3 months (to ensure sufficient time for impact of ART) after commencing ART. Ethnicity is inconsistently available in AHOD, with approximately half of the cohort with known ethnicity, of whom more than 90% report Caucasian. TAHOD has ethnicity consistently reported with approximately 95% known to be Asian, and less than 1% reported as White/Caucasian. Consistent with previous comparisons of AHOD and TAHOD populations [10], in these analyses, it is assumed that AHOD largely represents a Caucasian population, whilst TAHOD an Asian population. All Asian/Caucasian comparisons were therefore be defined by cohort.
Statistical analyses
For all analyses, baseline is defined as ART commencement date, and the closest measurement prior to the ART start date from herein will be referred to as the baseline measure. The primary outcomes of interest are pre-ART CD4:CD8 ratio and normal CD4:CD8 ratio at 12 months after ART initiation in both AHOD and TAHOD. Baseline characteristics were summarised by cohort. Median and interquartile range (IQR) for CD4:CD8 ratio were calculated by averaging individual patient measures at each time point, then averaging over the respective cohorts at each time point, and then illustrated graphically by month over 5 years stratified by cohort. These were estimated on available data only and there was no imputation of missing data or observation carried forward. Data were censored at 5 years, last follow-up visit or death (whichever came first). In both cohorts lost to follow-up (ltf) and death rates are low (AHOD: ltf 3.9/100 person years (py); mortality rate: 1.04/100 py. TAHOD: ltf 2.7/100 py; mortality rate: 0.9/100 py). The proportion of patients with an inverted CD4:CD8 ratio at baseline who achieve a normalised ratio (> 1) after commencing ART for both AHOD and TAHOD were also calculated.
Factors associated with an inverted CD4:CD8 ratio before commencing ART and for achieving a normal ratio (>1) at 12 and 24 months post treatment commencement were assessed using univariate and multivariate logistic regression methods. Only patients with follow-up measures within a 12 and 24 month time window (3 months on either side) were included, and taking the value closest to 12 and 24 months were used. For both outcomes factors assessed in addition to cohort included age (at time of ART start), sex, HBV and HCV coinfection status (ever), mode of HIV exposure (men who have sex with men (MSM), heterosexual contract, injecting drug use, and other/missing), AIDS diagnoses (prior to ART start) and ART class. ART class, defined as three or more antiretrovirals with regimens categorised as follows: a) non-nucleoside reverse transcriptase inhibitor (NNRTI) and excluding both protease inhibitors (PI) and integrase inhibitors (InSTI), b) PI excluding NNRTI and InSTI, c) InSTI, and d) Other. In addition, for the outcome normalisation of CD4:CD8 ratio analysis, baseline CD4+ and CD8+ count (cells/mm3), and CD4:CD8 ratio as a continuous variable, were also included. HIV viral load was not included as a factor for assessment for any of the regressions as it is differentially reported in TAHOD compared with AHOD (>30% missing viral load at baseline). For all regression analyses, factors with p<0.05 in univariate analysis were assessed in multivariate analysis. Multivariate models were built using backward stepwise regression methods and p value of <0.05 was used to determine factors independently associated with CD4:CD8 ratio. Guidelines for commencing ART at specific CD4+ count thresholds have changed over time and vary across countries. In these analyses, in addition to measuring and adjusting for CD4+ count, we also adjusted all regression analyses by calendar year to further account for changes in these guidelines.
Sensitivity analyses
We undertook additional sensitivity analyses where we limited the analysis population to 1. patients who commenced ART with CD4 counts above 250 cells/mm3 and 2. Patients whose baseline CD4 measure was within 6 months prior to ART start (as opposed to 12 months). We re-assessed factors associated with achieving a normal ratio 12 months and 24 months after commencing ART in these populations.
Results
A total of 4125 patients from AHOD and 8710 patients from TAHOD were recruited by March 2015 and September 2015, respectively. Of these, 591 patients from AHOD and 2620 patients from TAHOD met the inclusion criteria. The main reason for exclusion in AHOD (76%) was due to initiating ART prior to 1 January 2000 and/or previous treatment. In TAHOD the main reason for exclusion (54%) was due to the absence of the required CD4:CD8 measure before commencing ART or after 3 months of follow-up.
