Abstract
Aims/Introduction
To evaluate the diagnostic value of microribonucleic acid (miR) as biomarkers in patients with diabetic kidney disease (DKD).
Materials and Methods
A total of 230 diabetes mellitus patients and 53 healthy participants were enrolled, and the diabetes mellitus group was further divided into normoalbuminuria, microalbuminuria and large amount of albuminuria group. MiRs of serum and urine were quantificated using real‐time polymerase chain reaction. General clinical information was collected and analyzed for the risk factors. Cut‐off values of diagnosis sensitivity were determined by receiver operating characteristic curves and the Youden Index.
Results
Compared with the healthy participants, the expression of miR‐192 in serum decreased, whereas in urine it increased with the progression of DKD. The expression of both serum and urine miR‐126 increased in the diabetes mellitus group, but no significant change was obtained among the DKD groups. The area under the curve receiver operating characteristic of both serum and urine miR‐192 was higher than that of the albumin‐to‐creatinine ratio. Combined detection of urine and serum miR‐192 has a higher specificity and lower misdiagnosis rate.
Conclusions
Both serum and urinary miR‐192 could be a potential biomarker of DKD, playing a crucial role in the prevention and treatment of DKD.
Keywords: Biomarker, Diabetic kidney disease, Microribonucleic acid‐192
Introduction
Diabetic kidney disease (DKD) is the leading cause of end‐stage renal disease in Western countries, and its incidence is gradually increasing1, 2. Almost 50% of dialysis patients result from DKD3. The prevalence of diabetes mellitus accompanied by chronic kidney disease in various populations ranges from 15 to 64% according to a Chinese report4.
Microribonucleic acids (miRNAs) are a kind of non‐coding single‐stranded small ribonucleic acid (RNA) containing approximately 21–25 nucleotides that can regulate the expression of target genes by binding with messenger RNAs and degrading or silencing the translation of downstream proteins. More than 2,000 human miRNAs have been found to be involved with the pathological process, such as kidney diseases, and at least 60% of coding genes are regulated by miRNAs5.
Researchers have found a number of kidney‐enriched miRNAs, such as miR‐192, miR‐194, miR‐204 and miR‐215, in recent years6, from which, miR‐192 was found to be involved in the formation and progression of kidney diseases7, 8. Krupa et al.9 reported that the reduced expression of serum miR‐192 could escalate the severity of kidney diseases by promoting the progression of kidney fibrosis in patients with DKD. MiR‐126 plays an important role in endothelial cell biology, cardiovascular diseases and diabetes mellitus10, and some studies have found a significant difference of miR‐126 expression in the urine of type 2 diabetes mellitus patients with or without chronic kidney disease11. However, few studies of large samples regarding miRNAs expression have been carried out in serum and urine. The present study detected the expressions of miR‐192 and miR‐126 in serum and urine simultaneously for the first time, and analyzed their variations among different stages of DKD and healthy people, and then we investigated the clinical value in the early diagnosis of DKD.
Methods
Participants
In the present study, 283 participants were recruited, including 53 normal control participants (NC) and 230 type 2 diabetes mellitus inpatients at Tianjin Metabolic Diseases Hospital, Tianjin, China, during July 2015 and March 2016. The diagnosis of type 2 diabetes mellitus was in accordance with the criteria of the World Health Organization (1999)12. The diabetes mellitus group was divided into groups as follows: (i) the normal albuminuria group (NA; n = 92) – 24‐h urinary microalbumin (uMA) <30 mg, tested three times in the 6 months before admission; (ii) the microalbuminuria group (MA; n = 87) – 24‐h uMA 30–300 mg at least twice among three tests in the 6 months before admission; and (iii) the large amount of albuminuria group (LA; n = 51) – 24‐h uMA >300 mg or 24‐h urinary protein >0.5 g at least twice among three tests in the 6 months before admission. Exclusion criteria are as follows: special types of diabetes mellitus; secondary kidney diseases, such as Henoch–Schonlein purpura, systemic lupus erythematosus; acute cardiocerebral vascular disease; autoimmune disease; tumor; other diseases influencing glucose homeostasis, such as hyperthyroidism and Cushing syndrome; acute complications, such as diabetic ketoacidosis and hyperosmolar coma, occurred in 1 month; stress status, such as fever, acute infections and surgery; dialysis status or kidney transplantation; mental illness; and other kidney diseases. The study protocol obtained approval from the ethics committee of the Metabolic Diseases Hospital of Tianjin Medical University (IRB number: 3639), and all participants had signed informed consent.
