Abstract
Blood pressure abnormalities are frequently observed in patients with Parkinson's disease (PD), and are associated with cerebrovascular diseases such as white matter hyperintensities and carotid atherosclerosis. We assessed the relationship between blood pressure abnormalities and cerebral microbleeds (CMBs), a marker of cerebral small vessel disease, in 128 patients with PD. We examined supine and orthostatic blood pressures and used 24-hour ambulatory blood pressure monitoring to assess the presence or absence of orthostatic hypotension (OH), supine hypertension (SH), nocturnal hypertension (NH), and loss of nocturnal blood pressure dips (non-dipping). CMBs were found in 13 (10.2%) patients, and the median number of CMBs was 1 (range: 1 to 10). Six of these patients had deep or infratentorial CMBs, six had strictly lobar CMBs, and one had mixed CMBs. Linear regression analysis indicated that presence of both OH and SH was independently associated with greater numbers of CMBs in deep or infratentorial regions, independent of age, sex, cardiovascular risk factors, and white matter hyperintensities. NH and non-dipping were not associated with CMBs in deep or infratentorial regions, and there was no association between blood pressure and CMBs in lobar regions. Our results suggest that the presence of both OH and SH may be related to deep or infratentorial CMBs in patients with PD.
Keywords: Autonomic dysfunction, Cerebral microbleeds, Orthostatic hypotension, Parkinson's disease, Supine hypertension
Highlights
-
•
CMBs were found in 13 out of 128 (10.2%) patients with PD.
-
•
Presence of both OH and SH was associated with deep or infratentorial CMBs.
-
•
NH and non-dipping were not associated with deep or infratentorial CMBs.
-
•
Neither orthostatic nor nocturnal blood pressures were associated with lobar CMBs.
1. Introduction
Blood pressure abnormalities are common in Parkinson's disease (PD), and may manifest as orthostatic hypotension (OH), supine hypertension (SH), nocturnal hypertension (NH), or loss of nocturnal blood pressure dips (non-dipping), all of which frequently coexist [1]. In patients with PD, these blood pressure abnormalities are associated with cerebrovascular diseases, such as white matter hyperintensities [2], [3], [4] and carotid artery atherosclerosis [5].
Cerebral microbleeds (CMBs) are small round hypointense lesions seen on T2*-weighted magnetic resonance imaging (MRI) [6], and are associated with cognitive impairment [7] and recurrence of both ischemic and hemorrhagic stroke [8]. Histopathological studies indicate that CMBs represent previous extravasation of blood and are related to bleeding-prone microangiopathy [9]. Deep or infratentorial CMBs are associated with hypertension, while lobar CMBs reflect the presence of cerebral amyloid angiopathy [10], [11]. CMBs have mainly been studied in the general population, in patients with stroke, and in those with Alzheimer's disease [12], while few studies have investigated CMBs in patients with PD [13], [14], [15]. Nevertheless, it has been reported that CMBs occur more frequently in patients with PD with dementia compared to those without dementia. Indeed, patients with PD with CMBs have been found to have a poorer performance in the attention domain compared with those without CMBs [13]. Furthermore, comprehensive neuropsychological evaluation showed no significant association between the presence of CMBs and cognitive function in patients with PD without dementia [14]. We previously reported that OH is associated with deep or infratentorial CMBs in patients with PD [15]. However, whether coexisting SH, NH, or non-dipping is related to CMBs remains unanswered. Here, we evaluated supine and orthostatic blood pressure and used 24-hour ambulatory blood pressure monitoring (ABPM) in patients with PD to assess the relationship between blood pressure abnormalities and CMBs.
2. Patients and methods
2.1. Study population
We enrolled patients diagnosed with PD according to the U.K. Parkinson's disease Society Brain Bank criteria [16]. Participants were recruited consecutively between January 2014 and September 2016 from the Department of Neurology at Juntendo University School of Medicine. One hundred and forty-three patients underwent blood pressure measurements and brain MRI. Three patients were excluded due to unreliable ABPM data and twelve were excluded due to incomplete blood pressure evaluations. Therefore, 128 patients with PD were examined in the present study.
We collected clinical information regarding age, sex, disease duration, current medications, body mass index, laboratory data, hypertension, and diabetes mellitus. We also determined whether the patients had histories of stroke, coronary artery disease, or cigarette smoking. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mm Hg, a diastolic blood pressure (DBP) ≥ 90 mm Hg in the sitting position, or treatment with antihypertensive medications. Diabetes mellitus was defined as a fasting blood glucose level ≥ 126 mg/dL, a glycated hemoglobin level ≥ 6.5%, or the use of insulin or oral hypoglycemic agents. Subjects were considered current smokers if they had smoked at least one cigarette per day within the previous year. The mean levodopa equivalent daily dose was calculated for each participant [17]. This study was approved by the Institutional Review Board of Juntendo Hospital. Informed consent was obtained from all patients.
2.2. Blood pressure measurements
OH and SH were assessed using the Schellong test. Patients were studied after they had rested in the supine position for 15 min. The patients' blood pressure and heart rate were measured in the supine position, and then measured in the upright position using an electronic sphygmomanometer (ES-H55 or ES-H55B, Terumo). OH was defined as a fall in SBP ≥ 20 mm Hg, or a fall in DBP ≥ 10 mm Hg within 3 min after rising from a supine to a standing position according to the consensus statement [18]. SH was defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg according to the criteria of the American Heart Association [19].
ABPM was performed using an FB-270 device (Fukuda Denshi) every 30 min throughout the day (7:00 AM to 10:00 PM), and every 60 min during the night (10:00 PM to 7:00 AM). Mean SBP, DBP, and heart rate during the daytime, nighttime, and over a 24-hour period were evaluated. Nocturnal BP dip (%) was calculated as (mean daytime SBP – mean nighttime SBP) / mean daytime SBP × 100. Patients with < 10% nocturnal fall in mean BP were considered “non-dippers” according to the consensus guideline [20]. NH was defined as an average nighttime BP ≥ 120/70 mm Hg according to the 2007 European Hypertension Society/European Cardiology Society guidelines [21].
