Abstract
Despite great strides in pharmacotherapy for diabetes, there is increasing concern over the risk of hypoglycemia in patients with diabetes receiving pharmacotherapy as they become increasingly older. This has prompted the Japan Diabetes Society (JDS) to initiate a survey on the current status of severe hypoglycemia in clinical settings. In July 2015, following approval from the JDS Scientific Survey/Research Ethics Committee, the JDS extended an invitation to executive educators, who represented a total of 631 healthcare facilities accredited by the JDS for diabetes education, to participate in the proposed survey. Of these, those who expressed their willingness to participate in the survey were sent an application form required for obtaining ethical approval at these healthcare facilities and were then asked, following approval, to enter relevant clinical data on an unlinked, anonymous basis in a web‐based registry. The current survey was fully funded by the JDS Scientific Survey/Research Committee. A case registry (clinical case database) was launched after facility‐specific information (healthcare facility database) was collected from all participating facilities and after informed consent was obtained from all participating patients. With severe hypoglycemia defined as the “presence of hypoglycemic symptoms requiring assistance from another person to treat and preferably venous plasma glucose levels at onset/diagnosis of disease or at presentation clearly less than 60 mg/dL (capillary whole blood glucose, less than 50 mg/dL)”, the current survey was conducted between April 1, 2014 and March 31, 2015, during which facility‐specific information was collected from a total of 193 facilities with a total of 798 case reports collected from 113 facilities. Of the 193 respondent facilities, 149 reported having an emergency department as well, with the median number of patients who required emergency transportation services to reach these facilities totaling 4,962 annually, of which those with severe hypoglycemia accounted for 0.34% (17). The respondent facilities accommodated a total of 2,237 patients with severe hypoglycemia annually, with the number of patients thus accommodated being 6.5 patients per site. A total of 1,171 patients were admitted for severe hypoglycemia, with the number of patients thus admitted being 4.0 per site, who accounted for 52.3% of all patients visiting annually for severe hypoglycemia. A review of the 798 case reports collected during the survey revealed that 240, 480 and 78 patients had type 1 diabetes, type 2 diabetes, and other types of diabetes, respectively; those with type 2 diabetes were shown to be significantly older (median [interquartile range], 77.0 [68.0–83.0]) than those with type 1 diabetes (54.0 [41.0–67.0]) (P < 0.001); and the BMI was shown to be significantly higher for those with type 2 diabetes (22.0 [19.5–24.8] kg/m2) than for those with type 1 diabetes (21.3 [18.9–24.0] kg/m2) (P = 0.003). It was also found that the median estimated glomerular filtration rate (eGFR) was significantly lower among those with type 2 diabetes (50.6 mL [31.8–71.1]/min/1.73 m2) than among those with type 1 diabetes (73.3 [53.5–91.1] mL/min/1.73 m2) (P < 0.001). Again, the median HbA1c value at onset of severe hypoglycemia was shown to be 7.0 (6.3–8.1)% among all patients examined, 7.5 (6.9–8.6)% among those with type 1 diabetes, and 6.8 (6.1–7.6)% among those with type 2 diabetes, with the HbA1c value at onset of hypoglycemia being significantly lower among those with type 2 diabetes (P < 0.001). Antecedent symptoms of severe hypoglycemia were shown to be present, absent and unknown in 35.5, 35.6, and 28.9% of all patients, respectively, with the incidence of symptomatic hypoglycemia being significantly lower among those with type 1 diabetes (41.0%) than among those with type 2 diabetes (56.9%). The antidiabetic agents used in those with type 2 diabetes were insulin preparations (292 patients including 29 receiving concomitant sulfonylureas [SUs]) (60.8%), SUs (159 insulin‐naïve patients) (33.1%), and no insulin preparations or SUs (29 patients) (6.0%). Of the 798 patients surveyed, 296 patients (37.2%) were shown to have required emergency transportation services for severe hypoglycemia before. Thus, the survey revealed, for the first time, the current status of treatment‐related severe hypoglycemia in Japan and clearly highlights the acute need for implementing preventive measures against hypoglycemia not only through education on hypoglycemia but through optimization of antidiabetic therapy for those at high risk of severe hypoglycemia or those with a history of severe hypoglycemia.
Keywords: Insulin, Severe hypoglycemia, Sulfonylureas
Introduction
According to a survey conducted in 2016 on the causes of death among Japanese patients with diabetes mellitus (between 2001 and 2010), diabetic angiopathy accounted for 14.9% of all causes, a far lower rate than that reported in an earlier survey (26.8%; between 1991 and 2000)2, 3. This survey also revealed that the mean age at death was prolonged by 3.4 and 3.5 years, respectively, among the male and female patients, compared to that reported in the earlier survey2, 3. These results appear to reflect the ongoing successful implementation of diabetes treatment and education in the country that focused on preventing onset and/or progression of diabetic angiopathy in Japanese patients with diabetes.
On the other hand, the ever‐increasing number of elderly patients with diabetes, weight gain, and severe hypoglycemia or hypoglycemia unawareness are still among the major challenges in current pharmacotherapy for diabetes. Indeed, increasingly older patients with diabetes receiving antidiabetic therapy are likely to be associated not only with impaired hepatic/renal drug metabolism and/or poor medication adherence but also with weight gain, which leads to poor glycemic control, thus often constituting a vicious cycle necessitating the concurrent use of multiple antidiabetic drugs. Again, current research suggests that severe hypoglycemia or hypoglycemia unawareness is among the factors promoting the onset and/or progression of macroangiopathy or dementia4, 5, 6.
Against this background, the Japan Diabetes Society (JDS) passed a resolution at its May 2013 Board of Directors meeting initiating a survey on the status of treatment‐related severe hypoglycemia, which led to the JDS Committee for Surveys on Severe Hypoglycemia being launched, with the authors of the current report serving as principal members of the Committee.
Methods
As per the survey plan prepared by the JDS Committee for Surveys on Severe Hypoglycemia, the JDS extended an invitation in July 2015 to executive educators, who represented a total of 631 healthcare facilities accredited by the JDS for diabetes education, to participate in the proposed survey in which severe hypoglycemia was defined as the “presence of hypoglycemic symptoms requiring assistance from another person to treat and preferably venous plasma glucose levels at onset/diagnosis of disease or at presentation clearly less than 60 mg/dL (capillary whole blood glucose, less than 50 mg/dL)”. The current survey was conducted between April 1, 2014 and March 31, 2015, and facility‐specific data collected from a total of 193 facilities, as well as a total of 798 case reports collected from 113 facilities, were included for analysis.
Healthcare facility survey
All participating facilities that reported having an emergency department were inquired about the annual number of patients who required emergency transportation services to reach these facilities, as well as the proportion of those who did so for severe hypoglycemia.
Again, all participating facilities were inquired about the number of patients with diabetes mellitus, type 1 and type 2 diabetes currently presenting to these facilities, the number of patients with diabetes mellitus, type 1 and type 2 diabetes receiving insulin formulations and/or sulfonylureas (SU), as well as the annual number of patients presenting to or admitted to these facilities for severe hypoglycemia. All these numbers were analyzed as a whole and on a site‐by‐site basis.
Case‐based survey
The data collected from the 798 patients using a uniform case report form concerned the following items: (i) type of diabetes and sex; (ii) age (years); (iii) duration of diabetes (years); (iv) body mass index (BMI) (kg/m2); (v) estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2); (vi) glucose value at presentation (before treatment) (mg/dL); (vii) HbA1c (%); (viii) time‐period of onset of severe hypoglycemia; (ix) severity of diabetic microangiopathy; (x) presence or absence of antecedent symptoms of hypoglycemia; (xi) presence or absence of serious complications associated with severe hypoglycemia; (xii) prior history of hypoglycemia requiring medical intervention; (xiii) risk of traffic accidents due to hypoglycemia; and (xiv) factor(s) judged by attending physician(s) to have affected the onset of severe hypoglycemia.
As a first step, all reported patients were divided into those with type 1 and type 2 diabetes to allow comparison of data between the two groups. Then, all patients with type 2 diabetes were grouped into those receiving insulin (insulin group including those receiving both insulin and SUs), those receiving SUs (SU group), and those not receiving insulin or SUs (non‐insulin/SU group) to allow comparisons between the three groups. Distributions of variables used for analysis were expressed as mean ± standard deviation (SD) or median (interquartile range, 25th to 75th percentiles), and proportions (%). Statistical analyses were performed by using IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA), JMP 12 (SAS Institute, Cary, NC, USA) or SPSS ver22. (Japan IBM, Tokyo, Japan). The Pearson chi‐square test was used to compare proportions between the two groups and the Student/Welch t‐test and the Mann–Whitney U test was used to compare normally and non‐normally distributed means between the two groups, respectively. The Kruskal–Wallis test was used to examine whether the three groups were identical in distributions, and the Bonferroni correction was used to test all detected differences for significance. A P value of <0.05 was considered to indicate statistical significance.
