Abstract
Both glucagon and glucagon‐like peptide‐1 (GLP‐1) are produced from proglucagon through proteolytic cleavage. Blocking glucagon action increases the circulating levels of glucagon and GLP‐1, reduces the blood glucose level, and induces the proliferation of islet α‐cells. Glucagon blockade also suppresses hepatic amino acid catabolism and increases the serum amino acid level. In animal models defective in both glucagon and GLP‐1, the blood glucose level is not reduced, indicating that GLP‐1 is required for glucagon blockade to reduce the blood glucose level. In contrast, hyperplasia of α‐cells and hyperaminoacidemia are observed in such animal models, indicating that GLP‐1 is not required for the regulation of α‐cell proliferation or amino acid metabolism. These findings suggest that the regulation of amino acid metabolism is a more important specific physiological role of glucagon than the regulation of glucose metabolism. Although the effects of glucagon deficiency on glucose metabolism are compensated by the suppression of insulin secretion, the effects on amino acid metabolism are not. Recently, data showing a feedback regulatory mechanism between the liver and islet α‐cells, which is mediated by glucagon and amino acids, are accumulating. However, a number of questions on the mechanism of this regulation remain to be addressed. The profile of glucagon as a regulator of amino acid metabolism must be carefully considered for glucagon blockade to be applied therapeutically in the treatment of patients with diabetes.
Keywords: Amino acid catabolism, Amino acid transporter, Glutamine
Introduction
Glucagon, a hyperglycemic substance present in aqueous pancreas extracts, was first discovered in 19231. Nearly 60 years later, molecular cloning techniques were applied to identify glucagon precursors, and proglucagon and its derivative peptides, including glucagon‐like peptide‐1 (GLP‐1), were identified2. Whereas glucagon is mainly produced in islet α‐cells through the proteolytic cleavage of proglucagon by prohormone convertase 2 (Pcsk2), GLP‐1 is mainly produced in the intestinal endocrine L‐cells by Pcsk1. Both glucagon and GLP‐1 are involved in the regulation of the glucose level; however, these peptides apparently work in opposite directions. Glucagon stimulates glycogenolysis and gluconeogenesis in the liver to increase the blood glucose level. In contrast, GLP‐1, one of the major incretins, stimulates insulin secretion and β‐cell proliferation to reduce the blood glucose level3, 4, 5.
As both glucagon and GLP‐1 are derived from proglucagon, it is difficult to produce isolated glucagon deficiency without affecting the production of GLP‐1. The purpose of the present review was to provide an overview phenotype of animal models with glucagon deficiency, to underscore the regulation of amino acid metabolism as a specific physiological action of glucagon and to discuss recent advances in the understanding of the mechanisms that regulate islet α‐cell proliferation.
Animal Models Defective in the Production or Action of Glucagon
The first animal model deficient in the production of glucagon was generated by the disruption of the Pcsk2 gene. The Pcsk2 knockout mice lacked active glucagon production, and showed lower blood glucose levels and islet α‐cell hyperplasia6. The proteolytic processing of prosomatostatin and proinsulin is also attenuated in Pcsk2 knockout mice; thus, it remained elusive whether their lower blood glucose levels and α‐cell hyperplasia were simply attributable to the absence of active glucagon.
Several years later, animal models with glucagon receptor (Gcgr) deficiency were generated by two independent groups7, 8, and both groups reported lower blood glucose levels and marked hyperglucagonemia in these animals. As Gelling et al.8 also reported that Gcgr knockout mice showed islet α‐cell hyperplasia, it was confirmed that the absence of glucagon induces α‐cell proliferation. Gelling et al.8 also reported that in addition to glucagon, the pancreatic tissue concentration of GLP‐1 was increased in Gcgr knockout mice. Later, the plasma GLP‐1 levels of Pcsk2 knockout mice were also found to be increased9.
An increase in the serum levels of glucagon and GLP‐1, combined with islet α‐cell hyperplasia, has also been observed in mice with liver‐specific Gs α deficiency, in which glucagon‐induced cyclic adenosine monophosphate production in the liver is markedly attenuated10. Liver‐specific Gcgr knockout mice also showed a similar phenotype11.
The data obtained through the analyses of these models clearly showed that impaired glucagon action causes an increase in GLP‐1, a decrease in the blood glucose level and α‐cell proliferation. However, the causal relationship among these characteristics in these animal models remained elusive until the establishment of animal models that lack both glucagon and GLP‐1 action. The phenotypes of these animal models with deficient glucagon action are summarized in Table 1, together with those described in the following sections.
Table 1.