2.3. MRI protocol and assessment
Brain MRI was performed using a 3.0 T MR system (GE Healthcare, Discovery MR750w). The whole brain was scanned at a slice thickness of 5 mm with an interslice gap of 1 mm. Twenty-four axial images were obtained. The imaging protocol consisted of T1-weighted (repetition time [TR] = 2800 ms; echo time [TE] = 18 ms; field of view [FOV] = 22 × 22 cm, matrix = 288 × 352), T2-weighted (TR = 4427 ms; TE = 85 ms; FOV = 22 × 22 cm, matrix = 320 × 448), fluid-attenuated inversion recovery (FLAIR) (TR = 10,000 ms; TE = 120 ms, inversion time = 2570 ms; FOV = 22 × 22 cm, matrix = 224 × 288), and T2*-weighted gradient echo sequences (TR = 520 ms; TE = 15 ms; flip angle [FA] = 15°, FOV = 22 × 22 cm, matrix = 192 × 256).
CMBs were defined as small, homogeneous, round foci of low signal intensity on T2*-weighted images with diameters < 5 mm. Basal ganglia calcification and vascular flow voids were excluded. The Microbleed Anatomical Rating Scale [22] was used to guide this process and identify the locations of the CMBs. Deep and periventricular white matter hyperintensities were graded from zero to three according to the method described by Fazekas et al. [23]. Periventricular hyperintensities of grade 3 or deep white matter hyperintensities of grade 2 or 3 were defined as advanced white matter hyperintensities [24]. The images were analyzed by a trained observer who was blind to the patients' clinical data.
2.4. Statistical analysis
Continuous variables were compared using Student's t-tests or Mann-Whitney U tests, as appropriate. The frequencies of categorical variables were compared using χ2 tests. We performed linear regression analysis to assess the relationship between the number of CMBs and blood pressure abnormalities. Models were adjusted for age and sex (model 1), and additionally adjusted for hypertension, diabetes mellitus, history of stroke, antiplatelet treatment, and the presence of advanced white matter hyperintensities (model 2). The statistical analyses were performed using JMP Version 12.0 software (SAS Inc. Cary, NC, USA). P-values < 0.05 were considered to be statistically significant.
3. Results
One-hundred and twenty-eight patients with PD (mean age, 64.4 ± 9.7 years; 56 males) were included in this study. The mean duration of motor symptoms was 10.7 ± 6.0 years, and the mean Hoehn and Yahr stage score was 2.9 ± 0.9. The clinical characteristics of patients according to the presence of CMBs are shown in Table 1. Thirteen patients (10.2%) had CMBs, in whom the median number of CMBs was 1 (range: 1 to 10). Six of these patients had deep or infratentorial CMBs, six had strictly lobar CMBs, and one had mixed CMBs.
Table 1.
Clinical characteristics according to the presence of CMBs.
| Any CMBs |
Deep or infratentorial CMBs |
Lobar CMBs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Present |
Absent |
p value | Present |
Absent |
p value | Present |
Absent |
p value | |
| (n = 13) | (n = 115) | (n = 7) | (n = 121) | (n = 7) | (n = 121) | ||||
| Age, y | 72.3 (8.3) | 63.5 (9.5) | < 0.01 | 75.0 (7.3) | 63.8 (9.5) | < 0.01 | 70.0 (8.5) | 64.1 (9.7) | 0.12 |
| Male | 5 (38.5) | 51 (44.4) | 0.69 | 4 (57.1) | 52 (43.0) | 0.46 | 2 (28.6) | 54 (44.6) | 0.41 |
| BMI, kg/m2 | 20.7 (19.8–24.4) | 21.1 (18.5–24.0) | 0.59 | 20.5 (18.1–20.7) | 21.2 (18.5–24.1) | 0.37 | 23.5 (19.8–24.7) | 20.9 (18.5–23.9) | 0.15 |
| eGFR, mL/min per 1.73 m2 | 88.4 (25.6) | 88.3 (21.3) | 0.99 | 95.7 (29.6) | 87.9 (21.2) | 0.36 | 85.6 (23.0) | 88.5 (21.7) | 0.73 |
| LDL-cholesterol, mg/dL | 108.9 (32.3) | 106.5 (27.7) | 0.77 | 98.3 (26.9) | 107.3 (28.2) | 0.41 | 118.0 (33.9) | 106.1 (27.7) | 0.28 |
| HDL-cholesterol, mg/dL | 58.3 (17.5) | 60.7 (12.9) | 0.57 | 55.3 (14.0) | 60.7 (13.3) | 0.34 | 58.6 (20.9) | 60.5 (12.8) | 0.71 |
| HbA1c, % | 5.8 (5.6–6.1) | 5.7 (5.5–6.0) | 0.43 | 5.6 (5.4–6.1) | 5.7 (5.5–6.0) | 0.57 | 5.9 (5.7–6.1) | 5.7 (5.5–6.0) | 0.26 |
| Hypertension | 5 (38.5) | 26 (22.6) | 0.21 | 3 (42.9) | 28 (23.1) | 0.24 | 2 (28.6) | 29 (24.0) | 0.78 |
| Diabetes mellitus | 2 (15.4) | 10 (8.8) | 0.44 | 2 (28.6) | 10 (8.3) | 0.08 | 0 (0) | 12 (10.0) | 0.38 |
| Smoking status | |||||||||
| Non-smoker | 10 (76.9) | 79 (68.7) | 0.54 | 4 (57.1) | 85 (70.3) | 0.35 | 6 (85.7) | 83 (68.6) | 0.58 |
| Ex-smoker | 3 (23.1) | 26 (22.6) | 3 (42.9) | 26 (21.5) | 1 (14.3) | 28 (23.1) | |||
| Current smoker | 0 (0) | 10 (8.7) | 0 (0) | 10 (8.3) | 0 (0) | 10 (8.3) | |||
| History of stroke | 1 (7.7) | 3 (2.6) | 0.32 | 1 (14.3) | 3 (2.5) | 0.08 | 0 (0) | 4 (3.3) | 0.63 |
| Antiplatelet | 3 (23.1) | 6 (5.2) | 0.02 | 1 (14.3) | 8 (6.6) | 0.44 | 2 (28.6) | 7 (5.8) | 0.02 |
| Anticoagulant | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
BMI, body mass index; CAD, coronary artery disease; CMBs, cerebral microbleeds; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Values are number (%), mean (standard deviation), or median (interquartile range), as appropriate.
