Abstract
The genomic hypomethylation hypothesis of aging proposes that an overall decrease in global DNA methylation occurs with age, and it has been argued that the decrease in global DNA methylation could be an important factor in aging, resulting in the relaxation of gene expression regulation and abnormal gene expression. Since it was initially observed that DNA methylation decreased with age in 1974, 16 articles have been published describing the effect of age on global DNA methylation in various tissues from rodents and humans. We critically reviewed the publications on the effect of age on DNA methylation and the expression of the enzymes involved in DNA methylation to evaluate the validity of the hypomethylation hypothesis of aging. On the basis of the current scientific literature, we conclude that a decrease in the global methylation of the genome occurs in most if not all tissues/cells as an animal ages. However, age-related changes in DNA methylation in specific regions or at specific sites in the genome occur even though the global DNA methylation does not change.
Keywords: DNA Methylation, gene expression, DNA methylation enzymes, dietary restriction, hypomethylation, aging
Introduction
The presence of 5-methylcytosine (5mC) in DNA was first reported by Johnson and Coghill in 1925 as one of the hydrolysis products of tuberculinic acid.1 However, it was not until 1948 that Hotchkiss conclusively identified 5mC in calf thymus DNA using paper chromatography.2 Because of its relatively high abundance (~ 4% of the cytosines in the human genome are methylated3), 5mC is often referred as the fifth base. One of the interesting features of cytosine methylation is its enrichment in CpG dinucleotides. These palindromic sites have the unique potential for the symmetric methylation of cytosines (i.e., methylation can occur on both strands of DNA), which can then be copied during cell replication from the parent to daughter strand. Although CpG sites are enriched in 5mC, cytosines at CpH (H = A, T, or C) sites are also methylated, and because CpH sites are much more abundant that CpG sites, the majority of 5mC in the genome is found in CpH sites in some tissues, such as brain.4.
Cytosine methylation is catalyzed by a complex interplay of three independently encoded DNA methyltransferases (DNMTs) that use S-adenosyl methionine as the methyl donor: DNMT1, DNMT3a, and DNMT3b.5–7 DNMT1 is a maintenance methyltransferase capable of recognizing and functioning only on hemimethylated DNA8–10 through ubiquitin-like PHD and RING finger domains (UHRF1), which interact with DNMT1 and hemimethylated CpG.11,12 Thus, DNMT1 plays an important role in the heritability of epigenetic information.13 In contrast, DNMT3a and DNMT3b are de novo methyltransferases capable of recognizing and methylating both hemimethylated and unmethylated DNA,14,15 and these methyltransferases are responsible for changes in DNA methylation that occur at CpH sites. Because DNA methylation patterns are important in embryonic development, knocking out any of these three DNMTs in mice is embryonically lethal.10,15
The 5mC residues in the genome can be lost through passive and active mechanisms. The passive mechanism involves the loss of 5mC that occurs when the cytosines are not methylated during replication or from the repair of regions of the genome that contained 5mCs.16 The active mechanism involves DNA demethylation whereby the methyl group is modified and the resulting modified cytosine residue is removed and replaced. The process of demethylation of 5mC residues involves ten-eleven translocation (TET) proteins, also termed 5mC dioxygenases. The TET family of proteins includes the TET1, TET2, and TET3 proteins. These proteins catalyze the oxidation of the methyl group on 5mC to 5-hydroxymethyl cytosine (5hmC).17 Because the increase in 5hmC levels coincides with the reduction of 5mC,18,19 formation of 5hmC is believed to be the first step in the active demethylation of 5mC residues. However, 5hmC has also been shown to play an epigenetic role in gene regulation,20,21 especially in stem cells;22 therefore, 5hmC might have functional consequences in addition to being the first step in the demethylation of 5mC. Once 5hmC is formed, there are several pathways by which the 5hmC is removed. For example, 5hmC is more susceptible to deamination by activation-induced deaminase than 5mC,23 and the resulting product, 5-hyroxymethyluracil, is removed via the base-excision repair pathway and replaced with a non-methylated cytosine by the base-excision repair pathway via thymine DNA glycosylase.23 In addition, the TET family of proteins also catalyze the subsequent oxidation of 5hmC to 5-formylcytosine and 5-carboxylcytosine, which are also excised by the base-excision repair pathway.24 A critical area for further investigation is the targeting mechanisms by which 5mC is added or removed from specific sites. While transcription factors and histones are known to direct DNMTs to specific genomic regions, these mechanisms are poorly understood.25
Research over the past 2 decades has shown that changes in DNA methylation are an important component of epigenetic regulation in mammals, leading to alterations in gene expression and the maintenance of the differentiated state in cell lineages.26 The first report that DNA methylation changed with age was published in 1973, when Vanyushin et al.27 reported that the total levels of 5mC in the DNA isolated from various tissues of rats decreased with age. Subsequently, Wilson et al.28 reported that the levels of 5mC decreased with age in DNA isolated from various tissues or cells from mice, Peromyscus, and humans. These reports generated a great deal of interest, because they provided a mechanistic explanation for the relaxation of gene expression with age, a process termed dysdifferentiation.29 This concept was largely based on a study by Ono and Cutler,30 which reported that expression of globin genes in nonerythroid tissues, such as liver and brain, increased with age.30 It was argued that the loss of 5mC in the genome with increasing age was responsible for the abnormal expression of genes that are normally repressed in cells/tissues.29 However, in a recent study, White et al.31 did not observe any major relaxation of gene repression at old age, although some minor effect cannot be ruled out. Although investigators have not been able to replicate the study by Ono and Cutler30 showing a relaxation in the regulation of genes that are not normally expressed in differentiated cells, there have been several studies reporting a decrease, no change, or an increase in global methylation of the genome of tissues from animals as they age. Here, we critically evaluate the effect of age on the DNA methylating and demethylating enzymes and the global content of 5mC in the genomes of animals to determine the validity of the genomic hypomethylation hypothesis of aging.