Phenotype of animal models with deficient glucagon action
| Gene | Defect | Glucagon | GLP‐1 | Blood glucose levels | Islet α‐cell | References |
|---|---|---|---|---|---|---|
| Prohormone convertase 2 (Pcsk2−/−) | Processing of proglucagon | Decreased | Increased | Lower | Hyperplasia | 6, 9 |
| Glucagon receptor (whole body) (Gcgr−/−) | Glucagon action in whole body | Increased | Increased | Lower | Hyperplasia | 7, 8 |
| Gs α (liver specific) (Gs alpha‐LKO, GNAS‐LKO) | Hormone‐induced cAMP production in liver | Increased | Increased | Lower | Hyperplasia | 10 |
| Glucagon receptor (liver specific) (Gcgr‐LKO) | Glucagon action in liver | Increased | Increased | Lower | Hyperplasia | 11 |
| Glucagon (Gcggfp/gfp: GCGKO) | All the peptide derived from proglucagon | Absent | Absent | Normal | Hyperplasia (GFP‐positive islet cell) | 12 |
| Glucagon receptor and GLP‐1 receptor (Gcgr−/− × Glp1r−/−) | Both glucagon action and GLP‐1 action | Increased | Increased | Normal | Hyperplasia | 14 |
| NA (administration of glucagon receptor blocking antibody) | Glucagon action in tissues to which antibody accesses | Increased | Increased | Lower | Induction of proliferation | 36, 37 |
cAMP, cyclic adenosine monophosphate; GCGKO, mice homozygous for glucagon‐green fluorescent protein knock‐in allele; Gcgr, glucagon receptor; GFP, green fluorescent protein; GLP‐1, glucagon‐like peptide‐1; GNAS, guanine nucleotide binding protein, alpha stimulating; LKO, liver‐specific knock out; NA, not applicable; Pcsk2, prohormone convertase 2.
Animal Models with Defective Glucagon and GLP‐1 Action
Mice homozygous for glucagon‐green fluorescent protein (GFP) knock‐in allele (Gcg gfp/gfp [GCGKO]) lack all of the peptides derived from proglucagon, including glucagon and GLP‐1 (Figure 1). GCGKO mice are virtually normoglycemic and develop GFP‐positive α‐like cell hyperplasia12. As the plasma insulin concentration of ad libitum‐fed GCGKO mice is significantly lower than that of control littermates, the effects of glucagon deficiency on their blood glucose levels appear to be partially compensated by the suppression of insulin secretion13. Mice lacking both glucagon receptors and GLP‐1 receptors (Gcgr −/− × Glp1r −/−) also develop islet α‐cell hyperplasia under normoglycemic conditions14. These data show the pivotal importance of GLP‐1 in lowering the blood glucose level in the absence of glucagon action. In contrast, it is also clear that neither GLP‐1 nor lower blood glucose levels are prerequisites for the α‐cell proliferation induced by glucagon deficiency.
Figure 1.
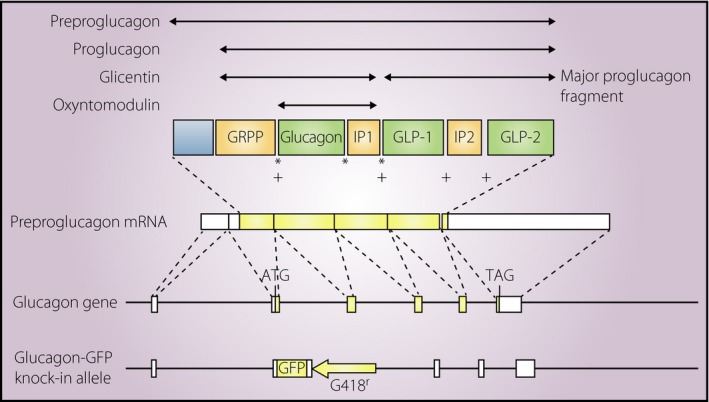
Structure of proglucagon and proglucagon‐derived peptides. Messenger ribonucleic acid (mRNA) transcribed from the glucagon gene encodes preproglucagon. The removal of the signal peptide from preproglucagon results in proglucagon, which serves as a precursor for multiple peptides, including glucagon and glucagon‐like peptides (GLPs). *Cleavage sites for prohormone convertase 2; +cleavage sites for prohormone convertase 1. The structure of the glucagon‐green fluorescent protein (GFP) knock‐in allele is also indicated at the bottom of the figure. ATG, initiation methionine codon; GRPP, glicentin‐related pancreatic polypeptide; IP, intervening peptide; TAG, termination codon.