Of the total 128 patients, 60 patients (46.9%) had OH and 20 patients (15.6%) had SH (Table 2). In patients with OH or SH, the mean ages were higher in those without such blood pressure abnormalities. The frequency of diuretic use was significantly higher in patients with OH than in those without OH. SH was found in 26.7% of patients with OH. Forty-four patients (34.3%) had OH only, four had SH only (3.1%), and sixteen (12.5%) had both OH and SH. Among the patients with SH, 80% of patients had OH. Mean SBP and DBP levels were higher in patients with OH or SH than those without, at daytime, nighttime, and over the entire 24-hour period. However, the frequencies of NH and non-dippers were not different between these groups.
Table 2.
Clinical characteristics according to postural blood pressure abnormalities.
| OH |
SH |
|||||
|---|---|---|---|---|---|---|
| Present |
Absent |
p value | Present |
Absent |
p value | |
| (n = 60) | (n = 68) | (n = 20) | (n = 108) | |||
| Age, y | 67.1 (8.8) | 62.0 (9.9) | < 0.01 | 68.6 (10.9) | 63.6 (9.4) | 0.04 |
| Male | 26 (43.3) | 30 (44.1) | 0.93 | 12 (60.0) | 44 (40.7) | 0.11 |
| Disease duration, y | 10 (6.3–15) | 10 (6–14) | 0.81 | 10 (6.3–14.8) | 9 (6–15.8) | 0.85 |
| Hoehn and Yahr stage | 3.1 (0.9) | 2.8 (0.8) | 0.04 | 3.4 (0.9) | 2.9 (0.8) | 0.01 |
| Hypertension | 13 (21.7) | 18 (26.5) | 0.53 | 11 (55.0) | 20 (18.5) | < 0.01 |
| Diabetes mellitus | 6 (10.2) | 6 (8.8) | 0.80 | 4 (21.1) | 8 (7.4) | 0.06 |
| Antihypertensive | 11 (18.3) | 16 (23.5) | 0.47 | 7 (35.0) | 20 (18.5) | 0.10 |
| ARB | 6 (10.0) | 9 (13.2) | 0.57 | 3 (15.0) | 12 (11.1) | 0.62 |
| Ca channel blockers | 4 (6.7) | 10 (14.7) | 0.15 | 4 (20.0) | 10 (9.3) | 0.16 |
| β-blockers | 1 (1.7) | 2 (2.9) | 0.63 | 2 (10.0) | 1 (0.9) | 0.02 |
| Diuretic | 4 (6.7) | 0 (0) | 0.03 | 1 (5.0) | 3 (2.8) | 0.60 |
| Antihypotensive | 6 (10.0) | 4 (5.9) | 0.39 | 1 (5.0) | 9 (8.3) | 0.61 |
| Midodrine | 0 (0) | 1 (1.5) | 0.35 | 0 (0) | 1 (0.9) | 0.67 |
| Droxidopa | 6 (10.0) | 3 (4.4) | 0.22 | 1 (5.0) | 8 (7.4) | 0.70 |
| LED, mg | 850 (721–1100) | 910 (604–1248) | 0.91 | 769 (596–917) | 922 (705–1129) | 0.06 |
| Supine SBP, mm Hg | 123.8 (21.4) | 112.9 (16.6) | < 0.01 | 152.2 (16.7) | 111.7 (12.3) | < 0.01 |
| Supine DBP, mm Hg | 72.3 (11.7) | 67.9 (11.3) | 0.03 | 85.0 (13.2) | 67.1 (9.0) | < 0.01 |
| ΔSBP, mm Hg | 32.5 (19.9) | 3.8 (10.8) | < 0.01 | 39.0 (29.6) | 13.2 (16.7) | < 0.01 |
| ΔDBP, mm Hg | 15.3 (10.3) | − 2.3 (6.9) | < 0.01 | 13.5 (14.8) | 4.5 (11.4) | < 0.01 |
| OH | 60 (100) | 0 (0) | – | 16 (80.0) | 44 (40.7) | < 0.01 |
| SH | 16 (26.7) | 4 (5.9) | < 0.01 | 20 (100) | 0 (0) | – |
| 24-hour SBP, mm Hg | 118.9 (11.8) | 112.3 (11.7) | < 0.01 | 126.3 (11.9) | 113.3 (11.1) | < 0.01 |
| 24-hour DBP, mm Hg | 78.3 (9.1) | 73.5 (7.1) | < 0.01 | 80.4 (10.5) | 74.9 (7.7) | < 0.01 |
| Daytime SBP, mm Hg | 120.3 (12.0) | 114.0 (11.4) | < 0.01 | 126.9 (12.0) | 115.1 (11.2) | < 0.01 |
| Daytime DBP, mm Hg | 80.6 (9.6) | 75.9 (7.8) | < 0.01 | 82.3 (10.5) | 77.3 (8.4) | 0.02 |
| Nighttime SBP, mm Hg | 117.8 (15.4) | 109.6 (14.7) | < 0.01 | 126.4 (14.9) | 111.0 (14.5) | < 0.01 |
| Nighttime DBP, mm Hg | 75.2 (11.0) | 69.7 (7.9) | < 0.01 | 77.8 (12.5) | 71.2 (8.9) | < 0.01 |
| NH | 40 (66.7) | 40 (58.8) | 0.36 | 16 (80.0) | 64 (59.3) | 0.08 |
| Non-dipping | 47 (78.3) | 49 (72.1) | 0.41 | 17 (85.0) | 79 (73.2) | 0.26 |
ARB, angiotensin-receptor blockers; DBP, diastolic blood pressure; LED, levodopa equivalent dose; NH, nocturnal hypertension; OH, orthostatic hypotension; SBP, systolic blood pressure; SH, supine hypertension. ΔS(D)BP = S(D)BP supine − S(D)BP standing. Values are number (%), mean (standard deviation), or median (interquartile range), as appropriate.
