Abstract
Purpose
As part of consolidative therapy in high-risk neuroblastoma, modern protocols recommend radiation therapy (RT) both to the primary site and to sites of metastatic disease that persist after induction chemotherapy. Although there are abundant data showing excellent local control (LC) with 21 Gy directed at the primary site, there are few data describing the feasibility and efficacy of RT directed at metastatic sites of disease as part of consolidation.
Methods and Materials
All patients with neuroblastoma who received RT to metastatic sites of disease as a part of consolidative therapy at a single institution between 2000 and 2015 were reviewed. Among 159 patients, 244 metastases were irradiated.
Results
The median follow-up period among surviving patients was 7.4 years. Over 85% of the irradiated metastases were treated with 21 Gy (range, 10.5-36 Gy). Tumor recurrence occurred in 43 of 244 irradiated metastases (18%). The 5-year LC rate of treated metastatic sites was 81%. Metastatic sites that cleared with induction chemotherapy had improved LC compared with sites with persistent uptake on metaiodobenzylguanidine scans (LC rate, 92% vs 67%; P <.0001). LC at irradiated metastatic sites did not differ based on total number of sites irradiated or site of disease irradiated (bone vs soft tissue). Patients with bulky, resistant disease who were treated with 30 to 36 Gy had worse LC (P = .02). However, on multivariate analysis, only persistence after induction chemotherapy remained a significant prognostic factor for LC (hazard ratio, 3.7; P < .0001). Patients who had LC at irradiated metastatic sites had improved overall survival compared with those who did not (overall survival rate, 71% vs 50%; P < .0001).
Conclusions
Response to chemotherapy is an important prognostic factor for LC at irradiated metastatic sites in neuroblastoma. Overall, consolidative RT appears to be an effective modality of LC. Long-term disease control can be achieved with such an approach.
Introduction
Neuroblastoma is the most common extracranial solid tumor in children, with approximately 650 cases per year in the United States. Over half of children with neuroblastoma present with metastatic disease at diagnosis. The general treatment paradigm for these children consists of induction chemotherapy, surgical resection, consolidative therapy, and treatment of minimal residual disease with biological agents. As part of consolidative therapy, radiation therapy (RT) is typically given both to the primary site and to persistent sites of metastatic disease after induction chemotherapy. There are many studies supporting the efficacy of consolidative RT to the primary site in achieving local control (LC) for patients with high-risk neuroblastoma (1-4). However, data describing the indications and efficacy of consolidative RT to metastatic sites of disease are scarce. As distant relapse remains the major obstacle to cure for patients with high-risk neuroblastoma (with rates >50%), it is imperative to assess the value of irradiation of metastatic sites of disease in achieving long-term control.
In an attempt to balance control of high-risk sites of metastatic disease with the toxicity of multisite irradiation, the current recommendation in modern protocols is that only sites of persistent metastatic disease after induction chemotherapy receive consolidative RT. However, recent evidence has shown that patients frequently have failure at anatomic sites of initial disease, even those that clear with chemotherapy (5). As such, it remains unclear whether RT to measurable sites of metastatic disease in complete response after induction chemotherapy may also be necessary for distant control. We sought to evaluate the role of consolidative RT to metastatic sites of disease in high-risk neuroblastoma.
Methods and Materials
This is a single-institution, retrospectively ascertained cohort of neuroblastoma patients treated at Memorial Sloan Kettering (MSK) with RT to sites of metastatic disease as a part of consolidative therapy with curative intent between 2000 and 2015. Patients undergoing palliative treatment for metastases and those receiving treatment for relapse were excluded. Metastatic sites that were irradiated included all sites of persistent disease (as assessed via imaging with 123I metaiodobenzylguanidine [MIBG]) as well as nonpersistent sites in patients who initially presented with oligometastatic disease (defined in general as ≤3 metastases). In addition, nonpersistent sites that were deemed at high risk of relapse were irradiated. High-risk sites included lesions in the skull given their often diffuse and extensive involvement of the calvaria. In addition, skull relapse is common and can lead to severe consequences including cranial nerve palsies. Additional criteria used to select high-risk metastatic lesions for RT included the gross size of the metastases (in relation to the bone or child) as well as the intensity of MIBG uptake. However, unlike fluorodeoxyglucose positron emission tomography, MIBG uptake is not quantified with standardized uptake values and therefore was based on clinical judgment of the treating physician. All included patients were classified as high risk: stage 4 disease with (any age) or without (>18 months old) MYCN amplification. We identified 159 patients, among whom 244 metastases were irradiated. Metastatic sites of involvement were imaged at diagnosis and subsequently followed with computed tomography (CT) scans and/or MIBG scans every 3 months for ≥24 months. The study was approved by the MSK Institutional Review Board/Privacy Board.
