Abstract
Objective
Several studies have reported the development of new molecular methods for the prognosis and diagnosis of male fertility based on biomarkers aimed at overcoming the limitations of conventional male fertility analysis tools. However, further studies are needed for the field application of these methods. Therefore, alternative methods based on existing semen analysis methods are required to improve production efficiency in the animal industry.
Methods
we examined the possibility of improving litter size in various pig breeds using combined Hoechst 33258/chlortetracycline fluorescence (H33258/CTC) staining. The correlation between field fertility and capacitation status by combined H33258/CTC staining in different ejaculates spermatozoa (n = 3) from an individual boar (20 Landrace, 20 Yorkshire, and 20 Duroc) was evaluated as well as overall accuracy.
Results
The acrosome reacted (AR) pattern after capacitation (%) was positively correlated with the litter size of Landrace, Yorkshire, and Duroc pigs and the overall accuracy was 75%, 75%, and 70% in Landrace, Yorkshire, and Duroc pigs, respectively. The difference (Δ) in AR pattern before and after capacitation was positively correlated with the litter size of Landrace, Yorkshire, and Duroc pigs and the overall accuracy was 80%, 65%, and 55% in Landrace, Yorkshire, and Duroc pigs, respectively. However, the difference (Δ) in capacitated (B) pattern before and after capacitation was negatively correlated with the litter size of Landrace pigs and the overall accuracy was 75%. Moreover, average litter size was significantly altered according to different combined H33258/CTC staining parameters.
Conclusion
These results show that combined H33258/CTC staining may be used to predict male fertility in various breeds. However, the selection of specific efficiency combined H33258/CTC staining parameters requires further consideration. Taken together, these findings suggest that combined H33258/CTC staining may constitute an alternative method for predicting male fertility until such time as fertility-related biomarkers are further validated.
Keywords: Litter Size, Capacitation Status, Boar Spermatozoa, Breeds, Fertility
INTRODUCTION
Prognosis of male fertility is very important and highly valuable in livestock industries because breeding system failures due to sire lead to huge economic loss. In pig farming, sire semen quality is the most common reason for replacing boars in European and North American countries [1]. Thus, selection of superior sires is important for increasing the productivity of domestic animal farms. To date, various conventional semen analysis methods have been developed to evaluate male fertility in the livestock industry [2–6]. However, it remains unclear whether conventional semen analyses are effective tools for predicting male fertility potential [7–11]. Recently, new approaches have been used to develop more accurate methods for predicting male fertility [8–11]. These studies have reported the identification of fertility-related biomarkers based on comprehensive proteomic approaches [9–11]. In particular, several studies have shown significantly increased litter sizes using these developed biomarkers in field trials [9–11]. However, additional studies and technical improvements are required for the field application of these new fertility-related biomarker based analysis tools for prognosis and diagnosis of male fertility.
Combined Hoechst 33258/chlortetracycline fluorescence (H33258/CTC) staining is widely used method to directly measure the capacitation status of spermatozoa by monitoring calcium-regulated changes using the fluorescent antibiotic chlortetracycline [12,13]. Recently, it has been reported that the H33258/CTC staining has been applied for evaluating boar fertility in order to overcome the limitations of current semen analyses [4]. Moreover, Kwon et al [13] have shown that litter size increased in a field trial following the prediction of litter size based on capacitation status evaluation when using combined H33258/CTC staining. Therefore, it is possible that combined H33258/CTC staining could be applied to predict male fertility in the field until it is replaced by more effective tools. However, to date, no studies have compared the use of combined H33258/CTC to increase production efficiency in various species and breeds. Therefore, the present study was performed to evaluate the correlation between field fertility and capacitation status by combined H33258/CTC staining in different ejaculates spermatozoa (n = 3) from an individual boar in different boar breeds (20 Landrace, 20 Yorkshire, and 20 Duroc). In addition, sensitivity, specificity, positive predictive value, and negative predictive value, and overall accuracy were evaluated.
MATERIALS AND METHODS
All processes were performed according to the guidelines for the ethical treatment of animals and were approved by the Institutional Animal Care and Use Committee of Chung-Ang University.