Patient characteristics of the included populations stratified by cohort are presented in Table 1 and Table 2. The cohorts differed in particular in terms of sex, 93% of the AHOD cohort were men compared to 80% in TAHOD. Median age at time of commencing ART (baseline) was 41 (IQR 34-48) years for AHOD patients and 36 years (IQR: 30-42) in TAHOD. Mode of HIV exposure was predominately male to male sex in AHOD (71%), whilst in TAHOD the main mode of exposure was heterosexual contact (52%) followed by male to male sex (36%). Median baseline CD4+ count (cells/mm3) was 290 (IQR: 197-446) for AHOD and 152 (IQR: 54-255) for TAHOD. Seven percent of AHOD patients had an AIDS-defining illness diagnosed prior to baseline compared to 40% of TAHOD patients. Starting ART combination varied between cohorts, the large majority of TAHOD patients (73%) commenced with an NNRTI compared with just over half (54%) of AHOD patients. A further 31% of AHOD patients commenced ART with a combination including a protease inhibitor (PI) or other antiretroviral (9%).
Table 1. Patient characteristics at baseline (commencing ART) by cohort.
| Total | AHOD | TAHOD | Total | |||
|---|---|---|---|---|---|---|
| 591 | % | 2620 | % | 3211 | % | |
| Male | 548 | 92.72 | 2088 | 79.69 | 2636 | 82.09 |
| Female | 40 | 6.77 | 531 | 20.27 | 571 | 17.78 |
| Transgender | 3 | 0.51 | 1 | 0.04 | 4 | 0.12 |
| HBV negative | 461 | 78.00 | 1739 | 66.37 | 2200 | 68.51 |
| HBV positive | 12 | 2.03 | 176 | 6.72 | 188 | 5.85 |
| Missing | 118 | 19.97 | 705 | 26.91 | 823 | 25.63 |
| HCV negative | 469 | 79.36 | 1730 | 66.03 | 469 | 14.61 |
| HCV positive | 55 | 9.31 | 146 | 5.57 | 55 | 1.71 |
| Missing | 67 | 11.34 | 744 | 28.40 | 811 | 25.26 |
| MSM* | 420 | 71.07 | 936 | 35.73 | 1356 | 42.23 |
| Heterosexual | 122 | 20.64 | 1386 | 52.90 | 1508 | 46.96 |
| IDU | 22 | 3.72 | 47 | 1.79 | 69 | 2.15 |
| Other/Missing | 27 | 4.57 | 251 | 9.58 | 278 | 8.66 |
| Prior AIDS | ||||||
| No | 551 | 93.23 | 1569 | 59.89 | 2120 | 66.02 |
| Yes | 40 | 6.77 | 1051 | 40.11 | 1091 | 33.98 |
| Baseline CD4:CD8 ratio** | ||||||
| Ratio <=0.20 | 159 | 26.90 | 1424 | 54.35 | 1583 | 49.30 |
| Ratio >0.20 | 432 | 73.10 | 1196 | 45.65 | 1628 | 50.70 |
| cART combination*** | ||||||
| NNRTI (no PI, no InSTI) | 321 | 54.31 | 1,912 | 72.98 | 2,233 | 69.54 |
| PI (no NNRTI, no InSTI) | 184 | 31.13 | 632 | 24.12 | 816 | 25.41 |
| Other (NRTI only, NNRTI+PI) | 57 | 9.64 | 33 | 1.26 | 90 | 2.80 |
| InSTI | 29 | 4.91 | 43 | 1.64 | 72 | 2.24 |
MSM: men who have sex with men, IDU: Injection drug users
Ratio of 0.2 chosen as cutpoint – median baseline value for combined cohort
NRTI: nucleos(t)ide reverse transcriptase inhibitor, NNRTI: non-nucleoside reverse transcriptase inhibitor, PI: protease inhibitor, InSTI: Integrase inhibitor
Table 2. Patient characteristics (continuous variables) at baseline by cohort.
| N | MEAN | SD | MEDIAN | LQ | UQ | |
|---|---|---|---|---|---|---|
| Age | ||||||
| OVERALL | 3211 | 37.91 | 10.09 | 36.38 | 30.61 | 43.46 |
| AHOD | 591 | 41.63 | 10.28 | 40.91 | 34.39 | 47.64 |
| TAHOD | 2620 | 37.07 | 9.86 | 35.55 | 30.09 | 42.19 |
| Baseline (BL) CD4:CD8 | ||||||
| OVERALL | 3211 | 0.26 | 0.25 | 0.20 | 0.10 | 0.33 |
| AHOD | 591 | 0.39 | 0.37 | 0.30 | 0.19 | 0.48 |
| TAHOD | 2620 | 0.22 | 0.20 | 0.18 | 0.09 | 0.30 |
| Baseline CD4 (cells/mm3) | ||||||
| OVERALL | 3211 | 207.34 | 186.69 | 178 | 68 | 284 |
| AHOD | 591 | 342.43 | 237.90 | 290 | 197 | 446 |
| TAHOD | 2620 | 176.87 | 157.87 | 152 | 54 | 255 |
| Baseline CD8 (cells/mm3) | ||||||
| OVERALL | 3211 | 883.30 | 503.79 | 792 | 514 | 1160 |
| AHOD | 591 | 1022.89 | 538.15 | 930 | 650 | 1273 |
| TAHOD | 2620 | 851.81 | 490.36 | 770 | 489 | 1124 |
The most recent CD4:CD8 ratio prior to commencing ART was low in both cohorts with a median of 0.30 (IQR: 0.19-0.48) in AHOD and a median of 0.18 (IQR: 0.09-0.30) in TAHOD respectively. The TAHOD cohort was almost 50% less likely to have a CD4:CD8 ratio above the overall cohort median of 0.2 (odds ratio (OR): 0.493, p<0.001) and having a prior AIDS diagnosis was also significantly associated with lower odds of having a baseline CD4:CD8 ratio above 0.2 (OR 0.361, p<0.001). Patients who report mode of HIV exposure as MSM were approximately 40% more likely to have a baseline ratio above 0.2 compared with either heterosexual contact group or IDU (p<0.001) (Table 3).