Again, multiple logistic regression analysis was performed to estimate the association between any of the patient attributes or clinical symptoms and the presence or absence of antecedent symptoms of hypoglycemia, as well as to explore significant factors contributing to the absence of antecedent symptoms of hypoglycemia. For each discrete (categorical) variable, a reference category was defined, and all the other categories were evaluated for their impact, against the reference category, on the absence of antecedent symptoms of hypoglycemia by creating a set of dummy variables, and any such impact was quantified in terms of odds ratios (OR) and 95% confidence intervals (CI) and tested for significance by using the Wald's test.
The proposed survey was implemented, and the survey details disclosed, following approval from the JDS Scientific Survey/Research Ethics Committee (JDSSSREC/H26‐001, 27 November 2014), the JDS extended an invitation to prospective participating facilities to participate in the proposed survey. Of these, those who expressed their willingness to participate in the survey were sent an application form required for obtaining ethical approval for survey participation to ensure that ethical approval was obtained at these facilities. All participating facilities were then asked to submit all survey data to a web‐based registry (with patient data entered on an unlinked, anonymous basis). The current survey was fully funded by the JDS Scientific Survey/Research Committee.
Results
Healthcare facility survey (Table 1)
Table 1.
Healthcare facility survey
| Number of patients presenting to the emergency departments at the respondent facilities (149 of the 193 facilities surveyed) | |
| Number of emergency transportation services ordered per facility | 4,962 (2073–9,910) |
| Number of emergency transportation services ordered for severe hypoglycemia per facility | 17.0 (8.0–32.0) |
| Proportion of emergency transportation services ordered for severe hypoglycemia per facility | 0.34% |
| Number of patients with diabetes mellitus receiving medical services at the respondent facilities and the treatments given to these patients | |
| Number of patients regularly receiving medical services | 346,939 |
| Number (proportion) of patients with type 1 diabetes | 20,553 (6.2%) |
| Number (proportion) of patients with type 2 diabetes | 292,638 (87.9%) |
| Number (proportion) of insulin users (including those receiving other antidiabetic drugs) | 106,645 (30.7%) |
| Number (proportion) of SU users without insulin | 71,280 (20.5%) |
| Number of patients presenting to/admitted to the respondent facilities for severe hypoglycemia | |
| Annual number of patients requiring medical services for severe hypoglycemia at these facilities | 2,237 |
| Number of patients requiring medical services for severe hypoglycemia per facility | 6.5 (3.0–16.0)a |
| Annual number of patients admitted for severe hypoglycemia | 1,171 |
| Annual number of patients admitted for severe hypoglycemia per facility | 4.0 (1.0–9.0)a |
Numbers, medians; numbers in parentheses, interquartile range (25th to 75th percentiles).
Number of patients presenting to the emergency departments at the respondent facilities Of the 193 respondent facilities, a total of 149 facilities (77.2%) reported having an emergency department. The median number of emergency transportation services ordered to reach these facilities totaled 4,962 per site annually; of these, those required for severe hypoglycemia accounted for 17.0 (0.34%).
Number of patients with diabetes mellitus receiving medical services at the respondent facilities and the treatments given to these patients A total of 346,939 patients with diabetes mellitus were currently receiving medical services at the respondent facilities. Of these, those with type 1 and type 2 diabetes accounted for 6.2% and 87.9%, respectively. Again, of these, insulin users (including those receiving other antidiabetic drugs as well) and SU users receiving no insulin accounted for 30.7% and 20.5%, respectively.
Number of patients with diabetes mellitus presenting to/admitted to the respondent facilities for severe hypoglycemia A total of 2,237 patients presented to the respondent facilities for severe hypoglycemia annually, with the per‐site patient number being 6.5. Again, a total of 1,171 patients were admitted to these facilities for severe hypoglycemia, with the per‐site patient number being 4.0; these patients accounted for 52.3% of those presenting to these facilities for hyperglycemia.
Case‐based survey
-
Survey results by type of diabetes
Type of diabetes and sex: The respondent facilities reported a total of 240 patients with type 1 diabetes (males/females, 121/119), 480 patients with type 2 diabetes (males/females, 283/197) and 78 patients with other types of diabetes (males/females, 60/18), and those with type 1, type 2 and other types of diabetes accounted for 30.1%, 60.2%, and 9.8%, respectively (Table 2).
Patient age: The median age of all patients with diabetes mellitus was 71.5 (58.0–81.0) years old, with that of those with type 1 and type 2 diabetes being 54.0 (41.0–67.0) and 77.0 (68.0–83.0) years old, respectively, demonstrating that patients with type 2 diabetes were significantly older than those with type 1 diabetes (P < 0.001) (Tables 2 and 3; Figure 1a).
Duration of diabetes: The mean duration of diabetes was 19.9 ± 11.9 years in all patients with diabetes mellitus, with that in those with type 1 and type 2 diabetes being 21.3 ± 11.7 and 20.4 ± 11.7 years, respectively, showing no significant difference between the groups.
BMI: The median BMI of all patients with diabetes mellitus, those with type 1 and type 2 diabetes was 21.6 (19.2–24.2), 21.3 (18.9–24.0) and 22.0 (19.5–24.8) kg/m2, respectively, showing that BMI was significantly higher for patients with type 2 diabetes than for all patients with diabetes or those with type 1 diabetes (P = 0.003) (Table 3).
eGFR: Patients with type 1 and type 2 diabetes had a median eGFR of 73.3 (53.5–91.1) and 50.6 (31.8–71.1) mL/min/1.73 m2, respectively, showing that those with type 2 diabetes had a significantly lower eGFR than those with type 1 diabetes (P < 0.001) (Table 3).
The median glucose value at presentation (before treatment) was 32.0 (24.0–40.0), 30.0 (22.0–40.0), and 32.0 (26.0–40.0) mg/dL for all patients with diabetes mellitus, those with type 1 diabetes and those with type 2 diabetes, respectively, with no significant difference shown between the groups (P = 0.15) (Tables 2 and 3).
The median HbA1c value at onset of severe hypoglycemia was 7.0 (6.3–8.1), 7.5 (6.9–8.6) and 6.8 (6.1–7.6) for all patients with diabetes mellitus, those with type 1 diabetes, and those with type 2 diabetes, respectively, with the HbA1c value shown to be significantly lower for those with type 2 diabetes (P < 0.001) (Tables 2, 3; Figure 1b).
Time‐period of onset of severe hypoglycemia: There was no particular trend noted in time‐period of onset of severe hypoglycemia in all patients with diabetes mellitus (Table 2).
Diabetic microangiopathy: Severe hypoglycemia was noted in all patients, irrespective of the severity/progression of diabetic microangiopathy (Table 4).
Presence or absence of antecedent symptoms of severe hypoglycemia: Antecedent symptoms were shown to be present in 35.5%, absent in 35.6%, and unknown in 28.9% of all patients (Table 2), with the rate of their occurrence being 41.0% and 56.9% in those with type 1 diabetes and those with type 2 diabetes, respectively, showing that these symptoms occurred significantly less frequently among those with type 1 diabetes than among those with type 2 diabetes (P < 0.001) (Table 3). The absence of antecedent symptoms, which likely represented hypoglycemia unawareness, was associated with lower glucose values at presentation (before treatment) than their presence (30.0 [23.0–40.0] vs. 34.5 [27.5–43.0]), as well as a significantly higher prevalence of diabetic peripheral neuropathy (71.0% vs. 61.1%; P < 0.05) and diabetic autonomic neuropathy (59.7% vs. 47.9%; P < 0.05) (Table 5a). Furthermore, using the presence or absence of antecedent symptoms served as the outcome variables and age, duration of diabetes, HbA1c, glucose value at presentation (before treatment), BMI, stage of nephropathy, type of diabetes, and neuropathy as potential explanatory variables, logistic regression analysis identified type 1 diabetes, low BMI values, low glucose values at presentation (before treatment), and the presence of peripheral neuropathy as significant explanatory variables (Table 5b).