Artificial insemination
Artificial insemination (AI) was performed in commercial great-grandparent farms to produce purebred F1 offspring (Landrace and Yorkshire: Sunjin Co., Danyang, Korea; Duroc: Darby Co., Chungju, Korea). To rule out seasonal effects during the study period (1 year), the environment was controlled (temperature [20°C±5°C], ventilation, light [on: 16 h, off: 8 h]). Semen samples were collected a previously described method [14]. To ensure a wide fertility range, 20 Landrace (mean age: 28.4±1.28; range = 20 to 37 months), 20 Yorkshire (mean age: 24.95±1.05; range = 18 to 31 months), and 20 Duroc (mean age: 27.8±0.99; range = 22 to 35 months) boars were selected based on field fertility data from Sunjin Co. and Darby Co., respectively. The lower limit AI values were volume >150 mL, total concentration >200×106, motility >70%, and total abnormalities <20% of the sample [13]. The semen sample was diluted (3×109 sperm cells/100 mL) for AI and stored at 17°C until insemination. Twenty Landrace, Yorkshire, and Duroc sows (60 in total) were randomly selected (two to five pregnancies) for AI. Next, 3×106 spermatozoa were inseminated twice per estrus in the cervix by well-trained technicians [13]. Finally, diluted semen samples from an individual boar were inseminated to 20 randomly selected sows. Thus, total 400 sows per breed were mated by AI using semen samples from 20 same breed boars.
Sample preparation
Three different ejaculates semen samples from an individual boar were prepared to evaluate capacitation status (n = 3). It was equally applied to 60 boars to prepare 3 different ejaculates semen samples per boar. The samples were washed and divided in two (before and after capacitation) [13]. One portion of the extended semen sample was used for the capacitation experiment; capacitation was induced by a previous described method [10,13,15].
Combined Hoechst 33258/chlortetracycline fluorescence assessment of capacitation status (H33258/CTC)
Capacitation status was evaluated by dual staining according to a previously described method [10,13,15]. Briefly, 135 μL of sperm suspension was incubated with 15 μL of H33258 solution (150 μM in Dulbecco’s phosphate buffered saline, DPBS) for 10 min at room temperature. Next, 250 μL of 2% (w/v) polyvinylpyrrolidone (Sigma-Aldrich, St Louis, MO, USA) in DPBS was added to remove the excess dye. After centrifugation, the pellet was resuspended in 500 μL of DPBS; 500 μL of chlortetracycline (CTC) fluorescence solution (750 mM CTC in 5 μL buffer containing 20 mM Tris, 130 mM NaCl, and 5 mM cysteine [pH 7.4]) (Sigma-Aldrich, USA). Stained samples were observed with a Microphot-FXA microscope (Nikon, Tokyo, Japan) under epifluorescence illumination using ultraviolet BP 340–380/LP 425 and BP 450–490/LP 515 excitation/emission filters for H33258 and CTC, respectively. Capacitation status was categorized as one of the following four patterns: dead pattern (D, nuclei show bright blue fluorescence over the sperm head), live non-capacitated pattern (F, bright green fluorescence distributed uniformly over entire sperm head, with or without stronger fluorescent line at the equatorial segment), live capacitated pattern (B, green fluorescence over the acrosome region and a dark post acrosome), or live acrosome-reacted pattern (AR, sperm showing mottled green fluorescence overhead, green fluorescence only in the post-acrosome region, or no fluorescence above the head) (Figure 1) [9–11,13]. Two slides per sample were evaluated using at least 400 spermatozoa per slide.
Figure 1.
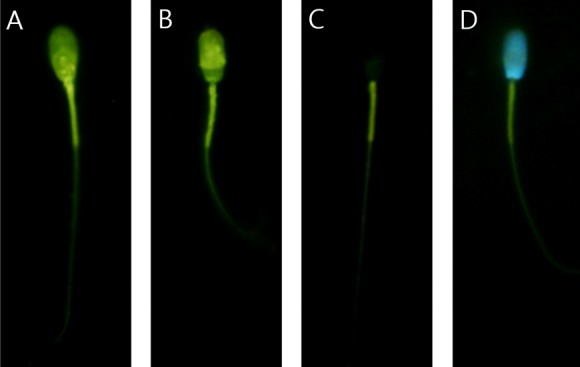
Different patterns of spermatozoa in combined H33258/CTC staining. (A) F pattern (live non-capacitated pattern, bright green fluorescence). (B) B Pattern (live capacitated pattern, green fluorescence over the acrosome region and a dark post acrosome). (C) AR pattern (live acrosome-reacted pattern, no fluorescence above the head). (D) D pattern (dead pattern, nuclei show bright blue fluorescence over the sperm head).
