Abstract
Aims/Introduction
Insulin‐treated diabetes patients are at high risk for lipohypertrophy (LH), but this clinical problem has been overlooked by some medical professionals. In addition, studies differed from each other significantly in regard to the prevalence of LH. The present systematic review aimed to determine pooled prevalence levels of LH among insulin‐injecting diabetes patients.
Materials and Methods
Four electronic databases (PubMed, EMBASE, The Cochrane Library and Scopus) were searched for eligible studies from their inception until April 2017, and reference lists were searched manually to identify additional studies. Studies containing data on LH in patients with diabetes mellitus were included. Meta‐analysis was carried out with a random effects model.
Results
A total of 26 studies with a total of 12,493 participants met the inclusion criteria. Meta‐analysis showed that the pooled prevalence of LH was 38% (95% confidence interval [CI] 29–46%, I 2 = 99.1%). The main influence on LH was the type of diabetes mellitus. The pooled prevalence of LH among patients with type 2 diabetes mellitus was higher than patients with type 1 diabetes mellitus (49%, 95% CI 23–74% vs 34%, 95% CI 19–49%). The pooled prevalence of LH of studies involving a mixed type of diabetes mellitus was 37% (95% CI 25–48%, I 2 = 98.3%).
Conclusion
The prevalence of LH was high in insulin‐treated diabetes patients. It showed that diabetes nurses should screen for LH regularly in their patients, and teach them how to prevent LH in their daily management of diabetes mellitus.
Keywords: Diabetes mellitus, Lipohypertrophy, Meta‐analysis
Introduction
Diabetes mellitus has been an epidemic worldwide, the number of patients with diabetes mellitus all over the world is estimated to reach 642 million by 20401. Patients with type 1 diabetes mellitus rely on exogenous insulin whether through continuous subcutaneous insulin infusion or multiple daily insulin injections to help control their blood glucose level. In addition, more and more individuals with type 2 diabetes mellitus start to use insulin because of failure of oral hypoglycemic medications and recommendations from updated guidelines2. Lipohypertrophy (LH) is a common complication of insulin therapy. It has been reported that patients with LH have an almost sixfold higher occurrence of unexplained hypoglycemia compared with patients without LH, and sevenfold higher occurrence of glycemic variability3. Suboptimal glycemic control also increases the risk of cardiovascular disease4, amputation5, retinal diseases6, kidney disease7 and a range of other diseases, as diabetes mellitus can affect multiple organs. Furthermore, LH can increase economic burden, as diabetes patients with LH consume more insulin8. As a consequence, it is crucial to discern LH from normal skin in diabetes patients through credible methods during their usual follow‐up visits, and give them some advice from professionals' perspective. However, present epidemiological data showed that the prevalence of LH in people with diabetes mellitus ranged widely from 1.9% to 73.4% in different studies9, 10. Various factors accounted for this vast difference, including study quality, not using the LH detection gold standard and the detection capacity of diverse screening staff involved across studies. In order to inform efforts to prevent, treat and identify influencing factors of LH among diabetes patients, dependable estimates of LH prevalence are required. To our knowledge, no systematic review and meta‐analysis has been found that quantified the prevalence of LH in patients with diabetes mellitus. The present systematic review, therefore, set out to establish pooled prevalence levels of LH among patients with diabetes mellitus, and to investigate the impacts of study variables on prevalence estimates.
Methods
Literature search
We searched four electronic data repositories (PubMed, EMBASE, The Cochrane Library and Scopus), and the main search terms were: “diabetes,” “diabetes mellitus,” “lipohypertrophy,” “insulin lipohypertrophy,” “subcutaneous induration,” “endermic induration” and “subcutaneous nodules.” The detailed search strategy is shown in Appendix S1. The search was limited to papers written in English published from the above databases' inception to April 2017. We also screened the reference lists of retrieved publications, and consulted experts in the field with the purpose of identifying relevant publications reporting the prevalence of LH among diabetes patients.
Study selection
Two authors independently searched four electronic databases, and browsed titles and read abstracts to decide whether the full text should be examined according to the established inclusion and exclusion criteria. Disagreement was resolved by discussing with a third party. Agreement between reviewers in relation to study relevancy was assessed using Cohen's kappa. We included articles that fulfilled the following criteria: (i) cross‐sectional design, baseline cross‐sectional data from a longitudinal study or baseline cross‐sectional data from a trial, before random allocation; (ii) detected LH by careful examination (at least observation and palpation), studies involving self‐report LH prevalence by patients were also included if the sample size was more than 500; (iii) participants were insulin‐treated patients with type 1 diabetes mellitus or type 2 diabetes mellitus. We excluded the following studies: commentaries, review articles, case reports, letters to the editor, studies in languages other than English, and studies with participants who did not have diabetes or were pregnant.