Table 3. Factors associated with CD4:CD8 ratio greater than 0.2 at baseline.
| OR | 95 % CI | p-value | p-overall* | OR | 95 % | p-value | p-overall | |
|---|---|---|---|---|---|---|---|---|
| AHOD | ||||||||
|
| ||||||||
| TAHOD | 0.309 | (0.254, 0.377) | <0.001 | 0.493 | (0.396, 0.614) | <0.001 | ||
|
| ||||||||
| Age per 10 years | 0.956 | (0.893, 1.024) | 0.202 | |||||
|
| ||||||||
| Female | 1.075 | (0.897, 1.289) | 0.433 | |||||
|
| ||||||||
| MSM** | ||||||||
| Heterosexual | 0.414 | (0.355, 0.481) | <0.001 | <0.001 | 0.580 | (0.488, 0.689) | <0.001 | <0.001 |
| IDU | 0.559 | (0.373, 0.837) | 0.005 | 0.614 | (0.401, 0.941) | 0.025 | ||
| Other/missing | 0.571 | (0.440, 0.740) | <0.001 | 0.734 | (0.556, 0.968) | 0.029 | ||
|
| ||||||||
| Never HBV | ||||||||
| HBV positive ever | 0.898 | (0.667, 1.210) | 0.480 | 0.734 | ||||
| Missing | 0.964 | (0.822, 1.132) | 0.656 | |||||
|
| ||||||||
| Never HCV | ||||||||
| HCV positive ever | 1.265 | (0.945, 1.693) | 0.114 | 0.021 | ||||
| Missing | 0.847 | (0.720, 0.995) | 0.043 | |||||
|
| ||||||||
| No Prior AIDS | ||||||||
| Prior AIDS | 0.302 | (0.259, 0.352) | <0.001 | 0.361 | (0.306, 0.426) | <0.001 | ||
P-values reported for test of homogeneity in nominal covariates and test for trend in ordinal covariates
MSM: men who have sex with men; IDU: injection drug users
Of the 538 AHOD and 2092 TAHOD patients that had an available CD4:CD8 ratio measure at 12 months following ART commencement, 17% of AHOD and 7% of TAHOD patients achieved a normal (>1.0) ratio. Factors independently associated with achieving a normal ratio at month 12 are shown in Table 4. In univariate analyses AHOD cohort (p<0.001), increasing age per 10 years (p=0.047), MSM as mode of HIV exposure (p=0.005), increased CD4 cell count (p<0.001) and decreased CD8 count (p=0.001), increasing baseline CD4:CD8 ratio (p<0.001) and commencing ART with a protease inhibitor (PI) (p=0.02) or an HIV integrase inhibitor (INSTI) (p<0.001) compared with an NNRTI had an increased odds of achieving a normal ratio by 12 months. In multivariate analyses, factors that remained independently significantly associated with achieving a normal ratio were increasing age per 10 years (p=0.041), increasing baseline CD4+ count (per 20 cells/mm3) (p=0.002), decreasing CD8 count (p=0.038), increasing baseline ratio (p<0.001) and not having a prior AIDS diagnosis (p=0.024). Only patients who commenced with an InSTI had increased odds of a normal ratio at 12 months compared to patients who commenced with an NNRTI (p=0.002). After adjustment for these confounders, cohort was no longer significant (p=0.475). At 24 months post ART, of the 460 and 1657 AHOD and TAHOD patients with an available CD4:CD8 measure, 21% and 10% respectively had achieved a normal ratio. By this time point, however, CD8+ count, age and ART class were no longer confounding factors for normalisation (data not shown).
