Abstract
Aims/Introduction
Impaired glucose tolerance (IGT) is a subtype of prediabetes, a condition having high risk for development to diabetes mellitus, but its pathophysiology is not fully understood. In the present study, we examined metabolic changes in IGT by using two types (D‐glucose [Glc] and partial hydrolysate of starch [PHS]) of oral glucose tolerance tests (OGTTs), with emphasis on serum incretins and metabolites.
Materials and Methods
We carried out the two types of OGTT (Glc/OGTT and PHS/OGTT) in 99 young Japanese individuals who had tested either positive (GU +; n = 48) or negative (GU −; n = 51) for glycosuria. After OGTT, they were sub‐grouped into five categories: normal glucose tolerance (NGT) in the GU − group (GU −/NGT; n = 49), NGT in the GU + group (GU +/NGT; n = 28), IGT (n = 12), diabetes mellitus (n = 1) and renal glycosuria (n = 9). Serum incretin and metabolites of GU −/NGT and IGT were then measured.
Results
When the serum insulin level at each time‐point during PHS/OGTT was expressed as its ratio relative to Glc/OGTT, it was increased time‐dependently in GU −/NGT, but not in IGT. Such an increase in the ratio was also detected of serum incretin levels in GU −/NGT, but not in IGT, suggesting a lack of deceleration of oligosaccharide absorption in IGT. Metabolome analysis showed a difference in the serum levels of two metabolites of unknown function in mammals, methylcysteine and sedoheptulose 1,7‐bisphosphate, between GU −/NGT and IGT.
Conclusions
Comparison of PHS/OGTT and Glc/OGTT showed that oligosaccharide absorption was accelerated in IGT. Methylcysteine and sedoheptulose 1,7‐bisphosphate could be novel markers for dysregulated glucose metabolism.
Keywords: Impaired glucose tolerance, Metabolome, Oral glucose tolerance test
Introduction
Diabetes mellitus is a metabolic disorder characterized by chronic elevation in blood glucose levels. The number of diabetes mellitus patients is rapidly increasing and has become an urgent social burden. In addition, recent clinical studies have shown that restoration of normal glucose regulation by lifestyle modification and/or pharmacological intervention in individuals with “pre”‐diabetes is required for preventing microvascular and macrovascular complications of diabetes mellitus1, 2. However, the progression to overt diabetes mellitus from impaired glucose tolerance (IGT), a subtype of prediabetes, can be significantly reduced by lifestyle interventions3. Accordingly, establishment of a diagnostic marker that can detect individuals at risk of diabetic vascular complications and a better understanding of the pathophysiology of IGT is essential.
Presently, diagnosis of diabetes mellitus is made principally on plasma glucose criteria, which requires either fasting plasma glucose or 2‐h plasma glucose levels after a 75‐g oral glucose tolerance test (OGTT) and/or glycated hemoglobin (HbA1c) criteria4, 5, 6. The 75‐g OGTT was initially carried out with 75 g anhydrous D‐glucose (Glc) dissolved in water. However, another type of test solution composed of partial hydrolysate of starch (PHS) was established in the 1960s7, 8 that reduced the sweetness and associated gastrointestinal symptoms. In 1983, Heine et al.9 compared OGTT with Glc (Glc/OGTT) and that with PHS (PHS/OGTT). They found that the area under the curve of plasma glucose and that of serum insulin for 0–15 min after ingestion were both higher in Glc/OGTT than in PHS/OGTT, when the test solution was ingested rapidly (over 1 min). In addition, differential responses in plasma glucose and serum insulin between Glc/OGTT and PHS/OGTT were reported in a case with possible abnormality in di‐ and oligosaccharide digestion10. Nevertheless, in general, Glc/OGTT and PHS/OGTT have been considered to be practically equivalent7, 8, without being compared thoroughly by repetitive, two‐round OGTT in a sufficient number of subjects.
In the ~50 years since the establishment of test solutions containing PHS, knowledge of gastrointestinal carbohydrate absorption and regulation of glucose homeostasis, especially that by incretins, has greatly advanced11, 12. We previously reported that inhibition of hydrolysis of disaccharides (sucrose or maltose) by α‐glucosidase inhibitors significantly increased secretion of glucagon‐like peptide‐1 (GLP‐1)13, 14. In addition, the incretin effect has been reported to be attenuated in prediabetes15. Therefore, we hypothesized that individuals with IGT and those with normal glucose tolerance (NGT) might show different glycemic responses to the two OGTTs. In addition to the progress in incretin studies, the recent technique of mass spectrometry has made measurement of a variety of molecules in blood samples during OGTT possible16, 17, 18, 19. Such metabolome analysis is becoming a powerful tool to unveil the pathophysiology of various metabolic disorders. In the present study, we examined metabolic alterations of IGT by the use of two types of OGTT (Glc/OGTT and PHS/OGTT), and searched for novel serum metabolites that might be useful as markers for IGT.