Presence or absence of serious complications associated with severe hypoglycemia: Central nervous system (CNS) sequelae were noted in 2.3% of all patients, 0.8% of those with type 1 diabetes and 2.9% of those with type 2 diabetes (Tables 2 and 3).
Prior history of hypoglycemia requiring medical intervention: 37.2% of all patients with diabetes mellitus reported having had hypoglycemia requiring medical intervention, 44.5% reported having no such history, and 18.3% reported being uncertain as to whether or not they had any such history. Again, 67.8% of patients with type 1 diabetes and 33.1% of patients with type 2 diabetes reported having visited their clinics again for severe hypoglycemia, with significantly more patients with type 1 diabetes shown to have visited their clinics again for severe hypoglycemia (P < 0.001) (Tables 2 and 3).
Risk of traffic accidents due to hypoglycemia: 1.9% of all patients with diabetes reported having had traffic accidents or near‐accidents, 71.1% reported having had none, and 27.0 reported being uncertain as to whether or not they had done. Again, 4.9% of those with type 1 diabetes and 1.3% of those with type 2 diabetes reported having had done, with significantly more patients with type 1 diabetes shown to have had done (P = 0.023) (Tables 2 and 3).
Factors judged by attending physicians to have affected the onset of severe hypoglycemia included: inappropriate diet or dietary timing (40%); drug overdose or incorrect drug use (27%); sick days (11%); excessive alcohol intake (8%); excessive exercise (4%); and others (10%) (Figure 2).
Comparison of factors associated with the onset of severe hypoglycemia in patients with type 2 diabetes by their drug use (Table 6)
Table 2.
Background characteristics of patients with severe hypoglycemia
| Characteristic | Distribution |
|---|---|
| Type of diabetes | |
| Type 1 diabetes, n (%) | 240 (30.1%) |
| Type 2 diabetes, n (%) | 480 (60.2%) |
| Other (including “unknown”), n (%) | 78 (9.8%) |
| Sex (males/females), n (%) | 464 (58.1%)/334 (41.9%) |
| Age (years), median (25th to 75th percentiles) | 71.5 (58.0–81.0) |
| Duration of diabetes (years), mean ± SD | 19.9 ± 11.9 |
| BMI (kg/m2), median (25th to 75th percentiles) | 21.6 (19.2–24.2) |
| eGFR (mL/min/1.73 m2), median (25th to 75th percentiles) | 58.4 (35.6–80.2) |
| Glucose value at presentation (before treatment), median (25th to 75th percentiles) | 32.0 (24.0–40.0) |
| HbA1c (%), median (25th to 75th percentiles) | 7.0 (6.3–8.1) |
| Time‐period of onset of severe hypoglycemia, n (%) | |
| 0–3 | 64 (8.3) |
| 3–6 | 41 (5.3) |
| 6–9 | 92 (11.9) |
| 9–12 | 114 (14.7) |
| 12–15 | 110 (14.2) |
| 15–18 | 105 (13.6) |
| 18–21 | 149 (19.3) |
| 21–24 | 99 (12.8) |
| Antecedent symptoms of hypoglycemia, n (%) | |
| Absent | 284 (35.6) |
| Present | 283 (35.5) |
| Unknown | 231 (28.9) |
| Recovery from impaired consciousness, n (%) | |
| Yes | 780 (97.5) |
| CNS sequelae, n (%) | |
| Present | 18 (2.3) |
| Serious complications of severe hypoglycemia, n (%) | |
| Absent | 768 (97.5) |
| Present | 30 (3.8) |
| Other complications present, n | |
| CAD | 1 |
| Arrhythmia | 1 |
| Death | 11 |
| Cognitive decline | 8 |
| Other | 14 |
| Prior history of severe hypoglycemia requiring visits to emergency departments, n (%) | |
| No | 354 (44.5) |
| Yes | 296 (37.2) |
| Unknown | 146 (18.3) |
| Number (proportion) of patients experiencing traffic accidents or near‐accidents, n (%) | |
| No | 567 (71.1) |
| Yes | 15 (1.9) |
| Unknown | 215 (27.0) |
BMI, body mass index; CAD, coronary artery disease; CNS, central nervous system; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Table 3.
Comparison of factors associated with severe hypoglycemia by type of diabetes
| Factor | Type 1 diabetes (n = 240) | Type 2 diabetes (n = 480) | P‐value |
|---|---|---|---|
| Age (years) | 54.0 (41.0–67.0) | 77.0 (68.0–83.0) | <0.0001 |
| Duration of diabetes (years) | 21.3 ± 11.7 | 20.4 ± 11.7 | 0.344 |
| BMI (kg/m2) | 21.3 (18.9–24.0) | 22.0 (19.5–24.8) | 0.003 |
| Glucose value at presentation (before treatment) (mg/dL) | 30.0 (22.0–40.0) | 32.0 (26.0–40.0) | 0.15 |
| HbA1c (%) | 7.5 (6.9–8.6) | 6.8 (6.1–7.6) | <0.0001 |
| Presence of antecedent symptoms (%) | 41.0 | 56.9 | <0.0001 |
| Presence of CNS sequelae (%) | 0.80 | 2.90 | 0.074 |
| Proportion of patients presenting for severe hypoglycemia (%) | 67.8 | 33.1 | <0.0001 |
| Total number of visits by patients with a history of prior visits (n) | 3.0 (1.0–5.0) | 1.0 (1.0–2.0) | <0.0001 |
| Proportion of patients experiencing traffic accidents or near‐accidents | 4.9 | 1.3 | 0.023 |
BMI, body mass index; CNS, central nervous system.
Figure 1.
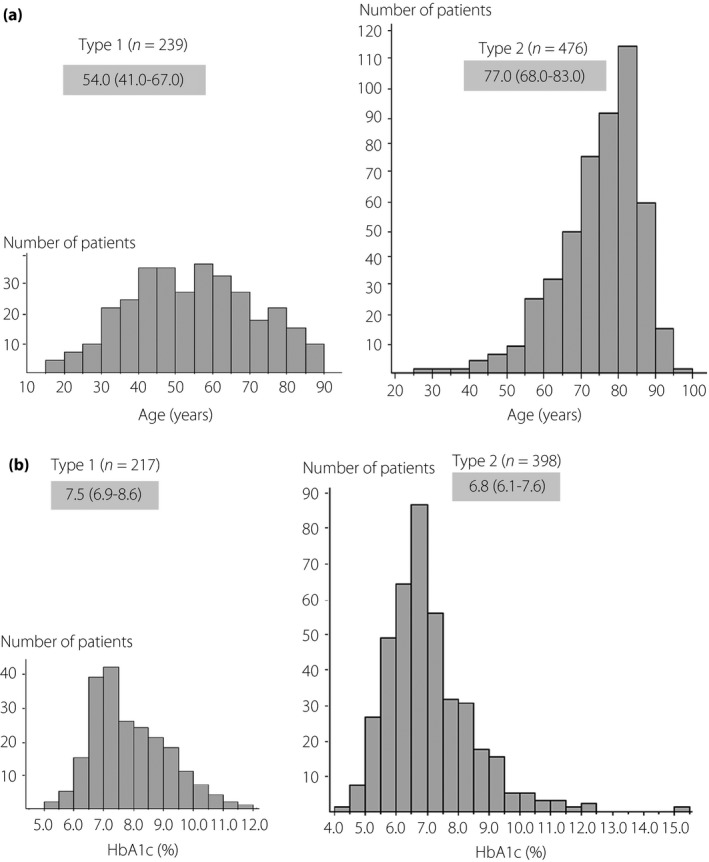
(a) Distribution of age in patients with severe hypoglycemia by type of diabetes. (b) Distribution of HbA1c values in patients with severe hypoglycemia by type of diabetes.
Table 4.
Severity/presence of complications in patients with diabetes mellitus
| Complication | Stage | No. of patients | (%) |
|---|---|---|---|
| Nephropathy | Stage 1 | 235 | 29.5 |
| Stage 2 | 124 | 15.6 | |
| Stage 3 | 105 | 13.2 | |
| Stage 4 | 78 | 9.8 | |
| Stage 5 | 44 | 5.5 | |
| Unknown | 211 | 26.5 | |
| Retinopathy | NDR | 182 | 22.8 |
| SDR | 106 | 13.3 | |
| PPDR | 56 | 7.0 | |
| PDR | 75 | 9.4 | |
| Blindness | 6 | 0.8 | |
| Unknown | 373 | 46.7 | |
| Peripheral neuropathy | Absent | 147 | 18.4 |
| Present | 306 | 38.4 | |
| Unknown | 344 | 43.2 | |
| Autonomic neuropathy | Absent | 175 | 22.0 |
| Present | 218 | 27.4 | |
| Unknown | 403 | 50.6 |
NDR, non‐diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPDR, pre‐proliferative diabetic retinopathy; SDR, simple diabetic retinopathy.