It is noteworthy that Gcgr −/− × Glp1r −/− mice develop hyperglycemia after streptozotocin‐induced β‐cell destruction, whereas Gcgr −/− mice with a similar degree of β‐cell damage do not15, 16. Based on the resistance to diabetes observed in Gcgr −/− mice, a glucagonocentric view of diabetes has been proposed, in which the hypersecretion of glucagon is as important as (if not more important than) insulin deprivation in the pathogenesis of diabetes15, 17. However, it is clear that GLP‐1 plays pivotally important roles in resistance to diabetes in Gcgr −/− mice, and that diabetes can develop in the absence of glucagon. Indeed, GCGKO mice that lack both glucagon and GLP‐1 show hyperglycemia and/or glucose intolerance on the administration of streptozotocin, high‐fat diet feeding or during pregnancy18, 19, 20. It has also been reported that Gcgr −/− mice develop diabetes after the near total ablation of β‐cells21. Thus, glucagon is not an absolute prerequisite for hyperglycemia and diabetes.
Glucagon as a Regulator of α‐Cell Proliferation and the Amino Acid Metabolism
The proliferation of cells in various endocrine organs and/or tissues, such as the thyroid, adrenal cortex and gonadal glands, is strictly regulated by corresponding tropic hormones that are secreted from the hypothalamo–pituitary axes. In contrast, far less is understood about the mechanisms regulating the proliferation of islet endocrine cells. Animal models, in which the glucagon action in the liver is specifically impaired, develop α‐cell hyperplasia10, 11. Thus, signals to stimulate α‐cell proliferation are considered to be derived from the liver. In addition, results from studies investigating the transplantation of islets or α‐like cells into the subrenal capsule of glucagon‐deficient animal models suggested that such signals are humoral rather than neural11, 22. Accordingly, the expression of genes encoding stimulators of α‐cell proliferation in the liver should be upregulated in animal models with glucagon deficiency, whereas the expression of suppressors should be downregulated. Thus, attempts were made to identify genes that were differentially expressed in animal models with glucagon deficiency; however, such attempts have not been able to identify the specific humoral factors that control α‐cell proliferation13 (and Y Hayashi unpublished data). In contrast, several genes involved in amino acid catabolism were found to be downregulated in GCGKO and Gcgr −/− mice13, 23. These changes in the gene expression were accompanied by increased amino acid concentrations in plasma and liver extract, as summarized in Figure 2, 13. Thus, the absence of glucagon action results in the alteration of the amino acid metabolism in the liver and increased plasma amino acid levels, regardless of the presence or absence of GLP‐1 action or lower blood glucose levels.
Figure 2.
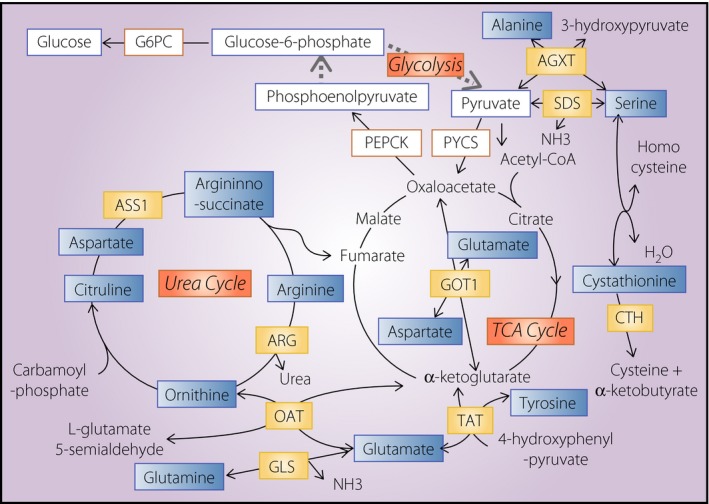
Schematic representation of the metabolic pathways regulated by glucagon. The enzymes and metabolites involved in glycolysis, gluconeogenesis, the tricarboxylic acid (TCA) cycle and the urea cycle are summarized. The expression levels of genes encoding enzymes in letters with yellow boxes are decreased in the glucagon‐green fluorescent protein knock‐in allele liver, whereas the expression levels of metabolites in letters in blue boxes are increased. Other enzymes and metabolites with boxes were also analyzed; however, no significant differences were observed in their expression between the glucagon‐green fluorescent protein knock‐in allele and control liver. AGXT, alanine‐glyoxylate aminotransferase; ARG, arginase; ASS1, arginosuccinate synthase 1; CTH, cystathionase; G6PC, glucose‐6‐phosphatase catalytic subunit; GLS, glutaminase; GOT1, glutamic‐oxaloacetic transaminase; OAT, ornithine aminotranspherase; PEPCK, phosphoenolpyruvate creatine kinase; PYCS, pyruvate carboxylase; SDS, serine dehydratase; TAT, tyrosine aminotransferase.
In the 1980s, it was reported that the serum amino acid levels increase in pancreatectomized patients with glucagon deficiency24. It has also been reported that the administration of glucagon reduces serum amino acid levels25. Human patients with glucagon receptor gene mutations show hyperglucagonemia and α‐cell hyperplasia26, 27, 28, 29, and hyperaminoacidemia has been documented in one such case29. These reports clearly show that glucagon is also required to downregulate the serum amino acid levels in humans.