Methods
Participants
From April 2013 to March 2015, 22,036 college students at Chiba University underwent a routine, annual, medical checkup including a test for glycosuria by urinary test strip (Figure 1). A total of 129 glycosuria‐positive (GU+; ≥1) participants were recommended to consult a doctor for detailed examination. A total of 52 of the 129 GU+ participants were recruited for the present study, which was a consecutive, two‐round OGTT; 48 participants completed the study (four withdrew after enrollment). As controls, 55 college students who tested negative (<1) for glycosuria (GU−) were examined; 51 of them completed the study (two were withdrawn due to difficulty in repeated blood sampling and two withdrew from the test). The GU− participants with medical illnesses, taking regular medication, or having a family history of diabetes within the second degree of kinship were excluded to minimize bias in the metabolic characteristics of normal controls. Although the GU− group did not precisely match the GU+ group, there were no significant differences in sex, age or body mass index. The HbA1c of the GU+ group was slightly, but significantly, higher than that of the GU− group. To reinforce our statistical analysis, we sought a sample size as large as possible. We therefore solicited all GU+ students who had participated in the health checkup at Chiba University over a 2‐year period (2013 April to 2015 March) to participate in the study. The study protocol was approved by the ethics committee of Chiba University, and written informed consent was obtained from all participants. The study conforms to the provisions of the Declaration of Helsinki.
Figure 1.
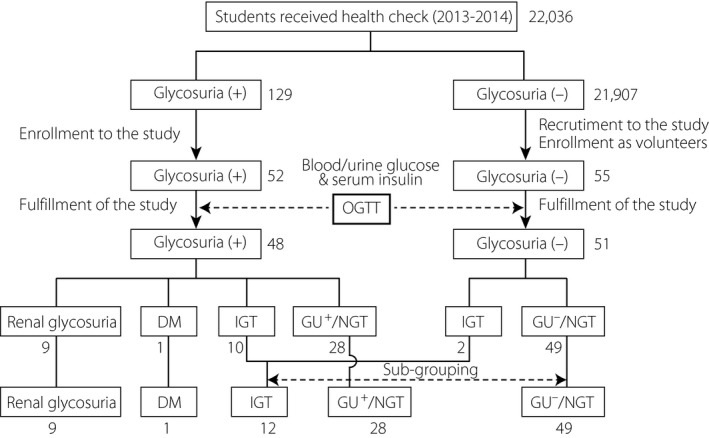
Study profile. Digits denote the numbers of the participants. DM, diabetes mellitus; GU +, glycosuria‐positive; GU −, glycosuria‐negative; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; NGT, normal glucose tolerance.
Study protocol
Each of the participants was subjected to the two types (75 g anhydrous D‐glucose or PHS equivalent to 75 g Glc) of OGTT on two separate days with an interval of ≥4 days but <6 months. For Glc/OGTT, 75 g anhydrous D‐glucose dissolved in commercially available sparkling water in a volume of 225 mL was used; 225 mL TRELAN®‐G75 (AY Pharmaceuticals Co. Ltd., Tokyo, Japan) was used for PHS/OGTT. Blood samples were withdrawn from a cubital vein before, and 30, 60, 90 and 120 min after ingestion of Glc or PHS.
Diagnosis of glucose tolerance (NGT, IGT and diabetes mellitus) was made in accordance with the current diagnostic criteria established by the American Diabetes Association4. IGT and diabetes mellitus were diagnosed when the results of either of the two OGTTs met the diagnostic criteria. As the diagnostic criteria of renal glycosuria have not been established, we used the appearance of glycosuria (≥50 mg/dL, at any time‐point) in the absence of hyperglycemia (≥170 mg/dL, for all time‐points) during OGTT. Based on the results of the OGTTs, the participants were classified into five categories: (i) GU+/NGT (NGT in GU+ group); (ii) GU−/NGT (NGT in GU− group); (iii) IGT; (iv) diabetes mellitus; and (v) renal glycosuria (Figure 1). Participants with renal glycosuria or diabetes mellitus were excluded from further evaluation, as renal glycosuria can influence glycemia in the former and, in the latter, the small sample size (n = 1) precluded statistical analysis.
Analytical procedures
Plasma concentrations of glucose and HbA1c were measured by the glucose oxidase method (Roche Diagnostics, Basel, Switzerland) and high‐performance liquid chromatography method (TSKgel® G8 His; Tosoh Corp., Tokyo, Japan). Serum levels of insulin, total GLP‐1 and total glucose‐dependent insulinotropic polypeptide (GIP) were measured by enzyme‐linked immunosorbent assay kits (Lumipulse Presto 2 [Fujirebio Inc., Tokyo, Japan], EZGLP1T‐36K [Merck Millipore, Darmstadt, Germany] and EZHGIP‐54K [Merck Millipore], respectively). The area under the curve (AUC) of each of the measurements was calculated according to the trapezoid rule. The insulinogenic index (an index of insulin secretory capacity) and Matsuda index (an index of insulin sensitivity) was calculated as previously described20.
Metabolome analysis by hydrophilic interaction chromatography tandem mass spectrometry
Serum concentrations of 263 metabolites were analyzed using hydrophilic interaction chromatography tandem mass spectrometry procedures reported previously21, 22. The serum levels of 42 metabolites found to be stable under our storage conditions (−20°C) were measured.
Statistical analysis
Data was presented as mean ± standard error of the mean. Difference in the measurement values (such as those for plasma glucose, serum hormones and serum metabolites) between the types of carbohydrate solution (PHS and Glc) or the time‐points (0 and 120 min) were evaluated using either the two‐way anova for repeated measures with the Bonferroni post‐hoc test or by the paired t‐test. For analysis of different groups (GU−/NGT, GU+/NGT and IGT), two‐way repeated‐measurement anova with Tukey's multiple comparison or one‐way anova with Tukey's multiple comparison was used. Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) was carried out to investigate metabolome profiles of different groups. Metabolites with a variable importance in projection value >1.0 were identified as potential markers.