Data collection
Baseline data, such as age, sex, diabetes mellitus duration and blood pressure status, were collected with detailed history taking. Weight, height, waist and hip circumference, and blood pressure were measured in the morning. Body mass index (weight [kg]/height2 [m2]) and waist‐to‐hip ration (WHR; waist [cm]/hip [cm]) were calculated afterwards. Blood samples were collected by venipuncture in the morning after an overnight fast (8–12 h). A 24‐h urine collection was carried out. Urine was collected in an appointed container, from 07.00 hours of the day until 07.00 hours the next day, discarding the first urine on the first day. The total volume was recorded and shaken, and 100 mL was extracted for testing. Fasting blood glucose (FBG) was measured by the glucose oxidase method using a Hitachi 7600 automated biochemistry analyzer (Hitachi, Tokyo, Japan). Fasting insulin (FINS) was measured by double antibody radioimmunoassay. Insulin resistance (IR) was quantified using homeostatic model assessment (HOMA) equations as follows (HOMA‐IS, HOMA of insulin secretion; HOMA‐β, HOMA of β‐cell function): HOMA‐IS = 1/FBG × FINS, HOMA‐IR = FBG × FINS/22.5, HOMA‐β = 20 × FINS/(FBG – 3.5)%. Hemoglobin A1c (HbA1c) was detected using an automated glycosylated Hemoglobin Analyzer (Bio‐Rad, Hercules, California, USA). Total cholesterol, triglyceride (TG), low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and serum creatinine (sCr) were measured by automated biochemistry analyzer (Beckman, Indianapolis, Indiana, USA). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation specific for Chinese people: eGFR (mL/[min × 1.73 m2]) = 186 × (sCr [μmol/L] × 0.011312)−1.154 × age−0.203, (×0.742, if female), (×1.233, if Chinese)13. Urine microalbumin was detected using an immunoturbidimetric assay, while urine creatinine was measured with the enzymatic method, then the urine albumin‐to‐creatinine ratio (ACR) was calculated.
Collection of blood and urine specimens
Blood (3 mL) was collected into tubes as mentioned above, and centrifuged at 4°C for 10 min at 1,500 g. Serum samples were separated into 200 μL and stored in 1.5 mL Eppendorf tubes at −80°C. Urine was collected in the morning into aseptic tubes, and centrifuged for 10 min at 4°C for 3,000 g (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The supernatants were removed and then centrifuged within 5 min at 4°C for 12,000 g. The debris was stored after removing the supernatants again.
Primer design and construction
Primers were designed using Primer Premier 5.0 software (Premier Biosoft, Palo Alto, California, USA), and synthesized by Nanjing Aoke Biological Technology Co, Ltd (Zhenjiang, Jiangsu, China). U6 was used as an internal control. The primers used were as follows: miR‐126, forward: 5′‐CATTATTACTTTTGCTACGCG‐3′; reverse: 5′‐CCAGTGCAGGGTCCGAGGT‐3′. miR‐192, forward: 5′‐CTGACCTATGAATTGACAGCC‐3′; reverse: 5′‐CCAGTGCAGG GTCCGAGGT‐3′. U6, forward: 5′‐TGGAACGCTTCACGAATTTGCG‐3′; reverse: 5′‐GGAACGATACAGAGAAGATTAGC‐3′.
Serum RNA extraction and Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was obtained from serum using Trizol LS Reagent (Invitrogen, Carlsbad, California, USA) following the manufacturer's instructions. The optical density values were detected using an ultraviolet spectrophotometer with A260/A280 ranging from 1.8 to 2.1. Reverse transcription was carried out with the Mir‐X miRNA First‐stand Synthesis Kit (Clontech Laboratories, Mountain View, California, USA) at 37°C for 60 min and then at 85°C for 5 min. The complementary deoxyribonucleic acid (cDNA) was diluted to 100 μL for further study. Quantitative reverse transcription polymerase chain reaction (qRT–PCR) was carried out by Mir‐X miRNA qRT–PCR SYBR Kit (SYBR Green; Clontech Takara, Otsu, Shiga, Japan) using the Roche Light Cycler Fluorescence Quantitative PCR System (ABI, Waltham, Massachusetts, USA) with 2 μL cDNA as the template under the following conditions: 95°C for 10 s, 40 cycles at 95°C for 5 s and 54°C for 20 s. All the PCR reactions were in a reaction volume of 25 μL and repeated three times. The results were represented as cycle threshold values.
Urinary miRNA extraction and QRT–PCR
Urinary miRNA was obtained from urine samples using a miRcute miRNA isolution Kit (Tiangen, Beijing, China). Optical density values were measured using an ultraviolet spectrophotometer. Reverse transcription was carried out with the miRNA first‐stand cDNA synthesis Kit (Tiangen) at 37°C for 60 min following the manufacturer's instructions. The 3′ terminals of miRNAs were treated with poly A. qRT–PCR was carried out by a miRcute miRNA qPCR Detection Kit (SYBR Green; Tiangen) with 2 μL cDNA as the template under the following conditions: 94°C for 2 min, 40 cycles of 94°C for 20 s and 54°C for 34 s. All the PCR reactions were in a reaction volume of 25 μL and repeated three times. The results were represented as cycle threshold values.
Receiver Operating Characteristic and Youden Index
Receiver operating characteristic (ROC) curves were constructed by Spss 21.0 (SPSS, Chicago, Illinois, USA) to evaluate the cut‐off values. The area under the curves presented the predictive power of biomarkers. The Youden Index (YI) was calculated to assess the facticity of these predictive values, the formula is as follows: YI = sensitivity + specificity − 1. The bigger value showed the better assessment value effect14. Parallel detection means one positive result in two tests, which can decrease the rate of missed diagnosis, whereas series detection means two positive results in two tests, which can lower the misdiagnosis rate. The positive and negative prediction values were calculated using the χ2‐test.