Effect of age on the levels of 5mC
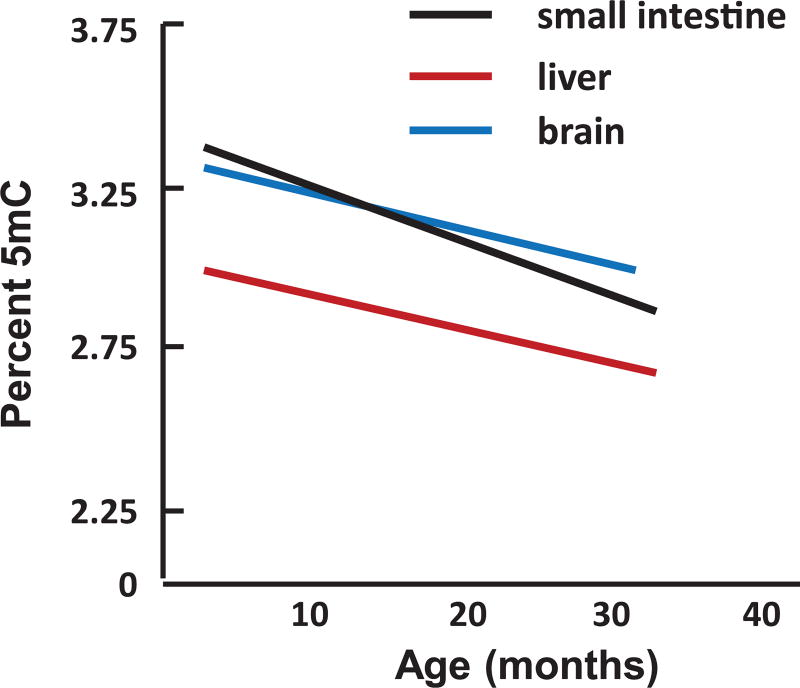
We have listed in Table 1 the published reports that have studied the effect of age on global DNA methylation (i.e., the content of 5mC in total DNA). We did not include studies that measured DNA methylation during development/maturation (e.g., the study by Romanov et al.45 showing that DNA methylation was lower in heart and thymus of 10- to 12-year-old cows compared with embryos;45 the study by Stubbs et al.46 showing that hypermethylation in DNA occurred between birth and 10 month of age in mouse liver, lung, heart and cortex; and the study by Heyn et al.47 that compared DNA methylation in blood from a newborn infant and a centenarian. In addition, we did not include reports in Table 1 where methylation was studied in only a portion of the genome. The initial study by Vanyushin et al.27 used thin-layer chromatography (TLC) to separate 5mC from other bases in formic acid hydrolysates of DNA and measured the level of 5mC eluting from the TLC by UV absorbance. They reported that the 5mC content of DNA hydrolysates from brain, heart, and spleen decreased over a range of ages between 1 and 28 months in male rats. However, they did not observe a significant change in 5mC content in DNA isolated from liver, lung, and kidney. In 1987, Wilson’s group developed a32P-postlabeling assay that was at the time the most sensitive and accurate method for measuring 5mC levels in DNA.28 After digesting DNA with micrococcal nuclease and calf spleen phosphodiesterase, the deoxynucleosides were radioactively labeled using T4 polynucleotide kinase and32P-ATP. The hydrolysates were then separated by 2-dimensional TLC, and the 5mC content was calculated based on the percent of radioactivity in 5-methyl-deoxycytidine compared with the radioactivity in deoxycytidine. In 1983, Wilson and Jones published a seminal paper in the area of DNA methylation and age showing that the levels of 5mC decreased with increasing population doublings in DNA isolated from skin fibroblasts from mice, hamsters, and humans.48 Although this paper is often cited as showing that hypomethylation of the genome occurred with age, this report only measured the effect of population doubling (i.e., replicative senescence) on DNA methylation, not aging. In 1987, Wilson et al. studied the effects of aging on DNA methylation when they measured the levels of 5mC in DNA from various tissues of laboratory mice and Peromyscus leucopus.28 As shown in Figure 1, they observed a decrease in the 5mC content of DNA isolated from the brain, liver, and small intestine of mice. They also observed a decrease in the 5mC content of DNA from the liver and small intestine of Peromyscus as well as a decrease in 5mC content in DNA from bronchial epithelial cells isolated from human donors of various ages. Interestingly, they observed that the rate of 5mC decrease with age was lower in Peromyscus, which live about twice as long as laboratory mice, and was the lowest in humans. Thus, they argued that the rate of 5mC loss from the genome was inversely correlated to life span––the genomes of shorter-lived animals had higher rates of 5mC loss. Subsequently, several investigators used high-performance liquid chromatography (HPLC) to analyze the 5mC content of DNA hydrolysates from cells/tissues from mice and humans of various ages. These studies, which used UV light to detect and quantify 5mC, reported a decrease in DNA methylation (Table 1). However, the decrease reported by Fuke et al.33 was very small. The fourth study, by Fasolino et al.35 used HPLC coupled with tandem mass spectrometry to identify and quantify 5mC levels in brain, and they failed to observe any significant change in DNA methylation with age.
Table 1.
Effect of age on global methylation of the genome
| Changes in methylation |
Animal model | Tissue or cell type | Age | References |
|---|---|---|---|---|
| Thin-layer chromatography | ||||
| Decrease | Rat–albino | Brain, heart, spleen | 1–28 months | 26 |
| No change | Rat–albino | Liver, lung, kidney | 1–28 months | 26 |
| Decrease | Mouse–C57BL/6 | Brain, liver, intestine | 2–35 months | 27 |
| Decrease | Peromyscus | Liver, intestine | 2–60 months | 27 |
| Decrease | Human | Lung fibroblasts | Various ages | 27 |
| High-pressure liquid chromatography | ||||
| Decrease | Mouse–C57BL/6 | Liver | 6 & 31 months | 30 |
| Decrease | Human | Leukocytes | 4–94 years | 31 |
| Decrease | Mouse–C57BL/6 | Hippocampus | 7–19 months | 32 |
| No change | Mouse–C57BL/6 | Striatum, substantia nigra | 3–18 months | 33 |
| Antibody based | ||||
| Increase | Mouse–C57BL/6 | Hippocampus | 12 & 24 months | 34 |
| No change | Mouse–C57BL/6 | Hippocampus | 2–24 months | 20 |
| No change | Mouse–unknown | Liver | 3–24 months | 35 |
| Decrease | Rat–SD | Hippocampus | 2–30 months | 36 |
| Decrease | Rat–SD | Hippocampus | 3–30 months | 37 |
| Next-generation sequencing | ||||
| No change | Human | Cortex | 16–64 years | 4 |
| No change | Mouse–C57BL/6 | Cortex | 1.5–22 months | 4 |
| No change | Human | Skin–epidemis | ~ 22 & ~ 72 years | 38 |
| No change | Mouse–C57BL/6 | Hematopoietic stem cells | 4 & 24 months | 39 |
| No change | Mouse–C57BL/6 | Hippocampus | 3–24 months | 40 |
| No change | Mouse–UMHET3 | Liver | 2 & 22 months | 41 |
| No change | Mouse–C3B6F1 | Liver | 5 & 27 months | 42 |
Figure 1.