Glucagon increases the expression of genes encoding enzymes that convert amino acids into substrates available for gluconeogenesis, such as pyruvate and oxaloacetate (Figure 2)13. In contrast, insulin serves as a growth factor and promotes the utilization of amino acids as substrates for protein synthesis. A hypothetical schematic illustration of the underlying mechanism of hyperaminoacidemia under glucagon deficiency is shown in Figure 3. Glucagon and insulin regulate the blood glucose levels in opposite directions (Figure 3a); thus, the amount of insulin required to control the blood glucose level is decreased under the condition of glucagon deficiency. In animal models with glucagon deficiency, both the utilization of amino acids for protein synthesis and the consumption of amino acids for gluconeogenesis are decreased (Figure 3b); thus, such animal models develop hyperaminoacidemia (Figure 3c). In contrast, hyperaminoacidemia might partially compensate for the reduction in phosphoinositide 3‐kinase–protein kinase B–mammalian target of rapamycin (mTOR) signaling activity, which is regulated by insulin, as amino acids directly activate mTOR complex 1 (mTORC1), which plays a critical role in the regulation of protein synthesis and cellular proliferation30, 31. Indeed, an increased lean body mass and/or body size has been documented in glucagon‐deficient models8, 12.
Figure 3.
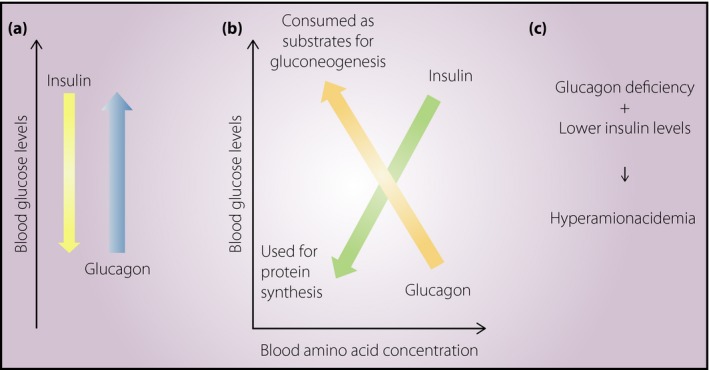
Effect of glucagon deficiency on serum amino acid concentrations. (a) Schematic illustration showing that the blood glucose levels are lowered by insulin and increased by glucagon. (b) Schematic illustration showing that both glucagon and insulin lower the blood amino acid concentration. (c) Hyperaminoacidemia in glucagon deficiency (see main text).
Transient Blockade of Glucagon Action and Amino Acid Metabolism
The blocking of glucagon action has been considered as a therapeutic approach to reduce the blood glucose level, and various approaches including antisense oligonucleotide for Gcgr messenger ribonucleic acid and small molecule glucagon antagonist, have been attempted32, 33, 34. Antisense oligonucleotide for Gcgr messenger ribonucleic acid successfully reduced blood glucose levels in rodent models of diabetes, including ob/ob mice and Zucker diabetic fatty rats32. Antisense oligonucleotide for Gcgr messenger ribonucleic acid also increased the plasma levels of glucagon and GLP‐1, and induced α‐cell hyperplasia; however, the data on the serum amino acid levels were not available in the study32. In contrast, an increase in the serum amino acid levels of rhesus monkeys treated with a small‐molecule glucagon receptor antagonist has been reported35.
The administration of REGN1193, a fully human monoclonal antibody that inhibits glucagon receptor signaling, has been shown to result in a threefold increase in the plasma total amino acid levels of diabetic cynomolgus monkeys36. An increase in the serum amino acid levels combined with the altered expression of genes encoding amino acid catabolism has also been shown in mice that were treated with mAb7, an allosteric antagonistic monoclonal antibody for glucagon receptor37. These studies clearly showed that the transient blockade of glucagon action is sufficient to remodel the hepatic amino acid metabolism in various mammalian species.
Feedback Regulation Between the Liver and Islet α‐Cells Mediated by Glucagon and Amino Acids
Resistance to glucagon, either as a result of genetic defects or pharmaceutical intervention to inhibit glucagon signaling, causes hyperaminoacidemia and hyperplasia of α‐cells. Attempts to identify genes that regulate α‐cell proliferation have been unsuccessful or provided controversial results38, 39. In contrast, amino acids themselves have emerged and attracted attention as candidate regulators of α‐cell proliferation, and it has been hypothesized that a feedback regulatory mechanism between the liver and islet α‐cells, which is mediated by glucagon and amino acids, exists37, 40, 41.