Treatment
Chemotherapy and other treatments
A total of 75 patients (47%) were treated on or according to MSK induction protocols N7 (n = 21) and N8 (n = 54) (6), and 81 patients (51%) were treated on or according to Children's Oncology Group (COG), Pediatric Oncology Group, International Society of Paediatric Oncology, or other institutional protocols. The remaining 3 patients received a combination of standard chemotherapy agents off protocol. Therapeutic MIBG with 131I was administered in 20 patients (13%) and was given after consolidative RT in all patients except 4. In addition, 54 patients (34%) underwent stem cell transplantation. The decision for myeloablative therapy and transplantation was based on whether systemic therapy was administered at MSK or at an outside institution; although routine elsewhere, stem cell transplantation has not been a part of the treatment paradigm at MSK for high-risk neuroblastoma since 2003. All but 3 patients (98%) also received 3F8 (anti-GD2 antibody) plus granulocyte-macrophage colony-stimulating factor.
Radiation therapy
All patients underwent RT to metastatic sites with curative intent after induction chemotherapy as part of consolidative therapy. RT to metastatic sites took place concurrently with primary-site RT in most cases (85%). The median interval from time of diagnosis to RT was 8 months. A hyperfractionated approach with 1.5-Gy fractions twice daily to a total dose of 21 Gy was used for the treatment of the majority of metastases (85%). Alternate doses and fractionation regimens (30-36 Gy) were used to treat bulky gross residual disease. Fourteen metastatic sites received <21 Gy (15-18 Gy) because of concerns for growth plate impairment. In general, the gross tumor volume consisted of the preinduction chemotherapy tumor volume. A 1-cm expansion along the length of the bone was used to create the clinical target volume, with careful attention to stay off of growth plates and to not expand outside of the involved bone. When there was diffuse involvement of the skull, we treated the entire skull with 21 Gy of hyperfractionated RT using a combined photon-electron skull RT technique that allows for full dose to the skull bones with maximal sparing of the brain parenchyma (7).
Statistical methods and design
Local failure was defined as recurrence of tumor within the RT field of a treated metastatic site. Event-free survival (EFS) was calculated as the time from initiation of treatment to the first event. Events were defined as local and/or distant failure or progression or death. Overall survival (OS) was calculated as the time from initiation of treatment to death from any cause. Patients without an event were censored at the time of last follow-up. The Kaplan-Meier method was used to assess LC, EFS, and OS. Survival curves were compared by the log-rank (Mantel-Haenszel) test, with P ≤ .05 considered significant. A Cox proportional hazards model with forward stepwise selection was used for multivariate analysis to assess for independent prognostic factors for LC; variables with P < .05 on univariate analysis were included in the multivariate analysis.
Results
Table 1 shows patient characteristics. The median age at RT was 4.0 years (range, 1.2-17.9 years). All patients had stage 4 neuroblastoma and were classified as high risk according to the COG risk classification and revised International Neuroblastoma Staging System. Among the 159 patients, 39% had MYCN-amplified disease. Metastatic involvement at diagnosis consisted of the bone and/or bone marrow in 94% of patients, with isolated soft tissue involvement in 6%. Of the patients, 62% received RT to 1 metastatic site as part of consolidation; 25%, to 2 sites; and 12%, to ≥3 sites (Table 2). Of the 244 metastatic sites irradiated, 166 (68%) were bony metastases, involving most commonly the skull (n = 76) and extremities (n = 59). Other common sites of irradiation included the neck and/or supraclavicular region (n = 45), mediastinum (n = 22), and liver (n = 10). Forty-five percent of patients had persistent disease after induction chemotherapy. The median follow-up period among surviving patients was 7.4 years (range, 1.4-17.4 years).