Statistical analysis
The data were analyzed with SPSS (v. 18.0; Chicago, IL, USA). Pearson correlation coefficients were calculated to determine the associations between capacitation status, variation of capacitation status, and litter size. The ability of individual analyzed parameters to predict litter size was evaluated by receiver-operating characteristic (ROC) curves (litter size ≥11 or <11 [based on average litter size of Landrace pigs], litter size ≥12 or <12 (based on average litter size of Yorkshire pigs), and litter size ≥8 or <8 [based on average litter size of Duroc pigs]).
The cut-off values of each breed were calculated based on ROC curves and determined in relation to the points of maximum sensitivity and specificity (Figure 2) [9,10,13]. Student’s two-tailed t test was used to compare the predicted litter sizes by ROCs. Less than 0.05 p value was considered a significant difference. All data were expressed as mean±standard error of the mean.
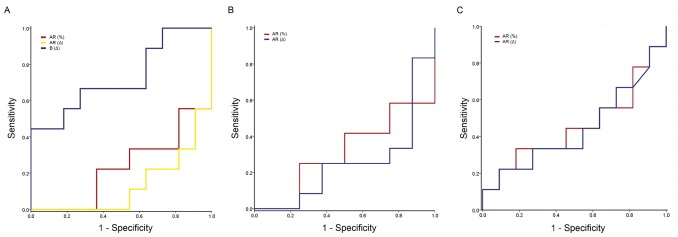
Figure 2.
Receiver operating characteristic (ROC) curves for different parameters using combined H33258/CTC staining in 20 individual samples of Landrace, Yorkshire, and Duroc spermatozoa. (A) ROC curve for the live acrosome reacted (AR) pattern after capacitation (%), difference (Δ) in AR pattern before and after capacitation, and difference (Δ) in B pattern before and after capacitation in 20 individual Landrace spermatozoa samples. (B) ROC curve for the AR pattern after capacitation (%) and difference (Δ) in AR pattern before and after capacitation in 20 individual Yorkshire spermatozoa samples. (C) ROC curve for the AR pattern after capacitation (%) and the difference (Δ) in AR pattern before and after capacitation in 20 individual Duroc spermatozoa samples.
Quality assessment of parameters
Four key parameters (sensitivity, specificity, positive predictive value, and negative predictive value) were used in the screening tests [4,5,9,10,13]. Sensitivity is defined as the percentage of boars that were correctly identified by the test based on litter size. Specificity is defined as the percentage of boars that tested as truly negative. The positive predictive value is defined as the percentage of boars that tested positive but actually had a litter size ≥11 or <11 (Landrace), litter size ≥12 or <12 (Yorkshire), or litter size ≥8 or <8 (Duroc). The negative predictive value is defined as the percentage of boars that tested negative but actually had a litter size ≥11 or <11 (Landrace), litter size ≥12 or <12 (Yorkshire), or litter size ≥8 or <8 (Duroc).
RESULTS
Correlation between capacitation status before and after capacitation, difference (Δ) in capacitation status before and after capacitation, and litter size
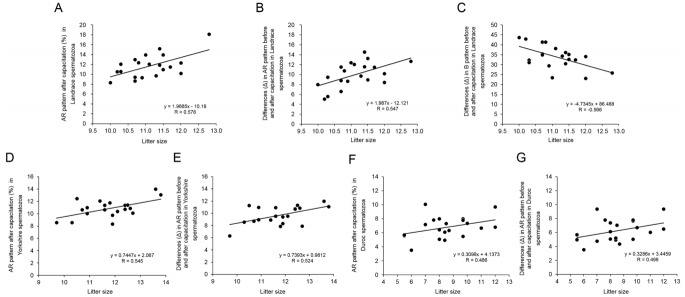
In Landrace pigs, the AR after capacitation (r = 0.578; p< 0.01, Table 1, Figure 3A) and the difference (Δ) in AR pattern before and after capacitation (r = 0.547; p<0.05, Table 1, Figure 3B) were positively correlated with litter size, while the difference (Δ) in the B pattern before and after capacitation was negatively correlated with litter size (r = −0.566; p<0.01; Table 1, Figure 3C). The AR after capacitation (r = 0.545; p< 0.05, Table 1, Figure 3D) and the difference (Δ) in AR pattern before and after capacitation (r = 0.524; p<0.05, Table 1, Figure 3E) in Yorkshire spermatozoa were positively correlated with litter size. The AR after capacitation (r = 0.486; p<0.05; Table 2, Figure 3F) and the difference (Δ) in AR pattern before and after capacitation (r = 0.498; p<0.05; Table 3, Figure 3G) in Duroc spermatozoa were also positively correlated with litter size.