Table 4. Factors associated with CD4:CD8 ratio normalisation (ratio >1) by month 12.
| OR | 95% CI | p-value | p-overall** | OR | 95% CI | p-value | p-overall | |
|---|---|---|---|---|---|---|---|---|
| AHOD | ||||||||
|
| ||||||||
| TAHOD | 0.401 | (0.303, 0.531) | <0.001 | 1.145 | (0.789, 1.661) | 0.475*** | ||
|
| ||||||||
| Female | 1.274 | (0.910, 1.785) | 0.158 | |||||
|
| ||||||||
| Age per 10 years | 1.134 | (1.002, 1.283) | 0.047 | 1.159 | (1.006, 1.335) | 0.041 | ||
|
| ||||||||
| MSM* | ||||||||
| Heterosexual | 0.606 | (0.455, 0.806) | 0.001 | 0.005 | ||||
| IDU | 0.547 | (0.217, 1.378) | 0.201 | |||||
| Other/missing | 0.744 | (0.444, 1.245) | 0.260 | |||||
|
| ||||||||
| BL CD4 per 20 mm3 | 1.088 | (1.075, 1.102) | <0.001 | 1.044 | (1.015, 1.073) | 0.002 | ||
| BL CD8 per 20 mm3 | 0.990 | (0.985, 0.996) | 0.001 | 0.988 | (0.976, 0.999) | 0.038 | ||
| BL ratio (continuous) | 102.783 | (58.553, 180.424) | <0.001 | 20.716 | (7.094, 60.499) | <0.001 | ||
|
| ||||||||
| Never HBV | ||||||||
| HBV positive ever | 0.594 | (0.298, 1.185) | 0.139 | 0.325 | ||||
| Missing | 0.935 | (0.681, 1.284) | 0.678 | |||||
|
| ||||||||
| Never HCV | ||||||||
| HCV positive ever | 1.294 | (0.773, 2.166) | 0.328 | 0.532 | ||||
| Missing | 0.934 | (0.672, 1.298) | 0.685 | |||||
|
| ||||||||
| Prior AIDS | 0.311 | (0.215, 0.449) | 0.000 | 0.629 | (0.420, 0.942) | 0.024 | ||
|
| ||||||||
| ART combination** | ||||||||
| NNRTI (no PI, no InSTI) | ||||||||
| PI (no NNRTI, no InSTI) | 1.555 | (1.168, 2.071) | 0.002 | 0.000 | 1.050 | (0.756, 1.458) | 0.771 | 0.016 |
| Other (NRTI only or NNRTI+PI, etc) | 1.524 | (0.745, 3.116) | 0.249 | 0.742 | (0.307, 1.795) | 0.508 | ||
| INSTI | 6.095 | (3.260, 11.396) | <0.001 | 3.151 | (1.523, 6.518) | 0.002 | ||
MSM: men who have sex with men; IDU: injection drug users, BL: baseline.
P-values reported for test of homogeneity in nominal covariates and test for trend in ordinal covariates.
NRTI: nucleos(t)ide reverse transcriptase inhibitor, NNRTI: non-nucleoside reverse transcriptase inhibitor, PI: protease inhibitor, InSTI: Integrase inhibitor.
In multivariate analysis COHORT is adjusted for the independent risk factors.
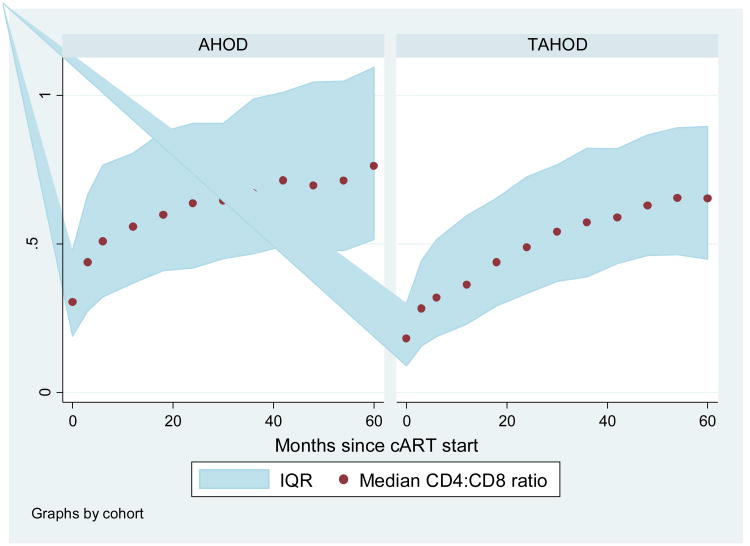
Over the entire follow-up period, 30% and 14% of all AHOD and TAHOD patients achieved a normal CD4:CD8 ratio. In Figure 1 the change in CD4:CD8 ratio over 60 months from commencing ART is presented for both AHOD and TAHOD. Despite commencing ART with varying baseline CD4:CD8 ratios, both cohorts show an increasing CD4:CD8 ratio over time. Figure 2a and 2b also show that the change in ratio is driven by increasing CD4+ counts and decreasing CD8+ counts. However, as also shown in Figure 1, the median ratio for both cohorts does not approach or exceed 1.