Results
Classification of the participants with or without glycosuria
The clinical characteristics of GU+ and GU− are listed in Table 1. Although OGTT was well tolerated in both GU+ and GU−, two GU− participants failed to complete the study because of technical difficulties in repeated blood sampling. We compared plasma glucose levels after PHS/OGTT and after Glc/OGTT (Figure 2a). The plasma glucose levels at 90 and 120 min after PHS/OGTT were slightly, but significantly, higher than those after Glc/OGTT. Serum insulin levels at 90 and 120 min after PHS/OGTT were also significantly higher than those after Glc/OGTT (Figure 2b). AUCs (from 0 to 120 min) of plasma glucose (AUCGlc) and serum insulin (AUCIns) in PHS/OGTT were both significantly higher than those in Glc/OGTT (Figure 2c,d).
Table 1.
Characteristics of participants to the study
| Glycosuria+ | Glycosuria− | P‐value | |
|---|---|---|---|
| n | 48 | 51 | |
| Male/female | 35/13 | 42/9 | NS† |
| Age (years) | 21.4 ± 0.5 | 22.1 ± 0.2 | NS‡ |
| BMI (kg/m2) | 20.8 ± 0.5 | 21.5 ± 0.4 | NS‡ |
| HbA1c (%) | 5.28 ± 0.08 | 5.08 ± 0.04 | 0.018‡ |
†Fisher's exact test. ‡Two‐tailed unpaired t‐test. HbA1c, glycated hemoglobin; NS, not significant.
Figure 2.
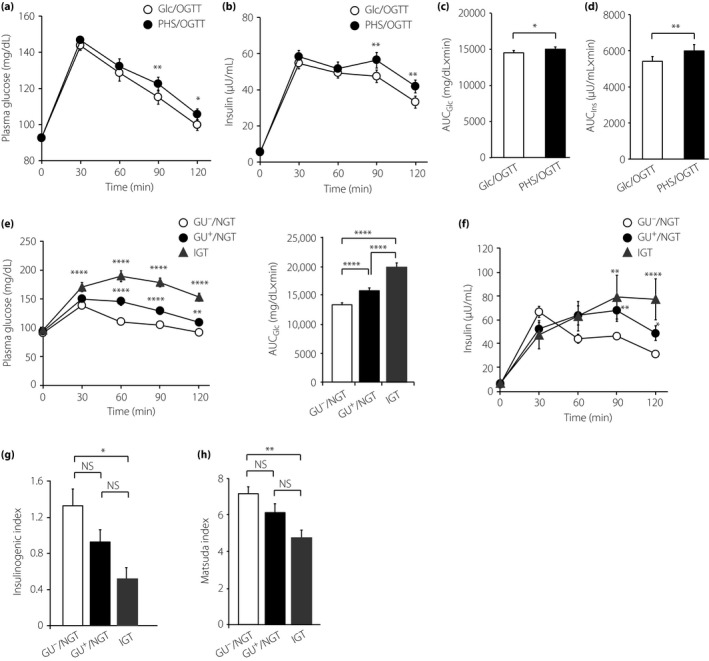
Comparison of plasma glucose and serum insulin between D‐glucose oral glucose tolerance test (Glc/OGTT) and partial hydrolysate of starch (PHS)/OGTT. (a,b) Time‐courses of (a) plasma glucose and (b) serum insulin during Glc/OGTT (filled circles) and PHS/OGTT (open circles). *P < 0.05, **P < 0.01 by two‐way repeated‐measurement anova with the Bonferroni post‐hoc test. Data are mean ± SE of the mean (n = 89). (c, d) Area under the curve from 0 to 120 min of (c) plasma glucose (AUCG lc) and (d) serum insulin (AUCI ns). *P < 0.05, **P < 0.01 by two‐tailed paired‐t test. (e,f) Time‐courses of (e) plasma glucose and (f) serum insulin in glycosuria‐negative/normal glucose tolerance (GU −/NGT; circle), glycosuria‐positive (GU +)/NGT (triangle) and impaired glucose tolerance (IGT; square). *P < 0.05, **P < 0.01, ****P < 0.0001 (GU −/NGT vs IGT). P‐value was calculated by two‐way repeated‐measurement anova with Tukey's multiple comparison test. (g) Insulinogenic index and (h) Matsuda index obtained during PHS/OGTT. *P < 0.05 by one‐way anova with Tukey's multiple comparison test. (e–h) Results of GU −/NGT (circle, n = 49), GU +/NGT (triangle, n = 28) and IGT (square, n = 12) are shown. NS, not significant.
Based on the results of OGTTs, GU+ participants (n = 48) and GU− participants (n = 51) were classified into five categories: GU+/NGT (n = 28), GU−/NGT (n = 49), IGT (n = 12), diabetes mellitus (n = 1) and renal glycosuria (n = 9; Figure 1). We discriminated GU−/NGT and GU+/NGT with an aim to clarify whether they have equivalent glucose tolerance, as in clinical settings, GU+/NGT individuals are liable to be judged to be “normal” and escape from follow‐up observation. The frequency of abnormal glucose tolerance (IGT or diabetes mellitus) in GU+ participants (11/48, 18.6%) was significantly higher (P = 0.019, Fisher's exact test) than that in GU− participants (2/51, 3.9%). Clinical characteristics of GU−/NGT, GU+/NGT and IGT are shown in Table 2. These participants were also young and lean, differing only in HbA1c levels (significantly higher in IGT vs GU−/NGT).
Table 2.