Table 1. Patient characteristics.
| Data | |
|---|---|
| Sex, n (%) | |
| Female | 69 (43) |
| Male | 90 (57) |
| Race, n (%) | |
| White | 128 (81) |
| Black | 14 (9) |
| Asian | 8 (5) |
| Other | 9 (6) |
| Age at RT, y | |
| Median | 4.0 |
| Range | 1.2-17.9 |
| Primary site location, n (%) | |
| Adrenal | 124 (78) |
| Central abdominal compartment | 28 (18) |
| Mediastinum and/or thorax | 7 (4) |
| MYCN, n (%) | |
| Nonamplified | 87 (55) |
| Amplified | 62 (39) |
| Unknown | 10 (6) |
| Shimada histopathology, n (%) | |
| Favorable | 8 (5) |
| Unfavorable | 111 (70) |
| Unknown | 40 (25) |
| Metastatic sites, n (%) | |
| Bone only | 76 (48) |
| Soft tissue only | 9 (6) |
| Bone and soft tissue | 74 (47) |
| Stem cell transplantation, n (%) | |
| Yes | 54 (34) |
| No | 105 (66) |
| MIBG therapy, n (%) | |
| Yes | 20 (13) |
| No | 139 (87) |
Abbreviations: MIBG = metaiodobenzylguanidine; RT = radiation therapy.
Table 2. Bone metastases and treatment characteristics.
| Data | |
|---|---|
| No. of bone metastases at diagnosis per patient,n (%) | |
| 0 | 20 (13) |
| 1 | 25 (16) |
| 2 | 14 (9) |
| 3 | 8 (5) |
| 4 | 11 (7) |
| 5 | 4 (3) |
| >5 | 77 (48) |
| No. of irradiated metastatic sites per patient, n (%) | |
| Median (range) | 1 (1-5) |
| 1 | 99 (62) |
| 2 | 41 (26) |
| 3 | 14 (9) |
| 4 | 3 (2) |
| 5 | 2 (1) |
| Site of metastatic irradiation, n (%) | |
| Bone | 166 (68) |
| Soft tissue | 78 (34) |
| Specific location of irradiated bone, n (%) | |
| Skull and/or orbits | 76 (46) |
| Extremity | 59 (36) |
| Pelvis | 10 (6) |
| Spine | 18 (11) |
| Other | 3 (2) |
| Radiation dose, n (%) | |
| <21 Gy | 15 (6) |
| 21 Gy (1.5 Gy twice per day) | 209 (86) |
| 21.6 Gy (1.8 Gy once per day) | 2 (1) |
| 24 Gy (8 Gy per day) | 1 (0.4) |
| 30 Gy (1.5 Gy twice per day) | 9 (4) |
| 36 Gy (1.5 Gy twice per day) | 8 (3) |
Local failure occurred in 43 of 244 irradiated metastases (18%) at a median time of 1.48 years from RT. The 5-year LC rate of treated metastatic sites was 81.0% (95% confidence interval [CI], 75%-86%). Table 3 shows prognostic factors for LC on univariate analysis. LC did not differ based on site of irradiated metastases (bone vs soft tissue), location of bony involvement, or number of bony sites of disease at diagnosis. However, response to induction chemotherapy was prognostic of LC: The 5-year LC rate was 92% in sites that cleared with chemotherapy versus 67% in persistent sites of disease (P < .0001, Fig. 1). In addition, patients who received 30 to 36 Gy because of resistant, bulky disease had worse local control (P = .02). However, on multivariate analysis, only persistence after induction chemotherapy remained significant (hazard ratio, 3.7; P < .0001).
