Abstract
Background
An aging population and a high incidence of colorectal cancer (CRC) in patients over the age of 80 make it important to understand survival times, hazard ratios and prognostic factors in this group. A better understanding of these factors will help clinicians determine appropriate therapeutic strategies for such patients, including when more aggressive treatment strategies may be preferred to palliative treatment.
Methods
A retrospective analysis of 619 CRC patients of ≥80 years of age from 1991–2010 at Baylor Scott & White Hospital in Temple, Texas. Twelve variables were analyzed through statistical analysis as potential prognostic factors for survival. Univariate and multivariate Cox proportional hazard models were used to determine hazard ratios. The elderly population was further stratified by age subgroup (80–84, 85–89, ≥90).
Results
Median survival time was 53.6, 30.0, and 11.3 months for age groups of 80–84, 85–89, and ≥90, respectively. Median survival time for stage 0/I, II, III, and IV patients was 72.4, 53.5, 28.0, and 5.9 months, respectively. Patients not receiving surgery had significantly higher mortality (hazard ratio 2.605; 95% CI, 1.826–3.694). For stage III CRC patients, those not receiving chemotherapy had significantly higher mortality (hazard ratio 1.808; 95% CI, 1.018–1.827).
Conclusions
Our study provides evidence to support the benefits of surgery and chemotherapy (for stage III) patients over 80, potentially contributing to improved clinical decisions in treating elderly CRC patients. Such patients are sometimes undertreated due to their underrepresentation in clinical trials. Additional prospective studies with a higher proportion of patients over 80 are needed.
Keywords: Geriatrics, aged, treatment, survival
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the U.S. of cancers affecting both men and women and the third most common cancer (1). It is estimated that 134,000 people were diagnosed with CRC and approximately 49,000 people died from CRC in 2016 (2). CRC primarily affects the elderly population, with 42% of cases occurring in patients >75 years of age (3) and a median age of diagnosis of 68 years in the U.S. (4). In particular, effective CRC management and treatment presents unique challenges in the elderly due to heterogeneity in the geriatric population with respect to clinical frailty and functional level, coexisting medical conditions, discrepancies between biological and chronological age, and social care issues (5-7). Elderly patients continue to remain underrepresented in clinical trials (8). A study of National Cancer Institute (NCI)-sponsored trials found a 54% elderly participation rate in early stage CRC clinical trials compared to a 78% incidence rate, and a 41% elderly participation rate in late stage CRC trials compared to a 73% incidence rate (9). This discrepancy between the ages of patients enrolled in clinical trials used to evaluate treatment options and the ages of patients seen more frequently in clinical practice limits the evidence-based guidance available to physicians in treating elderly CRC patients (10).
Despite the underrepresentation of older patients in clinical trials, successful outcomes among such patients have led researchers to conclude that age should not be used as a sole criterion to determine treatment options for older patients (11). However, as patients with CRC continue to get older, it becomes important to extend the definition from the elderly to the “oldest old” (12,13). Therefore, we study an underrepresented group even within the elderly population—patients 80 years and older. Since the definition of elderly remains unclear in the literature (14), with some papers considering 65+ as elderly (8,9,15), others considering a threshold of 70 years, and yet others using 75 years as the cutoff (16), the present study looks at patients over age 80, and divides them into three subgroups: 80–84, 85–89, and 90+. As life expectancies increase and the demographic proportion of the elderly continues to grow, the medical and societal burdens of CRC are only expected to increase (5), necessitating a better understanding of prognostic factors and treatment considerations in the oldest CRC patients (17).
Methods
Patients
A retrospective review of the Baylor Scott & White Memorial Hospital electronic medical system and tumor registry was performed to identify patients ≥80 years of age with CRC during the 20-year period between 1991 and 2010.
Factors analyzed
Twelve potential prognostic variables were identified for the purposes of this analysis—age (80–84, 85–89, ≥90), gender (male or female), race (white or non-white), ethnicity (Hispanic or non-Hispanic), stage (0, I, II, III, or IV), grade (I, II, III, or IV), site, histology, chemotherapy (yes or no), radiation (yes or no), surgery (yes or no), and surgery type.