Figure 1. Median CD4:CD8 ratio over 5 years by cohort.
*Numbers at month 0, 18, 36 and 60: AHOD - 591, 510, 361 and 204; TAHOD – 2620, 2238, 1371, 752.
IQR: Interquartile range
Figure 2. Median CD4 cells/mm3 and CD8 cells/mm3 over 5 years by cohort.
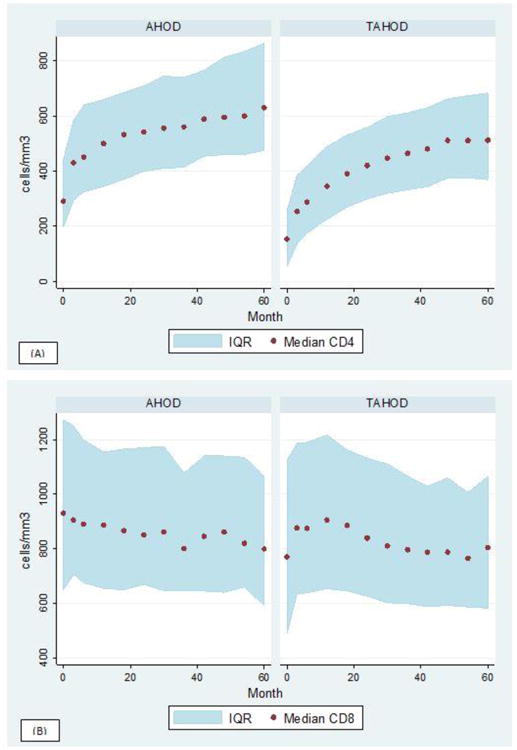
IQR: Interquartile range
Sensitivity analyses
When including only patients with a baseline above 250 cells/mm3 we found that baseline CD4 and CD8 counts were no longer significant, although female gender was now twice as likely to achieve a normal CD4:CD8 ratio. However, as with the analyses reported above, cohort was not statistically significant in multivariate analyses even after adjustment for the final significant model (OR: 1.134, confidence interval 0.750 – 1.712). Among patients with a baseline CD4 measure within 6 months prior to ART start, we found no difference in the final multivariate model to that reported above, and after adjustment cohort again was not an independently significant factor.
Conclusion
In this study we examined whether there were significant differences in CD4:CD8 ratio prior to commencing ART and in response to ART between Asian and Caucasian populations. We found a significantly lower CD4:CD8 ratio among Asian populations in TAHOD prior to commencing ART even after adjusting for absolute CD4+ and CD8+ counts and other confounders. Although CD4:CD8 ratio progressively increased for both AHOD and TAHOD for up to 5 years after commencing ART, only 30% of AHOD and fewer still of TAHOD (14%) achieved a normal ratio during follow-up. Despite fewer TAHOD patients reaching a normal ratio, after adjustment there was no significant difference between the cohorts in odds of achieving a normal ratio. Baseline CD4+ and CD8+ counts seem to be the main driver for this difference between these two populations.
TAHOD patients had lower CD4+ and CD8+ counts prior to commencing ART, and consequently a lower CD4:CD8 ratio at baseline. The significantly lower ratio compared to AHOD may be explained due to varying guidelines for commencing treatment within and across the region, often with recommended treatment commencement at lower CD4+ counts in Asia compared with Australia, as well as likely later presentation in the Asia region. However, even after adjustment for other significant factors, baseline CD4:CD8 ratio was significantly lower for TAHOD. In our study, prior AIDS diagnoses (indicator of later presentation), heterosexual mode of HIV exposure, and commencing ART with an NNRTI were also independently associated with having a lower CD4:CD8 ratio at baseline, and largely reflect the TAHOD cohort.
