Abstract
Introduction
We compared the efficacy of insulin detemir and biphasic insulin aspart‐30 given in the morning as an add‐on to oral hypoglycemic agents in type 2 diabetes patients.
Materials and Methods
The present study enrolled 30 patients with poorly controlled type 2 diabetes (8% ≤ glycated hemoglobin < 11%) being treated with oral hypoglycemic agent mono‐ or combination therapy with biguanides, sulfonylureas or thiazolidinediones. The patients were randomly assigned to insulin detemir (group D) or insulin aspart‐30 (group A) given in the morning as add‐on to oral hypoglycemic agents. After adjusting their insulin doses, the patients that underwent continuous glucose monitoring during a 3‐day hospitalization and with day 2 continuous glucose monitoring data were subjected to analysis.
Results
There was no significant difference in patient background, baseline glycated hemoglobin levels and insulin doses during continuous glucose monitoring between the two groups. The percent coefficient of variation of 24‐h glucose levels was significantly lower in group A (20.4 ± 7.6) than in group D (27.1 ± 6.5; P = 0.015). Similarly, mean amplitude of glycemic excursions was significantly smaller in group A (80 ± 32) than in group D (102 ± 14; P = 0.021). Postprandial glucose excursions were significantly smaller after breakfast in group A (65 ± 31 mg/dL) than in group D (106 ± 32 mg/dL; P = 0.002).
Conclusions
As once‐daily insulin injection therapy given before breakfast in type 2 diabetes patients, the biphasic insulin analog might represent a better insulin option in significantly lowering the percent coefficient of variation and mean amplitude of glycemic excursions than the long‐acting insulin preparation.
Keywords: Continuous glucose monitoring, Insulin aspart‐30, Insulin detemir
Introduction
To date, large‐scale clinical trials, such as the United Kingdom Prospective Diabetes Study, have shown that pancreatic β‐cell function is reduced by approximately 50% by the time of onset of type 2 diabetes, and that it decreases further over time as the disease progresses1. It has also been shown that an increasing proportion of diabetes patients is becoming less amenable to monotherapy with any of the oral hypoglycemic agents (OHAs) available, such as metformin, sulfonylurea or thiazolidinedione, over time2. Despite this, the Diabetes Attitudes, Wishes And Needs Japan Study showed that not only diabetes patients, but also physicians tend to show resistance to insulin therapy, leading to delays in its implementation3. It is critically important that insulin therapy be aggressively implemented in patients with poorly controlled diabetes despite the use of multiple OHAs, given that a wide array of insulin formulations that differ in their duration of action has become available for clinical use in recent years, thus allowing patients to be initiated into insulin therapy in a variety of ways. However, insulin therapy must be individualized for each patient, with adequate consideration given to each patient's disease condition, severity of disease and lifestyle.
Once‐daily insulin injection therapy (ODI) is intended to provide a once‐daily injection of long‐acting insulin as an add‐on to continued OHA therapy. The joint American Diabetes Association/European Association for the Study of Diabetes guidelines4, 5 recommend initiating insulin therapy first as ODI therapy. Of note, the 4‐T study showed that, of the insulin regimens to which 708 patients with type 2 diabetes receiving a sulfonylurea and biguanide were randomized – that is, ODI, twice‐daily biphasic insulin and thrice‐daily fast‐acting insulin – ODI was least associated with hypoglycemic episodes and weight gain6. Therefore, ODI appears to be relatively safe, particularly when initiated at a low dose; better accepted by patients starting on insulin therapy; and conveniently available for implementation in outpatient settings.
Long‐acting insulin formulations are often used in ODI, and include insulin detemir, insulin glargine and insulin degludec. Of note, a cross‐over study was carried out to compare insulin detemir and insulin glargine given at bedtime to determine patterns of glycemic variability in 29 patients with type 2 diabetes using continuous glucose monitoring (CGM)7. The findings showed no significant difference in the average glucose values measured at 1‐h intervals, demonstrating a similar pattern of glycemic variability with no significant difference in 24‐h mean glucose values.