Statistical analysis
Overall survival curves were estimated by the Kaplan-Meier method and statistical significance of variables were determined by log-rank tests. To evaluate the effect of prognostic factors on survival, multivariate hazard ratios were determined by a Cox proportional hazards model fitted to the data. Ninety-five percent profile likelihood confidence intervals were calculated for hazard ratios. Variables with a P<0.25 in a univariate Cox regression were considered as potential predictors to be included in the multivariate Cox model. Variable selection was then determined by forward selection, backward elimination, and stepwise process. The final multivariate model considered mortality as a function of the variables agreed upon by all three procedures. For CRC patients age 80 and over, the multivariate Cox proportional hazards model considers mortality as a function of age, stage, surgery, and chemotherapy. Patients were then stratified by age group and additional multivariate Cox regressions were performed, considering mortality as a function of stage and surgery in both the age 80–84 and age 85–89 groups, and as a function of stage, surgery, and gender in the age ≥90 group. For the multivariate analysis for stage III CRC patients only, the final model considered mortality as function of the variables determined by backward elimination—race, surgery, and chemotherapy. Residual analyses were performed to assess the fit of the data, check assumptions, and identify outliers, with a level of 0.05 considered statistically significant. In addition, comparison of variables by mortality was performed by Kruskal-Wallis, chi-square, or Wilcoxon-Sum-Rank tests, where appropriate. Statistical data analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). R software version 3.1.0 was used for the survival curves. StatXact software version 10 was used for exact Kruskal-Wallis tests.
Results
A total of 619 patients 80 years of age or older diagnosed with CRC between 1991 and 2010 were reviewed. Patient demographics and clinical characteristics of patients are shown in Table 1.
Table 1. Patient demographics and clinical characteristics.
| Characteristic | No. (%) of patients |
|---|---|
| Age, years | |
| 80–84 | 352 (56.9) |
| 85–89 | 205 (33.1) |
| 90 and older | 62 (10.0) |
| Gender | |
| Male | 253 (40.9) |
| Female | 366 (59.1) |
| Race (frequency missing =2) | |
| White | 576 (93.4) |
| Non-white | 41 (6.7) |
| Ethnicity (frequency missing =3) | |
| Hispanic | 26 (4.2) |
| Non-Hispanic | 590 (95.8) |
| Stage (frequency missing =21) | |
| Stage 0 | 33 (5.5) |
| Stage I | 160 (26.8) |
| Stage II | 169 (28.3) |
| Stage III | 140 (23.4) |
| Stage IV | 96 (16.1) |
| Grade (frequency missing =111) | |
| Grade I | 10 (2.0) |
| Grade II | 270 (53.1) |
| Grade III | 206 (40.6) |
| Grade IV | 22 (4.3) |
| Site | |
| Cecum, ascending colon, hepatic flexure | 290 (46.9) |
| Transverse colon | 48 (7.8) |
| Sigmoid, descending colon, splenic flexure | 127 (20.5) |
| Rectosigmoid junction | 29 (4.7) |
| Rectum | 107 (17.3) |
| Colon | 11 (1.8) |
| Appendix | 7 (1.1) |
| Histology (frequency missing =11) | |
| Adenocarcinoma | 581 (95.6) |
| Carcinoma | 25 (4.1) |
| Neuroendocrine carcinoma | 2 (0.3) |
| Chemotherapy (frequency missing =15) | |
| No | 514 (85.1) |
| Yes | 90 (14.9) |
| Radiation (frequency missing =2) | |
| No | 582 (94.3) |
| Yes | 35 (5.7) |
| Surgery (frequency missing =6) | |
| No | 92 (15.0) |
| Yes | 521 (85.0) |