Statistical analysis
Spss 21.0 statistical analysis software and GraphPad Prism 5 (La Jolla, California, USA) were used for data analysis and photo processing. The K‐S test and Levene's test were used to detect the homogeneity of variance. Quantitative data were analyzed using Pearson's correlation coefficient method, and expressed as mean ± standard deviation or median, whereas qualitative data were analyzed by Spearman's correlation analysis and the χ2‐test, and given as percentages. Differences between groups were analyzed by one‐way anova. Multivariable regression analysis was carried out with risk factors. Two‐tailed P‐values <0.05 were considered statistically significant.
Results
Characteristics of the study participants
Table 1 shows the clinical characteristics of 283 participants. There were no significant (P > 0.05) differences in age, sex, body mass index, uric acid, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol among groups. Duration, WHR, sCr, FINS, HOMA‐IS and HOMA‐IR were elevated (P < 0.05) in accordance with the increment of albuminuria; however, HOMA‐β was depressed (P < 0.05). Compared with the NC group, WHR, FBG, HbA1c, TG and ACR were elevated, but eGFR was depressed (P < 0.05) in the diabetes mellitus groups. Nevertheless, there was no significant difference (P < 0.05) between the LA group and MA group. Total cholesterol in the NA and MA groups, but not in the LA group, were significantly (P < 0.05) increased compared with the NC group. Furthermore, the prevalence of diabetic retinopathy was 32.6% (30/92), 47.1% (41/87), and 62.7% (32/57) in the NA, MA and LA groups.
Table 1.
Characteristics of participants in study groups
| Item | NC group (n = 53) | NA group (n = 92) | MA group (n = 87) | LA group (n = 51) |
|---|---|---|---|---|
| Age, years (SD) | 58.26 ± 9.50 | 55.19 ± 12.58 | 58.28 ± 11.68 | 58.40 ± 11.78 |
| Sex (male/female) | 26/27 | 53/39 | 45/42 | 29/22 |
| Duration, years (SD) | 0.00 | 4.60 (0.1–23)* | 11.30 (4–25)* | 14.70 (5–40)* |
| BMI, kg/m2 (SD) | 22.00 ± 1.41 | 25.86 ± 2.41* | 25.29 ± 2.34* | 23.39 ± 2.74‡ , † |
| WHR (SD) | 0.94 ± 0.06 | 1.02 ± 0.10* | 0.98 ± 0.12* , † | 0.94 ± 0.06* , ‡ |
| SBP, mmHg (SD) | 120.80 ± 5.60 | 128.20 ± 8.20 | 126.40 ± 12.30 | 120.30 ± 8.20 |
| FBG, mmol/L (SD) | 5.20 ± 0.90 | 8.70 ± 1.30* | 9.60 ± 2.40* | 6.20 ± 0.80* , ‡ , † |
| HbA1c, % (SD) | 5.37 ± 0.09 | 8.61 ± 0.23* | 8.71 ± 0.26* | 6.31 ± 0.40* |
| BUN, mmol/L (SD) | 4.48 ± 0.17 | 5.27 ± 0.16* | 4.83 ± 0.20 | 7.48 ± 0.56* , † , ‡ |
| sCr, umol/L (SD) | 56.73 ± 2.99 | 62.29 ± 2.12 | 64.59 ± 2.04 | 83.24 ± 4.96* , † , ‡ |
| UA, umol/L (SD) | 298.07 ± 6.69 | 302.90 ± 89.06 | 321.86 ± 11.43 | 320.49 ± 22.24 |
| TG, mmol/L (SD) | 0.96 ± 0.08 | 1.77 ± 0.14* | 2.35 ± 0.22* , † | 1.87 ± 0.21* |
| TC, mmol/L (SD) | 4.39 ± 0.13 | 4.95 ± 0.13* | 4.91 ± 0.16* | 4.49 ± 0.28 |
| HDL‐c, mmol/L (SD) | 1.37 ± 0.03 | 1.19 ± 0.05 | 1.35 ± 0.18 | 1.07 ± 0.03 |
| LDL‐c, mmol/L (SD) | 2.90 ± 0.09 | 3.03 ± 0.11 | 3.10 ± 0.12 | 2.85 ± 0.23 |
| FINS, mIU/L (SD) | 6.19 ± 0.20 | 10.75 ± 1.11* | 12.99 ± 0.96* | 15.60 ± 1.57* , † |
| HOMA‐IS (SD) | 1.29 ± 0.04 | 1.30 ± 0.16 | 1.45 ± 0.12 | 1.86 ± 0.18* , † , ‡ |
| HOMA‐β (SD) | 100.93 ± 5.00 | 48.47 ± 7.61* | 49.56 ± 4.60* | 71.89 ± 9.68* , † , ‡ |
| HOMA‐IR (SD) | 1.32 ± 0.56 | 4.20 ± 0.47* | 5.41 ± 0.50* | 6.54 ± 0.88* , † |
| ACR, mg/mmol (SD) | 1.50 ± 0.09 | 1.30 ± 0.07 | 8.48 ± 1.83* | 12.19 ± 2.29* |
| eGFR, mL/min × 1.73 m2 (SD) | 120.80 ± 12.99 | 105.64 ± 23.67** | 97.74 ± 24.15** , † | 77.40 ± 18.38** , † , ‡‡ |
| DR | 0 | 30 (32.6%) | 41 (47.1%) | 32 (62.7%) |
*P < 0.05, **P < 0.01, vs normal control (NC) group; † P < 0.05, †† P < 0.01, vs normal albuminuria (NA) group; ‡ P < 0.05, ‡‡ P < 0.01, vs microalbuminuria (MA) group. ACR, albumin‐to‐creatinine ratio; BMI, body mass index; BUN, blood urea nitrogen; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; FINS, fasting insulin; HbA1c, hemoglobin A1c; HDL‐c, high‐density lipoprotein cholesterol; HOMA‐β, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; HOMA‐IS, homeostatic model assessment of insulin secretion; LA, large amount of albuminuria group; LDL‐c, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; sCr, serum creatinine; SD, standard deviation; TC, total cholesterol; TG, triglyceride; UA, uric acid; WHR, waist‐to‐hip ratio.