In implementing ODI, the choice between long‐acting insulin and biphasic insulin should be made for each patient based on the pattern of glycemic variability seen in each patient. However, very few studies have reported an ODI approach involving once‐daily morning insulin injection therapy. Therefore, the present study was designed to compare long‐acting insulin detemir and biphasic insulin aspart‐30, each given once daily in the morning for 24‐h glycemic variability in patients with poorly controlled type 2 diabetes, despite the use of OHA monotherapy or combination therapy, using CGM.
Methods
The present study included 30 insulin‐naïve, type 2 diabetes patients (8% ≤ glycated hemoglobin [HbA1c] < 11%) despite monotherapy or combination therapy with biguanide, sulfonylurea or thiazolidinedione. After the patients gave informed consent, they were randomly assigned to receive insulin detemir or biphasic insulin aspart‐30 once daily in the morning. After insulin initiation, their insulin dose was adjusted to ensure their pre‐dinner glucose levels remained <130 mg/dL. After reviewing their pre‐dinner glucose levels for 3 days, their insulin dose was titrated upward or downward as required in accordance with the algorithm shown in Table 1. Again, after 2 months or more of insulin therapy, the patients were hospitalized to undergo CGM using CGMS GOLD, known as a ‘masked CGM’ (Medtronic, Inc., Northridge, CA, USA), for 3 days, with the CGM data obtained on day 2 of hospitalization used for analysis (Figure 1). During the days when the patients underwent CGM, the diet regimen given accounted for 25–30 kcal/kg (standard weight), 60% of which was derived from carbohydrates.
Table 1.
Algorithm for insulin dose adjustment and insulin dose during continuous glucose monitoring after hospitalization
| Mean pre‐dinner glucose level | Insulin dose (U) adjustment required |
|---|---|
| ≤80 mg/dL | Decrease the dose by 2 U |
| 80–130 mg/dL | No adjustment required |
| 131–180 mg/dL | Increase the dose by 1 U |
| 181–250 mg/dL | Increase the dose by 2 U |
| ≥251 mg/dL | Increase the dose by 4 U |
| Insulin detemir group | Insulin aspart‐30 group | P‐value | |
|---|---|---|---|
| Insulin dose during CGM after hospitalization (U/kg) | 0.116 ± 0.679 | 0.143 ± 0.102 | 0.418* |
| Changes in HbA1c by the time of admission (%) | −1.11 ± 0.73 | −1.01 ± 0.55 | 0.649* |
| Weight gain by the time of admission (kg) | 0.5 ± 2.6 | 2.2 ± 3.4 | 0.136* |
| The number of patients with hypoglycemia during insulin dose adjustment (n) | 4 | 1 | 0.157** |
Data are expressed as mean ± standard deviation. *The t‐test. **Fisher's exact test. The lower part of the table shows changes in glycated hemoglobin and weight gain from initiation of insulin therapy to hospitalization, and the number of patients with hypoglycemia during the insulin dose adjustment. CGM, continuous glucose monitoring.
Figure 1.
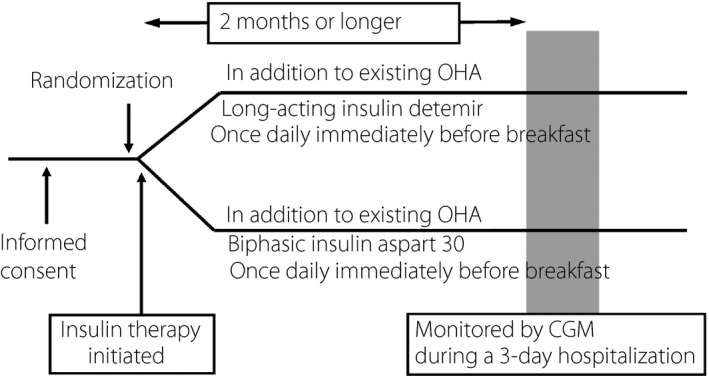
Study design. CGM, continuous glucose monitoring; OHA, oral hypoglycemic agent.