Once ART is commenced, both cohorts demonstrated an increasing CD4:CD8 ratio over 5 years. In absolute terms CD4:CD8 ratio remained lower in TAHOD and significantly fewer patients achieved a normal ratio by 12 and 24 months after commencing ART. We have previously shown when comparing TAHOD to the predominately Caucasian Aquitaine cohort that Asians tended to have lower total lymphocyte counts and correspondingly lower CD4+ count [8]; however, follow-up work showed that these differences were not clinically significant [10]. In the current analysis, once adjusted for key factors there was no significant difference between the cohorts. Immune status at baseline was the key contributing factor for normalisation. Patients with higher baseline CD4+ count, lower baseline CD8+ count, higher CD4:CD8 ratio at baseline, and not having a prior AIDS diagnosis were all significantly associated with normalisation at 12 months. Similar associated factors were also identified at month 24 except baseline CD8+ count was no longer significant. It has been suggested in the literature, in the presence of universal treatment and generally high CD4+ counts, that CD4:CD8 ratio might be more useful for predicting disease progression [6, 14]. However, our findings highlight the importance CD4+ count in explaining how CD4:CD8 ratio changes over time. Other studies have also suggested that older age, MSM and IDU as mode of HIV exposure, as well as starting ART regimen (AZT, 3TC or d4T compared with tenofovir and emtricitabine) also contribute to lower odds of achieving a normal ratio [5]. In our study older age and commencing ART with a PI or InSTI as opposed to an NNRTI were associated with increased likelihood of normalising by 12 months. It is likely that the treatment effects demonstrated in our study are due to the difference in treatment available as starting regimen in Asia compared to Australia. In Australia and among higher income countries of Asia, PI regimens and more recently InSTI regimens were used as first line therapy. PIs have been shown to boost CD4+ counts more than NNRTIs [15]. However, by 24 months starting ART regimen was not significant. Although other studies have found older age to be associated with not achieving a normal ratio [5, 16], we found the reverse. In our study older age was associated with increased odds of achieving a normal ratio at least in the first year. This might be due to an older AHOD cohort compared to TAHOD and also due to better treatment adherence, or monitoring of patients in AHOD.
Lower starting CD4:CD8 ratio is the likely reason CD4:CD8 ratio remained lower for the TAHOD cohort even after 5 years of ART. Studies have shown that early treatment initiation (early infection) compared to delayed treatment (chronic infection) allows for greater recovery of the CD4:CD8 ratio [17, 18], and that a low CD4:CD8 ratio prior to initiation of ART was associated with a decreased probability of achieving CD4+ counts above 500 cells/mm3 [6, 19]. Leung et al for instance reported normalisation in 6% of patients who commenced ART at a CD4+ count below 200 cells/mm3 compared to 21% of patients who commenced above 350 cells/mm3 [4]. Approximately 30% and 14% of AHOD and TAHOD participants achieved a normal ratio after 5 years. The proportion of patients with normal ratios reported in the ICONA cohort [5] (4% at 1 year, 12% by 2 years and 29% by 5 years) were similar to the AHOD cohort (most likely reflecting similar treatment availability and starting guidelines), while slightly lower rates (7%) over a median of 4 years were reported by Leung et [4]. Ongoing immune activation is suggested as underlying the low rates of normalisation among HIV+ patients [20]. Although CD4+ count in our study is shown to recover considerably over 5 years, CD8+ count only marginally declined over the same period which might explain the low rate of normalisation in our study. Failure of CD8+ count to adequately decrease after ART is initiated, suggests ongoing immune activation and inflammation, as an important factor for the failure of the CD4:CD8 to normalise. Helleberg et al 2015 examined the trajectories of CD8+ count before and after ART in 3882 HIV+ patients [21], and showed that CD8+ count was elevated during untreated HIV infection and remained elevated through 10 years of ART.
Lymphocyte activation, immune senescence and coinfections have also been attributed to failure to achieve a normal CD4:CD8 ratio. In our study ever being co-infected with HBV was associated with significantly decreased odds of achieving a normal ratio by 24 months, but there was no other significant association for either HBV or HCV coinfections for any of the other outcomes that we assessed. Saracino et al 2014 [16] reported no significant association with HBV, HCV or CMV infections. Whilst in the ICONA cohort, a negative CMV serology result was associated with the ratio normalising [5]. Similar associations with CMV positivity were also reported by Caby et al 2016 [22]. CMV serology was not available for assessment in our study.
A major strength of our study is that the cohorts were established using similar methodologies, with a significant number of years of data collection and follow-up. However, there are some limitations. First, the two cohorts are very heterogeneous, with varying treatment patterns and monitoring. AHOD is generally an older cohort in which the majority of patients commenced treatment pre-1996. In order to make the cohorts more comparable we limited the AHOD population to commencing treatment after 2000. However, this reduced the sample population of AHOD considerably. Viral load monitoring also differed considerably between the cohorts, with less frequent testing in TAHOD, as such we could not assess viral load as a covariate. We used cohort as a surrogate for whether patients were Caucasian or Asian as ethnicity data is incomplete in AHOD for several contributing sites. It is possible that some AHOD patients are of Asian ethnicity therefore resulting in misclassification; however, where ethnicity is recorded in AHOD (∼ 50%), more than 90% are Caucasians. Both cohorts are also select populations and whether these findings are generalizable to the wider population is not known. Finally, due to the observational nature of our study, we cannot exclude the risk of unmeasured confounding. It is also possible that in our analyses, patients lost to follow-up, or without available data at 12 and 24 months after ART initiation, might cause some selection bias. When we compared patients with and without data at these time points, there was some variability for some of the subcategories, though these differences mostly can be explained by smaller sample size of the populations without data at these time points.