Characteristics of the sub‐grouped population according to oral glucose tolerance test
| GU−/NGT | GU+/NGT | IGT | |
|---|---|---|---|
| n | 49 | 28 | 12 |
| Male/female | 40/9 | 20/8 | 8/4 |
| Age (years) | 22.12 ± 0.17 | 20.75 ± 0.52 | 21.92 ± 1.05 |
| BMI (kg/m2) | 21.33 ± 0.34 | 20.24 ± 0.30 | 21.35 ± 1.12 |
| HbA1c (%) | 5.07 ± 0.04* | 5.15 ± 0.06** | 5.43 ± 0.12 |
| Fasting PG (mg/dL) | 91.32 ± 0.76 | 93.83 ± 0.93 | 94.79 ± 3.00 |
| Fasting serum insulin (μU/mL) | 6.05 ± 0.28 | 6.33 ± 0.51 | 6.33 ± 0.76 |
The significance was evaluated by one‐way anova with Tukey's multiple comparison test. *P < 0.01 (vs impaired glucose tolerance [IGT]), **P < 0.05 (vs IGT). BMI, body mass index; GU+, glycosuria‐positive; GU−, glycosuria‐negative; HbA1c, glycated hemoglobin; NGT, normal glucose tolerance; NS, not significant; PG, plasma glucose.
Difference in glycemic and insulin excursion among subgroups
The changes in plasma glucose and serum insulin levels were compared among the GU−/NGT, GU+/NGT and IGT groups (Figure 2e,f). The plasma glucose levels after PHS/OGTT were highest in IGT and lowest in GU−/NGT (Figure 2e, left). When assessed by AUC of plasma glucose over 120 min after OGTT (AUCGlc), the value of GU−/NGT was significantly lower than that of GU+/NGT (P < 0.001), as well as that of IGT (P < 0.001; Figure 2e, right), suggesting that glucose tolerance in GU+/NGT is inferior to that in GU−/NGT, although both are diagnosed as normal. The same results were obtained by Glc/OGTT (data not shown).
We then compared the insulin secretory response among these groups. The temporal patterns of insulin secretion after PHS/OGTT differed among GU−/NGT, GU+/NGT and IGT; the secretory response was rapid (peaking at 30 min) in GU−/NGT, but was delayed (peaking at 90 min) in GU+/NGT and IGT (Figure 2f). The insulinogenic index (Figure 2g) and Matsuda index (Figure 2h) were highest in GU−/NGT and lowest in IGT.
Difference in insulin secretory profile between Glc/OGTT and PHS/OGTT in each subgroup
We next compared plasma glucose and serum insulin levels between PHS/OGTT and Glc/OGTT in GU−/NGT, GU+/NGT and IGT (Figure 3a,b). In accord with the higher plasma glucose in the late phase of PHS/OGTT compared with that in Glc/OGTT (Figure 2a), a very small time‐dependent increase in the plasma glucose ratio (the ratio of the value in PHS/OGTT to that in Glc/OGTT) was noticed (Figure 3a). However, there was not a significant difference in the ratio among GU−/NGT, GU+/NGT and IGT. By contrast, when the PHS/Glc ratio was plotted for serum insulin, there was a gradual increase in GU−/NGT, but not in IGT (Figure 3b).
Figure 3.
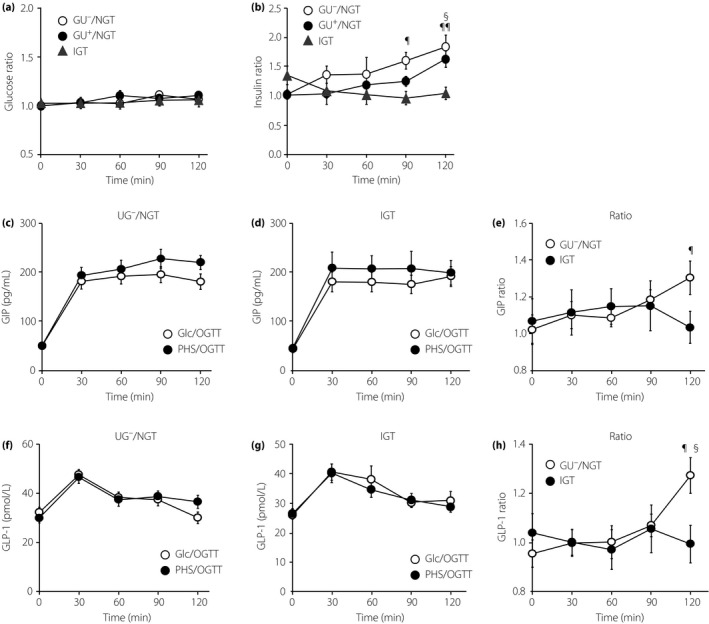
Time‐courses of plasma glucose, serum insulin and serum incretins during partial hydrolysate of starch oral glucose tolerance test (PHS/OGTT) expressed as their ratios to D‐glucose (Glc)/OGTT. (a,b) The ratios of (a) plasma glucose and (b) serum insulin during PHS/OGTT divided by their corresponding data during Glc/OGTT were shown for glycosuria‐negative/normal glucose tolerance (GU −/NGT; circle, n = 49), glycosuria‐positive (GU +)/NGT (triangle, n = 28) and IGT (square, n = 12). (b) ¶ P < 0.05 (vs 0 min) ¶¶ P < 0.01 (vs 0 min), § P < 0.05 (vs GU −/NGT). (c–h) Time‐courses of (c–e) serum glucose‐dependent insulinotropic polypeptide (GIP) and (f–h) glucagon‐like peptide‐1 (GLP‐1) in GU −/NGT (left) and IGT (middle). (e,h) Time‐courses of (c,d) GIP and (f,g) GLP‐1 during PHS/OGTT expressed as their ratios to Glc/OGTT are shown. The number of samples were n = 21 (GIP, GU −/NGT), n = 9 (GIP, IGT), n = 24 (GLP‐1, GU −/NGT) and n = 11 (GLP‐1, IGT), respectively. (e,h) ¶ P < 0.05 (vs 0 min), § P < 0.05 (vs GU −/NGT). (a–h) P‐value was calculated by two‐way repeated‐measurement anova with the Bonferroni post‐hoc test.