The present study was carried out with the approval of the institutional review board of Jikei University School of Medicine, Tokyo, Japan, and was registered with the UMIN Clinical Trials Registry (trial ID: UMIN000004396).
Variables compared in the study included such CGM‐derived variables as: 24‐h mean glucose levels; standard deviations (SDs) of 24‐h glucose levels; percent coefficient of variation (%CV) of 24‐h glucose levels; mean amplitude of glycemic excursions (MAGE); time in hypoglycemia (<70 mg/dL) and in hyperglycemia (>180 mg/dL) during a 24‐h period; preprandial glucose levels; peak glucose levels after each meal; the range of postprandial glucose increases; and time to postprandial peak glucose levels from before meals. In addition, the two groups were compared to determine the changes in HbA1c during the period from insulin initiation to hospitalization, and to determine the number of patients with associated hypoglycemia.
Results
At baseline, there was no significant difference between the two groups with regard to sex, age, body mass index, urinary C‐peptide immunoreactivity and HbA1c values (Table 2).
Table 2.
Patient background
| Insulin detemir group | Insulin aspart‐30 group | P‐value | |
|---|---|---|---|
| Sex (males/females) | 9/5 | 11/5 | 1.000* |
| Age (years) | 60.9 ± 6.8 | 59.0 ± 10.9 | 0.572** |
| BMI (kg/m2) | 24.4 ± 3.6 | 26.6 ± 4.0 | 0.140** |
| Urinary CPR (μg/day) | 69.0 ± 47.8 | 102.3 ± 63.0 | 0.118** |
| HbA1c at the initiation of insulin therapy (%) | 8.58 ± 0.43 | 8.88 ± 0.52 | 0.105** |
| Concomitant medication | |||
| Biguanides | 11 (79%) | 15 (94%) | 0.315* |
| Sulfonylureas | 13 (93%) | 16 (100%) | 0.467* |
| Thiazolidinediones | 7 (50%) | 9 (56%) | 1.000* |
Data are expressed as mean ± standard deviation. *Fisher's exact test. **The t‐test. BMI, body mass index; CPR, C‐peptide immunoreactivity; HbA1c, glycated hemoglobin.
As shown in Table 1, the insulin dose (mean ± SD) during CGM after hospitalization was not significantly different between the two groups at 0.116 ± 0.679 U/kg/day for insulin detemir versus 0.143 ± 0.102 for insulin aspart‐30 (P = 0.418). Again, the changes in HbA1c were not significantly different between the groups at −1.11 ± 0.73% with insulin detemir versus −1.01 ± 0.55% with insulin aspart‐30 (P = 0.649). Furthermore, insulin detemir was associated with more patients with hypoglycemia than was insulin aspart‐30, although this difference was not significant.
There was no significant difference between the two groups in terms of 24‐h mean glucose levels (155 ± 15 mg/dL with insulin detemir vs 165 ± 27 mg/dL with insulin aspart‐30; P = 0.241) and the SDs of 24‐h glucose levels (41.6 ± 9.0 with insulin detemir vs 33.9 ± 13.8 mg/dL with insulin aspart‐30; P = 0.088) (Table 3). However, %CV of 24‐h glucose levels as a measure of glycemic variability was shown to be significantly lower with insulin aspart‐30 at 20.4 ± 7.4 than with insulin detemir at 27.1 ± 6.5 (P = 0.015). Similarly, MAGE was also shown to be significantly smaller with insulin aspart‐30 at 80 ± 32 than with insulin detemir at 102 ± 14 (P = 0.021). The time in hypoglycemia (<70 mg/dL) during a 24‐h period tended to be shorter with insulin aspart‐30 at 0.3 ± 1.3 min than with insulin detemir at 15.4 ± 26.6 min (P = 0.055).