Solloway et al.37 cultured islets in media containing an amino acid concentration matched to Gcgr −/− serum or Gcgr +/+ serum (high or low amino acid media, respectively). They observed a two‐ to threefold increase in the number of α‐cells in islets cultured in high amino acid media in comparison to those in low amino acid media37. Dean et al.42 showed expression of Ki67, a proliferation marker, in primary α‐cells cultured in the presence of Gcgr −/− serum. Experiments involving size fractionated serum showed that molecules of <10 kDa in size have the potential to increase the Ki67 expression, and further experiments showed that amino acids, especially glutamine, play critical roles in promoting the proliferation of α‐cells42.
Kim et al.43 analyzed the effect of antibody‐mediated glucagon blockade on the gene expression in the liver and islets. They identified slc38a5 – which encodes an amino acid transporter with preference for neutral amino acids including glutamine – as the most upregulated gene in the islets. They also showed that in mice deficient of the slc38a5 gene, the proliferation of α‐cells in response to glucagon blockade was attenuated43.
The expression of the slc38a5 gene is regulated by mTORC1, and the inhibition of mTORC1 by rapamycin inhibited the proliferation of α‐cells and the induction of the slc38a5 expression in these studies42, 43. Thus, these studies clearly illustrated a feedback regulatory mechanism between the liver and islet α‐cells, which was mediated by glucagon and amino acids (Figure 4). Glucagon increases the amino acid catabolism in the liver, as well as controlling serum amino acid levels. Glucagon blockade results in an increase in the serum amino acid levels, which in turn activates the mTORC1 complex in α‐cells and promotes its proliferation. Among the various amino acid transporters, SLC38A5 is regulated by mTORC1 and plays a major role in the regulation of α‐cell proliferation. In contrast, GLP‐1 and lower blood glucose levels are not prerequisites for the proliferation of α‐cells induced by glucagon deficiency, as discussed in the section entitled, ‘Animal models defective in the action of both glucagon and GLP‐1’.
Figure 4.

Schematic representation of feedback regulation between the liver and the islet α‐cells. Glucagon is produced in islet α‐cells and the binding of glucagon‐to‐glucagon receptors in the liver increases the intracellular cyclic adenosine monophosphate level, which leads to an increase in hepatic glucose production and amino acid catabolism. Blockade of glucagon action results in an increase in the serum amino acid levels, and consequently promotes α‐cell proliferation. cAMP, cyclic adenosine monophosphate; GLP‐1, glucagon‐like peptide 1; GLP‐2, glucagon‐like peptide 2; GNAS, guanine nucleotide binding protein, alpha stimulating; GRPP, glicentin‐related pancreatic polypeptide; IP, intervening peptide.
Regulation of the α‐Cell Mass in Normal Development and Aristaless‐Related Homeobox
Although SLC38A5 appears to play an important role in the proliferation of α‐cells in response to glucagon blockade, the α‐cell mass in mice with slc38a5 gene deficiency was comparable with that in control mice. Thus, SLC38A5 is not required for the formation and maintenance of α‐cell mass43. Alternatively, how the α‐cell mass is controlled under normal development and/or physiological conditions remains largely elusive.
Mice with Aristaless‐related homeobox (Arx) gene deficiency lack α‐cells44, and the mature β‐cells acquire α‐ and PP cell‐like phenotypes after the misexpression of Arx 45. The expression of Arx is increased in the islets of GCGKO, which show α‐like cell hyperplasia12 (and Y Hayashi unpublished observation). While Arx null mice die shortly after birth, hypomorphic Arx mutants are viable46. Experiments using these hypomorphic Arx mutant and GCGKO mice showed that GFP‐positive α‐like cell hyperplasia in GCGKO mice with hypomorphic Arx was markedly attenuated47. Thus, Arx clearly plays important roles in the differentiation and proliferation of α‐cells. Recently, the long‐term administration of gamma amino butyric acid has been shown to reduce the expression of Arx in α‐cells and promote the transdifferentiation of α‐cells into β‐cells48.
Arx is also among the list of genes that are significantly upregulated in the islets of GCGR antibody‐treated mice43. However, it is not clear whether the increase in the Arx expression reflects the increased expression in each α‐cell or simply reflects an increase in the α‐cell mass. Further analyses are required to elucidate the mechanism involved in the regulation of the Arx expression and to determine whether Arx plays a role in the α‐cell proliferation induced by increased amino acid concentrations.
The Mechanism of the Selective Promotion of α‐Cell Proliferation by Amino Acids
An increase in the serum amino acid levels can be expected to have various effects on organs that consist of numerous types of cells. The response of cells to the alteration of the amino acid concentration is thought to be determined by the repertoire of amino acid transporters expressed in each cell30 and intracellular amino acid sensors, including mTORC149. The mechanisms involved in the selective proliferation of α‐cells in response to hyperaminoacidemia as a result of glucagon blockade remains to be elucidated. Both islet α‐cells and intestinal L‐cells express the glucagon gene and Arx 50. However, although α‐cells show hyperplasia, the number of L‐cells is not increased in glucagon‐deficient animal models12. Thus, comparing a single‐cell transcriptome profile of α‐cells with that of L‐cells might provide insights that can help to explore such mechanisms.