Difference in time‐courses of incretin secretion between Glc/OGTT and PHS/OGTT
We then measured the serum incretin levels of total GIP (Figure 3c–e) and total GLP‐1 (Figure 3f–h) in each of the groups. As expected, serum levels of GIP and GLP‐1 were increased time‐dependently in GU−/NGT, but not in IGT. Interestingly, insulin and incretin excursion after PHS ingestion differed from that of Glc in GU−/NGT, but was undistinguishable in IGT.
Difference in levels of serum metabolites during OGTT between GU−/NGT and IGT
To identify metabolic differences in the prediabetic condition, we quantified serum metabolites before and 120 min after OGTT in both GU−/NGT and IGT. Using hydrophilic interaction chromatography tandem mass spectrometry, we were able to quantify the serum levels of 42 metabolites, and OPLS‐DA analysis was then carried out to identify the significant differences.
First, we searched for the metabolites showing distinct differences between PHS/OGTT and Glc/OGTT; however, no such metabolites were found. Next, we attempted to identify metabolites with different serum levels before and 120 min after OGTT. Four sets of comparison were carried out: difference before vs after (i) Glc/OGTT in GU−/NGT, (ii) Glc/OGTT in IGT, (iii) PHS/OGTT in GU−/NGT and (iv) PHS/OGTT in IGT (Figure 4a–d). Using OPLS‐DA analysis, we obtained a model with a sufficient Q 2‐value (>0.3) to analyze Glc/OGTT in GU−/NGT and IGT and PHS/OGTT in IGT, but not for PHS/OGTT in GU−/NGT. Therefore, the result of OPLS‐DA of PHS/OGTT in GU−/NGT was used not for selecting the metabolites, but rather for evaluating their serum levels. Variable importance in projection scores was calculated for each metabolite based on its contribution to statistical discrimination. In total, 18 metabolites were found to meet the selection criteria (variable importance in projection > 1.0, r > 0.4 or r < −0.4; Table 3), and then were subjected to further evaluation.
Figure 4.
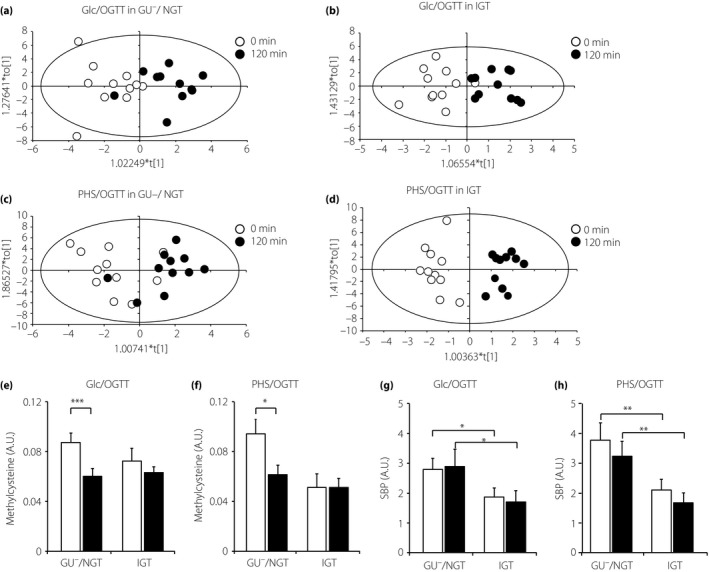
Changes in serum metabolites before and 120 min after oral glucose tolerance test (OGTT). (a–d) Orthogonal projections to latent structures discriminant analysis scores plots of data of (a) D‐glucose (Glc)/OGTT in glycosuria‐negative/normal glucose tolerance (GU −/NGT; R 2Y = 0.610, Q 2 = 0.354), (b) Glc/OGTT in impaired glucose tolerance (IGT; R 2Y = 0.721, Q 2 = 0.405) and (c) partial hydrolysate of starch (PHS)/OGTT in GU −/NGT (R 2Y = 0.509, Q 2 = 0.098). (d) PHS/OGTT in IGT (R 2Y = 0.909, Q 2 = 0.370). (e–h) Changes in serum methylcysteine and sedoheptulose 1,7‐bisphosphate (SBP) levels before and 120 min after OGTT. (e,f) Changes in serum methylcysteine at 0 (open columns) and 120 min (filled columns) during (e) Glc/OGTT and (f) PHS/OGTT. *P < 0.05, ***P < 0.001. NS, not significant by two‐tailed paired t‐test. (g,h) Changes in serum SBP at 0 (open columns) and 120 min (filled columns) during (g) Glc/OGTT and (h) PHS/OGTT. *P < 0.05, **P < 0.01 by two‐way repeated‐measurement anova with the Bonferroni post‐hoc test. (e–h) n = 11 for all groups. A.U., arbitrary unit.