Effect of age on DNA methylation in mouse tissues. Data for male C57BL/6 mice taken from Ref. 28.
Over the past 5 years, investigators have used antibodies that specifically bind to 5mC sites in the genome to measure the effects of aging on DNA methylation. However, antibody-based detection of 5mC preferentially detects regions with few CG sites, and its reactivity with mCH is unclear.49 In addition, antibody-based approaches only provide a relative quantification, and standards used in most 5mC enzyme-linked immunosorbent assays provide an inaccurate methylation level, as we have described previously.42 Five studies have used antibodies against 5mC to measure DNA methylation in the hippocampi of young and old mice and rats. The two studies with rats showed decrease in methylation with age, while the three studies with mice showed either an increase or no change in DNA methylation with age. Chouliaras et al.36 also measured the effect of dietary restriction on DNA methylation in the hippocampus, because dietary restriction has been shown to increase life span by delaying/retarding aging. They reported that lifelong dietary restriction prevented the age-related increase in DNA methylation, which they observed in the hippocampi of mice. Armstrong et al.37 and Brown-Borg et al.50 measured DNA methylation in Ames dwarf mice, which live ~ 50% longer than wild-type littermates. The level of global DNA methylation in the Ames dwarf mice was similar to wild-type mice and did not change with age from 3 to 24 months of age.
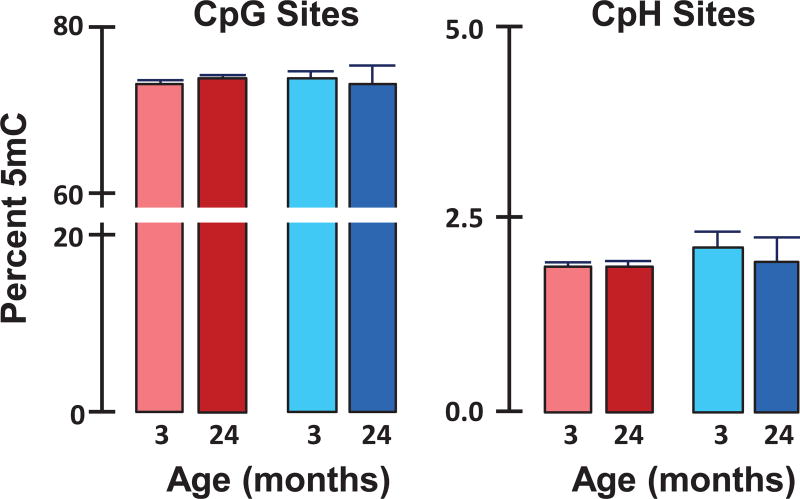
More recently, investigators have begun using bisulfite treatment, which converts unmethylated cytosines to uracil but not methylated cytosines, and next-generation-sequencing to quantify specific cytosines that are methylated in the genome. In a highly cited paper using this method, Heyn et al.47 reported that the genome of T cells from humans becomes hypomethylated with age. However, these data were generated with cord blood from one infant and blood from one centenarian. Recently, five groups, including ours, measured the effects of age on the 5mC content (percent of cytosines methylated) using next-generation sequencing/bisulfite, as shown in Table 1. Both Lister et al.4 and Hadad et al.42 measured 5mC in CpG and CpH sites, while Raddatz et al.,40 Sun et al.,41 Cole et al.,43 and Hahn et al.44 measured 5mC at only CpG sites. Lister et al.4 found no change in global DNA methylation with age in the genome of the cortex from humans or male mice. Our group (Hadad et al.42) also found no age-related change in 5mC in either CpG or CpH in the hippocampus from male and female mice. This observation was replicated in a subsequent experiment where we used high-depth whole-genome bisulfite sequencing to measure the 5mC content in ~ 20 million CpG sites and ~ 300 million CpH sites in DNA isolated from the hippocampi of male and female mice. As Figure 2 shows, the content of 5mC in both the CpG and CpH sites is similar in the hippocampus genomes of male and female mice and does not change with age. Raddatz et al.40 reported no change with age in the 5mC content at CpG sites in the genome of human epidermis. Sun et al.41 also reported that DNA methylation at CpG sites was similar in hematopoietic stem cells from 4- and 24-month-old mice (83.5% versus 84.6% methylation, respectively). Recently, Cole et al.43 and Hahn et al.44 found no change with age in the methylation of CpG sites in the liver genome from male and female mice, respectively. They also found that dietary restriction had no effect on the global methylation of CpG sites in the liver. It should be noted that Cruickshanks et al.51 reported a slight decrease (~ 7%) in the methylation of CpG sites in genome of senescent human lung fibroblasts (IMR90) compared with proliferating fibroblasts using next-generation sequencing/bisulfite.51
Figure 2.
Effect of age on the global methylation of the hippocampus genome. The 5mC content in CpG and CpH sites was measured in hippocampal DNA from young (3 months) and old (24 months) male (blue) and female (red) C57BL/6 mice by whole-genome bisulfite sequencing. Each value represents the mean ± SEM of three mice per group. There was no significant difference with age or sex in global DNA methylation at either the CpG or CpH sites.