Among the amino acids, leucine and arginine play major roles in the activation of mTORC1 through interaction with intracellular amino acid sensor molecules30, 51. Tan et al.52 recently addressed the role of glutamine in the regulation of mTORC1 activity under the condition of amino acid starvation. During the preparation of the present review, a new mouse model with the specific deletion of Raptor (an mTOR regulator) in α‐cells was reported53. In this model with the α‐cell‐specific loss of mTORC1 signaling, the α‐cell mass is normal at birth; however, it is gradually decreased after weaning. The authors concluded that mTORC1 signaling is dispensable for α‐cell development, but essential for α‐cell maturation during the transition from a milk‐based diet to a chow‐based diet. They also showed that inhibition of mTORC1 reduces the expression of FoxA2, Nkx2.2 and Pou3f4, but not the expression of Arx, in α‐TC‐1 cells53. These findings suggest that the mechanisms involved in the development of α‐cells and those involved in the regulation of the α‐cell mass in response to amino acid alterations are distinct.
Do any Factors Other than Amino Acids Regulate α‐Cell Proliferation?
Glucagon blockade results in a pleiotropic effect that affects most metabolic pathways, including those of amino acids, carbohydrates, fatty acids and nicotinamides. Although it has nearly been established that amino acids play a major role in regulating α‐cell proliferation, the possibility that other factors might regulate α‐cell proliferation has not been excluded.
The expression of FGF21 is regulated by glucagon, and it has been hypothesized that the physiological action of glucagon is partially mediated through an increase in the FGF21 level54. However, the proliferation of α‐cells in response to glucagon blockade was not attenuated in FGF21‐null mice. Thus, FGF21 is not required for the regulation39. In contrast, FGF21 serves as an endocrine signal for protein restriction55. Interestingly, a high‐protein diet increases glucagon secretion and suppresses FGF21 levels, uncoupling the upregulation of FGF21 by glucagon (Hayashi et al., manuscript in preparation).
Nicotinamide N‐methyltransferase is one of the genes whose expression levels are markedly diminished in GCGKO and other glucagon‐deficient models13, 41. As nicotinamide N‐methyltransferase has been reportedly involved in the regulation of the hepatic nutrient metabolism56, the relationship among glucagon, nicotinamide N‐methyltransferase, nutrient metabolism (including that of amino acids) and α‐cell proliferation should be further explored.
Is ‘Glucagon’ Appropriate Nomenclature?
Insulin and glucagon were discovered in the 1920s through exploratory research that aimed to treat diabetes by the administration of extracts from the pancreas; thus, the major physiological function of insulin and glucagon has been considered to be the regulation of the blood glucose level. Whereas insulin deficiency results in hyperglycemia and diabetes, glucagon deficiency results in hyperaminoacidemia rather than hypoglycemia. As discussed in the present review, data showing that the regulation of the amino acid metabolism is the most important specific physiological function of glucagon are accumulating. As a result, there might be questions as to whether ‘glucagon’ is the most appropriate nomenclature for the regulator of blood amino acid levels. As amino acids, especially glutamine, play a major role in the regulation of the α‐cell mass, it might be interesting to imagine possible alternative names for glucagon, such as ‘proteinon’, ‘aminoacidon’ or ‘glutaminon.’ It is also noteworthy that the plasma concentration of glutamine increases to approximately 6 mmol/L, a molar concentration comparable with that of glucose, through glucagon blockade43.
Protein‐derived calories accounted for one‐third of total caloric intake before the agricultural revolution, whereas they account for approximately 12% of the modern diet57. Glucagon appears to play a more important role as a regulator of the amino acid metabolism in protein‐rich diets, such as those of the preagricultural or paleolithic era. Indeed, protein‐rich food stimulates glucagon secretion, whereas carbohydrate‐rich food suppresses the plasma glucagon level in humans58.
Perspective
Glucagon deficiency results in the alteration of the amino acid metabolism and hyperaminoacidemia. Among the amino acids, the plasma concentration of glutamine is the highest, and glutamine can serve as an energy source through glutaminolysis, especially in cells with rapid turnover, such as those in the intestinal mucosa59. Thus, an increase in the plasma glutamine concentration might affect the metabolism of various types of cells. The homeostasis of plasma amino acid levels is not as well understood as that of glucose, and the impact of hyperaminoacidemia on the whole body is not fully understood. Thus, although glucagon blockade might have powerful therapeutic effects with regard to the control of the blood glucose level, the effects of glucagon blockade on the amino acid metabolism should be carefully evaluated in clinical trials.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
Our work is supported in part by Grants‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (24659451, 15K15356, 15H04681).