On ABPM, 80 patients (62.5%) had NH and 96 patients (75.0%) were non-dippers (Table 3). Mean age was similar between patients with and without NH and non-dipping. In patients with NH, mean supine SBP and DBP levels were higher, and the magnitude of the fall in SBP levels during orthostasis was greater compared to those without NH. The frequencies of OH and SH were, however, not different between patients with NH and those without NH.
Table 3.
Clinical characteristics according to nocturnal blood pressure abnormalities.
| NH |
Non-dipping |
|||||
|---|---|---|---|---|---|---|
| Present |
Absent |
p value | Present |
Absent |
p value | |
| (n = 80) | (n = 48) | (n = 96) | (n = 32) | |||
| Age, y | 64.0 (9.0) | 65.1 (10.9) | 0.56 | 64.6 (9.0) | 63.8 (11.7) | 0.71 |
| Male | 42 (52.5) | 14 (29.2) | 0.01 | 45 (46.9) | 11 (34.4) | 0.22 |
| Disease duration, y | 10 (7–16) | 10 (6–15) | 0.81 | 11 (6–13) | 10 (6.3–15) | 0.93 |
| Hoehn and Yahr stage | 2.9 (1.0) | 3.0 (0.8) | 0.59 | 3.0 (0.9) | 2.9 (0.9) | 0.76 |
| Hypertension | 19 (23.8) | 12 (25.0) | 0.87 | 24 (25.0) | 7 (21.9) | 0.72 |
| Diabetes mellitus | 8 (10.0) | 4 (8.5) | 0.81 | 9 (9.4) | 3 (9.4) | 0.99 |
| Antihypertensive | 16 (20.0) | 11 (22.9) | 0.70 | 20 (20.8) | 7 (21.9) | 0.90 |
| ARB | 9 (11.3) | 6 (12.5) | 0.83 | 10 (10.4) | 5 (15.6) | 0.43 |
| Ca channel blockers | 10 (12.5) | 4 (8.3) | 0.46 | 11 (11.5) | 3 (9.4) | 0.74 |
| β-blockers | 2 (2.5) | 1 (2.1) | 0.88 | 3 (3.1) | 0 (0) | 0.31 |
| Diuretic | 4 (5.0) | 0 (0) | 0.12 | 4 (4.2) | 0 (0) | 0.24 |
| Antihypotensive | 8 (10.0) | 2 (4.2) | 0.23 | 9 (9.4) | 1 (3.1) | 0.25 |
| Midodrine | 1 (1.3) | 0 (0) | 0.44 | 1 (1.0) | 0 (0) | 0.56 |
| Droxidopa | 7 (8.8) | 2 (4.2) | 0.33 | 8 (8.3) | 1 (3.1) | 0.32 |
| LED, mg | 910 (721–1244) | 786 (649–1100) | 0.24 | 910 (727–1128) | 783 (595–1150) | 0.15 |
| Supine SBP, mm Hg | 121.0 (21.5) | 113.5 (15.3) | 0.04 | 119.4 (20.3) | 114.6 (17.2) | 0.24 |
| Supine DBP, mm Hg | 71.6 (12.5) | 67.2 (9.6) | 0.04 | 70.4 (12.0) | 68.7 (10.6) | 0.48 |
| ΔSBP, mm Hg | 21.1 (23.0) | 10.6 (16.3) | < 0.01 | 18.7 (21.2) | 12.6 (21.4) | 0.17 |
| ΔDBP, mm Hg | 7.3 (12.9) | 3.4 (11.1) | 0.09 | 7.1 (12.3) | 2.1 (11.9) | 0.05 |
| OH | 40 (50.0) | 20 (41.7) | 0.36 | 47 (49.0) | 13 (40.6) | 0.41 |
| SH | 16 (20.0) | 4 (8.3) | 0.08 | 17 (17.7) | 3 (9.4) | 0.26 |
| 24-hour SBP, mm Hg | 120.0 (11.3) | 107.5 (9.4) | < 0.01 | 115.9 (12.7) | 113.7 (10.5) | 0.38 |
| 24-hour DBP, mm Hg | 79.3 (7.4) | 69.8 (6.5) | < 0.01 | 75.7 (8.6) | 76.0 (7.8) | 0.83 |
| Daytime SBP, mm Hg | 120.1 (11.2) | 111.6 (11.7) | < 0.01 | 115.8 (12.2) | 120.5 (11.2) | 0.06 |
| Daytime DBP, mm Hg | 80.7 (8.1) | 73.7 (8.6) | < 0.01 | 76.9 (8.9) | 81.8 (8.2) | < 0.01 |
| Nighttime SBP, mm Hg | 120.8 (13.8) | 101.0 (9.0) | < 0.01 | 117.3 (14.9) | 101.6 (11.0) | < 0.01 |
| Nighttime DBP, mm Hg | 77.4 (8.2) | 63.4 (4.8) | < 0.01 | 74.3 (9.4) | 66.1 (8.7) | < 0.01 |
| NH | 80 (100) | 0 (0) | – | 69 (71.9) | 11 (34.4) | < 0.01 |
| Non-dipping | 69 (86.3) | 27 (56.3) | < 0.01 | 96 (100) | 0 (0) | – |
ARB, angiotensin-receptor blockers; DBP, diastolic blood pressure; LED, levodopa equivalent dose; NH, nocturnal hypertension; OH, orthostatic hypotension; SBP, systolic blood pressure; SH, supine hypertension. ΔS(D)BP = S(D)BP supine − S(D)BP standing. Values are number (%), mean (standard deviation), or median (interquartile range), as appropriate.