Table 5.
(a) Comparison between patients with awareness of antecedent symptoms and those without. (b) Logistic regression analysis of factors predicting the absence of antecedent symptoms (n = 211)a
| Parameter | Awareness of antecedent symptoms | P‐value | |||
|---|---|---|---|---|---|
| Absent | n | Present | n | ||
| (a) | |||||
| Type of diabetes, type 1 (%)/type 2 (%) | 111 (44.2)/140 (55.8) | 251 | 77 (29.4)/185 (70.6) | 262 | <0.0001 |
| Age at onset of severe hypoglycemia | 70.0 (56.0–80.0) | 283 | 70.0 (57.0–80.0) | 282 | 0.565 |
| Duration of diabetes (years) | 19.0 (11.0–28.0) | 243 | 20.0 (11.0–29.0) | 233 | 0.815 |
| BMI (kg/m2) | 21.2 (18.9–24.1) | 266 | 22.1 (19.9–24.7) | 244 | 0.004 |
| Glucose value at presentation (before treatment) | 30.0 (23.0–40.0) | 233 | 34.5 (27.5–43.0) | 228 | 0.001 |
| HbA1c at onset of severe hypoglycemia (%) | 7.2 (6.5–8.3) | 255 | 7.2 (6.5–8.1) | 239 | 0.615 |
| Serum creatinine at onset of severe hypoglycemia (mg/dL) | 0.9 (0.7–1.4) | 261 | 0.9 (0.7–1.4) | 264 | 0.365 |
| eGFR at onset of severe hypoglycemia (mL/min/1.73 m2) | 59.7 (36.2–80.4) | 261 | 58.1 (35.3–79.1) | 264 | 0.608 |
| Peripheral neuropathy, present | 141 (71.0%) | 200 | 99 (61.1%) | 162 | 0.047 |
| Autonomic neuropathy, present | 108 (59.7%) | 181 | 67 (47.9%) | 140 | 0.035 |
| Factor | Odds ratio | 95% confidence interval | P‐value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| (b) | ||||
| Age at onset of severe hypoglycemia | 1.012 | 0.988 | 1.037 | 0.320 |
| Duration of diabetes | 0.975 | 0.944 | 1.008 | 0.139 |
| HbA1c at onset of severe hypoglycemia | 0.873 | 0.679 | 1.123 | 0.291 |
| Glucose value at presentation (before treatment) | 0.967 | 0.944 | 0.991 | 0.006 |
| BMI | 0.884 | 0.813 | 0.962 | 0.004 |
| Stage of nephropathy | 0.447 | |||
| Stage 1 | Reference value | |||
| Stage 2 | 0.763 | 0.321 | 1.811 | 0.539 |
| Stage 3 | 1.998 | 0.748 | 5.339 | 0.168 |
| Stage 4 | 0.883 | 0.245 | 3.173 | 0.848 |
| Stage 5 | 0.934 | 0.247 | 3.538 | 0.920 |
| Type of diabetes | ||||
| Type 1 | Reference value | |||
| Type 2 | 0.374 | 0.159 | 0.880 | 0.024 |
| Peripheral neuropathy | ||||
| Absent | Reference value | |||
| Present | 3.191 | 1.265 | 8.052 | 0.014 |
| Autonomic neuropathy | ||||
| Absent | Reference value | |||
| Present | 1.336 | 0.562 | 3.176 | 0.511 |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Patients with no missing data for any of the parameters compared (those with no antecedent symptoms, n = 119; those with antecedent symptoms, n = 92).
Figure 2.
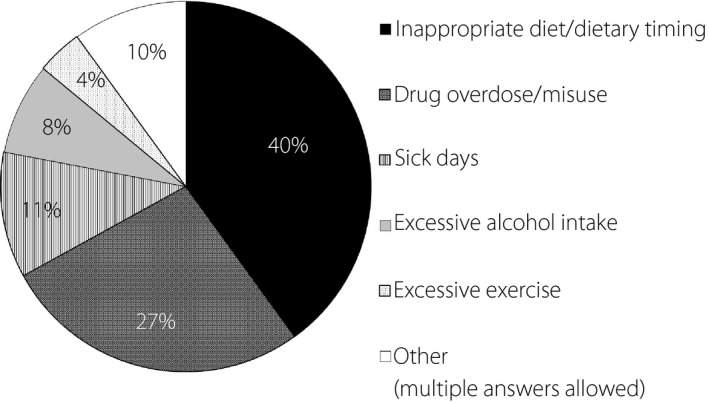
Factors affecting the onset of severe hypoglycemia.
Table 6.
Comparison of factors associated with severe hypoglycemia by drug use
| Parameter | Insulin group (n = 292) | SU group (n = 159) | Non‐insulin/SU group (n = 29) |
|---|---|---|---|
| Age (years) | 74.0 (65.0–81.0) | 81.0 (75.0–85.0)a | 77.0 (69.0–83.0)a |
| HbA1c (%) | 7.2 (6.5–8.1) | 6.4 (5.8–6.9)a | 6.0 (5.5–6.5)a |
| Glucose value at presentation (before treatment) | 32.0 (25.0–41.0) | 33.0 (27.0–38.0) | 31.5 (23.5–42.0) |
| eGFR (mL/min/1.73 m2) | 55.9 (36.1–75.4) | 42.3 (29.9–59.9)a | 39.5 (14.4–70.3) |
| BMI (kg/m2) | 22.7 (19.6–25.2) | 22.7 (19.7–24.8) | 20.6 (18.1–23.8) |
P < 0.001 vs. Insulin group
BMI, body mass index; eGFR, estimated glomerular filtration rate.
The antidiabetic agents used in patients with type 2 diabetes included insulin preparations (292 patients including 29 receiving concomitant SUs; insulin group) (60.8%), SUs (159 insulin‐naïve patients; SU group) (33.1%), and no insulin preparations or SUs (29 patients; non‐insulin/SU group) (6.0%) (Figure 3). Patients in the insulin, SU and non‐insulin/SU groups had a median age of 74.0 (65.0–81.0), 81.0 (75.0–85.0), 77.0 (69.0–83.0) years, respectively, with those in the SU and non‐insulin/SU groups shown to be significantly older than those in the insulin group (P < 0.001) (Table 6). Patients 65 years old or older and those 75 years old or older accounted for 76.4% and 47.3% of all patients in the insulin group and 96.2% and 77.4% of all patients in the SU group, respectively (Figure 4a). The patients in the insulin, SU and non‐insulin/SU groups had a median HbA1c value of 7.2 (6.5–8.1), 6.4 (5.8–6.9), 6.0 (5.5–6.5)%, respectively, with the HbA1c value shown to be significantly lower for those in the SU and non‐insulin/SU groups (P < 0.001) (Table 6). A comparison of patients with low HbA1c values showed that patients with HbA1c values 7% or lower and 7.5% or lower accounted for 46.8% and 63.6% of those in the insulin group and 80.6% and 89.9% of those in the SU group, respectively (Figure 4b).
Figure 3.
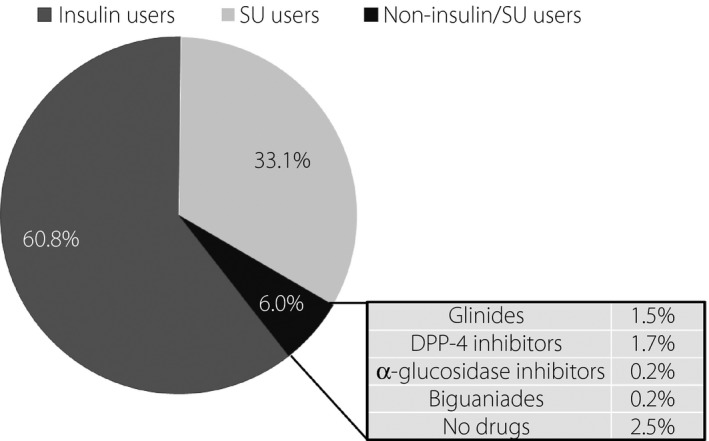
Causal agents of severe hypoglycemia in type 2 diabetic patients.