Table 1.
Correlation coefficient (r) from Pearson correlation analysis between capacitation status before and after capacitation, differences (Δ) before and after capacitation, and litter size in Landrace boar
| Item | Before capacitation (BC) | After capacitation (AC) | Differences (Δ) before and after capacitation | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| AR | F | B | AR | F | B | AR | F | B | |
| Litter size | −0.008 | −0.338 | 0.370 | 0.578** | 0.088 | −0.343 | 0.547* | 0.349 | −0.566** |
| AR (BC) | - | −0.386 | −0.020 | 0.297 | 0.123 | −0.242 | −0.448** | 0.416 | −0.197 |
| B (BC) | - | - | −0.915** | −0.195 | 0.233 | −0.107 | 0.098 | −0.644** | 0.564** |
| F (BC) | - | - | - | 0.081 | −0.306 | 0.222 | 0.090 | 0.515* | −0.525* |
| AR (AC) | - | - | - | - | 0.081 | −0.533** | 0.720** | 0.225 | −0.524* |
| B (AC) | - | - | - | - | - | −0.886** | −0.014 | 0.594** | −0.554* |
| F (AC) | - | - | - | - | - | - | −0.323 | −0.609** | 0.713** |
| AR (Δ) | - | - | - | - | - | - | - | −0.092 | −0.347 |
| B (Δ) | - | - | - | - | - | - | - | - | −0.902** |
AR, live acrosome-reacted sperm; F, live non-capacitated sperm; B, live capacitated sperm.
p<0.05;
p<0.01.
Figure 3.
Correlation between different parameters using combined H33258/CTC staining in Landrace, Yorkshire, and Duroc spermatozoa samples. (A) Correlation between the live acrosome reacted (AR) pattern after capacitation (%) and litter size of Landrace pigs. (B) Correlation between the difference (Δ) in AR pattern before and after capacitation and litter size of Landrace pigs. (C) Correlation between the difference (Δ) in B pattern before and after capacitation and litter size of Landrace pigs. (D) Correlation between the difference (Δ) in AR pattern before and after capacitation and litter size of Yorkshire pigs. (E) Correlation between the difference (Δ) in AR pattern before and after capacitation and litter size of Yorkshire pigs. (F) Correlation between the difference (Δ) in AR pattern before and after capacitation and litter size of Duroc pigs. (G) Correlation between the difference (Δ) in AR pattern before and after capacitation and litter size of Duroc pigs. Data represent mean±standard error of the mean, n = 3.
Table 2.
Correlation coefficient (r) from Pearson correlation analysis between capacitation status before and after capacitation, differences (Δ) before and after capacitation, and litter size in Yorkshire boar
| Item | Before capacitation (BC) | After capacitation (AC) | Differences (Δ) before and after capacitation | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| AR | F | B | AR | F | B | AR | F | B | |
| Litter size | 0.005 | 0.140 | −0.148 | 0.545* | −0.338 | 0.246 | 0.524* | −0.410 | 0.302 |
| AR (BC) | - | −0.330 | 0.298 | 0.356 | −0.468* | 0.377 | −0.425 | −0.307 | 0.213 |
| B (BC) | - | - | −0.853** | −0.015 | 0.258 | −0.203 | 0.240 | −0.236 | 0.216 |
| F (BC) | - | - | - | −0.042 | −0.171 | 0.128 | −0.269 | 0.251 | −0.357 |
| AR (AC) | - | - | - | - | −0.377 | 0.133 | 0.695** | −0.372 | 0.145 |
| B (AC) | - | - | - | - | - | −0.955** | −0.006 | 0.878** | −0.818** |
| F (AC) | - | - | - | - | - | - | −0.161 | −0.860** | 0.881** |
| AR (Δ) | - | - | - | - | - | - | - | −0.124 | −0.024 |
| B (Δ) | - | - | - | - | - | - | - | - | −0.930** |
AR, live acrosome-reacted sperm; F, live non-capacitated sperm; B, live capacitated sperm.
p<0.05;
p<0.01.
Table 3.