The results of blood pressure measurements according to the presence of CMBs are shown in Table 4. Mean supine (p = 0.03), 24-hour (p = 0.02), and daytime (p = 0.02) SBPs were higher in patients with CMBs than in those without CMBs. When we divided the patients into groups based on CMB location, the magnitudes of the falls in SBP (p = 0.02) and DBP (p = 0.02) during orthostasis were greater in patients with deep or infratentorial CMBs than in those without deep or infratentorial CMBs. When the patients were classified into 4 groups according to the presence or absence of OH and SH (non-OH and non-SH group, OH-only group, SH-only group, and both OH and SH group), the prevalence of these groups was significantly different between patients with deep or infratentorial CMBs and those without deep or infratentorial CMBs (p = 0.01). The mean 24-h SBP (p = 0.03), daytime SBP (p < 0.01), and daytime DBP (p = 0.02) were higher in patients with deep or infratentorial CMBs than in those without deep or infratentorial CMBs. There was no difference in the prevalence of non-dipping or NH between patients with and without CMBs at any location.
Table 4.
Results of blood pressure measurements according to the presence of CMBs.
| Any CMBs |
Deep or infratentorial CMBs |
Lobar CMBs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Present |
Absent |
p value | Present |
Absent |
p value | Present |
Absent |
p value | |
| (n = 13) | (n = 115) | (n = 7) | (n = 121) | (n = 7) | (n = 121) | ||||
| Schellong test | |||||||||
| Supine SBP, mm Hg | 129.3 (29.3) | 116.8 (18.0) | 0.03 | 131.3 (30.0) | 117.3 (18.8) | 0.07 | 112.7 (30.5) | 117.8 (19.0) | 0.52 |
| Supine DBP, mm Hg | 73.2 (14.2) | 69.6 (11.4) | 0.28 | 75.4 (12.7) | 69.6 (11.6) | 0.20 | 69.0 (15.7) | 70.0 (11.5) | 0.83 |
| ΔSBP, mm Hg | 24.9 (24.0) | 16.4 (20.9) | 0.17 | 35.1 (26.0) | 16.2 (20.6) | 0.02 | 15.6 (16.2) | 17.3 (21.6) | 0.83 |
| ΔDBP, mm Hg | 9.8 (14.4) | 5.5 (12.1) | 0.23 | 16.6 (11.8) | 5.3 (12.1) | 0.02 | 4.1 (13.9) | 6.0 (12.3) | 0.69 |
| Non-OH and non-SH | 3 (23.1) | 61 (53.0) | 0.07 | 0 (0) | 64 (52.9) | 0.01 | 3 (42.9) | 61 (50.4) | 0.37 |
| Only OH | 5 (38.5) | 39 (33.9) | 4 (57.1) | 40 (33.1) | 2 (28.6) | 42 (34.7) | |||
| Only SH | 1 (7.7) | 3 (2.6) | 0 (0) | 4 (3.3) | 1 (14.3) | 3 (2.5) | |||
| Both OH and SH | 4 (30.8) | 12 (10.4) | 3 (42.9) | 13 (10.7) | 1 (14.3) | 15 (12.4) | |||
| ABPM | |||||||||
| 24-hour SBP, mm Hg | 123.1 (9.4) | 114.5 (12.2) | 0.02 | 125.3 (11.2) | 114.8 (12.0) | 0.03 | 120.4 (6.3) | 115.1 (12.4) | 0.26 |
| 24-hour DBP, mm Hg | 79.0 (7.4) | 75.4 (8.4) | 0.14 | 81.6 (8.3) | 75.4 (8.3) | 0.06 | 76.7 (5.3) | 75.7 (8.6) | 0.76 |
| Daytime SBP, mm Hg | 124.5 (10.6) | 116.1 (12.0) | 0.02 | 129.3 (11.1) | 116.3 (11.8) | < 0.01 | 121.1 (8.8) | 116.7 (12.2) | 0.35 |
| Daytime DBP, mm Hg | 81.6 (9.3) | 77.7 (8.9) | 0.15 | 85.7 (10.0) | 77.7 (8.7) | 0.02 | 79.1 (8.4) | 78.1 (9.0) | 0.76 |
| Nighttime SBP, mm Hg | 122.5 (12.4) | 112.4 (15.5) | 0.03 | 119.3 (15.2) | 113.1 (15.5) | 0.31 | 122.6 (11.9) | 112.9 (15.6) | 0.11 |
| Nighttime DBP, mm Hg | 75.7 (7.9) | 71.9 (10.0) | 0.18 | 76.0 (9.3) | 72.0 (9.9) | 0.30 | 73.9 (7.3) | 72.2 (10.0) | 0.66 |
| NH | 10 (76.9) | 70 (60.9) | 0.26 | 4 (57.1) | 76 (62.8) | 0.76 | 6 (85.7) | 74 (61.2) | 0.19 |
| Non-dipping | 11 (84.6) | 85 (73.9) | 0.40 | 5 (71.4) | 91 (75.2) | 0.82 | 6 (85.7) | 90 (74.4) | 0.50 |
ABPM, ambulatory blood pressure monitoring; CMBs, cerebral microbleeds; DBP, diastolic blood pressure; NH, nocturnal hypertension; OH, orthostatic hypotension; SBP, systolic blood pressure; SH, supine hypertension. ΔS(D)BP = S(D)BP supine − S(D)BP standing. Values are number (%) or mean (standard deviation), as appropriate.