Data extraction
Two investigators extracted the data independently using a specific extraction form. The extracted data included the name of the first author, year of study publication, country, sample size, percentage of male participants, mean age of participants, number of participants with type 1 diabetes mellitus/type 2 diabetes mellitus, mean diabetes mellitus duration, mean insulin treatment duration, reported prevalence of LH and detection methods of LH. If there were multiple papers from longitudinal or cohort studies, publications were included according to their epidemiological quality.
Quality assessment
We used a modified version of the Newcastle–Ottawa Scale11 to assess the methodological quality of every study included in the present meta‐analysis. The total score ranges from 0 to 5, with ≥3 points indicating low risk of bias and <3 points indicating high risk of bias. The scale assesses quality in several domains: sample representativeness and size, comparability between respondents and non‐respondents, ascertainment of LH, and statistical quality. The detailed assessment process can be seen in Appendix S2.
Statistical analysis
Data analysis was carried out using the meta‐analysis software Stata version 12 (StataCorp, College Station, TX, USA). For evaluation of the pooled effect, a 95% confidence interval (CI) was considered, and statistical significance was set at a P < 0.05. We used random effects to pool studies reporting the prevalence of LH in patients with diabetes mellitus. Between‐study heterogeneity was assessed by the I 2 with thresholds of ≥25%, ≥50% and ≥75% indicating low, moderate and high heterogeneity, respectively. The influence of an individual study on the overall prevalence estimate was explored by consecutively excluding each study in sensitivity analyses. Subgroup analyses were undertaken based on overall study quality, sample size, country of origin, type of diabetes mellitus and publication year, when there was more than one study in the subgroup. Funnel plots and Egger's test were combined to explore the potential publication bias in this meta‐analysis.
Results
Characteristics of the participants in selected studies
The Reporting Items for Systematic reviews and Meta‐Analysis (PRISMA) statement12 was used to outline the selection process for eligible studies (Figure 1). The characteristics of the included studies are presented in Table 1. A total of 26 published studies matched the inclusion criteria, reporting on a total of 12,493 patients with diabetes mellitus. Interrater reliability of reviewers regarding study relevancy was high (Kappa = 0.86). Nine studies took place in Asia8, 10, 13, 14, 15, 16, 17, 18, 19, 14 in Europe2, 3, 9, 20, 21, 22, 23, and one each in North America31, Africa32 and a mix of different countries33. The median of the mean ages was 46 years (range 6.5–63.8 years), and the median percentage of males represented in the sample was 50% (range 27.3–59.1%). In addition, the median number of participants per study was 228 (range 54–4352), the median of mean disease duration was 10.0 years (range 2.8–17.0 years) and the median of mean insulin treatment duration was 9.3 years (range 3.0–15.0 years). When evaluated by the modified Newcastle–Ottawa quality assessment criteria, out of 5 possible points, one study received 5 points24, seven studies received 4 points3, 10, 15, 18, 26, 28, 29, 14 received 3 points2, 9, 14, 16, 17, 19, 20, 21, 22, 24, 27, 30, 31, 33, three received 2 points13, 30, 32 and one received 1 point25.
Figure 1.
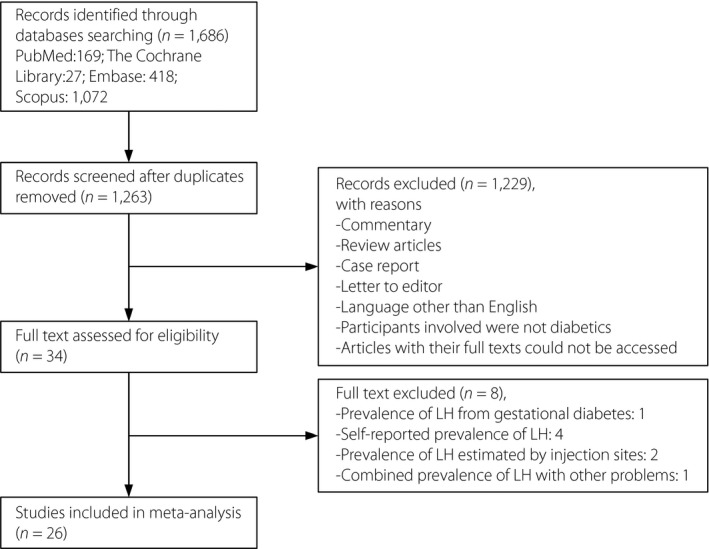
Flowchart of literature research. LH, lipohypertrophy.