In addition to the studies listed in Table 1 where DNA methylation was measured in the whole genome using next-generation sequencing/bisulfite, there are two studies using this technology where the 5mC content of specific regions and sites throughout the genome was measured as a function of age. Beerman et al.52 measured DNA methylation primarily in CpG islands and annotated gene promoters in hematopoietic stem cells from 3.5- and 25-month-old C57BL/6 male mice. They observed a slight but significant increase in global DNA methylation in these regions. More recently, our group measured 5mC content in ~ 120 Mb of regions across the hippocampus genome containing the most important gene regulatory regions (e.g., all gene promoters; all of the non-repeat region CpG islands, shores, and shelves; enhancers; and intragenic regions).53 We found no significant change in the 5mC content across the ~ 120 Mb of the hippocampal genome of 3- and 24-month-old male and female C57BL/6 mice. However, there were major sex and age differences in DNA methylation at thousands of specific CpG and CpH sites (both increases and decreases in methylation).
Effect of age on enzymes involved in DNA methylation
Because one would predict that age-related changes in one or more of the enzymes involved in DNA methylation could have a global impact on DNA methylation, we have also reviewed the scientific literature in this area. Table 2 lists the published articles that have studied the effects of age on the expression (either mRNA or protein levels) of the DNMT and TET families. The studies measuring the levels of DNMT and TET proteins are somewhat limited by the poor specificity of most of the antibodies that are currently available.42 As can be seen from Table 2, there is currently no consensus on how the expression of any of the DNMT proteins change with age; some studies show an increase, some a decrease, and others no change with age. This variability does not appear to be due to differences between tissues, because many of the studies using the same tissue reported different results. The effect of age on the expression of the TET proteins is not quite as variable, with the studies showing either no change or a decrease in expression. All of the current studies on TET2 show no change with age.
Table 2.
Effects of age on enzymes involved in DNA methylation
| Species | Sex | Agea (months/ years) |
Tissue | Level of expression |
Reference |
|---|---|---|---|---|---|
| Dnmt1 | |||||
| Mouse (C57BL/6) | M | 3 – 18 | Hippocampus | nc–mRNA | 52 |
| Rat (Wistar) | M | 3 – 20 | Hippocampus | ↑–protein | 53 |
| Mouse (Swiss albino) | M | 2.5 – 20 | Hippocampus & Cortex | nc–mRNA ↓–Protein | 54 |
| Mouse (C57BL/6) | M | 4–24 | BM–HSCs | ↓–mRNA | 39 |
| Rat (Sprague–Dawley) | ?b | 3 – 30 | Hippocampus | ↓–Protein | 37 |
| Humans | M/F | 35 – 75 | PMCs | ↓–mRNA | 55 |
| Mouse (C57BL/6) | M | 3–18 | Striatum | ↑–mRNA | 33 |
| Mouse (C57BL/6) | M | 3–18 | Substantia Nigra | nc–mRNA | 33 |
| Mouse (C57BL/6) | M/F | 3–24 | Hippocampus | nc–mRNA | 40 |
| Mouse | ? | 3–24 | Liver | nc–mRNA | 35 |
| Dnmt3a | |||||
| Humans | M/F | 16 – 78 | Liver | ↑–mRNA | 56 |
| Mouse (C57BL/6) | M | 12 –24 | Hippocampus | ↑–protein | 57 |
| Mouse (C57BL/6) | M | 3 –18 | Hippocampus | ↓–mRNA | 52 |
| Mouse (Swiss albino) | M | 2.5 – 20 | Hippocampus & Cortex | nc–mRNA nc–protein | 54 |
| Mouse (C57BL/6) | M | 4–24 | BM–HSCs | ↓–mRNA | 39 |
| Rat (Sprague–Dawley) | ? | 3 – 30 | Hippocampus | ↓–protein | 37 |
| Mouse (C57BL/6) | M | 3 – 18 | Striatum | ↑–mRNA | 33 |
| Mouse (C57BL/6) | M | 3 – 18 | Substantia Nigra | nc–mRNA | 33 |
| Mouse (C57BL/6) | M/F | 3 – 24 | Hippocampus | nc–mRNA | 40c |
| Mouse | ? | 3–24 | Liver | nc–mRNA | 35 |
| Dnmt3b | |||||
| Humans | M/F | 16–78 | Liver | ↑–mRNA | 56 |
| Mouse (C57BL/6) | M | 3–18 | Hippocampus | nc– mRNA | 52 |
| Rat | M | 3–20 | Hippocampus | nc–protein | 53 |
| Mouse (Swiss albino) | M | 2.5–20 | Hippocampus & Cortex | nc– mRNA nc–protein | 54 |
| Mouse (C57BL/6) | M | 4–24 | BM–HSCs | ↓–mRNA | 39 |
| Humans | M/F | 35–75 | Blood–PMCs | ↓–mRNA | 55 |
| Mouse (C57BL/6) | M/F | 3–24 | Hippocampus | nc–mRNA | 40 |
| Mouse | ? | 3–24 | Liver | nc–mRNA | 35 |
| Tet1 | |||||
| Mouse (C57BL/6) | M | 2–24 | Hippocampus | nc–mRNA | 20 |
| Mouse (C57BL/6) | M | 4–24 | BM–HSCs | ↓–mRNA | 39 |
| Mouse (C57BL/6) | ? | 3–30 | Hippocampus | ↓–mRNA | 58 |
| Humans | ? | 20–83 | Blood–T cells | ↓–mRNA | 59 |
| Humans | M/F | 34–74 | Blood–MNCs | ↓–mRNA | 60 |
| Mouse (C57BL/6) | M/F | 3–24 | Hippocampus | nc–mRNA | 40 |
| Tet2 | |||||
| Mouse (C57BL/6) | M | 2–24 | Hippocampus | nc–mRNA | 20 |
| Humans | ? | 20–83 | Blood–T cells | nc– mRNA | 59 |
| Mouse (C57BL/6) | M | 3–18 | Striatum | nc–mRNA | 33 |
| Mouse (C57BL/6) | M | 3–18 | Substantia Nigra | nc–mRNA | 33 |
| Humans | M/F | 34–74 | Blood–MNCs | nc–mRNA | 60 |
| Mouse (C57BL/6) | M/F | 3–24 | Hippocampus | nc–mRNA | 40 |
| Tet3 | |||||
| Mouse (C57BL/6) | M | 2–24 | Hippocampus | nc–mRNA | 20 |
| Mouse (C57BL/6) | M | 4–24 | BM–HSCs | ↓–mRNA | 39 |
| Humans | ? | 20–83 | T cells | ↓–mRNA | 59 |
| Humans | M/F | 34–74 | Blood–MNCs | ↓–mRNA | 60 |
| Mouse (C57BL/6) | M | 3–18 | Striatum | nc–mRNA | 33 |
| Mouse (C57BL/6) | M | 3–18 | Substantia migra | nc–mRNA | 33 |
| Mouse (C57BL/6) | M/F | 3–24 | Hippocampus | nc–mRNA | 40 |
Age of mice in months, age of humans in years
Article did not give sex of animals studied
Hadad et al. measured both Dnmt3a1 and Dnmt3a2; however, Dnmt3a2 was undetectable by qPCR.