Table 3.
List of metabolites that varied before and 120 min after oral glucose tolerance
| Type of OGTT | Glc/OGTT | PHS/OGTT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject group (Q2) | GU−/NGT (0.354) | IGT (0.405) | GU−/NGT (0.098) | IGT (0.370) | ||||||||
| VIP | r | VIP | r | VIP | r | VIP | r | |||||
| Acethyl‐carnitine | ○ | 1.558 | −0.673 | ○ | 1.224 | −0.435 | ||||||
| Adenine | ○ | 1.061 | −0.518 | ○ | 1.299 | −0.651 | ○ | 1.545 | −0.518 | |||
| Allantoate | ○ | 1.061 | −0.600 | |||||||||
| Asparagine | ○ | 1.000 | −0.454 | |||||||||
| Betaine‐aldehyde | ○ | 1.147 | 0.460 | |||||||||
| Carbamoyl aspartate | ○ | 1.109 | −0.605 | ○ | 1.446 | −0.499 | ||||||
| Dimethyl‐arginine | ○ | 1.138 | −0.612 | |||||||||
| Dopamin | ○ | 1.363 | −0.737 | ○ | 1.259 | −0.669 | ||||||
| Ethanolamine | ○ | ○ | 1.110 | −0.507 | ||||||||
| Histidine | ○ | 1.170 | −0.584 | ○ | 1.105 | −0.509 | ||||||
| Luecine/Isoluecine | ○ | 1.338 | −0.697 | ○ | 1.211 | −0.593 | ○ | 1.369 | −0.450 | |||
| Methylcysteine | ○ | 1.191 | −0.584 | ○ | 1.299 | −0.651 | ||||||
| N‐acetyl‐glutamine | ○ | 1.285 | −0.435 | |||||||||
| Quinolinate | ○ | 1.077 | −0.596 | |||||||||
| Phenylpropiolic acid | ○ | 1.384 | 0.565 | |||||||||
| Pipecolic acid | ○ | 1.531 | 0.681 | |||||||||
| Taurine | ○ | 1.363 | −0.737 | |||||||||
| Uracil | ○ | 1.170 | −0.584 | ○ | 1.058 | −0.525 | ||||||
All molecules selected as “altered by oral glucose tolerance test (OGTT) by orthogonal projections to latent structures discriminant analysis” in any of four sets of comparison (marked by circles [○]) are shown. The values of variable importance in projection (VIP) and correlation coefficient are given for comparisons that met the selection criteria (VIP > 1.0, r > 0.4 or r < −0.4). The metabolites are shown in alphabetical order. GU−, glycosuria‐negative; NGT, normal glucose tolerance.
We found that dopamine, histidine, methylcysteine and uracil were altered during both OGTTs in GU−/NGT, but not in IGT (Table3). Among these, we focused on methylcysteine, the serum levels of which were significantly decreased in both OGTTs (Glc/OGTT and PHS/OGTT) in GU−/NGT, but not in IGT (Figure 4e,f). These results suggest that glucose‐induced suppression of methylcysteine requires normal glucose metabolism and that it is compromised in IGT.
We also searched for metabolites differentially present in GU−/NGT and IGT, and we found that the serum level of sedoheptulose 1,7‐bisphosphate (SBP) at 120 min after PHS/OGTT was lower in IGT than that in GU−/NGT. After analyzing the other sets of comparison, we found that serum SBP levels were significantly lower in IGT compared with those in GU−/NGT (Figure 4g,h).
Discussion
Although Glc/OGTT is safe and is essential for diagnosing IGT, it has been recognized that it can cause discomfort as a result of too much sweetness and gastrointestinal symptoms (e.g., nausea and diarrhea) because of its high osmolality. To address these drawbacks, test solutions composed of partial hydrolysate of starch were established in the 1960s in the USA (Glucola®, 1965) and in Japan (TRELAN®‐G75, 1967), independently7, 8, and the results of Glc/OGTT and PHS/OGTT have been considered to be equivalent7; both Glc and PHS are authorized for use in OGTT in Japan5. However, considering the less‐frequent gastrointestinal adverse reactions during PHS/OGTT, we hypothesized that the dynamics of glucose absorption of the two OGTTs might differ. In the ~50 years since the introduction of Glucola® and TRELAN®‐G, there has been a great advance in understanding incretin physiology and in techniques for measuring metabolites using mass spectrometry. We therefore compared metabolites during OGTT in GU−/NGT and IGT with special emphasis on changes in incretins and serum metabolites.
As expected, excursions of plasma glucose and serum insulin of Glc/OGTT and PHS/OGTT were found not to be equal when all data (n = 99) were combined; they were significantly higher in PHS/OGTT than those in Glc/OGTT in the late phase (at 90 and 120 min). Because of the small difference seen between the two tests, this dissimilarity is unlikely to result in an erroneous diagnosis of individual cases in a clinical setting. Nevertheless, these results show a clear difference between mono‐ and oligosaccharide ingestions in terms of the kinetics of glucose metabolism after glucose loading.