Table 1.
Overview of prevalence studies of lipohypertrophy in patients with diabetes mellitus
| Study ID | Country | Sample size | Male (%) | Age, years (mean ± SD) | No. T1DM/T2DM | DM duration, years (mean ± SD) | Insulin treatment duration (years) | Prevalence of LH (%) | Methods for detection of LH | MNOS |
|---|---|---|---|---|---|---|---|---|---|---|
| McNally 1988 | UK | 281 | 53.7 |
Mean: 45.0 Range: 7.0–86.0 |
NS | NS | Mean: 11.0 | 27.1 | OAP by physicians | 3 |
| Hauner 1996 | Germany | 279 | 44.1 | 40.2 ± 18.1 | 223/56 | 14.1 ± 9.5 | ≥2.0 | 23.7 | OAP by a trained physician | 3 |
| Ibarra 1998 | Spain | 150 | 38.0 | 36.9 ± 17.9 | 113/37 | 13.3 ± 8.8 | 11.4 ± 7.9 | 52.0 | OAP by a trained diabetes nurse | 2 |
| Partanen 2000 | Finland | 100 | 44.0 | 32.0 ± 19.0 | 100/0 | 11.0 ± 9.0 | NS | 29.0 | OAP by a diabetes specialist nurse | 3 |
| Raile 2001 | Germany | 112 | NS |
Mean: 10.9 Range: 1.1–19.1 |
112/0 |
Mean: 4.6 Range: 0–15.3 |
NS | 43.8 | OAP by two investigators | 1 |
| Strauss 2002 | Seven European countries† | 1002 | 49.2 | 46.9 ± 18.4 | 581/421 | 14.7 ± 10.6 | ≥0.5 | 27.0 | OAP by trained diabetes nurses | 4 |
| Pavlovic 2007 | Serbia | 212 | 53.3 | 12.5 ± 3.7 | 212/0 | 4.2 ± 3.0 | NS | 1.9 | OAP by two experienced dermatologists | 3 |
| Vardar 2007 | Turkey | 215 | 36.3 | Mean: 59.6 | 31/184 | NS | ≥2.0 | 48.8 | Observation and palpation techniques | 4 |
| Schober 2009 | Austria | 78 | 52.6 | 6.5 ± 4.9 | 78/0 | NS | NS | 46.2 | OAP by researchers | 3 |
| Coninck 2010 | Sixteen countries‡ | 4352 | 49.4 | 48.4±20.1 | NS | 13.9 ± 10.6 | Mean: 11.0 | 47.9 | Self‐report by patients | 4 |
| Hajheydari 2011 | Iran | 220 | 27.3 | 49.0 ± 17.9 | 56/164 | 14 ± 8.5 | 5.4 ± 6.0 | 14.5 | OAP by one specialist physician | 3 |
| Cunningham 2013 | Ireland | 55 | 43.6 | 55.2 ± 16.6 | 41/14 | NS | 15.0 ± 12.6 | 51.0 | Observation and palpation techniques | 3 |
| Blanco 2013 | Spain | 430 | 52.2 | 49.0 ± 22.8 | 177/253 | 6.0–15.0 | 1.0–5.0 | 64.4 | Ultrasound examined, OAP by a diabetes nurse | 4 |
| Sawatkar 2014 | India | 500 | 54.4 | 16.9 ± 6.9 | 500/0 | 4.4 ± 4.4 | NS | 41.0 | OAP by one dermatologist | 3 |
| Ji 2014 | China | 380 | 50.0 | 54.6 ± 8.7 | 0/380 | NS | 3.6 ± 4.1 | 35.3 | OAP by trained diabetes nurses | 4 |
| Grassi 2014 | Italy | 346 | 51.9 | 55.5 ± 18.6 | NS | NS | 13.0 ± 9.8 | 48.7 | OAP by trained nurses | 4 |
| Munster 2014 | Netherlands | 231 | 50.2 | 14.0 ± 7.0 | 231/0 | 6.0 ± 8.0 | NS | 34.8 | OAP by experienced pediatric diabetes nurse practitioners | 4 |
| Binder 2015 | Austria | 54 | 51.9 |
Median: 9.6 Range: 7.1–13.8 |
54/0 |
Median: 3.9 Range: 2.6–6.2 |
NS | 20.0 | OAP by medical staff | 3 |
| Berard 2015 | Canada | 503 | 52.9 | 53.3 ± 19.7 | 125/378 | 14.7 ± 10.0 | ≥0.5 | 24.6 | Self‐report by patients | 4 |
| Ajlouni 2015 | Jordan | 1090 | 47.2 | 57.1 ± 10.3 | 0/1090 |