Table 3.
Indices for glycemic control as assessed by continuous glucose monitoring in the two groups
| Insulin detemir group | Insulin aspart‐30 group | P‐value* | |
|---|---|---|---|
| 24‐h mean glucose levels (mg/dL) | 155 ± 15 | 165 ± 27 | 0.241 |
| SD of 24‐h glucose levels | 41.6 ± 9.0 | 33.9 ± 13.8 | 0.088 |
| %CV of 24‐h glucose levels | 27.1 ± 6.5 | 20.4 ± 7.6 | 0.015 |
| Mean amplitude of glycemic excursions (MAGE) | 102 ± 14 | 80 ± 32 | 0.021 |
| Time in hypoglycemia, <70 mg/dL (min) | 15.4 ± 26.6 | 0.3 ± 1.3 | 0.055 |
| Time in hyperglycemia, >180 mg/dL (min) | 395 ± 169 | 433 ± 300 | 0.683 |
| Preprandial glucose levels (mg/dL) | |||
| Before breakfast | 137 ± 24 | 154 ± 30 | 0.110 |
| Before lunch | 128 ± 36 | 135 ± 30 | 0.552 |
| Before dinner | 113 ± 29 | 122 ± 29 | 0.393 |
| Postprandial peak glucose levels (mg/dL) | |||
| After breakfast | 243 ± 32 | 219 ± 46 | 0.110 |
| After lunch | 200 ± 37 | 206 ± 48 | 0.680 |
| After dinner | 225 ± 36 | 223 ± 60 | 0.939 |
| Range of postprandial glucose increases (mg/dL) | |||
| After breakfast | 106 ± 32 | 65 ± 31 | 0.002 |
| After lunch | 72 ± 32 | 71 ± 40 | 0.963 |
| After dinner | 112 ± 36 | 101 ± 47 | 0.494 |
| Time to postprandial peak glucose levels from before meals (min) | |||
| After breakfast | 109 ± 35 | 86 ± 24 | 0.044 |
| After lunch | 93 ± 42 | 98 ± 43 | 0.724 |
| After dinner | 100 ± 23 | 81 ± 34 | 0.095 |
Data are expressed as mean ± standard deviation. *The t‐test. %CV, percent coefficient of variation.
There was no significant difference in preprandial glucose levels and peak glucose levels after each meal between the two groups. The range of postprandial glucose increases with insulin aspart‐30 was significantly smaller only after breakfast at 65 ± 31 mg/dL than with insulin detemir at 106 ± 32 mg/dL (P = 0.002), which was thought likely to account for the significant difference in MAGE between the two groups. The time to postprandial peak glucose levels from before meals was significantly shorter after breakfast with insulin aspart‐30 than with insulin detemir (P = 0.044).
Figure 2 presents the mean ± SD for 24‐h CGM data for the two groups, which shows that the patients receiving insulin detemir might have had a higher risk of hypoglycemia during night‐time and before dinner, whereas those receiving insulin aspart‐30 had a nearly flat glycemic variability profile during night‐time and reduced glycemic excursions after breakfast.
Figure 2.
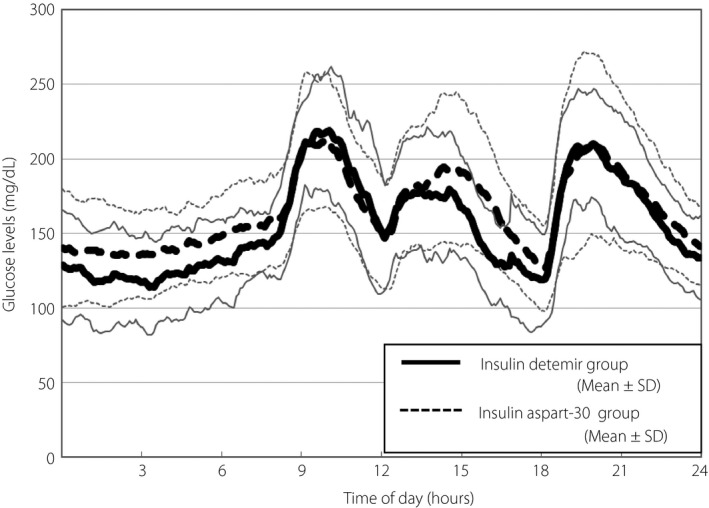
The 24‐h continuous glucose monitoring (CGM) data 2 months after initiation of insulin therapy. SD, standard deviation.