Interestingly, we found that the serum levels of insulin, GIP and GLP‐1 during PHS/OGTT expressed as the ratio to Glc/OGTT showed a time‐dependent increase in GU−/NGT, but not in IGT, suggesting that retardation of gastrointestinal absorption of oligosaccharides occurs in GU−/NGT, but is lost in IGT. Horowitz et al.23 reported that plasma glucose and serum insulin levels at 120 min after OGTT are related to gastric emptying in humans. In addition, levels of GLP‐1 and GIP were reported to be related to the gastric load of glucose24, and the existence of glucose in gut lumen was reported to be required for sustained GLP‐1 secretion14. Considering that nutrient digestion/absorption is minutely regulated25, it is tempting to hypothesize that undigested nutrients might slow the digestion/absorption process in NGT, and that this system becomes dysregulated in IGT. Regardless of the cause for the lack of delay in gastric absorption of oligosaccharide in IGT, our present study suggests that the dynamics of insulin and incretin secretions becomes dysregulated early in IGT.
We noticed an apparent difference in insulin secretory profiles during OGTT between GU+/NGT and GU−/NGT, despite their both being classified as NGT according to the diagnostic criteria. Hayashi et al.26 classified the temporal patterns of insulin secretion during OGTT in non‐diabetic individuals into five categories, the participants were monitored for 10–11 years for the development of diabetes. According to their classification system, the patterns of insulin secretion of GU−/NGT, GU+/NGT, and IGT in our group are classified as pattern 1, 3 and 4, respectively. They reported that the incidence of diabetes mellitus 10–11 years later was 3.2% in pattern 1, 15.4% in pattern 3 and 47.8% in pattern 4. Accordingly, GU+/NGT and GU−/NGT cannot be considered equivalent, and positive glycosuria at medical checkup, as well as the high (≥180 mg/dL) plasma glucose level at 1 h during OGTT5, might represent a risk factor for diabetes mellitus.
The present results on altered insulin secretory kinetics in IGT in response to oligosaccharide prompted us to explore physiological abnormalities of glucose and energy metabolism in IGT using hydrophilic interaction chromatography tandem mass spectrometry. By analyzing the metabolome in the serum before and 120 min after OGTT, we identified methylcysteine and SBP as showing a significant difference between GU−/NGT and IGT.
Serum methylcysteine levels were significantly reduced in both PHS/OGTT and Glc/OGTT in GU−/NGT, but not in IGT. Methylcysteine is a sulfur‐containing amino acid that is present in urine and blood at a low concentration in humans27, but its physiological function and dynamics in the blood remain largely unknown. In the protozoan parasite, Entamoeba histolytica, metabolome analysis showed an intracellular concentration of S‐methylcysteine that was markedly increased on deprivation of l‐cysteine, which is essential for the structure and stability of various proteins in the protozoa28. Methylcysteine is classified into S‐ and L‐methylcysteine, but we could not distinguish these forms in our present analysis. Entamoeba histolytica lives in anaerobic or microaerophilic conditions, and lacks aerobic metabolic pathways, such as the tricarboxylic acid cycle and oxidative phosphorylation, and generates energy by glycolysis29. To cope with the oxidative stress during infection to the host, Entamoeba histolytica has been shown to elicit a drastic change in energy metabolism, including an increase in S‐methylcysteine30.
SBP has been known as a sugar phosphate intermediate involved in the Calvin cycle, the carbon fixation pathway in photosynthetic eukaryotes31. Although it was reported previously that SBP can be generated from hexose 6‐P, triose 3‐P and sedoheptulose 7‐P using rat liver cytosolic enzyme preparation32, the physiological role of SBP in mammals remains unknown. Husain et al.30 reported that sedoheptulose 7‐P and DHAP (dihydroxyacetone phosphate), both of which can be generated from SBP, were increased in Entamoeba histolytica by treatment with paraquat, a widely‐used inducer of oxidative stress.
While metabolome analysis is a powerful tool, such analyses of IGT during OGTT have been carried out only in a few studies, so far16, 33, 34. Although the physiological role of methylcysteine and SBP in mammals is not elucidated in the present study, serum levels of methylcysteine and SBP may well reflect an early abnormality in glucose metabolism in prediabetes. However, it is difficult to determine a clear cut‐off value to discriminate IGT from GU−/NGT, as these values in GU−/NGT and IGT overlap considerably. Accordingly, these metabolites might not serve as diagnostic indicators of IGT, but rather be useful markers for subclinical glucose intolerance.
A limitation of the present study was the relatively small sample size of IGT. We wish to duplicate a similar analysis in a larger group in the future. In addition, to generalize our observation, we must examine whether the same results occur in an older population in which the incidence of diabetes mellitus is higher, and also in other ethnic groups. Furthermore, the participants in the present study were not obese, which has been shown to be critical in developing diabetes mellitus in the Japanese population35, 36.
In conclusion, the present study shows that glycemic excursion after Glc/OGTT and PHS/OGTT is similar, but is not identical. By focusing on the specific differences in insulin and incretin secretion between the two OGTTs, we were able to identify dysregulation of oligosaccharide absorption in IGT. Currently, only plasma glucose levels and serum insulin levels are used for diagnosis of diabetes mellitus and for calculating subsidiary diagnostic parameters. However, the present study shows that OGTT evokes drastic changes in various serum metabolites and that some of them, including methylcysteine and SBP, might be clinically and/or pathophysiologically useful biomarkers of dysregulated glucose metabolism.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank the staff in the Division of Endocrinology & Metabolism and the Division of Laboratory Medicine of Chiba University Hospital for their effort in the fulfillment of OGTT. The study was supported by Grants‐in‐Aid from the Ministry of Education, Science, Sports and Culture of Japan (16K08520 to TM, 16K00846 to EL, and 16K16198 to AE), and research support grants from MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co. Ltd. and Novo Nordisk Pharma Ltd.