J Diabetes Investig 2018;9: 464–472
References
- 1. Kimball CP, Murlin JR. Aqueous extracts of pancreas: III. Some precipitation reactions of insulin. J Biol Chem 1923; 58: 337–346. [Google Scholar]
- 2. Lund PK. The discovery of glucagon‐like peptide 1. Regul Pept 2005; 128: 93–96. [DOI] [PubMed] [Google Scholar]
- 3. Kieffer TJ, Habener JF. The glucagon‐like peptides. Endocr Rev 1999; 20: 876–913. [DOI] [PubMed] [Google Scholar]
- 4. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi Y. Metabolic impact of glucagon deficiency. Diabetes Obes Metab 2011; 13(Suppl 1): 151–157. [DOI] [PubMed] [Google Scholar]
- 6. Furuta M, Yano H, Zhou A, et al Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA 1997; 94: 6646–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker JC, Andrews KM, Allen MR, et al Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 2002; 290: 839–843. [DOI] [PubMed] [Google Scholar]
- 8. Gelling RW, Du XQ, Dichmann DS, et al Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 2003; 100: 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gagnon J, Mayne J, Chen A, et al PCSK2‐null mice exhibit delayed intestinal motility, reduced refeeding response and altered plasma levels of several regulatory peptides. Life Sci 2011; 88: 212–217. [DOI] [PubMed] [Google Scholar]
- 10. Chen M, Gavrilova O, Zhao WQ, et al Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver‐specific Gs alpha deficiency. J Clin Invest 2005; 115: 3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Longuet C, Robledo AM, Dean ED, et al Liver‐specific disruption of the murine glucagon receptor produces alpha‐cell hyperplasia: evidence for a circulating alpha‐cell growth factor. Diabetes 2013; 62: 1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi Y, Yamamoto M, Mizoguchi H, et al Mice deficient for glucagon gene‐derived peptides display normoglycemia and hyperplasia of islet {alpha}‐cells but not of intestinal L‐cells. Mol Endocrinol 2009; 23: 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe C, Seino Y, Miyahira H, et al Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon‐derived peptides. Diabetes 2012; 61: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ali S, Lamont BJ, Charron MJ, et al Dual elimination of the glucagon and GLP‐1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 2011; 121: 1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Wang MY, Du XQ, et al Glucagon receptor knockout prevents insulin‐deficient type 1 diabetes in mice. Diabetes 2011; 60: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jun LS, Millican RL, Hawkins ED, et al Absence of glucagon and insulin action reveals a role for the GLP‐1 receptor in endogenous glucose production. Diabetes 2015; 64: 819–827. [DOI] [PubMed] [Google Scholar]
- 17. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012; 122: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iida A, Seino Y, Fukami A, et al Endogenous GIP ameliorates impairment of insulin secretion in proglucagon‐deficient mice under moderate beta cell damage induced by streptozotocin. Diabetologia 2016; 59: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takagi Y, Kinoshita K, Ozaki N, et al Mice deficient in proglucagon‐derived peptides exhibit glucose intolerance on a high‐fat diet but are resistant to obesity. PLoS One 2015; 10: e0138322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugiyama C, Yamamoto M, Kotani T, et al Fertility and pregnancy‐associated ss‐cell proliferation in mice deficient in proglucagon‐derived peptides. PLoS One 2012; 7: e43745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damond N, Thorel F, Moyers JS, et al Blockade of glucagon signaling prevents or reverses diabetes onset only if residual beta‐cells persist. Elife 2016; 5: e13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takano Y, Kasai K, Takagishi Y, et al Pancreatic neuroendocrine tumors in mice deficient in proglucagon‐derived peptides. PLoS One 2015; 10: e0133812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, MacDougall ML, McDowell MT, et al Polyomic profiling reveals significant hepatic metabolic alterations in glucagon‐receptor (GCGR) knockout mice: implications on anti‐glucagon therapies for diabetes. BMC Genom 2011; 12: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boden G, Master RW, Rezvani I, et al Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. J Clin Invest 1980; 65: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boden G, Rezvani I, Owen OE. Effects of glucagon on plasma amino acids. J Clin Invest 1984; 73: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou C, Dhall D, Nissen NN, et al Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas 2009; 38: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sipos B, Sperveslage J, Anlauf M, et al Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab 2015; 100: E783–E788. [DOI] [PubMed] [Google Scholar]