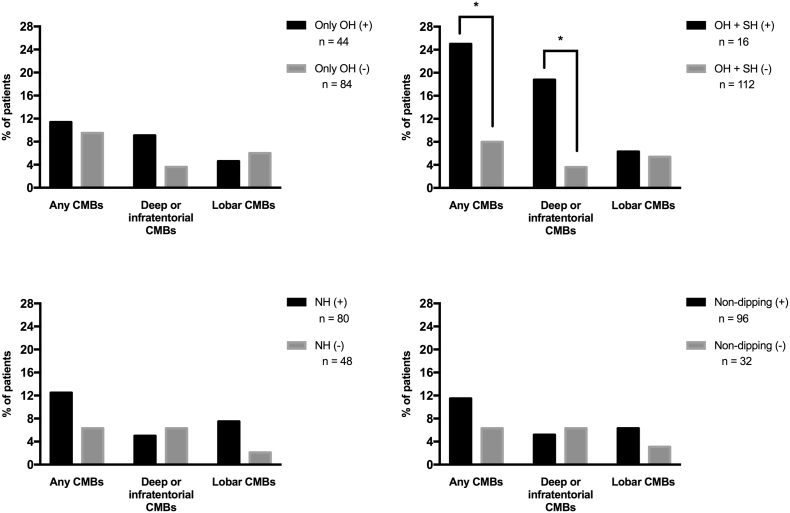
When CMBs were examined as a function of blood pressure abnormalities, the prevalence of any CMBs and that of deep or infratentorial CMBs were significantly higher in patients with both OH and SH than in others (p < 0.05). However, the prevalence of CMBs was not significantly different between patients with OH only and others (Fig. 1).
Fig. 1.
Prevalence of cerebral microbleeds. CMBs, cerebral microbleeds; NH, nocturnal hypertension; OH, orthostatic hypotension; SH, supine hypertension. *p < 0.05.
Using linear regression analysis, we found that supine SBP and the presence of both OH and SH were significantly correlated with an increased CMB count in any region (p < 0.05 and p < 0.01, respectively), and in deep or infratentorial regions (p < 0.05 and p < 0.01, respectively), but not in lobar regions (Table 5). The presence of both OH and SH remained significant after adjustment for age and sex (Table 5, model 1, p < 0.05). Additional adjustments for hypertension, diabetes mellitus, a history of stroke, antiplatelet treatment, and coexisting advanced white matter hyperintensities did not affect this association (Table 5, model 2, p < 0.05).
Table 5.
Linear regression analysis for blood pressure components in relation to CMBs count.
| Blood pressure component | Any CMBs |
Deep or infratentorial CMBs |
Lobar CMBs |
|||
|---|---|---|---|---|---|---|
| B (SE) | 95% CI | B (SE) | 95% CI | B (SE) | 95% CI | |
| Unadjusted | ||||||
| Supine SBP, per SD increase | 0.167 (0.082)a | 0.004 to 0.331 | 0.165 (0.080)a | 0.007 to 0.323 | 0.003 (0.024) | − 0.044 to 0.050 |
| ΔSBP, per SD increase | 0.074 (0.067) | − 0.058 to 0.207 | 0.082 (0.065) | − 0.047 to 0.210 | − 0.008 (0.019) | − 0.045 to 0.030 |
| ΔDBP, per SD increase | 0.088 (0.080) | − 0.070 to 0.247 | 0.089 (0.078) | − 0.065 to 0.243 | − 0.000 (0.023) | − 0.045 to 0.045 |
| Both OH and SH, present vs. absent | 0.383 (0.125)b | 0.136 to 0.632 | 0.384 (0.121)b | 0.145 to 0.623 | 0.000 (0.037) | − 0.073 to 0.073 |
| 24-hour SBP, per SD increase | 0.120 (0.082) | − 0.043 to 0.283 | 0.087 (0.080) | − 0.072 to 0.245 | 0.034 (0.023) | − 0.012 to 0.080 |
| Daytime SBP, per SD increase | 0.097 (0.087) | − 0.074 to 0.269 | 0.077 (0.084) | − 0.089 to 0.243 | 0.020 (0.025) | − 0.028 to 0.069 |
| Daytime DBP, per SD increase | 0.082 (0.085) | − 0.086 to 0.250 | 0.077 (0.082) | − 0.085 to 0.240 | 0.004 (0.024) | − 0.044 to 0.052 |
| Nighttime SBP, per SD increase | 0.023 (0.081) | − 0.137 to 0.183 | − 0.016 (0.078) | − 0.171 to 0.139 | 0.039 (0.023) | − 0.006 to 0.084 |
| Model 1 | ||||||
| Supine SBP, per SD increase | 0.094 (0.086) | − 0.076 to 0.263 | 0.095 (0.083) | − 0.071 to 0.260 | − 0.001 (0.025) | − 0.051 to 0.049 |
| ΔSBP, per SD increase | 0.029 (0.066) | − 0.102 to 0.160 | 0.041 (0.065) | − 0.087 to 0.169 | − 0.012 (0.019) | − 0.050 to 0.265 |
| ΔDBP, per SD increase | 0.120 (0.082) | − 0.043 to 0.283 | 0.109 (0.080) | − 0.050 to 0.268 | 0.011 (0.024) | − 0.037 to 0.059 |
| Both OH and SH, present vs. absent | 0.291 (0.127)a | 0.040 to 0.542 | 0.301 (0.123)a | 0.057 to 0.545 | − 0.010 (0.038) | − 0.085 to 0.038 |
| 24-hour SBP, per SD increase | 0.067 (0.081) | − 0.094 to 0.228 | 0.036 (0.080) | − 0.122 to 0.193 | 0.031 (0.024) | − 0.016 to 0.078 |
| Daytime SBP, per SD increase | 0.055 (0.085) | − 0.113 to 0.223 | 0.037 (0.083) | − 0.126 to 0.201 | 0.018 (0.025) | − 0.031 to 0.067 |
| Daytime DBP, per SD increase | 0.120 (0.082) | − 0.043 to 0.283 | 0.109 (0.080) | − 0.050 to 0.268 | 0.011 (0.024) | − 0.037 to 0.059 |
| Nighttime SBP, per SD increase | − 0.049 (0.081) | − 0.209 to 0.111 | − 0.086 (0.078) | − 0.241 to 0.070 | 0.036 (0.023) | − 0.010 to 0.083 |
| Model 2 | ||||||
| Supine SBP, per SD increase | 0.093 (0.072) | − 0.051 to 0.237 | 0.078 (0.070) | − 0.061 to 0.217 | 0.015 (0.024) | − 0.034 to 0.063 |
| ΔSBP, per SD increase | 0.039 (0.055) | − 0.071 to 0.148 | 0.050 (0.053) | − 0.056 to 0.155 | − 0.011 (0.018) | − 0.048 to 0.025 |
| ΔDBP, per SD increase | 0.055 (0.068) | − 0.080 to 0.190 | 0.063 (0.066) | − 0.066 to 0.193 | − 0.009 (0.023) | − 0.054 to 0.037 |
| Both OH and SH, present vs. absent | 0.253 (0.111)a | 0.036 to 0.472 | 0.249 (0.106)a | 0.038 to 0.459 | 0.004 (0.038) | − 0.071 to 0.079 |
| 24-hour SBP, per SD increase | 0.039 (0.069) | − 0.097 to 0.174 | 0.015 (0.066) | − 0.115 to 0.146 | 0.023 (0.023) | − 0.022 to 0.068 |
| Daytime SBP, per SD increase | 0.029 (0.071) | − 0.112 to 0.169 | 0.010 (0.068) | − 0.126 to 0.145 | 0.019 (0.024) | − 0.028 to 0.066 |
| Daytime DBP, per SD increase | 0.066 (0.070) | − 0.073 to 0.205 | 0.065 (0.068) | − 0.069 to 0.199 | 0.001 (0.0234) | − 0.045 to 0.048 |
| Nighttime SBP, per SD increase | − 0.068 (0.068) | − 0.201 to 0.066 | − 0.091 (0.065) | − 0.219 to 0.038 | 0.023 (0.023) | − 0.022 to 0.068 |
CMBs, cerebral microbleeds; DBP, diastolic blood pressure; OH, orthostatic hypotension; SBP, systolic blood pressure; SH, supine hypertension; SD, standard deviation; SE, standard error. ΔS(D)BP = S(D)BP supine − S(D)BP standing. Model 1: adjusted for age and sex; Model 2: additionally adjusted for hypertension, diabetes mellitus, history of stroke, antiplatelet treatment, and advanced white matter hyperintensities.