Median: 13.5 Range: 9.0–20.0 |
4.6 ± 5.0 | 37.3 | OAP by inspection and palpation | 3 |
| Li 2016 | China | 736 | 44.7 | 63.8 ± 8.8 | 0/736 | 10.0 ± 6.9 | >1.0 | 73.4 | OAP by trained diabetes nurses and nursing postgraduates | 4 |
| Hayek 2016 | Saudi Arabia | 174 | 51.7 | 15.4 ± 2.0 | 174/0 | 6.1 ± 4.5 | >1.0 | 52.3 | OAP by a trained diabetes educator | 2 |
| Youssef 2016 | Egypt | 152 | 48.7 | 8.4 ± 3.8 | 152/0 | 2.8 ± 2.9 | NS | 23.7 | OAP by the dermatology team | 2 |
| Patil 2016 | India | 225 | 59.1 | Mean: 50.0 | 30/195 | Median: 6.0 | Mean: 3.0 | 11.1 | OAP by investigators | 3 |
| Ji 2017 | China | 401 | 50.0 | 59.6 ± 11.5 | 26/375 | 11.8 ± 7.3 | 5.8 ± 4.5 | 53.1 | OAP by trained study stuff | 5 |
| Hernar 2017 | Norway | 215 | 51.6 |
Median: 36.0 Range: 18.0–82.0 |
215/0 |
Median: 17.0 Range: 1.0–57.0 |
>1.0 | 47.4 | OAP by trained diabetes specialist nurses | 3 |
†Seven European countries: Sweden, Belgium, Germany, France, Italy, Spain and the UK; ‡16 countries: USA, Russia, the Netherlands, Belgium, France, Spain, Italy, Switzerland, the UK and Ireland, Denmark, Sweden, Germany, China, Turkey, Portugal, and Finland. DM, diabetes mellitus; LH, lipohypertrophy; MNOS, Modified Newcastle Ottawa Scale; NS, not stated; OAP, observed and palpated; SD, standard deviation; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Sensitivity and subgroup analyses
Sensitivity analyses showed that the exclusion of studies with less sample representativeness (46%, 95% CI 36–55%), and fewer comparable respondents and non‐respondents [39%, 95% CI 25%–53%) tended to increase the prevalence of LH. The sensitivity analyses through omitting studies one‐by‐one showed no abnormalities, and the result can be seen in Appendix S3. The subgroup analyses were carried out according to sample size, overall quality, publication year, country of origin and type of diabetes mellitus. Table 2 suggests LH prevalence estimates according to subgroup analysis. The results showed that studies with sample sizes <200 had higher LH estimates (40%, 95% CI 30–49% vs 37%, 95% CI 26–47%). When evaluated by Newcastle–Ottawa criteria, studies with lower total overall quality scores yielded higher LH estimates (43%, 95% CI 28–57% vs 37%, 95% CI 27–46%). In contrast with clinical interviews, more recent publications tended to yield higher LH prevalence estimates. The subgroup analyses for country of origin showed that LH prevalence among Asians tended to be higher than Europeans (41%, 95% CI 27–55% vs 37%, 95% CI 25–49%). The subgroup analyses for diabetes mellitus type showed that LH prevalence among patients with type 2 diabetes mellitus (49%, 95% CI 23–74%) tended to be higher than type 1 diabetes mellitus (34%, 95% CI 19–49%) and a mixed type of diabetes mellitus (37%, 95% CI 25–48%; Figure 2).
Table 2.