Table 3. Prognostic factors for LC at irradiated sites.
| Factor | n | 5-y LC | P value |
|---|---|---|---|
| Site of irradiated metastasis | |||
| Bone | 166 | 79% | .65 |
| Soft tissue | 78 | 85% | |
| No. of bony sites of involvement at diagnosis | |||
| ≤5 | 102 | 83% | |
| >5 | 141 | 80% | .84 |
| Persistent disease in metastatic site after induction chemotherapy (bone and soft tissue) | |||
| Yes | 107 | 67% | |
| No | 137 | 92% | <.0001 |
| Persistent bone disease after induction chemotherapy | |||
| Yes | 88 | 66% | <.0001 |
| No | 78 | 93% | |
| Persistent soft tissue disease after induction chemotherapy | |||
| Yes | 18 | 71% | .13 |
| No | 60 | 91% | |
| Site of bone involvement | |||
| Skull and/or orbit | 76 | 86% | .36 |
| Extremity | 59 | 70% | |
| Pelvis | 10 | 77% | |
| Spine | 18 | 81% | |
| Other | 3 | 100% | |
| RT dose | |||
| <21 Gy | 15 | 88% | .02 |
| 21-24 Gy | 212 | 83% | |
| 30 Gy | 9 | 50% | |
| 36 Gy | 8 | 50% | |
Abbreviations: LC = local control; RT = radiation therapy.
Fig. 1.
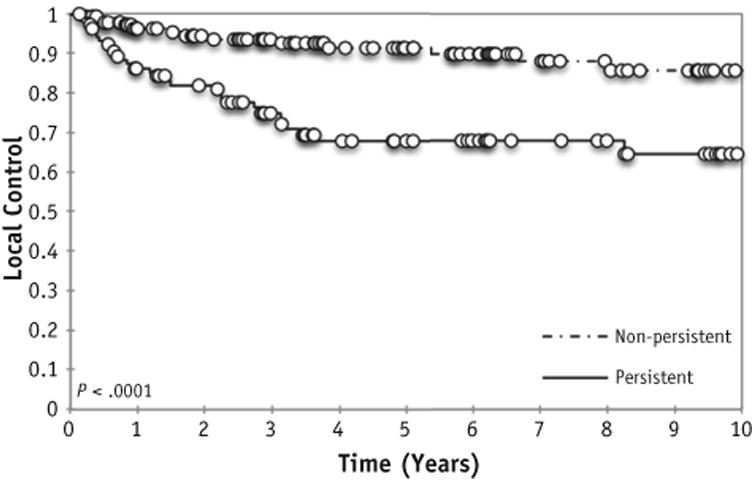
Local control at irradiated site based on response to induction chemotherapy.
Distant relapse occurred in 88 patients at a median time of 8.4 months from initiation of systemic therapy. The cumulative incidence of distant failure at 5 years was 57% (95% CI, 50%-60%). The 5-year EFS and OS rates were 47% (95% CI, 37%-58%) and 70% (95% CI, 60%-79%), respectively. Among the 78 patients with persistent disease at irradiated sites after induction chemotherapy, the 5-year EFS and OS rates were 34% and 61%, respectively. Patients who had local control at irradiated metastatic sites had improved EFS and OS compared with those who did not (5-year EFS rate of 54% vs 0%, P <.0001, and 5-year OS rate of 71% vs 50%, P < .0001; Fig. 2).
Fig. 2.
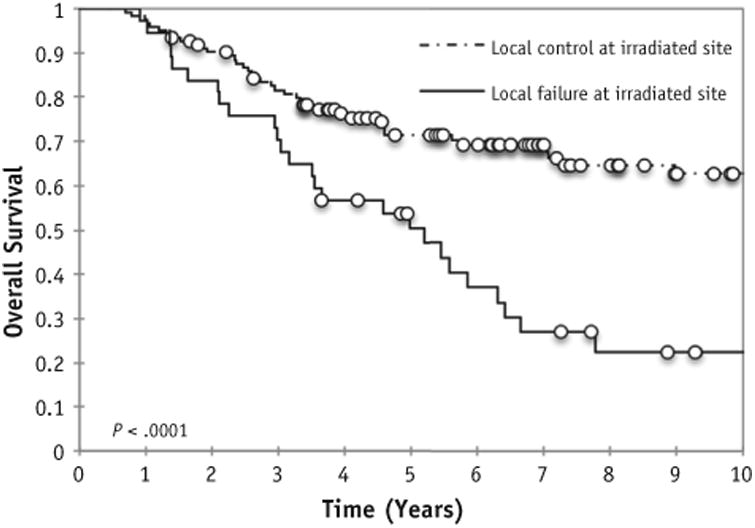
Overall survival based on local control at irradiated sites.