J Diabetes Investig 2018;9: 512–521
References
- 1. Perreault L, Pan Q, Mather KJ, et al Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012; 379: 2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cefalu WT, Buse JB, Tuomilehto J, et al Update and next steps for real‐world translation of interventions for type 2 diabetes prevention: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2016; 39: 1186–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kong AP, Luk AO, Chan JC. Detecting people at high risk of type 2 diabetes – how do we find them and who should be treated? Best Pract Res Clin Endocrinol Metab 2016; 30: 345–355. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40(Suppl 1): S11–S24.27979889 [Google Scholar]
- 5. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . WHO guidelines approved by the Guidelines Review Committee In: Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization Copyright (c), 2011, 25 pp. [PubMed] [Google Scholar]
- 7. Leonard S Jr, McCullagh EP, Christopher TC. A new carbohydrate solution for testing glucose tolerance. Diabetes 1965; 14: 96–99. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda Y, Saito H, Anzawa R, et al A new carbohydrate solution, SDT‐25 for testing oral glucose tolerance. J Jpn Diabetes Soc 1968; 11: 207–210 (Japanese). [Google Scholar]
- 9. Heine RJ, Hanning I, Morgan L, et al The oral glucose tolerance test (OGTT): effect of rate of ingestion of carbohydrate and different carbohydrate preparations. Diabetes Care 1983; 6: 441–445. [DOI] [PubMed] [Google Scholar]
- 10. Tokuyama Y, Ishizuka T, Kanatsuka A. A case of impaired glucose tolerance showing blunt response to oral glucose tolerance test loading partially hydrolyzed starch, Trelan‐G®, but acute response to glucose. J Jpn Diabetes Soc 2005; 48: 131–134 (Japanese). [Google Scholar]
- 11. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 12. Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol 2016; 4: 525–536. [DOI] [PubMed] [Google Scholar]
- 13. Sakurai K, Lee EY, Morita A, et al Glucagon‐like peptide‐1 secretion by direct stimulation of L cells with luminal sugar vs non‐nutritive sweetener. J Diabetes Investig 2012; 3: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee EY, Kaneko S, Jutabha P, et al Distinct action of the alpha‐glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J Endocrinol 2015; 224: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michaliszyn SF, Mari A, Lee S, et al Beta‐cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 2014; 63: 3846–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaham O, Wei R, Wang TJ, et al Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 2008; 4: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho JE, Larson MG, Vasan RS, et al Metabolite profiles during oral glucose challenge. Diabetes 2013; 62: 2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menni C, Fauman E, Erte I, et al Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013; 62: 4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giesbertz P, Padberg I, Rein D, et al Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia 2015; 58: 2133–2143. [DOI] [PubMed] [Google Scholar]
- 20. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 21. Soga T, Igarashi K, Ito C, et al Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem 2009; 81: 6165–6174. [DOI] [PubMed] [Google Scholar]
- 22. Yuan M, Breitkopf SB, Yang X, et al A positive/negative ion‐switching, targeted mass spectrometry‐based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012; 7: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horowitz M, Edelbroek MA, Wishart JM, et al Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993; 36: 857–862. [DOI] [PubMed] [Google Scholar]
- 24. Pilichiewicz AN, Chaikomin R, Brennan IM, et al Load‐dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 2007; 293: E743–E753. [DOI] [PubMed] [Google Scholar]
- 25. Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology 1983; 85: 76–82. [PubMed] [Google Scholar]
- 26. Hayashi T, Boyko EJ, Sato KK, et al Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care 2013; 36: 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong MD. N‐delta‐acetylornithine and S‐methylcysteine in blood plasma. Biochim Biophys Acta 1979; 587: 638–642. [DOI] [PubMed] [Google Scholar]
- 28. Husain A, Sato D, Jeelani G, et al Metabolome analysis revealed increase in S‐methylcysteine and phosphatidylisopropanolamine synthesis upon L‐cysteine deprivation in the anaerobic protozoan parasite Entamoeba histolytica . J Biol Chem 2010; 285: 39160–39170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loftus B, Anderson I, Davies R, et al The genome of the protist parasite Entamoeba histolytica . Nature 2005; 433: 865–868. [DOI] [PubMed] [Google Scholar]
- 30. Husain A, Sato D, Jeelani G, et al Dramatic increase in glycerol biosynthesis upon oxidative stress in the anaerobic protozoan parasite Entamoeba histolytica . PLoS Negl Trop Dis 2012; 6: e1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang YH, Wang DY, Wen JF. The independent prokaryotic origins of eukaryotic fructose‐1, 6‐bisphosphatase and sedoheptulose‐1, 7‐bisphosphatase and the implications of their origins for the evolution of eukaryotic Calvin cycle. BMC Evol Biol 2012; 12: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams JF, Blackmore PF, Arora KK. The significance of sedoheptulose 1,7‐bisphosphate in the metabolism and regulation of the pentose pathway in liver. Biochem Int 1985; 11: 599–610. [PubMed] [Google Scholar]
- 33. Deo RC, Hunter L, Lewis GD, et al Interpreting metabolomic profiles using unbiased pathway models. PLoS Comput Biol 2010; 6: e1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gall WE, Beebe K, Lawton KA, et al alpha‐hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010; 5: e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moller JB, Pedersen M, Tanaka H, et al Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 36. Araneta MR, Kanaya AM, Hsu WC, et al Optimum BMI cut points to screen asian americans for type 2 diabetes. Diabetes Care 2015; 38: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]