Impact of study characteristics on prevalence estimates for lipohypertrophy in diabetes mellitus patients: Subgroup analyses
| Subgroup analysis | n | 95% CI | I 2 (%) | P‐value |
|---|---|---|---|---|
| Sample size | ||||
| <200 | 8 | 0.40 (0.30–0.49) | 89.1 | 0.000a |
| ≥200 | 18 | 0.37 (0.26–0.47) | 99.4 | 0.000a |
| Overall quality | ||||
| <3 points (low quality) | 4 | 0.43 (0.28–0.57) | 92.8 | 0.000a |
| ≥3 points (high quality) | 22 | 0.37 (0.27–0.46) | 99.2 | 0.000a |
| Publication year | ||||
| 1990s | 3 | 0.34 (0.19–0.48) | 94.5 | 0.000a |
| 2000s | 6 | 0.32 (0.16–0.49) | 98.9 | 0.000a |
| 2010– | 17 | 0.40 (0.32–0.48) | 98.5 | 0.000a |
| Country of origin | ||||
| Europe | 14 | 0.37 (0.25–0.49) | 98.8 | 0.000a |
| Asia | 9 | 0.41 (0.27,0.55) | 99.0 | 0.000a |
| Africa | 1 | – | ||
| North America | 1 | – | ||
| Mixed | 1 | – | ||
| DM type | ||||
| T1DM | 10 | 0.34 (0.19–0.49) | 98.6 | 0.000a |
| T2DM | 3 | 0.49 (0.23–0.74) | 99.4 | 0.000a |
| T1DM and T2DM | 10 | 0.37 (0.25–0.48) | 98.3 | 0.000a |
| NS | 3 | 0.41 (0.29–0.54) | 96.6 | 0.000a |
P < 0.001. I 2 ≥25% (low), ≥50% (moderate), ≥75% (high). CI, confidence interval; DM, diabetes mellitus; LH, lipohypertrophy; NS, not stated; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Figure 2.
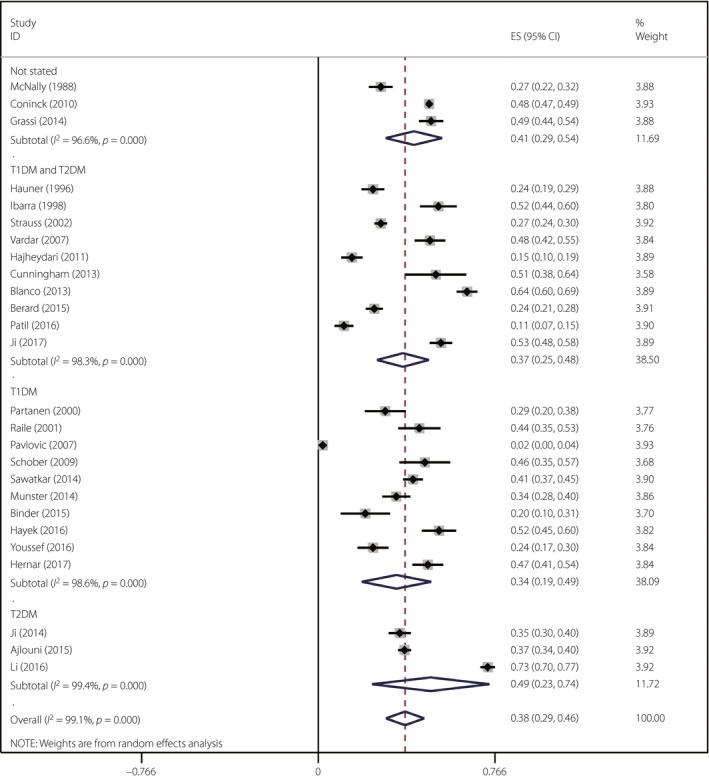
Forest plot of subgroup analysis by type of diabetes mellitus. T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Assessment of publication bias
Assessment of publication bias showed no publication bias, according to the Egger's test (Egger: bias = 2.35, 95% CI –5.93–10.62, P = 0.56) and the funnel plot (Figure 3).
Figure 3.