Correlation coefficient (r) from Pearson correlation analysis between capacitation status before and after capacitation, differences (Δ) before and after capacitation, and litter size in Duroc boar
| Item | Before capacitation (BC) | After capacitation (AC) | Differences (Δ) before and after capacitation | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| AR | F | B | AR | F | B | AR | F | B | |
| Litter size | −0.076 | 0.126 | −0.334 | 0.486* | −0.385 | 0.267 | 0.498* | −0.400 | 0.389 |
| AR (BC) | - | 0.094 | −0.218 | 0.098 | −0.322 | 0.350 | −0.252 | −0.327 | 0.362 |
| B (BC) | - | - | −0.701** | −0.325 | 0.075 | 0.042 | −0.348 | −0.550** | 0.499* |
| F (BC) | - | - | - | 0.114 | 0.131 | −0.206 | 0.186 | 0.540* | −0.801** |
| AR (AC) | - | - | - | - | −0.643** | 0.370 | 0.938** | −0.340 | 0.149 |
| B (AC) | - | - | - | - | - | −0.949** | −0.514* | 0.792** | −0.669** |
| F (AC) | - | - | - | - | - | - | 0.238 | −0.821** | 0.751** |
| AR (Δ) | - | - | - | - | - | - | - | −0.217 | 0.019 |
| B (Δ) | - | - | - | - | - | - | - | - | −0.866** |
AR, live acrosome-reacted sperm; F, live non-capacitated sperm; B, live capacitated sperm.
p<0.05;
p<0.01.
Quality assessment of parameters
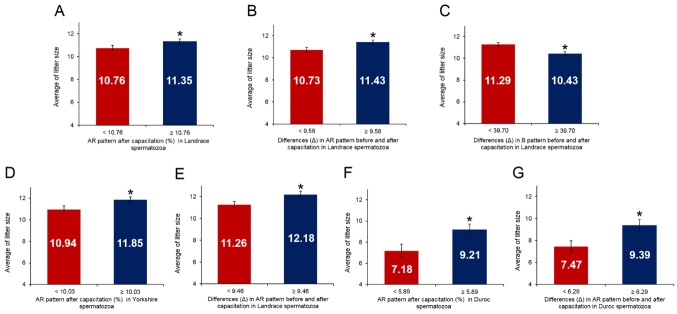
The breed litter cut-off values were determined by ROCs (Landrace: 11 pigs; Yorkshire: 12 pigs; Duroc: 8 pigs). The cut-off values of the AR pattern after capacitation (%) were 10.76% for Landrace, 10.03% for Yorkshire, and 5.89% for Duroc (Table 4). The differences (Δ) in AR pattern before and after capacitation were 9.58%, 9.46% and 6.29% for Landrace, Yorkshire, and Duroc, respectively (Table 4). In addition, the difference (Δ) in the B pattern before and after capacitation was 39.7% for Landrace pigs (Table 4). The overall accuracy of the AR pattern after capacitation was 75.00%, 75.00%, and 70.00% in Landrace, Yorkshire, and Duroc, respectively (Table 4). The average litter size of Landrace boars with less than 10.76% AR pattern after capacitation was 10.76 piglets, whereas the average litter size of boars with ≥10.76% AR pattern after capacitation was 11.35 piglets (p<0.05, Figure 4A). Yorkshire boars with <10.03% AR pattern after capacitation produced 10.94 piglets, whereas Yorkshire boars with ≥10.03% AR pattern after capacitation produced 11.85 piglets (p<0.05, Figure 4D). In Duroc, the average litter size of boars with <5.89% AR pattern after capacitation was 7.18 piglets, whereas the average litter size of boars with ≥5.89% AR pattern after capacitation was 9.21 piglets (p<0.05, Figure 4F). The overall accuracy of the difference (Δ) in AR pattern before and after capacitation were 80.00%, 65.00%, and 55.00% for Landrace, Yorkshire, and Duroc, respectively (Table 4). While Landrace boars with <9.58% difference (Δ) in AR pattern before and after capacitation produced 10.73 piglets, Landrace boars with ≥9.58% difference (Δ) in AR pattern before and after capacitation produced 11.43 piglets (p<0.05, Figure 4B). The average litter size of Yorkshire boars with < 9.46% difference (Δ) in AR pattern before and after capacitation was 11.26 piglets, whereas that of those with ≥9.46% was 12.18 piglets (p<0.05, Figure 4E). In addition, Duroc boars with <6.29% difference (Δ) in AR pattern before and after capacitation produced 7.47 piglets, whereas Duroc boars with ≥6.29% difference (Δ) in AR pattern before and after capacitation produced 9.39 piglets (p<0.05, Figure 4G). The overall accuracy of the difference (Δ) in B pattern before and after capacitation was 75.00% in Landrace boars. In addition, the sensitivity, specificity, negative predictive value, and positive predictive value were 100.00%, 44.44%, 100.00%, and 68.75%, respectively (Table 4). The average litter size of Landrace boars with <39.70% difference (Δ) in B pattern before and after capacitation was 11.29 piglets, whereas the average litter size of those with ≥39.70% difference (Δ) in B pattern before and after capacitation was 10.43 piglets (p< 0.05, Figure 4C).