Expression of miR‐192 and miR‐126 in serum and urine
The expression of serum miR‐192 in the LA group was significantly diminished compared with the MA and NA groups, and the level in the MA group was lower than that in NA group, as shown in Figure 1, but they were all depressed in contrast with the NC group (P < 0.05). The expression of urinary miR‐192 was elevated in the MA and LA groups, along with the increment of albuminuria (P < 0.05). The levels of miR‐126 in serum of the LA, MA and NA groups were higher than the NC group, but the variations among the different groups showed no significant differences (P > 0.05). MiR‐126 in the urine of the NA, MA and LA groups was higher than the NC group, whereas the MA group presented the highest value. The variations among different groups showed significant differences (P < 0.05) .
Figure 1.
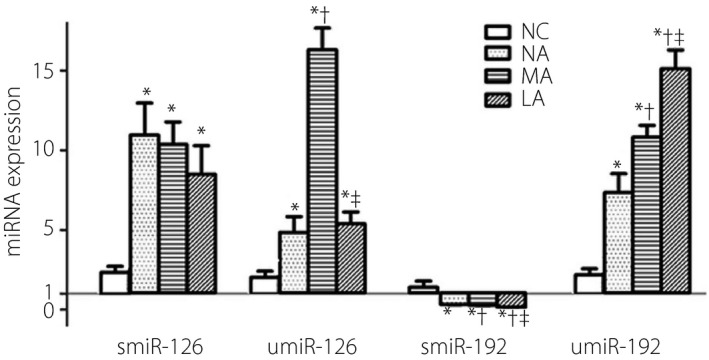
Expressions of microribonucleic acid (miR)‐192 and miR‐126 in serum (smiR) and urine (umiR). *P < 0.05 vs normal control group (NC); † P < 0.05 vs normal albuminuria group (NA); ‡ P < 0.05 vs microalbuminuria group (MA). Microribonucleic acids were detected using quantitative reverse transcription polymerase chain reaction. LA, large amount of albuminuria group.
Relationship between miR‐192 and other clinical parameters
The expressions of serum miR‐192 were found to have no obvious association with age, sex, smoking, body mass index, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and HOMA‐IS in DKD patients using Spearman's and Pearson's correlation analysis, but were positively associated with HOMA‐β (r = 0.264, P < 0.01), whereas negatively correlated with history of drinking, duration, WHR, HbA1c, systolic blood pressure, TG, FINS and HOMA‐IR (r = −0.160, −0.364, −0.249, −0.390, −0.376, −0.155, −0.404, −0.360, respectively; P < 0.01). Among the renal function biomarkers, serum miR‐192 was negative associated with sCr, blood urea nitrogen and ACR (r = −0.159, −0.242, −0.233, respectively; P < 0.05; Table 2).
Table 2.
Analysis of serum microribonucleic acid‐192 and clinical parameters
| Parameters | r | P | Adjusted age and sex | |
|---|---|---|---|---|
| r | P | |||
| Sex† | 0.011 | 0.885 | – | – |
| Age | 0.003 | 0.986 | – | – |
| Smoking† | −0.045 | 0.556 | −0.066 | 0.386 |
| Drinking† | −0.160* | 0.034 | −0.064 | 0.402 |
| Duration | −0.364** | 0 | −0.407** | 0 |
| BMI | −0.118 | 0.121 | −0.118 | 0.123 |
| WHR | −0.249** | 0.001 | −0.259** | 0.001 |
| HbA1c | −0.390** | 0 | −0.393** | 0 |
| SBP | −0.376** | 0 | −0.379** | 0 |
| TG | −0.155* | 0.041 | −0.155* | 0.041 |
| TC | −0.123 | 0.105 | −0.123 | 0.108 |
| HDL‐c | 0.092 | 0.228 | 0.091 | 0.233 |
| LDL‐c | −0.009 | 0.909 | −0.008 | 0.917 |
| FINS | −0.404** | 0 | −0.409** | 0 |
| ACR | −0.233** | 0.003 | −0.234** | 0.003 |
| HOMA‐IS | −0.154 | 0.117 | −0.159 | 0.108 |
| HOMA‐IR | −0.360** | 0 | −0.362** | 0 |
| HOMA‐β | 0.264** | 0.007 | 0.261** | 0.008 |
| sCr | −0.163* | 0.031 | −0.173* | 0.023 |
| BUN | −0.159* | 0.036 | −0.161* | 0.034 |
| uMA | −0.242** | 0.001 | −0.243** | 0.001 |
ACR, albumin‐to‐creatinine ratio; BMI, body mass index; BUN, blood urea nitrogen; FINS, fasting insulin; HbA1c, hemoglobin A1c; HDL‐c, high‐density lipoprotein cholesterol; HOMA‐β, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; HOMA‐IS, homeostatic model assessment of insulin secretion; LDL‐c, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; sCr, serum creatinine; TC, total cholesterol; TG, triglyceride; uMA, urinary microalbumin; WHR, waist‐to‐hip ratio. †Spearman's correlation analysis, others using Pearson's correlation analysis. *P < 0.05, **P < 0.01.