p < 0.05.
p < 0.01.
4. Discussion
Here, we demonstrated that the presence of both OH and SH is associated with increased CMB count in deep or infratentorial regions, independently of age, sex, cardiovascular risk factors, and coexisting cerebrovascular disease. However, NH and non-dipping, as assessed by ABPM, were not associated with CMBs. Furthermore, there were no associations between any blood pressure abnormalities and CMBs in lobar regions.
Recent research has suggested a negative impact of blood pressure abnormalities on the cerebrovascular system in patients with PD. For instance, the presence of OH, SH, or both have been associated with advanced white matter lesions [2]. Furthermore, orthostatic blood pressure changes and supine blood pressure [3], and the presence of NH and nighttime SBP [4], have been found to be positively correlated with the severity of white matter lesions. In addition, increased intima media thickness of the carotid artery has been associated with OH, SH, and supine and overnight blood pressure [5].
It has been suggested that autonomic dysfunction might share some pathophysiological aspects with target organ damage in essential hypertension [25]. SH is associated with left ventricular hypertrophy [26] and renal impairment [27] in patients with primary autonomic failure. Our study showed that OH was associated with deep or infratentorial CMBs in combination with SH, but not when it existed alone. These findings indicate that cerebral hypoperfusion by OH alone is not sufficient to cause deep or infratentorial CMBs, and that SH contributes to the development of such lesions. This is consistent with evidence that deep or infratentorial CMBs may reflect hypertensive microangiopathy in general [9], [10], [11], [12]. In our study, it was not possible to analyze the relative contributions of OH and SH to CMBs separately because most patients with SH (85%) had coexisting OH. Whether SH alone is sufficient to cause CMBs thus remains unknown. However, it should be noted that vasodilation of cerebral vessels occurs to ensure cerebral hypoperfusion during OH [28], and Indelicato et al. [29] have suggested that such adaptations of autoregulation to chronic OH may be directly related to increased susceptibility to hypertensive peaks.
Associations between blood pressure abnormalities, as assessed by ABPM, and CMBs have been found in other patient groups. For example, the presence of CMBs is associated with daytime, nighttime, and 24-hour blood pressure levels and NH, but not with nocturnal dipping in hypertensive subjects without a history of cerebrovascular disease [24]. Nocturnal reverse dipping (blood pressure increase during nighttime) is associated with CMBs in patients with both hypertension and ischemic stroke [30]. However, CMBs were associated with neither NH nor nocturnal dipping in our study. Thus, the presence of both OH and SH in particular may be more strongly associated with CMBs than nocturnal blood pressure abnormalities in patients with PD.
Our study has some limitations. First, due to the cross-sectional design of the study, we were unable to make causal inferences. In addition, the number of patients with CMBs was small in the present study, which could weaken the statistical power. Indeed, the prevalence of CMBs in our present study (10.2%) was lower than that in other and our previous studies [14], [15] (approximately 17%), which might be explained by a difference in age. Second, whether the risk of CMBs in patients with PD is greater than that of age-matched healthy controls was not determined. Further studies are needed to determine whether the association between orthostatic blood pressure abnormalities and CMBs is specific to patients with PD or if it also applies to non-PD individuals. Third, 24-h blood pressure monitoring and hospitalization may interfere with quality of sleep and affect the accuracy of the nighttime blood pressure measurements. Data on sleep quality during hospitalization was not obtained in our study. Another limitation is the possible influence of medications on blood pressure abnormalities. Namely, OH may be associated with antihypertensive and anti-Parkinson drugs. Our study showed that the frequency of diuretic use was significantly higher in patients with OH than in those without OH. Thus, diuretic use might be associated with the occurrence of OH in our patients. Meanwhile, anti-hypotensive drugs are widely used in patients with OH and may cause SH. However, the prevalence of anti-hypotensive use was not different in patients with and without SH. Thus, SH was not merely the result of anti-hypotensive treatment in patients with OH. SH is frequently found in patients with PD with OH; Umehara et al. reported that 55% of patients with OH had SH [31]. In our study, the prevalence of SH was 26.7% in patients with OH. This difference may be related to the lower prevalence of hypertension and the lower age of patients in our study than in theirs, which was associated with the prevalence of SH [31]. Although the mechanisms of co-occurrence of OH and SH in PD remain poorly understood, baroreflex failure and abnormal natriuresis may be implicated [32].