Table 4.
Correlations between capacitation status before and after capacitation, differences (Δ) in capacitation status before and after capacitation, and litter size1)
| Item | Species | Cut-off value (%) | Sensitivity (%) | Specificity (%) | Negative predictive value (%) | Positive predictive value (%) | Overall accuracy (%) |
|---|---|---|---|---|---|---|---|
| AR pattern after capacitation | Landrace | 10.76 | 81.82 | 66.67 | 75.00 | 75.00 | 75.00 |
| Yorkshire | 10.03 | 85.71 | 50.00 | 60.00 | 80.00 | 75.00 | |
| Duroc | 5.89 | 78.57 | 50.00 | 50.00 | 75.57 | 70.00 | |
| Differences (Δ) in AR pattern before and after capacitation | Landrace | 9.58 | 81.82 | 77.78 | 77.78 | 81.82 | 80.00 |
| Yorkshire | 9.46 | 64.29 | 66.67 | 44.44 | 81.82 | 65.00 | |
| Duroc | 6.29 | 50.00 | 66.67 | 36.36 | 77.78 | 55.00 | |
| Differences (Δ) in B pattern before and after capacitation | Landrace | 39.70 | 100.00 | 44.44 | 100.00 | 68.75 | 75.00 |
Sensitivity: the percentage of boars that were correctly identified by the test based on litter size. Specificity: the percentage of boars that tested as truly negative. The positive predictive value: the percentage of boars that tested positive but actually had a litter size ≥11 or <11 (Landrace), litter size ≥12 or <12 (Yorkshire), or litter size ≥8 or <8 (Duroc). The negative predictive value: the percentage of boars that tested negative but actually had a litter size ≥11 or <11 (Landrace), litter size ≥12 or <12 (Yorkshire), or litter size ≥8 or <8 (Duroc).
Figure 4.
Average litter size according to different parameters using combined H33258/CTC staining in spermatozoa. (A) Average litter size according to live acrosome reacted (AR) pattern after capacitation (%) in Landrace spermatozoa. (B) Average litter size according to the difference (Δ) in AR pattern before and after capacitation in Landrace spermatozoa. (C) Average litter size according to the difference (Δ) in B pattern before and after capacitation in Landrace spermatozoa. (D) Average litter size according to AR pattern after capacitation (%) in Yorkshire spermatozoa. (E) Average litter size according to the differences (Δ) in AR pattern before and after capacitation in Yorkshire spermatozoa. (F) Average litter size according to AR pattern after capacitation (%) in Duroc spermatozoa. (G) Average litter size according to the difference (Δ) in AR pattern before and after capacitation in Duroc spermatozoa. Data represent the mean±standard error of the mean, n = 3. * p<0.05.
DISCUSSION
Various conventional semen analysis methods have been developed to evaluate male fertility both in humans and animals. However, the clinical value of these conventional semen analysis methods is uncertain [7]. To overcome the shortcomings of conventional semen analysis methods, recent studies have attempted to identify fertility related biomarkers using proteomic analysis approaches [8–11,16,17]. In addition, several studies have validated these newly identified fertility-related biomarkers by application to field trials [9,10]. However, further studies are required to commercialize field application in the economic animal industry. Moreover, these methods require much effort and time. Therefore, alternative methods for evaluating male fertility in the livestock industry are being considered.
Sperm cells have to undergo special events to penetrate oocytes during their journey in the female reproductive tract. This process is known as ‘capacitation’ [18,19]. Capacitation is initiated by various ions and chemicals such as Ca2+, HCO3−, bovine serum albumin, and heparin [20–23]; cAMP level is in turn increased by the calcium influx. Production of ATP is induced by the increased intracellular calcium level, changing sperm motility and motion kinematics [20–23]. Moreover, tyrosine phosphorylation occurs through a protein kinase activated pathway by cAMP [22–25]. Finally, the sperm cells undergo a morphological change and are then ready to penetrate the oocyte membrane (the acrosome reaction) [22–28]. Several studies have analyzed various sperm parameters during capacitation; in particular, sperm motility and capacitation status, which are unique phenotypes during capacitation [29–33]. Unfortunately, simple analysis of sperm motility and capacitation status may not be sufficient for the prognosis and diagnosis of male fertility potential [7–11].