Diagnosis efficacy of serum and urinary miR‐192 in different groups and its cut‐off points in DKD
We regrouped the participants as follows according to different situations in clinical practice and aimed to adjust confounding factors: A group: NC + NA + MA + LA groups; B group: NC + NA + MA groups; C group: NC + NA + LA groups; D group: NA + MA groups; E group: MA + LA group.
ROC curves were constructed to evaluate the diagnostic performance as determined, the sensitivity and specificity of miR‐192 in serum and urine among groups were all >50%. The YI was between 0 and 1, and the best value was 0.929. Urinary miR‐192 shared the same accuracy in the diagnosis of DKD and the early stage of diabetic kidney disease. When the optimal cut‐off values according to the YI of serum and urinary miR‐192, as derived from the ROC curve analysis, were set at 0.4067 and 3.1271, the type 2 diabetes mellitus patients could be separated from normal control participants. Once the values were set at 0.3373 and 5.5147, the type 2 diabetes mellitus patients with albuminuria or without albuminuria could be distinguished from each other. When the values of serum and urinary miR‐192 were set at 0.1097 and 9.3778, patients with macroalbuminuria could be screened out. Thus, we could distinguish the different stages of DKD by the different cut‐off values, which suggests that miR‐192 is valuable in the diagnosis in DKD (Table 3).
Table 3.
Efficacy of serum and urinary microribonucleic acid‐192 in type 2 diabetes mellitus and diabetic kidney disease
| Groups | Sensitivity (%) | Specificity (%) | YI | Cut‐off values | ||||
|---|---|---|---|---|---|---|---|---|
| S | U | S | U | S | U | S | U | |
| A: NC + NA + MA + LA | 93.3 | 92.9 | 74.5 | 100 | 0.678 | 0.929 | 0.4067 | 3.1271 |
| B: NC + NA + MA | 64.6 | 90.6 | 78.5 | 77.3 | 0.431 | 0.679 | 0.3373 | 5.5147 |
| C: NC + NA + LA | 85.5 | 96.2 | 56.7 | 59.8 | 0.422 | 0.56 | 0.1097 | 9.3778 |
| D: NA + MA | 70.8 | 97.4 | 65.3 | 68.2 | 0.361 | 0.656 | 0.2916 | 4.1065 |
| E: MA + LA | 69.4 | 80.8 | 56.7 | 47.4 | 0.261 | 0.282 | 0.1097 | 9.4381 |
Youden Index (YI) = sensitivity + specificity‐1. LA, large amount of albuminuria; MA, microalbuminuria; NA, normal albuminuria; NC, normal controls; S, serum; U, urine.
Comparison of miR‐192 and ACR in the Diagnosis of Diabetic kidney injury
The participants were divided into three groups according to their eGFR values: group I: eGFR ≥ 90 mL/min/1.73 m2, group II: 60 mL/min/1.73 m2 ≤ eGFR < 90 mL/min/1.73 m2 and group III: eGFR <60 mL/min/1.73 m2. We found serum miR‐192 was significantly diminished with the decrease of eGFR and positively related to eGFR (r = 0.423, P < 0.01), whereas ACR was negatively related to eGFR (r = −0.406, P < 0.01). We defined patients with eGFR <90 mL/min/1.73 m2 as kidney injury and constructed the ROCs. The areas under the curve of serum and urinary miR‐192 and ACR were 0.780 (95% confidence interval: 0.712–0.847), 0.804 (95% CI: 0.739–0.870) and 0.746 (95% CI: 0.681–0.838), respectively (Figure 2).
Figure 2.