Additionally, in order to determine which of the patients’ background factors might correlate with the CGM data after insulin therapy in the 30 patients in whom ODI had been initiated, multiple linear regression analysis was carried out, with the CGM data serving as dependent variables, and age, BMI at initiation of ODI, urinary CPR, HbA1c at initiation of ODI and insulin dose serving as independent variables (Table 4). Multiple linear regression analysis showed that the lower the HbA1c value at initiation of ODI, the significantly lower the 24‐h mean glucose value after treatment (R 2 = 0.34, β = 0.43, P = 0.036); and that the higher the BMI at initiation of ODI, the significantly lower the SD and %CV of the 24‐h glucose levels and the MAGE (R 2 = 0.23, β = −0.60, P = 0.019; R 2 = 0.18, β = −0.53, P = 0.041; R 2 = 0.22, β = −0.57, P = 0.024).
Table 4.
Multiple linear regression analysis carried out to evaluate the correlation between patient background characteristics and continuous glucose monitoring parameters among those receiving once‐daily insulin injection therapy
| 24‐h mean glucose levels, mg/dL (R 2 = 0.34) | SD of 24‐h glucose levels (R 2 = 0.23) | %CV of 24‐h glucose levels (R 2 = 0.18) | MAGE (R 2 = 0.22) | |||||
|---|---|---|---|---|---|---|---|---|
| β | P‐value | β | P‐value | β | P‐value | β | P‐value | |
| Age (years) | 0.06 | 0.745 | −0.01 | 0.969 | −0.03 | 0.873 | −0.13 | 0.948 |
| BMI at initiation of ODI (kg/m2) | −0.24 | 0.292 | −0.60 | 0.019 | −0.53 | 0.041 | −0.57 | 0.024 |
| Urinary CPR (μg/day) | −0.20 | 0.329 | 0.24 | 0.280 | 0.29 | 0.207 | 0.17 | 0.446 |
| HbA1c at the initiation of ODI (%) | 0.43 | 0.036 | 0.25 | 0.252 | 0.10 | 0.662 | 0.27 | 0.211 |
| Insulin dose during CGM after hospitalization (U/kg) | −0.05 | 0.806 | −0.36 | 0.118 | −0.31 | 0.187 | −0.27 | 0.229 |
| Time in hypoglycemia, min (R 2 = 0.21) | Time in hyperglycemia, min (R 2 = 0.36) | |||
|---|---|---|---|---|
| β | P‐value | β | P‐value | |
| Age (years) | −0.58 | 0.778 | 0.12 | 0.519 |
| BMI at initiation of ODI (kg/m2) | −0.32 | 0.194 | −0.42 | 0.063 |
| Urinary‐CPR (μg/day) | −0.01 | 0.972 | −0.11 | 0.597 |
| HbA1c at the initiation of ODI (%) | 0.43 | 0.054 | 0.39 | 0.051 |
| Insulin dose during CGM after hospitalization (U/kg) | −0.30 | 0.194 | −0.15 | 0.453 |
%CV, percent coefficient of variation; BMI, body mass index; CGM, continuous glucose monitoring; CPR, C‐peptide immunoreactivity; HbA1c, glycated hemoglobin; MAGE, mean amplitude of glycemic excursions; ODI, once‐daily insulin injection therapy.