Figure 4.
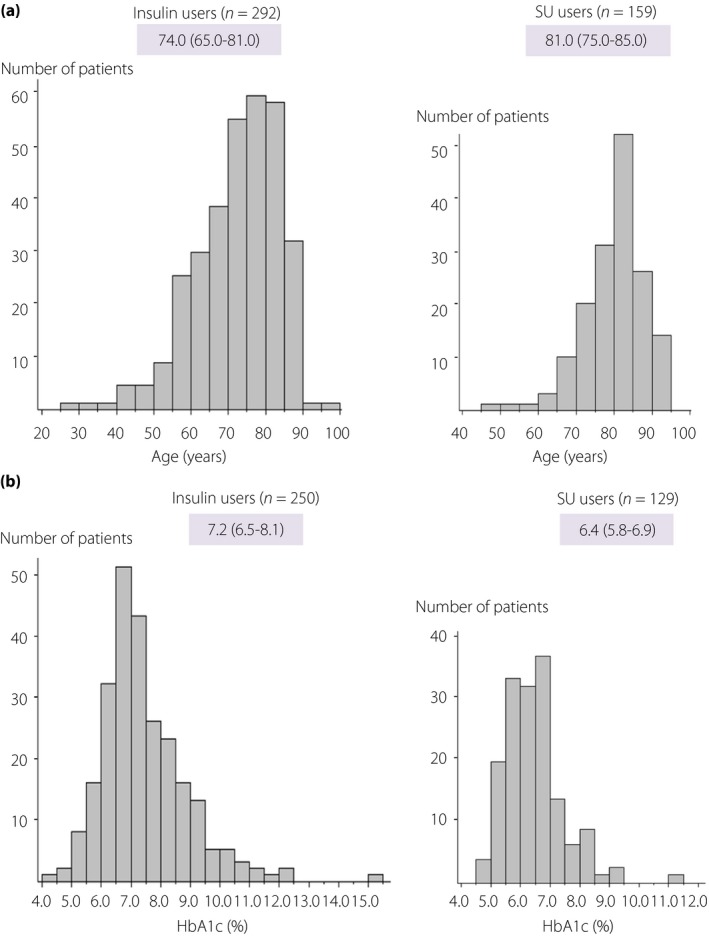
(a) Distribution of age in type 2 diabetic patients by drug use. (b) Distribution of HbA1c values in type 2 diabetic patients.
Of all patients in the non‐insulin/SU group, severe hypoglycemia was noted 7 patients receiving glinides (including 4 receiving DPP‐4 inhibitors) and 8 glinide‐naïve patients receiving DPP‐4 inhibitors, who had a median age of 84.0 (81.0–86.0) and 75.0 (71.0–81.0) years, a median HbA1c value of 6.5 (6.2–6.6) and 6.3 (5.8–6.4)%, and a median BMI of 21.2 (17.9–24.2) and 22.4 (19.5–24.1) kg/m2, respectively. The remaining 14 patients in the non‐insulin/SU group had a median age of 67.5 (60.0–78.0) years, a median HbA1c value of 5.5 (4.9–5.8)%, and a median BMI value of 18.4 (17.1–21.6) with the HbA1c and BMI values tending to be lower.
A review of the stages of nephropathy as well as the use of insulin/SUs in all patients (Figure 5) showed that nephropathy was far more advanced in those with type 2 diabetes than in those with type 1 diabetes and that, among those with type 2 diabetes, there was no difference in stage of nephropathy between the SU and insulin groups, with 50% of those receiving SUs shown to have ≥ stage 3 nephropathy.
Figure 5.
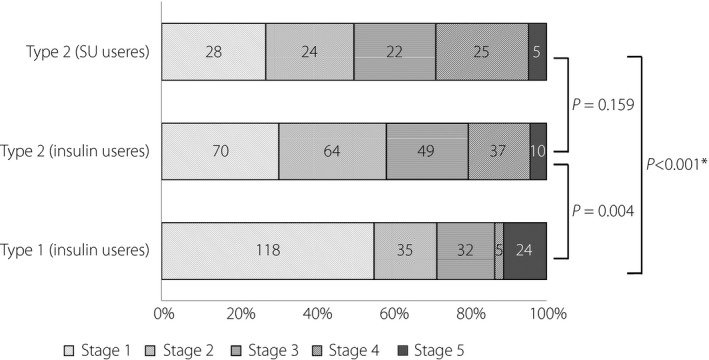
Proportion of stages of nephropathy among patients with diabetes mellitus by type of diabetes comparisons using the Bonferroni correction. All P‐values with the chi‐square test for comparisons between patients with stage 1 to 2 and those with stages 3 to 5. *Refers to combinations shown to be significant at an alpha level of 0.05 in multiple comparisons using the Bonferroni correction.
Discussion
The current survey sought to investigate the current status of treatment‐related severe hypoglycemia by inviting executive educators, who represented 631 healthcare facilities accredited by the JDS for diabetes education, to participate in the survey, with severe hypoglycemia defined as the “presence of hypoglycemic symptoms requiring assistance from another person to treat and preferably venous plasma glucose levels at onset/diagnosis of disease or at presentation clearly less than 60 mg/dL (capillary whole blood glucose, less than 50 mg/dL)”.
Of the 631 facilities approached, a total of 193 facilities responded with facility‐specific information, thus making the current survey on severe hypoglycemia utterly unprecedented in scale in Japan. The median number of patients who required emergency transportation services to reach these facilities totaled 4,962 annually, of which those with severe hypoglycemia accounted for 0.34% (Table 1). In contrast to the number of emergency transportation services ordered for 2014 that totaled some 5.4 million, according to the Statistics Bureau, Ministry of Internal Affairs and Communications7, with the current survey conducted in JDS‐accredited healthcare facilities, our figure (4,962 services) represented an estimate based on the reported number of patients transported to emergency departments at these facilities, which translated into an estimated total of some 20,000 patients transported annually for severe hypoglycemia in Japan. Of note, of the roughly 346,939 patients enrolled in the current survey, a total of 2,237 patients (0.64%) were shown to have experienced severe hypoglycemia, which, coupled with the reported number of diabetic patients receiving treatment that totaled 3.166 million in the 2014 Ministry of Health, Labor and Welfare survey8, again translated into an estimated total of some 20,000 patients transported annually for severe hypoglycemia in Japan.
Of all surveys on severe hypoglycemia available overseas, one on all drug‐associated adverse events including hypoglycemia at 58 emergency departments in the US is of particular note9, which reported that a total of 42,585 patients had presented to these emergency departments during the 2013–2014 period, and 27.3% of these patients had been admitted. Of all drugs used in these patients, anticoagulants were reported to be most frequently associated with adverse events (17.6%), followed by antibiotics (16.1%) and antidiabetic drugs (13.3%). Of these, insulin and metformin were reported to be the second and eighth most common cause of drug‐related adverse events (4,859 and 766 events, respectively). While SUs were not among the top 15 common causes of these events, a review of drug‐associated adverse events in patients 65 years old or older revealed that the SUs glipizide (not commercially available in Japan), glibenclamide and glimepiride ranked eighth, fourteenth, and fifteenth among the causes of drug‐associated adverse events. It was also found that insulin, oral hypoglycemic agents and other antidiabetic agents were the causes of hospital admissions in 34.8%, 53.0%, and 38.5% of these patients, respectively. Of note, while not readily available for direct comparison, the proportion of hospital admissions accounted for by oral hypoglycemic agents reported in the US (53.0%) appears to be nearly consistent with that for severe hypoglycemia reported in the current survey (52.3%).
Another report from the US is also of interest, in that it demonstrated the status of insulin‐associated adverse events resulting in visits to emergency departments on a nationwide basis by drawing on data from a total of 8,100 patients presenting to these departments for such reasons as insulin‐associated hypoglycemia, insulin product name confusion, and insulin overdose, reporting that a total of 97,648 patients had presented to emergency departments for these reasons annually, with 29.3% of these patients shown to have been admitted, 60.6% shown to be associated with serious neurological symptoms, and 53.4% shown to have an estimated glucose value of 50 mg/dL or lower10.