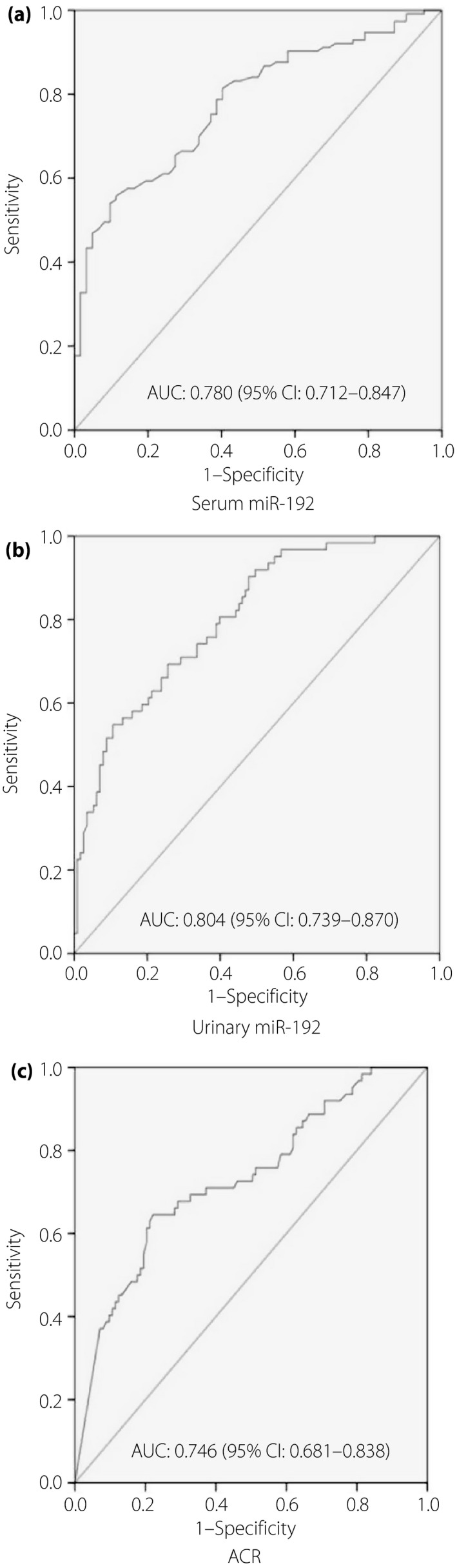
Receiver operating characteristic curves for (a) serum microribonucleic acid (miR)‐192, (b) urinary miR‐192 and (c) albumin‐to‐creatinine ratio (ACR) in diabetic kidney injury. Areas under the curve (AUC) and 95% confidence intervals (CI) are shown.
Diagnostic efficiency of serum miR‐192 compared with urinary miR‐192 in DKD
We found urinary miR‐192 had a higher sensitivity (97.4%), whereas serum miR‐192 had a higher specificity (72.5%) in single detection. Simultaneously, the positive and negative predictive value of urinary miR‐192 was 52.1 and 97.1%, suggesting that urinary miR‐192 is a better single detected biomarker in kidney injury induced by diabetes. We simultaneously detected serum and urinary miR‐192 and found the specificity was increased to 81.2%, leading to the reduction of the false positive rate. The positive and negative prediction values were increased to 66.7 and 81.2%, but the sensitivity was decreased to 66.7%, showing that each indicator tends to be balanced. The YI of series detection was higher than other detections (Table 4).
Table 4.
Efficiency of serum and urinary microribonucleic acid‐192 single or combined detected in diabetic kidney injury
| Biomarker | Sensitivity | Specificity | PPV | NPV | YI |
|---|---|---|---|---|---|
| Serum miR‐192 | 56.4 | 72.5 | 53.7 | 74.6 | 0.289 |
| Urinary miR‐192 | 97.4 | 49.3 | 52.1 | 97.1 | 0.467 |
| Parallel detection | 97.4 | 42 | 48.7 | 96.7 | 0.394 |
| Series detection | 66.7 | 81.2 | 66.7 | 81.2 | 0.479 |
Youden Index (YI) = sensitivity + specificity‐1. miR‐192, microribonucleic acid‐192; NPV, negative prediction value; Parallel detection, one positive result in two tests; PPV, positive prediction value; Series detection, two positive results in two tests.
Discussion
MiRNAs are involved in the regulation of gene expression through monitoring the post‐transcriptional production15, 16, and they also play an important role in the epithelial–mesenchymal transition, which is an important pathological mechanism in chronic kidney disease including DKD17, 18. An increasing number of studies have found miRNAs regulate the progression of kidney disease, such as glomerulosclerosis and end‐stage glomerular disease19, 20. Researchers have found that downregulation of miR‐192 in cultured proximal tubular cells promotes kidney fibrosis in DKD9, but the study only focused on kidney tissue samples, which is not easy for clinical practice. Ma et al.21 reported that miR‐192 in the serum was decreased in DKD patients compared with healthy people, but there is little research on large samples, and no research has been carried out to detect the expression of miR‐192 in both serum and urine simultaneously to evaluate the efficiency in the early diagnosis of DKD.