| Surgery type (frequency missing =6) | |
| None | 92 (15.0) |
| Polypectomy | 29 (4.7) |
| Proctocolectomy, hemicolectomy, colectomy | 474 (77.3) |
| Transanal excision | 18 (2.9) |
| Mortality status | |
| Alive | 143 (23.1) |
| Dead | 476 (76.9) |
Univariate analyses
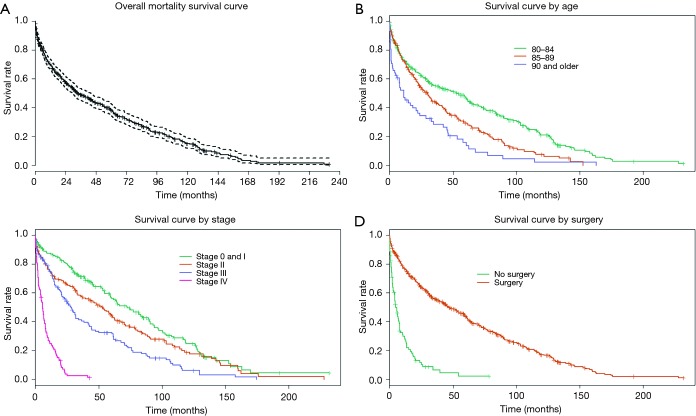
Kaplan-Meier curves were generated to estimate survival times. Median survival time across all CRC patients age 80 and above was 33.3 (95% CI, 29.0–43.3) months (Figure 1A).Median survival in age groups of 80–84, 85–89, and ≥90 was 53.6 (95% CI, 34.2–61.9), 30.0 (95% CI, 22.6–37.4), and 11.3 (95% CI, 4.9–22.2) months, respectively (Figure 1B). Median survival for stage 0/I, II, III, and IV patients was 72.4 (95% CI, 59.4–89.3), 53.5 (95% CI, 35.5–62.5), 28.0 (95% CI, 20.9–34.2), and 5.9 (95% CI, 4.0–7.4) months, respectively (Figure 1C). Median survival was 44.0 (95% CI, 34.2–56.1) months for patients with grade I or II CRC, and 26.8 months for patients with grade III or IV CRC (95% CI, 20.5–33.6).
Figure 1.
Overall survival curve as well as survival by age, stage, and surgery: (A) overall mortality survival curve; median survival time across all CRC patients age 80 and above was 33.3 (95% CI, 29.0–43.3) months; (B) survival curve by age; median survival in age groups of 80–84, 85–89, and ≥90 was 53.6 (95% CI, 34.2–61.9), 30.0 (95% CI, 22.6–37.4), and 11.3 (95% CI, 4.9–22.2) months, respectively; (C) survival curve by stage; median survival for stage 0/I, II, III, and IV patients was 72.4 (95% CI, 59.4–89.3), 53.5 (95% CI, 35.5–62.5), 28.0 (95% CI, 20.9–34.2), and 5.9 (95% CI, 4.0–7.4) months, respectively; (D) Kaplan-Meier survival curves were also generated based on treatment type. Treatment by surgery appeared to have the most dramatic effect on median survival time with 42 additional months of survival, from 4.7 (95% CI, 3.1–7.7) without surgery to 46.7 (95% CI, 39.2–58.0) months with surgery. CRC, colorectal cancer.
Kaplan-Meier survival curves were also generated based on treatment type. Treatment by surgery appeared to have the most dramatic effect on median survival time with 42 additional months of survival, from 4.7 (95% CI, 3.1–7.7) without surgery to 46.7 (95% CI, 39.2–58.0) months with surgery (Figure 1D). For stage III CRC patients, median survival time increased by 13.7 months—from 29.0 (95% CI, 21.0–41.8) with surgery versus 15.3 (95% CI, 4.0–25.2) months for patients who did not receive surgery. Stage IV CRC patients had a median survival of 8.3 (95% CI, 6.0–11.3) months with surgery versus 2.6 (95% CI, 1.8–4.7) months for patients who did not receive surgery. For stage III CRC patients, chemotherapy was associated with increased median survival time of 20.5 months, from 22.1 (95% CI, 14.8–28.9) months without chemotherapy to 42.6 (95% CI, 29.0–67.6) months for those receiving chemotherapy. For stage IV CRC patients, median survival times were 4.4 (95% CI, 2.2–6.6) months without chemotherapy versus 7.9 (95% CI, 5.9–12.9) months for patients receiving chemotherapy. Treatment by radiation had no impact on survival times; although it must be kept in mind that only 35/617 patients received radiation.
Demographic variables—race, gender, and ethnicity—did not influence survival times; with the exception of gender for patients over 90 (see multivariate results for “age” below). There was no relationship between either locations of CRC or histology and mortality in our study (see Table 1 for a description of these variables).