Conventionally, the diagnosis of hypoglycemia is generally assumed to be consistent with glucose values less than 70 mg/dL11. Of note, a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes in 2016 recommended that “criteria used in clinical studies for hypoglycemia should include self‐monitoring of whole blood glucose as converted to venous plasma glucose, interstitial glucose concentration in continuous glucose monitoring or venous plasma glucose values less than 54 mg/dL, which do not occur under physiological conditions in non‐diabetic individuals and serious hypoglycemia resulting in impaired consciousness and requiring the assistance of another to treat”12. In this survey, which focused on treatment‐associated severe hypoglycemia, however, severe hypoglycemia was defined as preferably venous plasma glucose levels at onset/diagnosis of disease or at presentation clearly less than 60 mg/dL (capillary whole blood glucose, less than 50 mg/dL), with priority given to the supposed presence of symptoms of hypoglycemia not amenable to self‐management in affected patients. Indeed, given that patients with glucose values 60 mg/dL or higher accounted for 3.9% of both type 1 and type 2 diabetic patients, suggesting that severe hypoglycemia may not be diagnosed on the basis of glucose values alone, the diagnostic approach to severe hypoglycemia in this study which involved assessment of both glucose values and symptoms appears to be valid.
Risk factors for severe hypoglycemia are reported to include type of diabetes13, aging14, SUs15, 16, insulin therapy10, 13, 17, low HbA1c values16, 18, and long duration of diabetes13, 14. In this survey as well, severe hypoglycemia was noted in as many as 30% of patients with type 1 diabetes who accounted for 6.2% of all patients with diabetes mellitus presenting to outpatient clinics (Tables 1 and 2). Indeed, severe hypoglycemia was noted in type 1 diabetic patients across all age brackets but was noted frequently in type 2 diabetic patients with their median age being 77.0 years and 83.4% of these patients being 65 years old or older (Figure 1a). The HbA1c value at onset of severe hypoglycemia was shown to be significantly lower in type 2 diabetic patients than in type 1 diabetic patients, and those with HbA1c 7% or lower and 7.5% or lower accounted for 59.8% and 73.4%, respectively, of all type 2 diabetic patients, as well as 31.8% and 50.0%, respectively, of all type 1 diabetic patients. Again, severe hypoglycemia was shown to have occurred frequently in type 1 diabetic patients, including those with HbA1c values 9% or higher (Figure 1b). It was also found that the distributions of age and HbA1c values varied depending on the type of diabetes.
Of the antidiabetic drugs used in type 2 diabetic patients, insulin was shown to be most commonly used (60.8%) followed by SUs (33.1%), which, together, accounted for 94% of all drugs used (Figure 3). Of note, Iwakura and colleagues examined a total 135 type 2 diabetic patients transported to their emergency department for severe hypoglycemia over a 3‐year period starting in 2008 and reported that the use of SUs was a far more common cause of severe hypoglycemia than insulin therapy, thus highlighting concern over severe hypoglycemia associated with SUs19. By the same token, by 2010, there emerged concern over severe hypoglycemia associated with combined use of SUs and DPP‐4 inhibitors, which prompted the JDS to issue precautions for use of SUs combined with DPP‐4 inhibitors20. In this regard, the current 1‐year survey (started in 2014) showed that more patients were receiving insulin therapy than SUs, suggesting that the use of SUs has come under scrutiny in recent years. On the other hand, consistently with earlier reports, this survey also revealed that SU users were older and had lower HbA1c values than insulin users, suggesting that, when using SUs in diabetic patients, adequate care needs to be exercised to ensure that the glycemic control goals set for these patients are not too low, while taking their age into account. In this survey, patients 65 years old or older and 75 years old or older accounted for 76.4 and 47.3%, respectively, of those receiving insulin and 96.2 and 77.4%, respectively, of those receiving SUs (Figure 4a). Again, patients with HbA1c values 7.0% or lower and 7.5% or lower accounted for 46.8 and 63.6%, respectively, of those receiving insulin and 80.6 and 89.9%, respectively, of those receiving SUs (Figure 4b). The current survey results also appear to support the glucose (HbA1c) control goals for elderly patients with diabetes mellitus proposed by the JDS/Japan Geriatrics Society in 2016, which recommended that the lower HbA1c limit should range between 6.5 and 7.5% depending on the degree of cognitive and ADL impairment in elderly patients21. Of note, however, insulin users and SU users were shown to vary with regard to their age and HbA1c distributions in this survey, suggesting that insulin and SU users need to be addressed separately and their glycemic control goals need to be established separately rather than uniformly. Again, given that patients receiving glinides, DPP‐4 inhibitors and no antidiabetic drugs accounted for 1.5, 1.7, and 2.5%, respectively, of all non‐insulin/SU users, who, in turn, accounted for 6% of all patients with severe hypoglycemia in this survey (Figure 3), it remains a challenge to elucidate the causal relationship between these insulin‐promoting drugs and the onset of severe hypoglycemia. Further in‐depth analysis is required to clarify whether or not undernutrition may have contributed to the BMI and HbA1c values in non‐insulin/SU users that tended to be lower.
In this survey, renal function (eGFR) was shown to be decreased in type 2 diabetic patients to a greater degree than that in type 1 diabetic patients, and many of these patients were shown to have never presented to their clinics for hyperglycemia before (Table 3). Half of type 2 diabetic patients receiving SUs were shown to be in ≥ stage 3 nephropathy, thus highlighting the need for appropriate use of SUs in these patients, given that SUs are to be used judiciously or contraindicated depending on the progression of nephropathy (Figure 5). In this regard, this survey did investigate the types of SUs used but fell short of determining the impact of SUs by type on the onset of severe hypoglycemia as it related to renal function, given the lack of back ground information on their frequency of use.
According to a diabetes case registry launched by diabetologists in Japan, Japanese patients with type 2 diabetes are reported to have a mean age of approximately 65 years, a mean BMI value of approximately 25 kg/m2, and a mean HbA1c value of 7.1%22. Compared to these typical patients, those type 2 diabetic patients who were shown to have been associated with severe hypoglycemia in this survey were clearly older, had lower BMI values, and slightly lower HbA1c values; given that nearly all of these patients were being treated with insulin or SUs, many of these patients were thought to be non‐obese patients with impaired endogenous insulin secretion. Also, elderly type 2 diabetic patients with low BMI values were thought to include those with frailty‐ or sarcopenia‐associated weight loss. In this regard, research suggests that ultra‐rapid‐acting insulin is likely to be rapidly absorbed in insulin patients with low BMI values, thus potentially placing these patients at high risk of postprandial hypoglycemia23. Furthermore, it was also suggested in this survey that renal impairment associated with aging and prolonged duration of diabetes is likely to lead to the pharmacological action of SUs being chronically enhanced, thus contributing not only to decreases in HbA1c but to the worsening of hypoglycemia in SU users.
Thus, given the ever‐increasing population of elderly patients with type 2 diabetes, it appears imperative that care be exercised in reviewing their current treatment regimens, as well as in optimizing education for these patients, whether or not they have a history of severe hypoglycemia, with consideration also given to their age, HbA1c value, renal function or their use of insulin or insulin secretion‐promoting agents.
Of all factors examined in the survey for association with the onset of severe hypoglycemia, inappropriate diet or dietary timing, excessive alcohol intake, sick days were found to be associated with the onset of severe hypoglycemia in the majority of patients, in addition to factors associated with human errors, such as drug misuse or drug name confusion, which were shown to be associated with the onset of severe hypoglycemia in some (Figure 2). It was also found, however, that severe hypoglycemia could be avoided through appropriate treatment choice and patient education in a majority of cases. Of note, the Dose Adjustment for Normal Eating—Hypoglycemia Awareness Restoration Training (DAFNE‐HART) study24 reported that no episodes of severe hypoglycemia were noted, with decreased episodes of moderate hypoglycemia, in type 1 diabetic patients with reduced awareness of hypoglycemia for 1 year after they were provided with a basic knowledge of hypoglycemia, its symptomatic diversity, and patient‐specific hallmark features of its symptoms, as well as instructions on how to deal with hypoglycemia. This appears to suggest an acute need to establish in Japan a similar approach to instructing patients or generating patient educational material on how to prevent hypoglycemia to that used in this study.