As noted above, knocking out genes involved in DNA methylation is lethal; however, there is a study that has looked at the effect of reduced levels of DNMT1 on life span using heterozygous Dnmt1 knockout mice (Dnmt1+/−). The Dnmt1+/− mice showed a 33% decrease in DNMT1 expression and no change in the expression of DNMT3a or DNMT3b; however, the life span of the Dnmt1+/− mice similar to wild-type mice.63
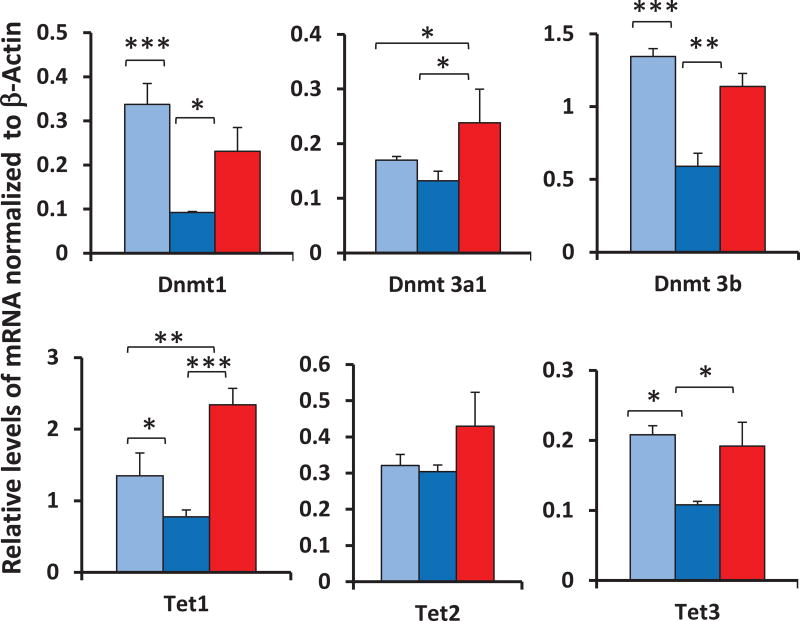
There has been only one published study that examined the effects of dietary restriction (DR) on some of the proteins involved in DNA methylation. Chouliaras et al.59 reported that the age-related increase in the level of DNMT3a protein in the hippocampus was attenuated by DR. We recently studied the effects of aging and DR on the expression (transcript levels) of the DNMT and TET genes in colon mucosa isolated from mice. As shown in Figure 3, we observed an age-related decrease in the expression of Dnmt1, Dnmt3b, Tet1, and Tet3, but no change in the expression of Dnmt3a1 or Tet2. Interestingly, DR prevented the age-related decline in the expression of all of the six genes we studied compared with old mice fed ad libitum; however, the increase was not statistically significant for Tet2. Armstrong et al.37 measured the expression of the DNMT genes in male Ames dwarf and wild-type mice from 3 to 24 months of age. They observed that the transcripts for DNMT1 and DNMT3a were significantly higher in young dwarf mice compared with young wild-type mice; however, with age this difference disappeared.
Figure 3.
Effect of age and dietary restriction on the expression of genes involved in DNA methylation. The levels of the various transcripts were measured by qPCR for colon mucosa isolated from young (9 months, light blue) and old (24 months, dark blue) mice fed ad libitum and old (24 months, red) DR mice. Each bar represents the mean ± SEM of five mice per group. * P < 0.05; **P < 0.01; ***P < 0.001.
Summary
A great deal of interest in the role of DNA methylation in aging was recently sparked by Horvath and others when they showed that DNA methylation at a few hundred CpG sites in the human genome was a quantitative biomarker of chronological age.64,65 Similar epigenetic clocks have been developed for 90–329 CpG sites in mice.46,66,67 These observations have led to the re-emergence of the hypomethylation hypothesis of aging. However, a critical examination of the current scientific literature calls into question this hypothesis, as shown in Table 1. There are currently 17 publications that have reported on the effects of aging on global DNA methylation in tissues from rodents and cells from humans. Seven of the 17 studies reported a decrease in DNA methylation with age, while nine reported no change in global DNA methylation and one study reported a significant increase in methylation. The most likely explanation for the varied results is improved technology and increased sensitivity currently available to measure the 5mC content of DNA isolated from the tissues/cells. For example, all of the recent studies, which have used next-generation sequencing/bisulfite to measure the 5mC content of DNA, have consistently failed to observe significant age-related changes in global DNA methylation. These observations have been made in the cortex (Lister et al.4), hematopoietic stem cells (Lister et al.4), hippocampus (Hadad et al.42), and liver (Cole et al.;43 Hahn et al.44) from mice, and the cortex (Lister et al.4) and skin (Raddatz et al.40) from humans. A review of the current literature with respect to the effect of age on the enzymes involved in DNA methylation also demonstrates no obvious overall effect of age on the expression of the DNMT and TET proteins, as shown in Table 2.