Multivariate analyses
To identify prognostic factors that are predictive of survival in elderly CRC patients, a multivariate Cox regression analysis was conducted assuming a proportional hazard rate. The multivariate model considered mortality as a function of four variables found to be statistically significant: age, stage, surgery, and chemotherapy (Table 2).
Table 2. Parameters that correlate with survival of stage 0/I–IV CRC patients ages ≥80 in multivariate analysis.
| Parameter | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age, years | |||
| 80–84 | 1.000 | − | − |
| 85–89 | 1.483 | 1.199–1.828 | 0.0003 |
| ≥90 | 1.964 | 1.404–2.695 | <0.0001 |
| Stage | |||
| 0&I | 1.000 | − | − |
| II | 1.398 | 1.089–1.795 | 0.0086 |
| III | 2.315 | 1.761–3.036 | <0.0001 |
| IV | 6.462 | 4.500–9.240 | <0.0001 |
| Chemotherapy | |||
| Yes | 1.000 | − | − |
| No | 1.355 | 1.018–1.827 | 0.0413 |
| Surgery | |||
| Yes | 1.000 | − | − |
| No | 2.605 | 1.826–3.694 | <0.0001 |
CRC, colorectal cancer.
Age
Compared with patients aged 80–84, higher mortality was observed in patients with advancing age: 85–89 years (hazard ratio 1.483; 95% CI, 1.199–1.828), and 90 and over (hazard ratio 1.964; 95% CI, 1.404–2.695) (Table 2). A stratified regression analysis by age was also performed to assess if a Cox proportional hazard model by age group would attain a better fit with the data (Table 3). The stratification exercise shows that, with one exception, predictors of mortality and the effect on hazard ratios do not vary by age—all age groups shared stage and surgery as predictors of mortality. However, for patients 90 and older, gender was also a predictor of mortality in addition to stage and surgery, with women having lower hazard ratios than men (0.373; 95% CI, 0.187–0.766). This may be because, on average, women have longer life expectancies as compared to men.
Table 3. Parameters that correlate with survival of stage 0/I–IV CRC patients in multivariate analysis.
| Parameter | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Ages 80–84 | |||
| Stage | |||
| 0 & I | 1.000 | − | − |
| II | 0.977 | 0.684–1.387 | 0.8990 |
| III | 2.336 | 1.678–3.250 | <0.0001 |
| IV | 6.462 | 4.500–9.240 | <0.0001 |
| Surgery | |||
| Yes | 1.000 | − | − |
| No | 2.673 | 1.702–4.124 | <0.0001 |
| Ages 85–89 | |||
| Stage | |||
| 0 & I | 1.000 | − | − |
| II | 1.969 | 1.287–3.041 | 0.0019 |
| III | 1.823 | 1.115–2.959 | 0.0154 |
| IV | 6.348 | 3.489–11.417 | <0.0001 |
| Surgery | |||
| Yes | 1.000 | − | − |
| No | 2.414 | 1.263–4.510 | 0.0064 |
| Ages ≥90 | |||
| Stage | |||
| 0 & I | 1.000 | − | − |
| II | 2.462 | 1.150–5.386 | 0.0209 |
| III | 1.809 | 0.726–4.340 | 0.1879 |
| IV | 3.361 | 1.271–8.913 | 0.0142 |
| Surgery | |||
| Yes | 1.000 | − | − |
| No | 2.792 | 1.161–6.497 | 0.0191 |
| Gender | |||
| Male | 1.000 | − | − |
| Female | 0.373 | 0.187–0.766 | 0.0057 |
CRC, colorectal cancer.
Stage
Compared with patients with stage 0 and I CRC, higher mortality was observed in patients with advancing stage: stage II (hazard ratio 1.398; 95% CI, 1.089–1.795), stage III (hazard ratio 2.315; 95% CI, 1.761–3.036), and stage IV (hazard ratio 6.462; 95% CI, 4.500–9.240) (Table 2).