The present study selected two target genes – miR‐192 and miR‐126 – miR‐192 is located in 11q13.1 and specifically expressed in the kidney, whereas miR‐126 is located in 9q34.3 and associated with microvascular endothelial, which shows a high value in the detection of kidney disease4, 22. Oghbaei et al.23 found that 60 days of moderate activity can significantly increase the expression of miR‐192 in streptozotocin‐induced rats, and no change occurred in healthy rats, thus showing a potential value of therapy. Transforming growth factor‐β is a cytokine that functions in cell growth and extracellular matrix accumulation, and is widely implicated in the pathogenesis of renal fibrosis in human disease. The repression of E‐cadherin is an early event in tubular epithelial cells during epithelial‐to‐mesenchymal transition in kidney fibrosis24, 25. Krupa et al.9 found that stimulating tubular cells with transforming growth factor‐β apparently decreased miR‐192 and E‐cadherin expression in vitro. In the present study, we showed that the expression of serum miR‐192 decreased with the progression of DKD, which is in line with the results of Krupa et al.9, 10 and partly supports the hypothesis that transforming growth factor‐β somehow reduced the expression of miR‐19226. In contrast, after increasing the expression of miR‐192, the expression of the E‐Box repressors, ZEB1 and ZEB2, was suppressed. These two repressors are targets of miR‐192 and further amplify the fibrotic response27, thereby encouraging the expression of E‐cadherin for 17 folds and finally ameliorating the kidney fibrosis9. We found that miR‐126 showed no significance among different groups in serum, and conferred that miR‐126 might not be an efficient biomarker in diabetic kidney disease.
In the present study, we also analyzed the relationship between serum miR‐192 and metabolic indicators, and found that miRNA was positively correlated with HOMA‐IS and negatively correlated with some indicators reflecting glucose control or kidney function, such as HbA1c, systolic blood pressure, TG, FINS, HOMA‐IR, HOMA‐β, sCr, blood urea nitrogen and ACR, which showed that miRNA might be involved in the pathway of insulin synthesis and release, and might be useful in the evaluation of plasma glucose control in diabetes mellitus patients. Poor control of plasma glucose can accelerate the progression of DKD, thus we propose that miR‐192 is involved in this acceleration. We also suggest the close relationship between expression of miR‐192 in different groups and the severity of kidney disease. As shown in the present study, serum miR‐192 decreased with the development of diabetic kidney injury, whereas the urinary miR‐192 increased, as shown in Figure 1. Several studies have reported that miR‐192 was expressed in kidney cortical tissue specifically, and stored and excreted in urine as microvesicles15, 28. Thus, miR‐192 has become a biomarker to assess the control of diabetes and the severity of kidney injury.
In the present study, we found the expression of urinary miR‐192 was upregulated with the progression of DKD, and serum miR‐192 was downregulated with the progression of DKD, suggesting that miR‐192 might be a novel biomarker in the diagnosis and monitoring of DKD. Jia et al.29 analyzed multiple miRNAs in extracellular vesicles in urine, and found urinary miR‐192 was expressed increasingly in DKD. The present study lies on the basis of the aforementioned studies showing that when the optimal cut‐off values of serum and urinary miR‐192 were set at 0.4067 and 3.7271, as derived from the ROC curve analysis, diabetes mellitus patients can be separated from healthy people. Although the cut‐off values were set at 0.3373 and 5.5147, we can separate the patients with and without albuminuria. Furthermore, once the serum miR‐192 is <0.1097 and urinary miR‐192 is >9.3778, we can distinguish the patients with macroalbuminuria. All these values are of great clinical significance for the detection of and therapy in different stages of DKD, showing that miR‐192 might serve as a powerful biomarker in the diagnosis and monitoring of DKD.
In contrast, although the expression of serum and urinary miR‐126 were enhanced in DKD patients, there was no significant difference among the three diabetes mellitus groups. Al‐Kafaji et al.30 reported a reduced expression of miR‐126 in type 2 diabetes mellitus and DKD patients, which differs from the present results, and might partly be because of the racial differences among difference case groups, and their sample (102 patients) was smaller than ours (230 patients). As we know, miRNA‐126 plays an important role in angiogensis and vascular inflammation. Considering that it is not a kidney‐specific miRNA and is easily influenced by systemic conditions, miR‐126 might not be an ideal biomarker in the diagnosis of DKD.
The area under the curve of miR‐192 was larger compared with that of ACR, indicating that miR‐192 can better evaluate the degree of kidney injury. Although urinary protein is mainly related to increased filtration membrane permeability resulting from glomerular sclerosis31, it is influenced by various factors, such as intracorporal total protein, organs function, body conditions and emotions. That was different with serum miR‐192, which is totally stable. In addition, it was regulated by renal tubular resorption. MicroRNA is stable in serum and urine, hardly degraded, and it is easy to detect in urinary debris32. Thus, these results have showed that detection of urinary miR‐192 reduced the false negative rate, whereas a combined detection of serum and urinary miR‐192 reduced the false positive rate. Thus, different methods can be chosen for specific aims in clinical practice.
In conclusion, the present study investigated miRNA expression in both serum and urinary samples in Tianjin, China. We found serum miR‐192 was decreased and urinary miR‐192 was increased with the progression of kidney disease. The cut‐off values of serum and urinary miR‐192 were of great clinical importance. In future, we can enlarge the sample size, follow up the cases and further explore its underlying mechanism. We propose that urinary miR‐192 might be a new valuable biomarker to detect and diagnose kidney injury in DKD, and plays a vital role in the early prevention and treatment of DKD.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81600743), Tianjin Health Industry Key Research Projects (no. 15KG101), and Tianjin Science and Technology Support Project (no. 13ZCDSY01300).