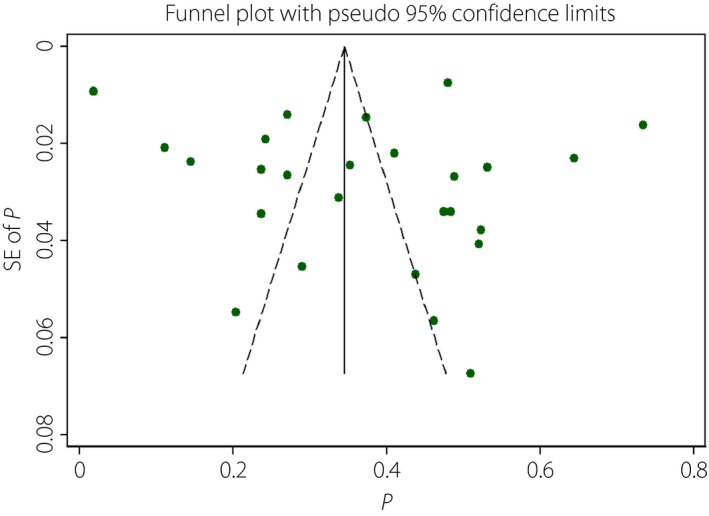
Funnel plot of the meta‐analysis. SE, standard error.
Discussion
The present systematic review and meta‐analysis of 26 studies involved 12,493 patients with diabetes mellitus. Different studies had roughly the same definition of LH, namely, visible and palpable fatty swellings of subcutaneous adipose tissue at insulin injection or infusion sites8. The gold standard for detecting of LH is skin ultrasound scans34. The value of ultrasound examination can be seen in the case of a study carried out by Volkova et al.35, which showed that just eight of 50 participants had clinically evident LH, but 33 of the remaining showed ultrasound evidence of LH.
However, instead of detecting LH using ultrasound examination, most studies detected LH by observing and palpating the injection sites of patients using insulin. Up to now, there is no unified method of visual inspection and palpation. Just three8, 10, 20 of the included studies described the methods further, and the methods referred to by Ji et al.8 are more preferable. They took into consideration the body positions of patients when they evaluated the injection sites. For abdominal examinations, patients lay supine; for the thigh, they sat with knees bent and feet on the floor; for buttock, patients stood; and for arms, patients could sit or stand. The concrete method was that examiners washed their hands and kept them warm, then they daubed ultrasound gel on their hands and the injection sites, and the patients were then examined in a specific position by trained staff in a warm environment to avoid shivering, with oblique lighting to assist visual inspection. Light‐to‐moderate pressure with small sweeps of the fingertips was used to detect LH.
The reason why the researchers prefer observation and palpation is that it is expensive and time‐consuming to investigate LH by ultrasound scans just for the purpose of screening3. In addition, carrying out biopsies for histopathological examination to detect LH is a reliable method36, and it can avoid the misdiagnosis of amyloid lumps as LH, because they are hard to distinguish from each other by physical examination, but it is not practical or economical. Sandro et al.34 reported a suitable palpation technique to identify LH, which reached a 97% consistency rate as compared with the gold standard. Future studies can take advantage of this approach to detect LH in a cost‐effective way. At present, patients are not competent to identify LH by themselves, so we discarded studies involving this condition unless the samples were large31, 33. Furthermore, not all studies mentioned that trained medical professionals were responsible for the detection of LH, which gave implications for future studies, as non‐professionals are likely to overestimate or underestimate the prevalence of LH. We found that the prevalence of LH ranged from 1.9% to 73.4%, and the overall prevalence was 38% (95% CI 29–46%).
Subgroup analysis revealed some interesting findings. The present study found that the prevalence of LH among Asians tended to be higher than among Europeans. This inconsistency might have something to do with social and cultural elements. However, we also found that most studies carried out in Asia were published later, which was in line with the outcome that recent publications were associated with increased LH prevalence among diabetes mellitus patients. In addition, the result of subgroup analysis by type of diabetes mellitus showed that patients with type 2 diabetes mellitus were more likely to develop LH than patients with type 1 diabetes mellitus. Among studies dealing with a mixed type of diabetes mellitus, some of them2, 16, 26, 30 showed that participants with type 1 diabetes mellitus developed LH more easily, though other studies failed to come to such a conclusion3, 8. This discrepancy might be due to the number of patients with different types of diabetes mellitus in those articles being unbalanced. Typically, one study8 had a total sample of 401 participants, but there were just 26 patients living with type 1 diabetes mellitus, the rest of the sample were all patients with type 2 diabetes mellitus. Although studies varied widely in terms of quality, our sensitivity analyses suggested that LH prevalence estimates were reasonably stable. Furthermore, studies with lower total overall quality scores yielded higher LH estimates. The present study also showed that studies with sample size <200 had higher LH estimates.