In the 80–84 age group (Table 3), stage III patients (hazard ratio 2.336; 95% CI, 1.678–3.250) and stage IV patients (hazard ratio 6.462; 95% CI, 4.500–9.240) experienced significantly higher mortality compared to stage 0 and I patients. Interestingly, there was no difference in survival between stages 0, I, and stage II patients (hazard ratio 0.977; 95% CI, 0.684–1.387). In the 85–89 age group mortality was observed to rise with advancing stage—relative to stages 0 and I, stage II (hazard ratio 1.969; 95% CI, 1.287–3.041), stage III (hazard ratio 1.823; 95% CI, 1.115–2.959) and stage IV (hazard ratio 6.348; 95% CI, 3.489–11.417) had higher mortality. In the ≥90 age group, stage II (hazard ratio 2.462; 95% CI, 1.150–5.386), and stage IV (hazard ratio 3.361; 95% CI, 1.271–8.913) patients had higher mortality than stage 0 and I patients. There was no significant difference in mortality between stage III patients and stage 0 and I patients (hazard ratio 1.809; 95% CI, 0.726–4.340). Overall, the model for patients age 80–84 and 85–89 tended to overestimate some survival times, whereas the model for patients 90 years and older underestimated survival times. Although the model adequately describes the retrospective data, the residual analyses indicate that the model is not predictive.
Treatment
Most patients in our study received surgery (521/619 patients). Patients who did not undergo surgery had higher mortality than patients who did (hazard ratio 2.605; 95% CI, 1.826–3.694), with every age group (Table 3) experiencing higher mortality—the 80–84 age group (hazard ratio 2.673; 95% CI, 1.702–4.124), the 85–89 age group (hazard ratio 2.414; 95% CI, 1.263–4.510), and the ≥90 age group (hazard ratio 2.792; 95% CI, 1.161–6.497).
A Cox proportional hazard model fitted to data for stage III CRC patients only (Table 4) indicated that patients who did not receive chemotherapy had higher mortality than patients who received chemotherapy (hazard ratio 1.808; 95% CI, 1.018–1.827). This was the only subgroup by age or stage for which chemotherapy lowered mortality hazard in the present study, suggesting that stage III CRC patients is the group most likely to benefit from chemotherapy. The impact of chemotherapy for stages 0, I, and II could not be assessed to due to a small sample size of patients receiving chemotherapy (n=21). Likewise, a Cox proportional hazard model on the impact of chemotherapy for stage IV patients could not be estimated due to the small number of surviving patients (n=4).
Table 4. Parameters that correlate with survival of stage III CRC patients ages ≥80 in multivariate analysis.
| Parameter | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Racea | |||
| White | 1.000 | − | − |
| Non-white | 0.321 | 0.096–0.792 | 0.0350 |
| Chemotherapy | |||
| Yes | 1.000 | − | − |
| No | 1.808 | 1.018–1.827 | 0.0102 |
| Surgery | |||
| Yes | 1.000 | − | − |
| No | 2.724 | 1.826–3.694 | 0.0346 |
a, results regarding race must be viewed with caution as only 41/617 patients were non-white. CRC, colorectal cancer.
Discussion
Our results suggest that patients over 80 are likely to benefit from treatment that clinicians may sometimes be hesitant to provide today given the lack of studies in this subgroup (17). This finding is important because CRC is primarily considered a disease of the elderly (18) as the risk of getting CRC increases with age (19,20). However, undertreatment of the elderly persists because of lack of data on whether the benefits and duration of survival justify the potential complications of aggressive treatment in this population (21).
In our study, the benefit of surgery in treating CRC was clear across all age subgroups in the elderly and across stages, providing additional support for recent findings that age over 80 alone should not preclude surgical intervention in patients with acceptable operative risk (22). Surgery is widely regarded to be the most successful treatment for colorectal tumors (14). Surgical resection is considered a curative treatment for CRC (23) and can also be used palliatively to avoid complications such as obstruction and perforation (18). However, in practice, the treatment received by the elderly population remains suboptimal—with a lower percentage of patients operated on, a lower rate of curative surgery, and a higher rate of emergency surgery as compared to younger patients (6). Patients over 80 receive emergency surgery at a rate that is 1.7 times higher than those under 65, and are also less likely to be screened (23). A caveat to our result is the 90+ age group, in which careful decision is required by patients and medical practitioners as to whether surgery is warranted because median survival increased by less than 6 months (from 2.6 to 8.3 months). Prior research indicates that postoperative mortality for CRC patients increases from 8% in the 80–84 age group to 13% in the 85–89 age group to 20% in the 90+ age group (24). Additionally, in the 90+ age group, our results suggest that females may be better candidates for surgery given their higher survival rates.