Recently, new analysis methods based on sperm motility, motion kinematics, and capacitation status have been introduced to evaluate the correlation with litter size [5,13]. Sperm motility and motion kinematics have not been correlated with litter size, while capacitation status, evaluated using combined H33258/CTC staining, has been correlated with litter size in boars [5,13]. However, these studies were performed with specific breeds and the application of the combined H33258/CTC staining method for the prediction of male fertility in various species/breeds was not examined. Therefore, the present study was designed to validate the applicability of a method for predicting male fertility based on combined H33258/CTC staining analysis before and after capacitation in various breed boars. In addition, to rule out seasonal effects during the study period, the environment was controlled and seasonal effects on conception rate was not observed.
In the present study, the AR pattern after capacitation (Table 1, Figure 3A) and the difference (Δ) in AR pattern before and after capacitation (Table 1, Figure 3B) were positively correlated with litter size in the Landrace breed. However, the difference (Δ) in B pattern before and after capacitation was negatively correlated with litter size in the Landrace breed (Table 1, Figure 3C). These finding are similar to previously reported results [13]. Moreover, it appears that analysis of capacitation status using the combined H33258/CTC staining analysis was well optimized in the present study. The AR pattern after capacitation (Table 1, 2; Figure 3D, 3F) and the difference (Δ) in AR pattern before and after capacitation (Tables 1, 3; Figure 3E, 3G) were also positively correlated with litter size in the Yorkshire and Duroc breeds. However, no correlation was observed between the difference (Δ) in B pattern before and after capacitation and litter size in the Yorkshire and Duroc breeds. These findings suggest that the correlation between litter size and capitation status or difference (Δ) following capacitation differ by breed. In addition, the efficiency of parameters, such as AR pattern after capacitation and difference (Δ) in AR pattern before and after capacitation, differed by breed (Table 4). The difference (Δ) in AR pattern before and after capacitation was the most efficient parameter for predicting male fertility in the Landrace breed according to ROCs (Table 4). In addition, the AR pattern after capacitation (%) was the most efficient parameter for predicting male fertility in the Yorkshire and Duroc breeds according to ROCs (Table 4). These findings indicate that the difference (Δ) in AR pattern before and after capacitation can be applied to more efficiently predict male fertility in the Landrace breed, while the AR pattern after capacitation (%) is better for providing more accurate results in the Yorkshire and Duroc breeds. The highest increase in average litter size of the Yorkshire breed was obtained when the difference (Δ) in B pattern before and after capacitation was used to predict litter size in a field trial (Δ = 0.86 pigs, Figure 4). Although the other breeds also demonstrated differences in average litter size when the AR pattern after capacitation (%) and the difference (Δ) in AR pattern before and after capacitation were used to predict litter size in a field trial, these changes were slight (Figure 4). Present study was performed to these results suggest that selection of the parameter applied to predict male fertility should be determined by breed.
CONCLUSION
In the present study, we performed combined H33258/CTC staining analysis to predict litter size in various pig breeds. In addition, the predictability of litter size in various pig breeds was validated through AI in field trials. To the best of our knowledge, this is the first study to apply combined H33258/CTC staining analysis for the diagnosis and prognosis of male fertility in various breeds. This study suggests a new option for diagnosis and prognosis of male fertility in various breeds using advanced conventional sperm analysis. Moreover, we propose that combined H33258/CTC staining analysis could be used as an alternative for diagnosis and prognosis of male fertility until sufficient development and field application validation of fertility-related biomarker methods have been performed.