The overall objective of our study was to determine whether CD4:CD8 ratio in Asian populations are similar to Caucasian populations. In previous work by our group, lower CD4+ counts in TAHOD compared to AHOD did not translate to an increase in mortality risk [10]. Several studies have investigated CD4:CD8 ratio as a useful independent predictor for disease progression, and have shown that a lower CD4:CD8 ratio in ART treated populations increases the risk of non-AIDS mortality and morbidity [7, 14, 17]. However, our data suggest that the main reason for the lower CD4:CD8 ratio in the Asian cohort is lower CD4+ count when ART is commenced. As treatment availability and access improves across the region and patients commence treatment earlier, the lower starting CD4:CD8 ratio in Asia is likely to disappear. Nevertheless, future work will compare these cohorts to investigate the impact of CD4:CD8 ratio on mortality and serious non-AIDS clinical endpoints.
Acknowledgments
The TREAT Asia HIV Observational Database and the Australian HIV Observational Database are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
AHOD Investigators
D Ellis, Coffs Harbour Medical Centre, Coffs Harbour;
M Bloch, S Agrawal, T Vincent, Holdsworth House Medical Practice, Sydney;
D Allen, Holden Street Clinic, Gosford;
D Smith, A Rankin, Lismore Sexual Health & AIDS Services, Lismore
D Baker*, East Sydney Doctors, Surry Hills
DJ Templeton*†, CC O'Connor, O Thackeray, RPA Sexual Health, Camperdown;
E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba;
N Ryder, G Sweeney, Clinic 468, HNE Sexual Health, Tamworth;
D Cooper, A Carr, K Macrae, K Hesse, St Vincent's Hospital, Darlinghurst;
R Finlayson, S Gupta, D Ninham, Taylor Square Private Clinic, Darlinghurst;
J Langton-Lockton, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith;
K Brown, S Idle, N Arvela, Illawarra Sexual Health Service, Warrawong;
R Varma, H Lu, Sydney Sexual Health Centre, Sydney;
D Couldwell, S Eswarappa, Western Sydney Sexual Health Clinic;
DE Smith*, V Furner, D Smith, G Cabrera, Albion Street Centre;
M Gunathilake, R Payne, Communicable Disease Centre, Darwin.
M O'Sullivan, A Croydon, Gold Coast Sexual Health Clinic, Miami;
D Russell, C Cashman, C Roberts, Cairns Sexual Health Service, Cairns;
D Sowden, K Taing, P Marshall, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour;
D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill;
D Rowling, N Latch, E Warzywoda, Sexual Health and HIV Service in Metro North, Brisbane;
R Moore, S Edwards, S Boyd, Northside Clinic, North Fitzroy;
NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne;
J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne;
I Woolley, M Giles*, T Korman, J Williams*, Monash Medical Centre, Clayton.
D Nolan, A Allen, G Guelfi. Department of Clinical Immunology, Royal Perth Hospital, Perth.
G Mills, C Wharry, Waikato District Hospital Hamilton;
N Raymond, K Bargh, Wellington Hospital, Wellington.
A Cogle*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Boyd*, University of Adelaide; B Dickson*, CaraData. W Donohue, O'Brien Street General Practice, Adelaide. M Law*, K Petoumenos*, R Puhr*, R Huang* The Kirby Institute, UNSW Australia.
CoDe reviewers: D Templeton, M Giles, K Brown and J Hoy.
*AHOD Steering Committee member - 2016; † Steering Committee Chair
TAHOD Investigators
PS Ly* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee* †, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
S Pujari*, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India;
TP Merati*, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
E Yunihastuti*, D Imran and A Widhani, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
BLH Sim*, YM Gani, and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco* E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines;
WW Wong*‡, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore;
A Avihingsanon*, S Gatechompol, P Phanuphak and C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul*, S Sungkanuparph, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand;
P Kantipong* and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
W Ratanasuwan* and R Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand;
KV Nguyen*, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
DD Cuong*, NV An and NT Luan, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn*, JL Ross* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, A Jiamsakul* and DC Boettiger, The Kirby Institute, UNSW Australia, Sydney, Australia.
*TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair
Footnotes
Conflicts of interests: The no authors have no conflict of interest relevant to this article to declare
References
- 1.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009 Sep-Oct;17(4):118–23. [PubMed] [Google Scholar]
- 2.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. Journal of immunology. 2006 Feb 15;176(4):2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 3.Strindhall J, Skog M, Ernerudh J, Bengner M, Lofgren S, Matussek A, et al. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age. 2013 Jun;35(3):985–91. doi: 10.1007/s11357-012-9400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung V, Gillis J, Raboud J, Cooper C, Hogg RS, Loutfy MR, et al. Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PloS one. 2013;8(10):e77665. doi: 10.1371/journal.pone.0077665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015 Mar;2(3):e98–106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PloS one. 2014;9(1):e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrano-Villar S, Moreno S, Fuentes-Ferrer M, Sanchez-Marcos C, Avila M, Sainz T, et al. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV medicine. 2014 Jan;15(1):40–9. doi: 10.1111/hiv.12081. [DOI] [PubMed] [Google Scholar]
- 8.Achhra AC, Zhou J, Dabis F, Pujari S, Thiebaut R, Law MG, et al. Difference in absolute CD4+ count according to CD4 percentage between Asian and Caucasian HIV-infected patients. Journal of AIDS & clinical research. 2010 Oct 8;1(1):1–4. doi: 10.4172/2155-6113.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger S, Petoumenos K, Kamarulzaman A, Hoy J, Sungkanuparph S, Chuah J, et al. Long-term patterns in CD4 response are determined by an interaction between baseline CD4 cell count, viral load, and time: The Asia Pacific HIV Observational Database (APHOD) Journal of acquired immune deficiency syndromes. 2009 Apr 15;50(5):513–20. doi: 10.1097/qai.0b013e31819906d3. Epub 2009/05/02.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achhra AC, Zhou J, Choi JY, Hoy J, Zhang F, Templeton DJ, et al. The Clinical Significance of CD4 Counts in Asian and Caucasian HIV-Infected Populations: Results from TAHOD and AHOD. J Int Assoc Physicians AIDS Care (Chic) 2011 May-Jun;10(3):160–70. doi: 10.1177/1545109711402213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network TTAHODT. A Decade of Combination Antiretroviral Treatment in Asia: The TREAT Asia HIV Observational Database Cohort. AIDS research and human retroviruses. 2016 Aug;32(8):772–81. doi: 10.1089/aid.2015.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV medicine. 2002 Jan;3(1):28–36. doi: 10.1046/j.1464-2662.2001.00094.x. Epub 2002/06/13.eng. [DOI] [PubMed] [Google Scholar]
- 13.Duda SN, Farr AM, Lindegren ML, Blevins M, Wester CW, Wools-Kaloustian K, et al. Characteristics and comprehensiveness of adult HIV care and treatment programmes in Asia-Pacific, sub-Saharan Africa and the Americas: results of a site assessment conducted by the International epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. Journal of the International AIDS Society. 2014;17:19045. doi: 10.7448/IAS.17.1.19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS pathogens. 2014 May;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Leth F, Wit FW, Reiss P, Schattenkerk JK, van der Ende ME, Schneider MM, et al. Differential CD4 T-cell response in HIV-1-infected patients using protease inhibitor-based or nevirapine-based highly active antiretroviral therapy. HIV medicine. 2004 Mar;5(2):74–81. doi: 10.1111/j.1468-1293.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 16.Saracino A, Bruno G, Scudeller L, Volpe A, Caricato P, Ladisa N, et al. Chronic inflammation in a long-term cohort of HIV-infected patients according to the normalization of the CD4:CD8 ratio. AIDS research and human retroviruses. 2014 Dec;30(12):1178–84. doi: 10.1089/aid.2014.0080. [DOI] [PubMed] [Google Scholar]
- 17.Thornhill J, Inshaw J, Kaleebu P, Cooper D, Ramjee G, Schechter M, et al. Enhanced normalisation of CD4/CD8 ratio with earlier antiretroviral therapy at Primary HIV Infection. Journal of acquired immune deficiency syndromes. 2016 Apr 6; doi: 10.1097/QAI.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. The Journal of antimicrobial chemotherapy. 2013 May;68(5):1169–78. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 19.Torti C, Prosperi M, Motta D, Digiambenedetto S, Maggiolo F, Paraninfo G, et al. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012 May;18(5):449–58. doi: 10.1111/j.1469-0691.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. Journal of the International AIDS Society. 2015;18:20052. doi: 10.7448/IAS.18.1.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and Clinical Significance of CD8+ T-Cell Counts in a Large Cohort of HIV-Infected Individuals. The Journal of infectious diseases. 2015 Jun 1;211(11):1726–34. doi: 10.1093/infdis/jiu669. [DOI] [PubMed] [Google Scholar]
- 22.Caby F, Guihot A, Lambert-Niclot S, Guiguet M, Boutolleau D, Agher R, et al. Determinants of a Low CD4/CD8 Ratio in HIV-1-Infected Individuals Despite Long-term Viral Suppression. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 May 15;62(10):1297–303. doi: 10.1093/cid/ciw076. [DOI] [PubMed] [Google Scholar]