Antecedent symptoms of severe hypoglycemia were noted in 41.0% of patients with type 1 diabetes and 56.9% of patients with type 2 diabetic patients in this survey (Table 3). Thus, those with hypoglycemia unawareness as no antecedent symptoms of severe hypoglycemia accounted for a high proportion of both type 1 and type 2 diabetic patients (59.0 and 43.1%, respectively), suggesting that hypoglycemia unawareness is likely an important background factor for severe hypoglycemia. In this regard, Geddes and colleagues reported that hypoglycemia unawareness is noted in 19.5% of all patients with type 1 diabetes, with their risk for onset of severe hypoglycemia increased by 6‐fold25. It is also reported that hypoglycemia unawareness is noted in 9.8% of type 2 diabetic patients receiving insulin therapy as well, with their risk for onset of severe hypoglycemia increased by 17‐fold26. Of note, hypoglycemia unawareness is assumed to represent an adaptive response to hypoglycemia to protect the brain against its recurrent episodes and thus a decrease in the threshold for counterregulatory response to hypoglycemia27. In this survey, the glucose level at onset of severe hypoglycemia (before treatment) was shown to be low in patients without antecedent symptoms of hypoglycemia, likely reflecting a decrease in the threshold for glycemic response resulting in an associated decrease in hyperglycemic response. Again, in our survey, type 1 diabetes, low BMI value and peripheral neuropathy were identified as further factors associated with hypoglycemia unawareness (Table 5B). Given that low BMI value was identified as a factor associated with hypoglycemia unawareness in the survey, partly because type 1 diabetic patients tend to have low BMI values but also because type 2 diabetic patients with low BMI values are thought likely to be associated with worsening of hypoglycemia, further detailed study is required to clarify the association between BMI, worsening of hypoglycemia, and hypoglycemia unawareness. Again, while the association between neuropathy and asymptomatic hypoglycemia remains controversial28, 29, our survey results demonstrated that peripheral neuropathy is more strongly associated with asymptomatic hypoglycemia than autonomic neuropathy or autonomic failure. Since our survey employed no consistent or quantitative criteria for diagnosis of autonomic neuropathy, peripheral neuropathy, a factor that is readily amenable to early diagnosis in clinical settings, was chosen as an explanatory factor for asymptomatic hypoglycemia.
Severe hypoglycemia is shown to lead to CNS sequelae in some patients with impaired consciousness. In this survey as well, these CNS sequelae were noted in 18 of 798 patients, with cognitive decline also noted in 8 of these patients, thus highlighting the fact that these sequelae are among the most frequent of complications associated with severe hypoglycemia (Table 2). Indeed, it is reported that severe hypoglycemia is also associated with cognitive decline in elderly patients with diabetes mellitus5, and that the more frequent the onset of severe hypoglycemia in elderly patients with diabetes mellitus, the greater their future risk for dementia30. Thus, given that severe hypoglycemia is shown to be common in elderly patients with diabetes mellitus, further study is acutely required in a large population of elderly patients to examine in depth the impact of severe hypoglycemia on their cognitive function and CNS symptoms as well as to establish countermeasures against severe hypoglycemia, including preventive measures. Again, severe hypoglycemia is shown to be associated with arrhythmia, coronary artery disease (CAD) and mortality31, 32, 33. In this regard, while eleven deaths were reported in the current survey (Table 2), arrhythmia and CAD was reported in as few as 1 patient each. However, the retrospective nature of the survey involving a limited number of patients made it rather difficult to establish any association between the direct causes of death and arrhythmia or CAD.
Prior history of severe hypoglycemia and low HbA1c values are known to be associated with increased risk for traffic accidents34. Therefore, the current survey investigated traffic accidents or near‐accidents in patients with severe hypoglycemia and demonstrated, while being not a patient self‐administered but physician‐mediated survey, that 15 patients reported having had traffic accidents or near‐accidents (Table 2). Given that the survey questionnaire featured “near‐accidents” alongside “traffic accidents” as a single answer choice, our figure may suggest that traffic accidents due to severe hypoglycemia occur less frequently than assumed but the fact remains that traffic accidents lead to enormous social losses. Thus, the survey results appear to point to the need for instructing patients at high risk of severe hypoglycemia, particularly those with hypoglycemia unawareness on how to prevent or deal with hypoglycemic episodes occurring while driving.
The limitations of the current survey were that it was primarily directed at healthcare facilities accredited by the JDS for diabetes education where diabetologists were available and that it only involved 193 of the 631 facilities approached (30.5%) and a total of 798 patients reported from 113 of the 193 respondent facilities. Furthermore, the survey results on severe hypoglycemia may not necessarily reflect those in routine diabetes care, where patients with type 2 diabetes are usually being cared for by their family physicians. Again, diagnostic accuracy may have suffered for awareness of antecedent symptoms, complications such as neuropathy or retinopathy, and impact of severe hypoglycemia on cognitive function, in this retrospective, physician‐mediated questionnaire survey. Despite these limitations, this first multicenter survey may have much to offer, in that it provided insights into the current status of severe hypoglycemia, its social impact, and background factors, thus highlighting issues of current interest in diabetes treatment in Japan, while extensive and detailed studies are required to build on the findings reported.
In conclusion, the survey revealed, for the first time, the current status of treatment‐related severe hypoglycemia in Japan and demonstrated that patients with severe hypoglycemia accounted for 0.34% of all patients requiring emergency transportation services to reach emergency departments; that, of these, type 1 and type 2 diabetic patients accounted for approximately 30 and 60%, respectively; that severe hypoglycemia occurred in type 1 diabetic patients across a relatively wide age range, while, in contrast, it occurred frequently in elderly type 2 diabetic patients with reduced renal function and low HbA1c values, the majority of whom were receiving insulin or SUs. Again, the survey revealed that severe hypoglycemia was associated with dementia, CNS sequelae and even deaths, thus highlighting the acute need for implementing preventive measures against hypoglycemia not only through education on hypoglycemia but through optimization of antidiabetic therapy for those at high risk of severe hypoglycemia or those with a history of severe hypoglycemia.
Disclosure
Mitsuyoshi Namba: speaker fees (Sanwa Kagaku Kenkyuusho Co., Ltd, Sanofi K. K., Novo Nordisk Co., Ltd., AstraZeneca, Novartis Pharma K.K., Nippon Eli Lilly Japan K.K., Mitsubishi Tanabe Corporation, and Kowa Pharmaceutical Co., Ltd.); clinical research grants (funding for clinical trials, clinical studies, contracted research, and collaborative research) (Arkray Inc.); endowed scholarships/donations (Astellas Pharm Inc., Novartis Pharma K.K., Sanwa Kagaku Kenkyuusho Co., Ltd, Kyowa Hakko Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Teijin Pharma Co., Ltd., Mitsubishi Tanabe Corporation, Nippon Boehringer Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sanofi K. K., Sumitomo Dainippon Pharma Co., Ltd., AstraZeneca, and Pfizer Japan Inc.) and endowed lectures (Mitsubishi Tanabe Corporation, Nippon Eli Lilly Japan K.K., and Sanwa Kagaku Kenkyuusho Co.). Toshio Iwakura: none. Rimei Nishimura: speaker fees (Sanofi K. K., Medtronic Japan Co., Ltd., Nippon Boehringer Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Eli Lilly Japan K.K., Novartis Pharma K.K., and Astellas Pharm Inc.). Kohei Akazawa: endowed scholarships/donations (Japanese Foundation for Multidisciplinary Treatment for Cancer, Medical Informatics Study Group, and Japan Diabetes Society). Munehide Matsuhisa: speaker fees (Sanofi K. K., Novartis Pharma K.K., Novo Nordisk Co., Ltd., Mitsubishi Tanabe Corporation, Astellas Pharm Inc., Takeda Pharmaceutical Co., Ltd., Nippon Eli Lilly Japan K.K.); clinical research grants (funding for clinical trials, clinical studies, contracted research, and collaborative research) (Daiichi Sankyo Co., Ltd., Nippon Boehringer Co., Ltd., Mitsubishi Tanabe Corporation, Tokushima Data Service Co., Ltd., and Astellas Pharm Inc.). Yoshihito Atsumi: speaker fees (Arkray Inc., Astellas Pharm Inc., Nippon Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Sanofi K. K., Taisho Toyama Pharmaceutical Co., Ltd., Mitsubishi Tanabe Corporation, Novo Nordisk Co., Ltd., Novartis Pharma K.K., and Nippon Becton Dickinson Co., Ltd.). Jo Satoh: speaker fees (Astellas Pharm Inc., Nippon Eli Lilly Japan K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., AstraZeneca, Sanofi K. K., Mitsubishi Tanabe Corporation, Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Co., Ltd., and MSD K.K.). Toshimasa Yamauchi: speaker fees (Takeda Pharmaceutical Co., Ltd., MSD K.K., AstraZeneca); endowed scholarships/donations (Nippon Boehringer Co., Ltd., Novo Nordisk Co., Ltd., Astellas Pharm Inc., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Corporation, Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., MSK K.K., and Sanofi K. K.)