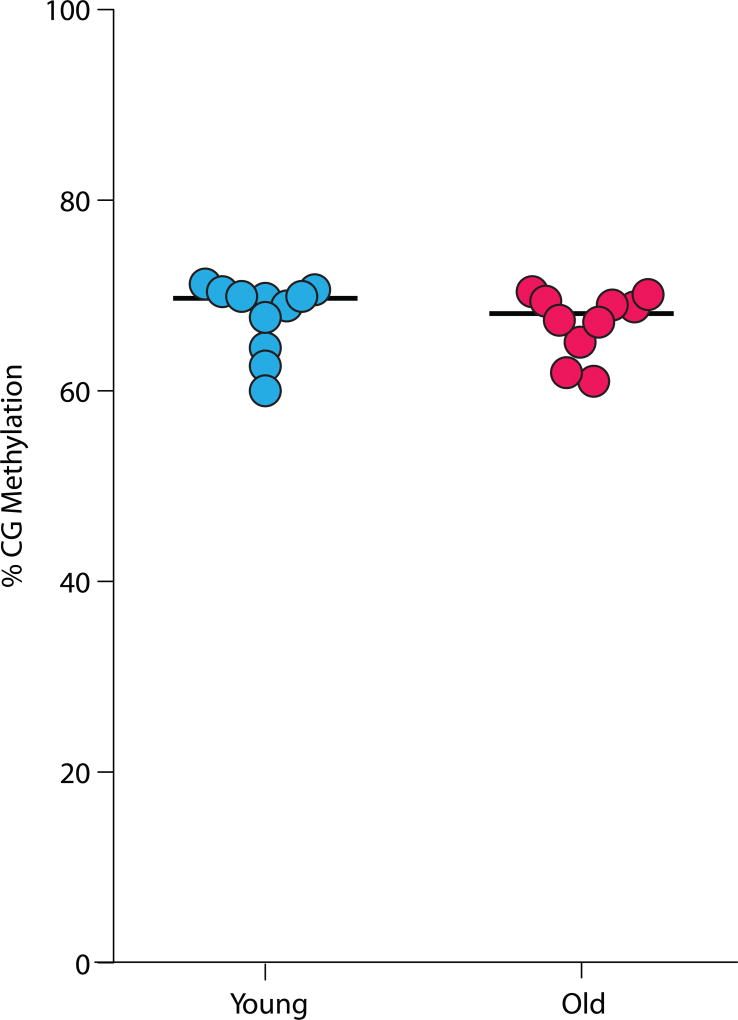
On the basis of the current literature, we conclude that a decrease in methylation is not a universal process that occurs in the genomes of all tissues/cells as an animal ages. While the studies reviewed above have been of methylation levels in tissues, the possibility exists that specific cells (e.g., certain cell types, or senescent cells) could be undergoing hypomethylation. With new methods to analyze single-cell methylation profiles, it is now possible to gain single-cell resolution.68,69 We re-analyzed the data from Gravina et al.70 and examined genome-wide methylation levels from young and old hepatocytes and found no differences in genome-wide methylation levels in this specific cell type (Fig. 4). Thus, the current data do not support the hypothesis that a global, random loss of 5mC from the genome is a hallmark of aging and contributes to some of the phenotypes that occur with age. However, it is important to emphasize that all of the studies using next-generation sequencing/bisulfite that did not observe a change in global DNA methylation did observe age-related changes in DNA methylation in specific regions or at specific sites in the genome. For example, we observed major age differences in DNA methylation at thousands of specific CpG and CpH sites (both increases and decreases in methylation) in the ~ 120 Mb of the hippocampal genome analyzed, even though there was no change in the total 5mC content of the genome.53 Raddatz et al.40 found age-related changes in DNA methylation in promoter and enhancer regions of the genome of human epidermis that were correlated with changes in transcription, and Lister et al.4 also observed age-related changes in methylation in specific CpG and CpH sites in the cortex of mice and humans. Sun et al.41 observed a decrease in methylation with age in CpG islands and promoters and increases in CpG sites in gene bodies and repetitive elements at CpG sites in hematopoietic stem cells, even though global methylation of the genome of these cells did not occur with age. Both Cole et al.43 and Hahn et al.44 found hypo- and hypermethylation events occurring with age and DR as distinct regulatory features in the liver genomes of mice. even though the global content of 5mC did not change with either age or DR.
Figure 4.
Effect of age on the genome-wide methylation profiles of single hepatocytes. Analysis done on the data reposited by Gravina et al.70
In conclusion, it is clear from the current literature that aging has a major effect on the patterns of methylation at specific sites in the genome; however, these changes in DNA methylation are not due to a general loss of methylation in the genome leading to the relaxation of gene expression with age and dysdifferentiation, as proposed 40 years ago by Nagy et al.29 Rather, the changes in DNA methylation appear to occur at specific CpG and CpH sites and regions in the genome, indicating that the changes in methylation that occur with age are regulated and may be functionally important.4,42,53 Future studies examining methylation changes with aging will need to utilize these new methodologies to fully describe the patterns of differential methylation across the genome with aging. These patterns, including CpH methylation and other cytosine modifications (5hmC, 5-formylcytosine, and 5-carboxylcytosine) need to be fully described and integrated with chromatin/histone marks to understand the network of epigenomic changes occurring with aging. Through these types of systems analyses, the role of the epigenome in altered gene expression with aging can be understood and interventional approaches to maintain a youthful epigenome tested.
Materials and methods
Animals
Male C57BL/6 mice were obtained from the NIA aging and caloric-restricted colony at 9 and 24 months of age, housed on arrival in the animal facility at the University of Oklahoma Health Sciences Center, and maintained under SPF conditions in a HEPA barrier environment until sacrifice. The animals were fed irradiated NIH-31 mouse/rat diet from Teklad (Envigo, Madison, WI). Both DR and AL mice were individually housed. DR was initiated at 14 weeks of age at 10% restriction and increased to 25% restriction at 15 weeks and to 40% restriction at 16 weeks, where it is maintained throughout the life of the animal. Mice were then sacrificed and colon mucosa was harvested, snap frozen in liquid nitrogen, and stored at –80 ±C until used. For the methylation data, 3- and 24-month-old male and female C57BL/6 mice were obtained from the NIA aging colony. All animal experiments were performed according to protocols approved by the OUHSC Institutional Animal Care and Use Committee.