Chemotherapy is standard care in stage III and IV CRC patients. The effectiveness of adjuvant chemotherapy observed in this study for stage III patients is consistent with prior research (14). Unfortunately, we found that only 32% of stage III patients in our study received adjuvant chemotherapy, similar to the Sanoff et al. finding that only 43% of colon cancer patients aged 80–84 and 14% aged ≥85 received postoperative adjuvant chemotherapy compared with 63% aged 75–79 (16). A systematic review of 25 articles covering 39 studies on the safety and efficacy of chemotherapy in patients over 65 years of age concluded that the relative safety and efficacy is similar for older versus younger patients with stage III colon cancer, and that the significant underrepresentation of elderly patients in clinical trials has led to uncertainty around and underuse of chemotherapy in patients over 65 (15). In particular, there are concerns around increased toxicity and drug interactions in polymedicated elderly patients (5). However, our results indicate that stage III CRC patients may not be receiving adjuvant chemotherapy as often as the evidence suggests they should.
Limitations of the study include those inherent in any retrospective study. Future research would benefit from further granularity of data, for example, examining colon cancer and rectal cancer separately. Also, this study lacks racial diversity (n=41 non-white versus n=576 white) and ethnic diversity (n=26 Hispanic versus n=590 non-Hispanic). While the implications of race and ethnicity on CRC survival are unclear across the general population, this is especially true in the elderly population. In patients 50 years and older, CRC incidence rates were found to be highest among black patients (25), and across age groups in stage III colon cancer treated with adjuvant chemotherapy, 5-year relative survival has been consistently found to be lower in blacks than whites by approximately 3% to 4% per cohort (26). In addition, given the benefits of surgery in treating CRC in the elderly, further analysis of outcomes in emergency versus elective surgery would be helpful. In the elderly, prior research has found an increased rate of postoperative mortality in emergency CRC cases, with older age being a predictive factor of increased mortality when associated with emergency rather than elective surgery (27). Disentangling the effect of comorbidities from the effect of age on survival would also help further refine appropriate evidence-based treatment protocols in the geriatric CRC population (17). In an analysis of 137,536 CRC patients aged 66 years or older, approximately 16% of patients presented with 2 or more comorbidities and 25% presented with one comorbidity, with the most common comorbidities in this population found to be diabetes and sequelae (17.2%), chronic obstructive pulmonary disease (COPD) (12.9%), and congestive heart failure (11.6%) (28). Finally, it was difficult to assess the impact of chemotherapy on stage 0 & I, II, and IV CRC cases due to sample limitations, with a small sample size of stage 0, I, and II patients receiving chemotherapy (n=21), and a small sample size of stage IV survivors (n=4).
Conclusions
CRC treatment by surgery across all ages and stages and by chemotherapy in stage III cases was shown to increase median survival time in elderly patients, indicating that selected geriatric CRC patients can benefit from these treatments. Age alone should not preclude “oldest old” patients from receiving more aggressive treatments for CRC, particularly those patients with acceptable risk tolerance and without conflicting comorbidities. Additional prospective studies focused on the elderly will enable physicians to more precisely select the appropriate treatment modality, in conjunction with geriatric assessment of patient fitness and the patient’s own preference. Studies of elderly CRC patients have used 65, 70 or sometimes 75 years of age as a cutoff for defining the elderly. However, as the population of patients with CRC ages, clinicians are likely to see many more patients over the age of 80, adding to the importance of establishing evidence-based treatment guidelines for this group.
Acknowledgements
The authors thank Baylor Scott & White Health for its support in making this research possible.