Supporting information
Table S1 | Participated physicians at the JDS‐accredited educational facilities.
Acknowledgments
The authors would like to thank Ms. Mari Watanabe, Department of Medical Informatics, Niigata University Medical & Dental Hospital, for her assistance with in‐depth analysis of the survey data. The authors also owe enormous thanks to Mr. Masayasu Yamada, a legal advisor for the JDS, for his expert advice and counsel on the ethical aspects of the survey and to Ms. Maki Sato and Mr. Hitomi Shibasaki, Secretariat of the JDS, for their superb administrative assistance throughout the survey. The authors’ sincere thanks are also due to all physicians at the JDS‐accredited educational facilities in Table S1 for their generous help in sharing their valuable data.
J Diabetes Investig 2018;9: 642–656
In 2013, the Japan Diabetes Society established a Committee for Surveys on Severe Hypoglycemia, which published its final committee report in 20171. This is the English version of that report with some revisions, which is published here to enhance our non‐Japanese colleagues’ and other interested parties’ understanding of this topic. This is the official published version of that report, which is jointly published in Diabetology International (the official English journal of the Japan Diabetes Society: doi: 10.1007/s13340‐018‐0346‐2) and the Journal of Diabetes Investigation (the official journal of the Asian Association for the Study of Diabetes).
References
- 1. Namba M, Iwakura T, Nishimura R, et al The current status of treatment‐related severe hypoglycemia in Japanese patients with diabetes mellitus: A report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. J Japan Diabet Soc 2017; 60: 826–842 (Japanese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakamura J, Kamiya H, Haneda M, et al Cause of death in Japanese patients with diabetes based on the results of survey of 45,708 cases during 2001–2010. J Japan Diabet Soc 2016; 59: 667–684 (Japanese). [Google Scholar]
- 3. Hotta N, Nakamura J, Iwamoto Y, et al A questionnaire survey on the causes of death in 18,385 Japanese patients with diabetes mellitus during the 10 years between 1991 and 2000. J Japan Diabet Soc 2007; 50: 47–61 (Japanese). [Google Scholar]
- 4. Skyler JS, Bergenstal R, Bonow RO, et al Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaffe K, Falvey CM, Hamilton N, et al Association between hypoglvcemia and dementia in a Biracial cohort of older adults with diabetes mellitus. JAMA Int Med 2013; 173: 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aung PP, Strachan WJ, Frier BM, et al Severe hypoglycemia and late‐life cognitive ability in older people with type 2 diabetes mellitus: the Edinburgh Type 2 Diabetes Study. Diabet Med 2012; 29: 328–336. [DOI] [PubMed] [Google Scholar]
- 7. Statistics Bureau, Ministry of Internal Affairs and Communication of Japan (2015) Number of emergency transportation services ordered for 2014 (flash report)Available from: http://www.fdma.go.jp/neuter/topics/houdou/h27/03/270331_houdou_2.pdf. Accessed June 29, 2017 (Japanese).
- 8. Ministry of Health, Labor and Welfare of Japan (2015) Fact sheets from the 2014 Patient Survey. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/14/index.html. Accessed June 29, 2017 (Japanese).
- 9. Shehab N, Lovegrove MC, Geller AI, et al US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016; 316: 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geller AI, Shehab N, Lovegrove MC, et al National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014; 174: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Japan Diabetes Society . Guidelines for the Management of Diabetes Mellitus 2016. Tokyo: Nankodo, 2016. (Japanese). [Google Scholar]
- 12. The International Hypoglycaemia Study Group . Glucose concentration of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2017; 60: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hypoglycaemia UK. Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 14. Huang ES, Laiteerapong N, Liu JY, et al Rates of complications and mortality in older patients with diabetes mellitus. The Diabetes and Aging Study. JAMA Intern Med 2010; 174: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holstein A, Hammer C, Hahn M, et al Severe sulfonylurea‐induced hypoglycemia: a problem of uncritical prescription and deficiencies of diabetes care in geriatric patients. Expert Opin Drug Saf 2010; 9: 675–681. [DOI] [PubMed] [Google Scholar]
- 16. Greco D, Pisciotta M, Gambina F, et al Severe hypoglycaemia leading to hospital admission in type 2 diabetic patients aged 80 years or older. Exp Clin Endocrinol Diabetes 2010; 118: 215–219. [DOI] [PubMed] [Google Scholar]
- 17. Yau CK, Eng C, Cenzer IS, et al Glycosylated hemoglobin and functional decline in community‐dwelling nursing home‐eligible elderly adults with diabetes mellitus. J Am Geriatr Soc 2012; 60: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bramlage P, Gitt AK, Binz C, et al Oral antidiabetic treatment in type‐2 diabetes in the elderly: balancing the need for glucose control and the risk of hypoglycemia. Cardiovasc Diabetol 2012; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwakura T, Sasaki S, Fujiwara Y, et al Analysis of 135 type 2 diabetic patients with treatment‐associated severe hypoglycemia. J Japan Diabet Soc 2012; 55: 857–865 (Japanese). [Google Scholar]
- 20. Japan Diabetes Society Committee on the Proper Use of Incretin‐based Drugs (GLP‐1 Receptor Agonists and DPP‐4 Inhibitors): On the Proper Use of Incretin‐based Drugs and Sulfonylureas (SUs). Available from: http://www.nittokyo.or.jp/kinkyu_incretin100408m.html Accessed April 15, 2010 (Japanese).
- 21. Japan Diabetes Society (2017) Japan Diabetes Society/Japan Geriatrics Society Joint Committee for Improving Treatments for Elderly Patients with Diabetes Mellitus: Glycemic Control Goals for Elderly Patients with Diabetes Mellitus . Diabetes Treatment Guide 2016–2017. Tokyo; Bunkodo, 2016. (Japanese) [Google Scholar]
- 22. Oishi M, Yamazaki K, Okuguchi F, et al Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002–2011 in Japan (JDDM32). J Diab Invest 2014; 5: 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuroda A, Kaneto H, Kawashima S, et al Regular insulin, rather than rapid‐acting insulin, is a suitable choice for premeal bolus insulin in lean patients with type 2 diabetes mellitus. J Diab Invest 2013; 4: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeZoysa N, Roqers H, Standler M, et al A psychoeducational program to restore hypoglycemia awareness: the DAFNE‐HART pilot study. Diabetes Care 2014; 37: 863–866. [DOI] [PubMed] [Google Scholar]
- 25. Geddes J, Schopman JE, Zammitt NN, et al Prevalence of impaired awareness of hypoglycemia in adults with type 1 diabetes. Diabet Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 26. Schopman JE, Geddes J, Fisher BM. Prevalence of impaired awareness of hypoglycemia and frequency of hypoglycemia in insulin‐treated type 2 diabetes. Diabetes Res Clin Pract 2010; 87: 64–68. [DOI] [PubMed] [Google Scholar]
- 27. Cryer PE. Mechanism of hypoglycemia‐associated autonomic failure and its component syndromes in diabetes. Diabetes 2005; 54: 3592–3601. [DOI] [PubMed] [Google Scholar]
- 28. Meyer C, Grossmann R, Mitrakou A, et al Effects of autonomic neuropathy on counterregulation and awareness of hypoglycemia in type 1 diabetic patients. Diabetes Care 1998; 21: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 29. Olsen SE, Biorgaas MR, Asvoid BO, et al Impaired awareness of hypoglycemia in adults with type 1 diabetes is not associated with autonomic dysfunction or peripheral neuropathy. Diabetes Care 2016; 39: 426–433. [DOI] [PubMed] [Google Scholar]
- 30. Feinkohl I, Aung PP, Keller M, et al Severe hypoglycemia and cognitive decline in older patients with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014; 37: 507–515. [DOI] [PubMed] [Google Scholar]
- 31. Gruden G, Giunti S, Barutta F, et al QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM Complications Study. Diabetes Care 2012; 35: 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zoungas S, Patel A, Chalmers J, et al Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 33. Hsu PF, Sung SH, Cheng HM, et al Association of clinical symptomatic hypoglycemia with cardiovascular events and total‐mortality in type 2 diabetes. Diabetes Care 2013; 36: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population‐based case control analysis. PLoS Med 2009; 12: e1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Participated physicians at the JDS‐accredited educational facilities.