5. Conclusion
Our results suggest the importance of coexisting OH and SH in cerebrovascular injury in patients with PD. Myriad episodes of cerebral hypo- and hyperperfusion related to OH and SH may contribute to CMBs in deep or infratentorial regions. Future studies should be carried out to address whether modifying blood pressure abnormalities is beneficial in preventing such lesions.
Funding
This study was supported by the Health and Labour Sciences Research Grant (H26-Nanchito(Nan)-Ippan-085).
Disclosure of conflicts of interest
The authors have no financial or other conflicts of interest to declare.
Contributor Information
Kazuo Yamashiro, Email: kazuo-y@juntendo.ac.jp.
Nobutaka Hattori, Email: nhattori@juntendo.ac.jp.
References
- 1.Espay A.J., LeWitt P.A., Hauser R.A. Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15:954–966. doi: 10.1016/S1474-4422(16)30079-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.S., YS Oh., Lee K.S. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology. 2012;79:1323–1331. doi: 10.1212/WNL.0b013e31826c1acd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.YS Oh., Kim J.S., Lee K.S. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J. Mov. Dis. 2013;6:23–27. doi: 10.14802/jmd.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.YS Oh., Kim J.S., Yang D.W. Nighttime blood pressure and white matter hyperintensities in patients with Parkinson disease. Chronobiol. Int. 2013;30:811–817. doi: 10.3109/07420528.2013.766618. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.S., Oh Y.S., Lee K.S. Carotid artery thickening and neurocirculatory abnormalities in de novo Parkinson disease. J. Neural Transmission (Vienna) 2014;121:1259–1268. doi: 10.1007/s00702-014-1203-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S.M., Vernooij M.W., Cordonnier C. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashiro K., Tanaka R., Okuma Y. Cerebral microbleeds are associated with worse cognitive function in the nondemented elderly with small vessel disease. Cerebrovascul. Dis. Extra. 2014;4:212–220. doi: 10.1159/000369294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charidimou A., Kakar P., Fox Z. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F., Kleinert R., Roob G. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. Am. J. Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 10.Vernooij M.W., van der Lugt A., Ikram M.A. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 11.Yamashiro K., Tanaka R., Okuma Y. Associations of durations of antiplatelet use and vascular risk factors with the presence of cerebral microbleeds. J. Stroke Cerebrovasc. Dis. 2014;23:433–440. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Yates P.A., Villemagne V.L., Ellis K.A. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front. Neurol. 2014;4(205) doi: 10.3389/fneur.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham J.H., Yi H., Sunwoo M.K. Cerebral microbleeds in patients with Parkinson's disease. J. Neurol. 2014;261:1628–1635. doi: 10.1007/s00415-014-7403-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.H., Park J., Kim Y.H. Characterization of cerebral microbleeds in idiopathic Parkinson's disease. Eur. J. Neurol. 2015;22:377–383. doi: 10.1111/ene.12584. [DOI] [PubMed] [Google Scholar]
- 15.Yamashiro K., Tanaka R., Hoshino Y. The prevalence and risk factors of cerebral microbleeds in patients with Parkinson's disease. Parkinsonism Relat. Disord. 2015;21:1076–1081. doi: 10.1016/j.parkreldis.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Hughes A.J., Daniel S.E., Kilford L. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlinson C.L., Stowe R., Patel S. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 18.Freeman R., Wieling W., Axelrod F.B. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian A.V., Bakris G.L., Black H.R. Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Staessen J.A., Asmar R., De Buyzere M. Task force II: blood pressure measurement and cardiovascular outcome. Blood Press Monit. 2001;6:355–370. doi: 10.1097/00126097-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G., De Backer G., Dominiczak A. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur. Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 22.Gregoire S.M., Chaudhary U.J., Brown M.M. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F., Chawluk J.B., Alavi A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 24.Henskens L.H., van Oostenbrugge R.J., Kroon A.A. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68. doi: 10.1161/HYPERTENSIONAHA.107.100610. [DOI] [PubMed] [Google Scholar]
- 25.Milazzo V., Di Stefano C., Milan A. Cardiovascular complications in patients with autonomic failure. Clin. Auton. Res. 2015;25:133–140. doi: 10.1007/s10286-015-0275-0. [DOI] [PubMed] [Google Scholar]
- 26.Vagaonescu T.D., Saadia D., Tuhrim S. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. doi: 10.1016/S0140-6736(99)05320-9. [DOI] [PubMed] [Google Scholar]
- 27.Garland E.M., Gamboa A., Okamoto L. Renal impairment of pure autonomic failure. Hypertension. 2009;54:1057–1061. doi: 10.1161/HYPERTENSIONAHA.109.136853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz D.R., Kaufmann H. Autoregulatory cerebral vasodilation occurs during orthostatic hypotension in patients with primary autonomic failure. Clin. Auton. Res. 2001;11:363–367. doi: 10.1007/BF02292768. [DOI] [PubMed] [Google Scholar]
- 29.Indelicato E., Fanciulli A., Poewe W. Cerebral autoregulation and white matter lesions in Parkinson's disease and multiple system atrophy. Parkinsonism Relat. Disord. 2015;21:1393–1397. doi: 10.1016/j.parkreldis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Kwon H.M., Lim J.S., Kim Y.S. Cerebral microbleeds are associated with nocturnal reverse dipping in hypertensive patients with ischemic stroke. BMC Neurol. 2014;14(8) doi: 10.1186/1471-2377-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umehara T., Matsuno H., Toyoda C. Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin. Auton. Res. 2016;26:15–21. doi: 10.1007/s10286-015-0324-8. [DOI] [PubMed] [Google Scholar]
- 32.Sharabi Y., Goldstein D.S. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J. Neurol. Sci. 2011;310:123–128. doi: 10.1016/j.jns.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]