Ethical Statement: The study was approved by the Scott and White Institutional Review Board of #IRB00000706. The IRB committee waived informed consent as patient information was de-identified prior to analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Colorectal cancer statistics. Atlanta, GA: Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, 2017. Available online: https://www.cdc.gov/cancer/colorectal/statistics/
- 2.US Preventive Services Task Force , Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315:2564-75. 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002;94:2766-92. 10.1002/cncr.10593 [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al. Previous Version: SEER cancer statistics review, 1975-2013. Bethesda, MD: National Cancer Institute, 2016. Available online: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 5.Köhne CH, Folprecht G, Goldberg RM, et al. Chemotherapy in elderly patients with colorectal cancer. Oncologist 2008;13:390-402. 10.1634/theoncologist.2007-0043 [DOI] [PubMed] [Google Scholar]
- 6.Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204-20. 10.4251/wjgo.v7.i10.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decoster L, Vanacker L, Kenis C, et al. Relevance of geriatric assessment in older patients with colorectal cancer. Clin Colorectal Cancer 2017;16:e221-9. 10.1016/j.clcc.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. 10.1056/NEJM199912303412706 [DOI] [PubMed] [Google Scholar]
- 9.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383-9. 10.1200/JCO.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 10.McCleary NJ. Treatment considerations in elderly colorectal cancer patients. Clin Adv Hematol Oncol 2010;8:337-9. [PMC free article] [PubMed] [Google Scholar]
- 11.Chu E. Equal opportunity for the elderly. Clin Colorectal Cancer 2008;7:356. 10.3816/CCC.2008.n.046 [DOI] [PubMed] [Google Scholar]
- 12.Al-Refaie WB, Parsons HM, Habermann EB, et al. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg 2011;253:947-52. 10.1097/SLA.0b013e318216f56e [DOI] [PubMed] [Google Scholar]
- 13.Neuman HB, O’Connor ES, Weiss J, et al. Surgical treatment of colon cancer in patients aged 80 years and older: analysis of 31,574 patients in the SEER-Medicare database. Cancer 2013;119:639-47. 10.1002/cncr.27765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol 2009;20:5-16. 10.1093/annonc/mdn532 [DOI] [PubMed] [Google Scholar]
- 15.Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage III colon cancer: a systematic review. Oncologist 2013;18:54-63. 10.1634/theoncologist.2012-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624-34. 10.1200/JCO.2011.41.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko JJ, Kennecke HF, Lim HJ, et al. Reasons for underuse of adjuvant chemotherapy in elderly patients with stage III colon cancer. Clin Colorectal Cancer 2016;15:179-85. 10.1016/j.clcc.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968-74. 10.1016/S0140-6736(00)02713-6 [DOI] [PubMed] [Google Scholar]
- 19.Simmonds PD, Best L, George S, et al. Colorectal cancer risk by age. Atlanta, GA: Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, 2015. Available online: https://www.cdc.gov/cancer/colorectal/statistics/age.htm [Google Scholar]
- 20.Pedrazzani C, Cerullo G, De Marco G, et al. Impact of age-related comorbidity on results of colorectal cancer surgery. World J Gastroenterol 2009;15:5706-11. 10.3748/wjg.15.5706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paksoy M, Ipek T, Colak T, et al. Influence of age on prognosis and management of patients with colorectal carcinoma. Eur J Surg 1999;165:55-9. 10.1080/110241599750007513 [DOI] [PubMed] [Google Scholar]
- 22.Kiran RP, Pokala N, Dudrick SJ. Long-term outcome after operative intervention for rectal cancer in patients aged over 80 years: analysis of 9,501 patients. Dis Colon Rectum 2007;50:604-10. 10.1007/s10350-006-0802-0 [DOI] [PubMed] [Google Scholar]
- 23.Jafari MD, Jafari F, Halabi WJ, et al. Colorectal cancer resections in the aging US population: a trend toward decreasing rates and improved outcomes. JAMA Surg 2014;149:557-64. 10.1001/jamasurg.2013.4930 [DOI] [PubMed] [Google Scholar]
- 24.Damhuis RA, Meurs CJ, Meijer WS. Postoperative mortality after cancer surgery in octogenarians and nonagenarians: results from a series of 5,390 patients. World J Surg Oncol 2005;3:71. 10.1186/1477-7819-3-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-73. 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 2005;294:2703-11. 10.1001/jama.294.21.2703 [DOI] [PubMed] [Google Scholar]
- 27.Pirrera B, Vaccari S, Ciucchi D, et al. Impact of octogenarians on surgical outcome in colorectal cancer. Int J Surg 2016;35:28-33. 10.1016/j.ijsu.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290-314. 10.1002/cncr.28509 [DOI] [PMC free article] [PubMed] [Google Scholar]