Gene expression
The levels of mRNA transcripts of genes involved in DNA methylation (Dnmt1, Dnmt3a1, Dnmt3b, Tet1, Tet2, and Tet3)) were measured in the individual samples (n = 5/group) with three technical replicates using real-time PCR. Briefly, RNA was isolated using RNeasy RNA isolation kit from Qiagen (Germantown, MD, USA). The first strand cDNA was synthesized from 1µg RNA using random primers (Promega, Madison, WI) and purified using the QIAquick PCR purification kit (Qiagen, Germantown, MD). Expression of the candidate genes were quantified using real-time PCR with pre-designed Taqman probes (Thermo Fisher, Waltham, MA). Relative gene expression was quantified as comparative ct analysis using the 2−ΔΔct analysis method with β-actin as endogenous control. One-way ANOVA design with Tukey’s multiple test correction was used to statistically analyze individual samples.
Whole-genome methylation analysis
Whole-genome bisulfite sequencing was performed as previously described in Hadad et al.42 in the hippocampi of 3- and 24-month-old animals. In this instance, bisulfite conversion and library construction were performed according to the manufacturer’s protocol (Accel-NGS Methyl-Seq, Swift Biosciences, Ann Arbor, MI). Libraries were sequenced (HiSeq 2500) to an average coverage depth of > 10× across the entire genome. Alignment and quantitation was as previously described by Hadad et al.42 Quantitation at each CG and CH site covered at ≥10 × was then computed, and an average level of methylation across the genome for each mouse was calculated.
Acknowledgments
The efforts of authors were supported by NIH Grants R01 AG045693 (A.U. and A.R.) R01EY021716, R21EY024520 (W.M.F., D.R.M., D.R.M., and N.H.), T32EY023202, and T32AG052363 (D.R.M.), a Senior Career Research Award from the Department of Veterans Affairs (A.R.), the Oklahoma Center for Advancement of Science and Technology (HR14-174) (W.M.F., D.R.M., D.R.M., and N.H.), the Donald W. Reynolds Foundation (A.R. and W.M.F.), and the Oklahoma Nathan Shock Aging Center (P30 AG050911).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Wyatt GR. Occurrence of 5-methylcytosine in nucleic acids. Nature. 1950;166(4214):237–8. doi: 10.1038/166237b0. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RD. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem. 1948;175(1):315–32. [PubMed] [Google Scholar]
- 3.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 4.Lister R, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Li E. Structure and function of eukaryotic DNA methyltransferases. Curr Top Dev Biol. 2004;60:55–89. doi: 10.1016/S0070-2153(04)60003-2. [DOI] [PubMed] [Google Scholar]
- 6.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20(24):3139–55. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 7.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 8.Hermann A, Goyal R, Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem. 2004;279(46):48350–9. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 9.Groth A, et al. Chromatin challenges during DNA replication and repair. Cell. 2007;128(4):721–33. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 11.Bostick M, et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317(5845):1760–4. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 12.Sharif J, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450(7171):908–12. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 13.Breiling A, Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin. 2015;8:24. doi: 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12(7):647–56. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okano M, et al. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 16.Kastan MB, Gowans BJ, Lieberman MW. Methylation of deoxycytidine incorporated by excision-repair synthesis of DNA. Cell. 1982;30(2):509–16. doi: 10.1016/0092-8674(82)90248-3. [DOI] [PubMed] [Google Scholar]
- 17.Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139(11):1895–902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal K, et al. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A. 2011;108(9):3642–7. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wossidlo M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 20.Song CX, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29(1):68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Dzitoyeva S, Manev H. Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus. Restor Neurol Neurosci. 2012;30(3):237–45. doi: 10.3233/RNN-2012-110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, et al. DNA methylation and hydroxymethylation in stem cells. Cell Biochem Funct. 2015;33(4):161–73. doi: 10.1002/cbf.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo JU, et al. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell Cycle. 2011;10(16):2662–8. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14(6):341–56. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchal C, Miotto B. Emerging concept in DNA methylation: role of transcription factors in shaping DNA methylation patterns. J Cell Physiol. 2015;230(4):743–51. doi: 10.1002/jcp.24836. [DOI] [PubMed] [Google Scholar]
- 26.Riggs AD. DNA methylation and late replication probably aid cell memory, and type I DNA reeling could aid chromosome folding and enhancer function. Philos Trans R Soc Lond B Biol Sci. 1990;326(1235):285–97. doi: 10.1098/rstb.1990.0012. [DOI] [PubMed] [Google Scholar]
- 27.Vanyushin BF, et al. The 5-methylcytosine in DNA of rats. Tissue and age specificity and the changes induced by hydrocortisone and other agents. Gerontologia. 1973;19(3):138–52. [PubMed] [Google Scholar]
- 28.Wilson VL, et al. Genomic 5-methyldeoxycytidine decreases with age. J Biol Chem. 1987;262(21):9948–51. [PubMed] [Google Scholar]
- 29.Zs-Nagy I, Cutler RG, Semsei I. Dysdifferentiation hypothesis of aging and cancer: a comparison with the membrane hypothesis of aging. Ann N Y Acad Sci. 1988;521:215–25. doi: 10.1111/j.1749-6632.1988.tb35280.x. [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Cutler RG. Age-dependent relaxation of gene repression: increase of endogenous murine leukemia virus-related and globin-related RNA in brain and liver of mice. Proc Natl Acad Sci U S A. 1978;75(9):4431–5. doi: 10.1073/pnas.75.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White RR, et al. Comprehensive transcriptional landscape of aging mouse liver. BMC Genomics. 2015;16:899. doi: 10.1186/s12864-015-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhal RP, Mays-Hoopes LL, Eichhorn GL. DNA methylation in aging of mice. Mech Ageing Dev. 1987;41(3):199–210. doi: 10.1016/0047-6374(87)90040-6. [DOI] [PubMed] [Google Scholar]