Discussion
The results of the present study suggest that the use of biphasic insulin aspart‐30 rather than long‐acting insulin detemir as ODI in the morning might contribute to improving glycemic variability in type 2 diabetes patients. In contrast, insulin detemir was associated with a significantly wider range of glucose increases after breakfast and a significantly longer time from pre‐breakfast to post‐breakfast peak glucose levels, and tended to cause more hypoglycemia, thus leading to significantly higher %CV of glucose levels and greater MAGE than insulin aspart‐30.
Of note, Monnier et al.8 presented 24‐h glycemic variability profiles by HbA1c category for 130 type 2 diabetes patients, clearly demonstrating that their glucose levels showed a marked decreasing trend during night‐time (from 00.00 to 06.00 h) compared with daytime levels, and a marked increasing trend during morning hours, suggesting that less insulin is required during night‐time than during daytime, especially after breakfast. Therefore, biphasic insulin given as ODI in the morning might be effective in improving glycemic variability among those with poorly controlled diabetes; that is, those with HbA1c ≥8%.
Of interest, the Once Mix Study9, which randomly assigned 433 patients with type 2 diabetes on OHAs to biphasic insulin aspart‐30 given once daily before dinner or insulin glargine given once before bedtime, showed a mean decrease in HbA1c of 1.25% with insulin glargine, but an even higher, albeit non‐significant, HbA1c decrease of 1.41% with insulin aspart‐30 after 26 weeks of insulin therapy. In addition, an analysis of eight glucose measurements carried out on a daily basis (before and after each meal, at bedtime and at approximately 03.00 h) in the study showed that the use of insulin aspart‐30 was associated with significantly lower postprandial 2‐h glucose levels and bedtime glucose levels than insulin glargine (P = 0.04 and P = 0.0078, respectively).
Clinical studies of insulin aspart‐30, such as the 1‐2‐3 Study10 and the Sapporo 1‐2‐3 Study11, demonstrate that glycemic control is shown to definitely improve with insulin aspart‐30 started as ODI given before dinner, because its dosing frequency increases over time. This, coupled with the present study findings, could suggest that a stepwise approach to the use of insulin aspart‐30 that involves starting it as ODI given before breakfast and then increasing its dosing over time might represent the gold standard for insulin therapy.
Again, a biphasic insulin formulation (IDegAsp) consisting of 70% long‐acting insulin degludec and 30% fast‐acting insulin aspart has recently become available for clinical use. In this regard, a randomized trial compared IDegAsp and insulin glargine once daily in 296 type 2 diabetes patients on OHAs. and reported significantly greater decreases in HbA1c values among those randomized to IDegAsp after 26 weeks of therapy (P < 0.01), with fewer, albeit not significantly different, episodes of hypoglycemia and nocturnal hypoglycemia reported for those receiving IDegAsp than for those receiving insulin glargine12. Again, a CGM‐based study of IDegAsp and insulin glargine given before dinner showed smaller glucose excursions after dinner among those given IDegAsp than among those given insulin glargine13. Therefore, IDegAsp given before breakfast is likely to become a mainstay of ODI in the years to come.
The limitations of the present study are that it included a relatively small sample size and that the CGM data obtained in an in‐patient setting for the study might have yielded different results than those obtained in an outpatient setting.
Multiple linear regression analysis showed that the lower the HbA1c value at initiation of ODI, the lower the 24‐h mean glucose value after treatment, suggesting that ODI is effective when initiated as early as possible before the HbA1c value becomes elevated. It was also found that the higher the BMI value at initiation of ODI, the lower the indices for glucose variability, this might be accounted for by the fact that the higher the BMI value, the lower the patient age (P = 0.030, Pearson's correlation coefficient) and the higher the urinary CPR (P = 0.006, Pearson's correlation coefficient).