Because LH is associated with erratic glucose control3, 10, increased risk of chronic complications4, 5, 6, 7 and increased economic burden3, 8, these findings stressed that it is vital that diabetes nurses recognize this condition by inspecting and palpating insulin injecting sites regularly, and draw up a plan for patients to avoid the development of LH. Not only does LH have an influence on disease management, but it can also affect the appearance of a person. Furthermore, there is no established therapeutic method for LH, and people with severe LH must have these parts of the body removed by surgery37, therefore it is important that we discover these sites early so as to let them disappear slowly when the degree of LH is not that serious.
The present review had several limitations. First, the heterogeneity of both total population and subgroup was high, part of which could not be explained. Unexamined factors, such as age, sex, diabetes mellitus duration, insulin treatment duration and methods for detecting of LH might also contribute to the risk for LH, but we could not analyze these factors because of incomplete data. Second, the studies searched were restricted to articles published in English. Third, most studies did not use gold standard for detecting of LH, so there might be significant interobserver variation in the reporting of this condition.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 ¦ The detailed search strategy.
Appendix S2 ¦ Quality assessment.
Appendix S3 ¦ Sensitivity analyses through consecutively excluding each study.
J Diabetes Investig 2018;9: 536–543
Clinical Trial Registry
PROSPERO
CRD 42017065540
References
- 1. International Diabetes Federation . IDF diabetes atlas. 7th ed. http://www.diabetesatlas.org/. [accessed August 21, 2016].
- 2. Cunningham MT, Mckenna M. Lipohypertrophy in insulin‐treated diabetes: Prevalence and associated risk factors. J Diabetes Nur 2013; 17: 340–343. [Google Scholar]
- 3. Blanco M, Hernández MT, Strauss KW, et al Prevalence and risk factors of lipohypertrophy in insulin‐injecting patients with diabetes. Diabetes Metab 2013; 39: 445–453. [DOI] [PubMed] [Google Scholar]
- 4. Smith KJ, Rabasa‐Lhoret R, Strychar I, et al Good vs. poor self‐rated diabetes control: differences in cardiovascular risk and self‐care activities. Exp Clin Endocrinol Diabetes 2014; 122: 236–239. [DOI] [PubMed] [Google Scholar]
- 5. Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr 2017; 11: 149–156. [DOI] [PubMed] [Google Scholar]
- 6. Mowatt L. Diabetic retinopathy and its risk factors at the University Hospital in Jamaica. Middle East Afr J Ophthalmol 2013; 20: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boer IHD, Group FE. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ji L, Sun Z, Li Q, et al Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther 2017; 19: 61–67. [DOI] [PubMed] [Google Scholar]
- 9. Pavlovic MD, Milenkovic T, Dinic M, et al The prevalence of cutaneous manifestations in young patients with Type 1 diabetes. Diabetes Care 2007; 30: 1964–1967. [DOI] [PubMed] [Google Scholar]
- 10. Li FF, Fu SM, Liu ZP, et al Injection sites lipohypertrophy among 736 patients with type 2 diabetes of different‐grade hospitals. Int J Clin Exp Med 2016; 9: 13178–13183. [Google Scholar]
- 11. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 12. Panic N, Leoncini E, Belvis GD, et al Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta‐analysis (PRISMA) statement on the quality of published systematic review and meta‐analyses. PLoS ONE 2013; 8: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al Hayek AA, Robert AA, Braham RB, et al Frequency of lipohypertrophy and associated risk factors in young patients with Type 1 diabetes: a cross‐sectional study. Diabetes Ther 2016; 7: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al Ajlouni M, Abujbara M, Batieha A, et al Prevalence of lipohypertrophy and associated risk factors in insulin‐treated patients with type 2 diabetes mellitus. Int J Endocrinol Metab 2015; 13: e20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vardar B, Kızılcı S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract 2007; 77: 231–236. [DOI] [PubMed] [Google Scholar]
- 16. Hajheydari Z, Kashi Z, Akha O, et al Frequency of lipodystrophy induced by recombinant human insulin. Eur Rev Med Pharmacol Sci 2011; 15: 1196–1201. [PubMed] [Google Scholar]