J Diabetes Investig 2018;9: 602–609
References
- 1. Collins AJ, Foley RN, Chavers B, et al ‘United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end‐stage renal disease in the United States. Am J Kidney Dis 2012; 59(1 Suppl 1):A7, e1–e420. [DOI] [PubMed] [Google Scholar]
- 2. Shabankhani B, Kazemnejad A, Zaeri F, et al Survival Factors in Patients With End‐stage Renal Disease in Mazandaran Province, Iran. Iran J Kidney Dis 2016; 10: 79–84. [PubMed] [Google Scholar]
- 3. Nacak H, Bolignano D, Van Diepen M, et al Timing of start of dialysis in diabetes mellitus patients: a systematic literature review. Nephrol Dial Transplant 2016; 31: 306–316. [DOI] [PubMed] [Google Scholar]
- 4. Guo K, Zhang L, Zhao F, et al Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: cross‐sectional study. J Diabetes Complications 2016; 30: 803–810. [DOI] [PubMed] [Google Scholar]
- 5. Wu H, Kong L, Zhou S, et al The role of microRNAs in diabetic nephropathy. J Diabetes Res 2014; 2014: 920134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Y, Koo S, White N, et al Development of a micro‐array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 2004; 32: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung AC, Huang XR, Meng X, et al miR‐192 mediates TGF‐beta/Smad3‐driven renal fibrosis. J Am Soc Nephrol 2010; 21: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 2009; 4: 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krupa A, Jenkins R, Luo DD, et al Loss of MicroRNA‐192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 2010; 21: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asgeirsdottir SA, van Solingen C, Kurniati NF, et al MicroRNA‐126 contributes to renal microvascular heterogeneity of VCAM‐1 protein expression in acute inflammation. Am J Physiol Renal Physiol 2012; 302: F1630–F1639. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Gao G, Yang C, et al Stability of miR‐126 in Urine and Its Potential as a Biomarker for Renal Endothelial Injury with Diabetic Nephropathy. Int J Endocrinol 2014; 2014: 393109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 13. Ma YC, Zuo L, Chen JH, et al Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944. [DOI] [PubMed] [Google Scholar]
- 14. Fang F, Xiao H, Li C, et al Fasting glucose level is associated with nocturnal hypoglycemia in elderly male patients with type 2 diabetes. The Aging Male 2013; 16: 132–136. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt K, Kato M, Natarajan R. Mini‐review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 2016; 310: F109–F118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rudnicki M, Beckers A, Neuwirt H, et al RNA expression signatures and posttranscriptional regulation in diabetic nephropathy. Nephrol Dial Transplant 2015; 30(Suppl 4): iv35–iv42. [DOI] [PubMed] [Google Scholar]
- 17. Zaravinos A. The regulatory role of MicroRNAs in EMT and cancer. J Oncol 2015; 2015: 865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neilson EG. Mechanisms of disease: fibroblasts–a new look at an old problem. Nat Clin Pract Nephrol 2006; 2: 101–108. [DOI] [PubMed] [Google Scholar]
- 19. Schena FP, Sallustio F, Serino G. microRNAs in glomerular diseases from pathophysiology to potential treatment target. Clin Sci (Lond) 2015; 128: 775–788. [DOI] [PubMed] [Google Scholar]
- 20. Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 2015; 11: 23–33. [DOI] [PubMed] [Google Scholar]
- 21. Ma X, Lu C, Lv C, et al The Expression of miR‐192 and its significance in diabetic nephropathy patients with different urine albumin creatinine ratio. J Diabetes Res 2016; 2016: 6789402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorenzen JM. Vascular and circulating microRNAs in renal ischaemia‐reperfusion injury. J Physiol 2015; 593: 1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oghbaei H, Ahmadi Asl N, Sheikhzadeh F, et al The effect of regular moderate exercise on miRNA‐192 expression changes in kidney of streptozotocin‐induced diabetic male rats. Adv Pharm Bull. 2015; 5: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Border WA, Okuda S, Languino LR, et al Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 1990; 346: 371–374. [DOI] [PubMed] [Google Scholar]
- 25. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 2001; 159: 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang B, Herman‐Edelstein M, Koh P, et al E‐cadherin expression is regulated by miR‐192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor‐beta. Diabetes 2010; 59: 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kato M, Arce L, Wang M, et al A microRNA circuit mediates transforming growth factor‐beta1 autoregulation in renal glomerular mesangial cells. Kidney Int 2011; 80: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delic D, Eisele C, Schmid R, et al Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS One 2016; 11: e0150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia Y, Guan M, Zheng Z, et al miRNAs in urine extracellular vesicles as predictors of early‐stage diabetic nephropathy. J Diabetes Res 2016; 2016: 7932765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al‑Kafaji G, Al‑Mahroos G, Al‑Muhtaresh H, et al Decreased expression of circulating microRNA‐126 in patients with type 2 diabetic nephropathy: a potential blood‐based biomarker. Exp Therap Med 2016; 12: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin‐4 encodes small RNAs with antisense complementarity to lin‐14. Cell 1993; 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 32. Martens RJ, Henry RM, Houben AJ, et al Capillary rarefaction associates with albuminuria: the Maastricht study. J Am Soc Nephrol 2016; 27: 3748–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]