The current comparative study showed that although insulin detemir led to greater, albeit non‐significant, decreases in 24‐h mean glucose levels than insulin aspart‐30, insulin aspart‐30 led to significantly better outcomes in such measures of glycemic variability as %CV of glucose levels and MAGE than did insulin detemir. Long‐term study is required to clarify which might have a greater role in the onset of hard end‐points (mortality and cardiovascular events), high mean glucose levels with smaller %CV (or MAGE) or low mean glucose levels with greater %CV (or MAGE).
Disclosure
Daisuke Tsujino has participated in speaker's bureau/advisory panels for Novo Nordisk, and received research support from Boehringer Ingelheim and Takeda. Rimei Nishimura has participated in speaker's bureau/advisory panels for Astellas, Astra Zeneca, Boehringer Ingelheim, Daiichi‐Sankyo, Eli Lilly, Johnson & Johnson, Kissei, Kowa, Medtronic, Novo Nordisk, Ono, Sanofi, Taisho, Takeda and Tanabe‐Mitsubishi, and served as a consultant for Abbott, Boehringer Ingelheim, Eli Lilly and Taisho. Kiyotaka Ando has participated in speaker's bureau/advisory panels for Novo Nordisk and Sanofi. Kazunori Utsunomiya has received research support from Terumo, Kowa, Taisho, Arkray, Kyowa Kirin, MSD, Astellas, Boehringer Ingelheim, Ono, Novo Nordisk, Kissei and Tanabe‐Mitsubishi, and has participated in speaker's bureau/advisory panels for Astellas, Astra Zeneca, Kowa, MSD, Eli Lilly, Taisho, Novo Nordisk and Sanofi. All the other authors declare no conflict of interest.
Acknowledgments
This study was funded by Japan Diabetes Foundation. We thank all the study participants and Kimie Shida for their assistance.
J Diabetes Investig 2018;9: 573–578
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000004396
References
- 1. Prospective UK, Diabetes Study Group . U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 2. Kahn SE, Haffner SM, Heise MA, et al Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 3. Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes And Needs (DAWN) JAPAN study. PLoS One 2012; 7: e36361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58: 429–442. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Thorne KI, Farmer AJ, et al Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007; 357: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 7. King AB. Once‐daily insulin detemir is comparable to once‐daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double‐blind, randomized, crossover study. Diabetes Obes Metab 2009; 11: 69–71. [DOI] [PubMed] [Google Scholar]
- 8. Monnier L, Colette C, Dunseath GJ, et al The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007; 30: 263–269. [DOI] [PubMed] [Google Scholar]
- 9. Strojek K, Bebakar WM, Khutsoane DT, et al Once‐daily initiation with biphasic insulin aspart 30 versus insulin glargine in patients with type 2 diabetes inadequately controlled with oral drugs: an open‐label, multinational RCT. Curr Med Res Opin 2009; 25: 2887–2894. [DOI] [PubMed] [Google Scholar]
- 10. Garber AJ, Wahlen J, Wahl T, et al Attainment of glycaemic goals in type 2 diabetes with once‐, twice‐, or thrice‐daily dosing with biphasic insulin aspart 70/30 (The 1‐2‐3 study). Diabetes Obes Metab 2006; 8: 58–66. [DOI] [PubMed] [Google Scholar]
- 11. Yoshioka N, Kurihara Y, Manda N, et al Step‐up therapy with biphasic insulin aspart‐70/30–Sapporo 1‐2‐3 study. Diabetes Res Clin Pract 2009; 85: 47–52. [DOI] [PubMed] [Google Scholar]
- 12. Onishi Y, Ono Y, Rabøl R, et al Superior glycaemic control with once‐daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab 2013; 15: 826–832. [DOI] [PubMed] [Google Scholar]
- 13. Liebl A, Davidson J, Mersebach H, et al A novel insulin combination of insulin degludec and insulin aspart achieves a more stable overnight glucose profile than insulin glargine: results from continuous glucose monitoring in a proof‐of‐concept trial. J Diabetes Sci Technol 2013; 7: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]