- 17. Sawatkar GU, Kanwar AJ, Dogra S, et al Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol 2014; 171: 1402–1406. [DOI] [PubMed] [Google Scholar]
- 18. Ji J, Lou Q. Insulin pen injection technique survey in patients with type 2 diabetes in mainland China in 2010. Curr Med Res Opin 2014; 30: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 19. Patil M, Sahoo J, Kamalanathan S, et al Assessment of insulin injection techniques among diabetes patients in a tertiary care centre. Diabetes Metab Syndr 2016. https://doi.org/10.1016/j.dsx.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Hernar I, Haltbakk J, Broström A. Differences in depression, treatment satisfaction and injection behaviour in adults with type 1 diabetes and different degrees of lipohypertrophy. J Clin Nurs 2017. https://doi.org/10.1111/jocn.13801 [DOI] [PubMed] [Google Scholar]
- 21. Mcnally PG, Jowett NI, Kurinczuk JJ, et al Lipohypertrophy and lipoatrophy complicating treatment with highly purified bovine and porcine insulins. Postgrad Med J 1988; 64: 850–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hauner H, Stockamp B, Haastert B. Prevalence of lipohypertrophy in insulin‐treated diabetic patients and predisposing factors. J Clin Nurs 1996; 104: 106–110. [DOI] [PubMed] [Google Scholar]
- 23. Ibarrac SD, Bsc FG. Factors related to lipohypertrophy in insulin‐treated diabetic patients: role of educational intervention. Practical Diabetes Int 1998; 15: 9–11. [Google Scholar]
- 24. Partanen TM, Rissanen A. Insulin injection practices. Practical Diabetes Int 2000; 17: 252–254. [Google Scholar]
- 25. Raile K, Noelle V, Landgraf R, et al Insulin antibodies are associated with lipoatrophy but also with lipohypertrophy in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes 2001; 109: 393–396. [DOI] [PubMed] [Google Scholar]
- 26. Kenneth Strauss MD, Gols HD, Hannet I, et al A pan‐European epidemiologic study of insulin injection technique in patients with diabetes. Practical Diabetes 2002; 19: 71–76. [Google Scholar]
- 27. Schober E, Rami B. Dermatological side effects and complications of continuous subcutaneous insulin infusion in preschool‐age and school‐age children. Pediatr Diabetes 2009; 10: 198–201. [DOI] [PubMed] [Google Scholar]
- 28. Grassi G, Scuntero P, Trepiccioni R, et al Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol 2014; 1: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Munster HE, Van PM, Voorhoeve PG, et al Dermatological complications of insulin therapy in children with type 1 diabetes. Eur Diabetes Nurs 2015; 11: 79–84. [Google Scholar]
- 30. Binder E, Lange O, Edlinger M, et al Frequency of dermatological side effects of continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes 2015; 123: 260–264. [DOI] [PubMed] [Google Scholar]
- 31. Berard L, Cameron B. Injection technique practices in a population of Canadians with diabetes: results from a recent patient/diabetes educator survey. Can J Diabetes 2015; 39: 146–151. [DOI] [PubMed] [Google Scholar]
- 32. Youssef RM, Ibrahim A, Amin IM, et al Cutaneous manifestations among Egyptian children and adolescents with type 1 diabetes. Egypt Pediatr Assoc Gaz 2016; 64: 44–49. [Google Scholar]
- 33. De Coninck C, Frid A, Gaspar R, et al Results and analysis of the 2008–2009 insulin injection technique questionnaire survey. J Diabetes 2010; 2: 168–179. [DOI] [PubMed] [Google Scholar]
- 34. Gentile S, Guarino G, Giancaterini A, et al A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin‐treated people with diabetes. SpringerPlus 2016; 5: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Volkova NYI, Davidenko IYYE. Lypohypertrophy in patients receiving insulin therapy: state of the art. Diabetes Mellitus. Saharnyj Diabet 2011; 14: 86–89. [Google Scholar]
- 36. Nilsson MR. Insulin amyloid at injection sites of patients with diabetes. Amyloid 2016; 23: 139–147. [DOI] [PubMed] [Google Scholar]
- 37. Brun A, Comparin JP, Voulliaume D, et al Insulin‐induced lipohypertrophy treated by liposuction. Ann Chir Plast Esthet 2007; 52: 218–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 ¦ The detailed search strategy.
Appendix S2 ¦ Quality assessment.
Appendix S3 ¦ Sensitivity analyses through consecutively excluding each study.