- 28. Miller HC, Kidd M, Modlin IM, et al Glucagon receptor gene mutations with hyperglucagonemia but without the glucagonoma syndrome. World J Gastrointest Surg 2015; 7: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larger E, Wewer Albrechtsen NJ, Hansen LH, et al Pancreatic α‐cell hyperplasia and hyperglucagonemia due to a glucagon receptor splice mutation. Endocrinol Diabetes Metab Case Rep 2016. https://doi.org/10.1530/EDM-16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Broer S, Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J 2017; 474: 1935–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 2017; 26: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sloop KW, Cao JX, Siesky AM, et al Hepatic and glucagon‐like peptide‐1‐mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 2004; 113: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27‐9955) on glucagon‐stimulated glucose production in humans. Diabetologia 2001; 44: 2018–2024. [DOI] [PubMed] [Google Scholar]
- 34. Bagger JI, Knop FK, Holst JJ, et al Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab 2011; 13: 965–971. [DOI] [PubMed] [Google Scholar]
- 35. Mu J, Qureshi SA, Brady EJ, et al Anti‐diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small‐molecule glucagon receptor antagonist. PLoS One 2012; 7: e49572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okamoto H, Kim J, Aglione J, et al Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 2015; 156: 2781–2794. [DOI] [PubMed] [Google Scholar]
- 37. Solloway MJ, Madjidi A, Gu C, et al Glucagon couples hepatic amino acid catabolism to mTOR‐dependent regulation of alpha‐cell mass. Cell Rep 2015; 12: 495–510. [DOI] [PubMed] [Google Scholar]
- 38. Ben‐Zvi D, Barrandon O, Hadley S, et al Angptl4 links alpha‐cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci USA 2015; 112: 15498–15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okamoto H, Cavino K, Na E, et al Angptl4 does not control hyperglucagonemia or alpha‐cell hyperplasia following glucagon receptor inhibition. Proc Natl Acad Sci USA 2017; 114: 2747–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holst JJ, Wewer Albrechtsen NJ, Pedersen J, et al Glucagon and amino acids are linked in a mutual feedback cycle: the liver‐alpha‐cell axis. Diabetes 2017; 66: 235–240. [DOI] [PubMed] [Google Scholar]
- 41. Dean ED, Unger RH, Holland WL. Glucagon antagonism in islet cell proliferation. Proc Natl Acad Sci USA 2017; 114: 3006–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dean ED, Li M, Prasad N, et al Interrupted glucagon signaling reveals hepatic alpha cell axis and role for L‐glutamine in alpha cell proliferation. Cell Metab 2017; 25: 1362–1373 e1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J, Okamoto H, Huang Z, et al Amino acid transporter Slc38a5 controls glucagon receptor inhibition‐induced pancreatic alpha cell hyperplasia in mice. Cell Metab 2017; 25: 1348–1361 e1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collombat P, Mansouri A, Hecksher‐Sorensen J, et al Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003; 17: 2591–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collombat P, Hecksher‐Sorensen J, Krull J, et al Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest 2007; 117: 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitamura K, Itou Y, Yanazawa M, et al Three human ARX mutations cause the lissencephaly‐like and mental retardation with epilepsy‐like pleiotropic phenotypes in mice. Hum Mol Genet 2009; 18: 3708–3724. [DOI] [PubMed] [Google Scholar]
- 47. Xu S, Hayashi Y, Takagishi Y, et al Aristaless‐related homeobox plays a key role in hyperplasia of the pancreas islet alpha‐like cells in mice deficient in proglucagon‐derived peptides. PLoS One 2013; 8: e64415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ben‐Othman N, Vieira A, Courtney M, et al Long‐term GABA administration induces alpha cell‐mediated beta‐like cell neogenesis. Cell 2017; 168: 73–85 e11. [DOI] [PubMed] [Google Scholar]
- 49. Wang S, Tsun ZY, Wolfson RL, et al Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015; 347: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beucher A, Gjernes E, Collin C, et al The homeodomain‐containing transcription factors Arx and Pax4 control enteroendocrine subtype specification in mice. PLoS One 2012; 7: e36449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wyant GA, Abu‐Remaileh M, Wolfson RL, et al mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017; 171: 642–654 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan HWS, Sim AYL, Long YC. Glutamine metabolism regulates autophagy‐dependent mTORC1 reactivation during amino acid starvation. Nat Commun 2017; 8: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bozadjieva N, Blandino‐Rosano M, Chase J, et al Loss of mTORC1 signaling alters pancreatic alpha cell mass and impairs glucagon secretion. J Clin Invest 2017; 127: 4379–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Habegger KM, Stemmer K, Cheng C, et al Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 2013; 62: 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Laeger T, Henagan TM, Albarado DC, et al FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014; 124: 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong S, Moreno‐Navarrete JM, Wei X, et al Nicotinamide N‐methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med 2015; 21: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312: 283–289. [DOI] [PubMed] [Google Scholar]
- 58. Kuwata H, Iwasaki M, Shimizu S, et al Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomised, controlled crossover, exploratory trial. Diabetologia 2016; 59: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 2016; 16: 619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]