- 33.Fuke C, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68(Pt 3):196–204. doi: 10.1046/j.1529-8817.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, et al. Insufficient DNA methylation affects healthy aging and promotes age-related health problems. Clin Epigenetics. 2011;2(2):349–60. doi: 10.1007/s13148-011-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasolino M, et al. Distinct cellular and molecular environments support aging-related DNA methylation changes in the substantia nigra. Epigenomics. 2017;9(1):21–31. doi: 10.2217/epi-2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chouliaras L, et al. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiol Aging. 2012;33(8):1672–81. doi: 10.1016/j.neurobiolaging.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong VL, et al. Expression of DNA methyltransferases is influenced by growth hormone in the long-living Ames dwarf mouse in vivo and in vitro. J Gerontol A Biol Sci Med Sci. 2014;69(8):923–33. doi: 10.1093/gerona/glt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mei Y, et al. Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell. 2015;14(4):659–68. doi: 10.1111/acel.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong Z, et al. Age-related formaldehyde interferes with DNA methyltransferase function, causing memory loss in Alzheimer's disease. Neurobiol Aging. 2015;36(1):100–10. doi: 10.1016/j.neurobiolaging.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Raddatz G, et al. Aging is associated with highly defined epigenetic changes in the human epidermis. Epigenetics Chromatin. 2013;6(1):36. doi: 10.1186/1756-8935-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14(5):673–88. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadad N, et al. Absence of genomic hypomethylation or regulation of cytosine-modifying enzymes with aging in male and female mice. Epigenetics Chromatin. 2016;9:30. doi: 10.1186/s13072-016-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole JJ, et al. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. 2017;18(1):58. doi: 10.1186/s13059-017-1185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn O, et al. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017;18(1):56. doi: 10.1186/s13059-017-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanov GA, Vanyushin BF. Methylation of reiterated sequences in mammalian DNAs. Effects of the tissue type, age, malignancy and hormonal induction. Biochim Biophys Acta. 1981;653(2):204–18. doi: 10.1016/0005-2787(81)90156-8. [DOI] [PubMed] [Google Scholar]
- 46.Stubbs TM, et al. Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 2017;18(1):68. doi: 10.1186/s13059-017-1203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heyn H, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A. 2012;109(26):10522–7. doi: 10.1073/pnas.1120658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson VL, Jones PA. DNA methylation decreases in aging but not in immortal cells. Science. 1983;220(4601):1055–7. doi: 10.1126/science.6844925. [DOI] [PubMed] [Google Scholar]
- 49.Nair SS, et al. Comparison of methyl-DNA immunoprecipitation (MeDIP) and methyl-CpG binding domain (MBD) protein capture for genome-wide DNA methylation analysis reveal CpG sequence coverage bias. Epigenetics. 2011;6(1):34–44. doi: 10.4161/epi.6.1.13313. [DOI] [PubMed] [Google Scholar]
- 50.Brown-Borg HM, et al. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 51.Cruickshanks HA, et al. Senescent cells harbour features of the cancer epigenome. Nat Cell Biol. 2013;15(12):1495–506. doi: 10.1038/ncb2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beerman I, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12(4):413–25. doi: 10.1016/j.stem.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yung R, et al. Unexpected effects of a heterozygous dnmt1 null mutation on age-dependent DNA hypomethylation and autoimmunity. J Gerontol A Biol Sci Med Sci. 2001;56(6):B268–76. doi: 10.1093/gerona/56.6.b268. [DOI] [PubMed] [Google Scholar]
- 54.Oliveira AM, Hemstedt TJ, Bading H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci. 2012;15(8):1111–3. doi: 10.1038/nn.3151. [DOI] [PubMed] [Google Scholar]
- 55.Elsner VR, et al. Exercise induces age-dependent changes on epigenetic parameters in rat hippocampus: a preliminary study. Exp Gerontol. 2013;48(2):136–9. doi: 10.1016/j.exger.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh P, Thakur MK. Reduced recognition memory is correlated with decrease in DNA methyltransferase1 and increase in histone deacetylase2 protein expression in old male mice. Biogerontology. 2014;15(4):339–46. doi: 10.1007/s10522-014-9504-5. [DOI] [PubMed] [Google Scholar]
- 57.Ciccarone F, et al. Age-dependent expression of DNMT1 and DNMT3B in PBMCs from a large European population enrolled in the MARK-AGE study. Aging Cell. 2016;15(4):755–65. doi: 10.1111/acel.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao Y, et al. Age and gender affect DNMT3a and DNMT3b expression in human liver. Cell Biol Toxicol. 2008;24(3):265–72. doi: 10.1007/s10565-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 59.Chouliaras L, et al. Caloric restriction attenuates age-related changes of DNA methyltransferase 3a in mouse hippocampus. Brain Behav Immun. 2011;25(4):616–23. doi: 10.1016/j.bbi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Stilling RM, et al. De-regulation of gene expression and alternative splicing affects distinct cellular pathways in the aging hippocampus. Front Cell Neurosci. 2014;8:373. doi: 10.3389/fncel.2014.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truong TP, et al. Age-Dependent Decrease of DNA Hydroxymethylation in Human T Cells. J Clin Exp Hematop. 2015;55(1):1–6. doi: 10.3960/jslrt.55.1. [DOI] [PubMed] [Google Scholar]
- 62.Valentini E, et al. Analysis of the machinery and intermediates of the 5hmC-mediated DNA demethylation pathway in aging on samples from the MARK-AGE Study. Aging (Albany NY) 2016;8(9):1896–1922. doi: 10.18632/aging.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray D, et al. Aging in heterozygous Dnmt1-deficient mice: effects on survival, the DNA methylation genes, and the development of amyloidosis. J Gerontol A Biol Sci Med Sci. 2006;61(2):115–24. doi: 10.1093/gerona/61.2.115. [DOI] [PubMed] [Google Scholar]
- 64.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weidner CI, et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15(2):R24. doi: 10.1186/gb-2014-15-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petkovich DA, et al. Using DNA Methylation Profiling to Evaluate Biological Age and Longevity Interventions. Cell Metab. 2017;25(4):954–960. e6. doi: 10.1016/j.cmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang T, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. doi: 10.1186/s13059-017-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clark SJ, et al. Genome-wide base-resolution mapping of DNA methylation in single cells using single-cell bisulfite sequencing (scBS-seq) Nat Protoc. 2017;12(3):534–547. doi: 10.1038/nprot.2016.187. [DOI] [PubMed] [Google Scholar]
- 69.Farlik M, et al. Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep. 2015;10(8):1386–97. doi: 10.1016/j.celrep.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gravina S, et al. Single-cell genome-wide bisulfite sequencing uncovers extensive heterogeneity in the mouse liver methylome. Genome Biol. 2016;17(1):150. doi: 10.1186/s13059-016-1011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]