ACKNOWLEDGMENTS
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116172-3).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Robinson JA, Buhr MM. Impact of genetic selection on management of boar replacement. Theriogenology. 2005;63:668–78. doi: 10.1016/j.theriogenology.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Budworth PR, Amann RP, Chapman PL. Relationships between computerized measurements of motion of frozen-thawed bull spermatozoa and fertility. J Androl. 1988;9:41–54. doi: 10.1002/j.1939-4640.1988.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonde JP, Ernst E, Jensen TK, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 4.Oh SA, You YA, Park YJ, Pang MG. The sperm penetration assay predicts the litter size in pigs. Int J Androl. 2010;33:604–12. doi: 10.1111/j.1365-2605.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 5.Oh SA, Park YJ, You YA, Mohamed EA, Pang MG. Capacitation status of stored boar spermatozoa is related to litter size of sows. Anim Reprod Sci. 2010;121:131–8. doi: 10.1016/j.anireprosci.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Milardi D, Grande G, Vincenzoni F, Castagnola M, Marana R. Proteomics of human seminal plasma: identification of biomarker candidates for fertility and infertility and the evolution of technology. Mol Reprod Dev. 2013;80:350–7. doi: 10.1002/mrd.22178. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SE. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134:31–40. doi: 10.1530/REP-07-0152. [DOI] [PubMed] [Google Scholar]
- 8.Park YJ, Kwon WS, Oh SA, Pang MG. Fertility-related proteomic profiling bull spermatozoa separated by percoll. J Proteome Res. 2012;11:4162–8. doi: 10.1021/pr300248s. [DOI] [PubMed] [Google Scholar]
- 9.Kwon WS, Rahman MS, Lee JS, et al. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol Cell Proteomics. 2015;14:1230–40. doi: 10.1074/mcp.M114.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon WS, Rahman MS, Ryu DY, Park YJ, Pang MG. Increased male fertility using fertility-related biomarkers. Sci Rep. 2015;5:15654. doi: 10.1038/srep15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon WS, Oh SA, Kim YJ, et al. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci Rep. 2015;5:13821. doi: 10.1038/srep13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillan L, Evans G, Maxwell WM. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology. 2005;63:445–57. doi: 10.1016/j.theriogenology.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Kwon WS, Rahman MS, Lee JS, You YA, Pang MG. Improving litter size by boar spermatozoa: application of combined H33258/CTC staining in field trial with artificial insemination. Andrology. 2015;3:552–7. doi: 10.1111/andr.12020. [DOI] [PubMed] [Google Scholar]
- 14.Almond G, Britt J, Flowers B, et al. The awine A.I. book. Raleigh, NC, USA: Morgan Morrow Press; 1998. [Google Scholar]
- 15.Kwon WS, Rahman MS, Lee JS, et al. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genomics. 2014;15:897. doi: 10.1186/1471-2164-15-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mateo S, Martínez-Heredia J, Estanyol JM, et al. Marked correlations in protein expression identified by proteomic analysis of human spermatozoa. Proteomics. 2007;7:4264–77. doi: 10.1002/pmic.200700521. [DOI] [PubMed] [Google Scholar]
- 17.Secciani F, Bianchi L, Ermini L, et al. Protein profile of capacitated versus ejaculated human sperm. J Proteome Res. 2009;8:3377–89. doi: 10.1021/pr900031r. [DOI] [PubMed] [Google Scholar]
- 18.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 19.Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1995;4:581–96. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- 20.Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod. 1992;46:686–91. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- 21.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–57. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 22.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA. 2009;6:667–8. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman MS, Kwon WS, Pang MG. Calcium influx and male fertility in the context of the sperm proteome: an update. Biomed Res Int. 2014;2014 doi: 10.1155/2014/841615. Article ID 841615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon WS, Park YJ, Kim YH, et al. Vasopressin effectively suppresses male fertility. PLoS ONE. 2013;8:e5419. doi: 10.1371/journal.pone.0054192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon WS, Park YJ, Mohamed el-SA, Pang MG. Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril. 2013;99:354–61. doi: 10.1016/j.fertnstert.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Naz RK. Involvement of protein tyrosine phosphorylation of human sperm in capacitation/acrosome reaction and zona pellucida binding. Front Biosci. 1996;1:206–13. doi: 10.2741/a126. [DOI] [PubMed] [Google Scholar]
- 27.Fraser LR. Sperm capacitation and the acrosome reaction. Hum Reprod. 1998;13:9–19. doi: 10.1093/humrep/13.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 28.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263–8. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- 29.Fraser LR, Quinn PJ. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil. 1981;61:25–35. doi: 10.1530/jrf.0.0610025. [DOI] [PubMed] [Google Scholar]
- 30.Zaneveld LJ, De Jonge CJ, Anderson RA, Mack SR. Human sperm capacitation and the acrosome reaction. Hum Reprod. 1991;6:1265–74. doi: 10.1093/oxfordjournals.humrep.a137524. [DOI] [PubMed] [Google Scholar]
- 31.Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact,moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci USA. 1993;90:4660–4. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol. 2003;49:321–7. [PubMed] [Google Scholar]
- 33.Marquez B, Suarez SS. Soluble adenylyl cyclase is required for activation of sperm but does not have a direct effect on hyperactivation. Reprod Fertil Dev. 2008;20:247–52. doi: 10.1071/rd07146. [DOI] [PubMed] [Google Scholar]