Among the 86 patients in whom failure occurred distantly, the site of first relapse most commonly involved the bone (n = 51) and central nervous system (n = 15). Of the 51 patients whose site of first relapse was the bone, 33 (65%) had failure in a bone that was initially involved at diagnosis but was not irradiated because of clearance after induction chemotherapy. Of the patients with failure at a nonirradiated site that was initially present at diagnosis, 82% had widespread osseous metastases at the time of first presentation. Relapse at a nonirradiated, previously involved metastatic site did not differ based on MYCN amplification or the receipt of stem cell transplantation.
All patients were able to complete RT without significant acute toxicity or delay. A second hematologic malignancy developed in 6 patients at a median time of 5.1 years from diagnosis. There were no second solid neoplasms. Regarding other late effects, among the 94 survivors, the most common irradiation-related late toxicity was musculoskeletal abnormalities (n = 27). Neurocognitive delay was also observed in 6 survivors treated with whole-skull RT, although this was likely multifactorial in etiology.
Discussion
The combination of myeloablative therapy, immunotherapy, and differentiation therapy with 13-cis-retinoic acid has led to an improvement in survival for high-risk neuroblastoma. However, >50% of children will still undergo relapse systemically (1). Although RT is routinely used to prevent local relapse, it is unknown whether irradiation to metastatic sites at risk of relapse can prevent disease recurrence and thereby increase long-term disease control. With the goal of improving disease-free control, the current COG protocol ANBL0532 recommends irradiation to sites of metastatic disease that persist after induction chemotherapy. At our institution, we have used 21 Gy of hyperfractionated RT to treat not only persistent sites of disease but also previous sites of measurable or bulky disease in complete response, especially those in critical locations such as the skull or weight-bearing bones. As discussed in more detail later, the rationale for the latter approach stems from prior experiences showing that the majority of failures occur at previous sites of involvement, even those in complete response to induction therapy (5, 8-10).
We found the local control rate at sites of irradiated metastatic disease at 5 years to be 81%. Response to induction chemotherapy was a significant prognostic factor for disease control: The local control rate was 92% at sites that cleared with chemotherapy versus 67% at persistent sites of disease. Our local control rate of >90% at nonpersistent sites of metastatic disease is similar to the local control rate >90% at the primary site after gross total resection and 21 Gy of hyperfractionated RT. Given the similarity between local control rates—and without a prospective trial analyzing the optimal dose—21 Gy appears sufficient for control of microscopic sites of metastatic neuroblastoma.
However, for patients with persistent disease after induction chemotherapy (comprising 45% of the cohort), the local control rate at irradiated metastatic sites was <70%. Similarly, the control rate in patients with gross disease at the primary site after induction chemotherapy and surgical resection is inferior (11). More recent data have suggested that 21 Gy to the primary site may not be a sufficient dose for these patients (12, 13). As such, modern protocols currently recommend 36 Gy postoperatively to the primary site for all patients with gross residual disease after resection. Of the 107 metastatic sites of persistent disease treated in our cohort, only 8 were treated with 36 Gy; in all cases this was because of concern for resistant and aggressive disease (that had progressed despite induction therapy). Although local control was not improved in these 8 patients, this was an especially unfavorable subset of patients in whom higher doses were purposefully chosen. In addition, while persistence after induction chemotherapy remained a significant prognostic factor for local control on multivariate analysis, dose did not. It remains unclear whether >21 Gy is needed for persistent sites of metastatic disease. Given the inferior local control rates, this is an area that should be further explored.
Other smaller series have evaluated local control after RT to metastatic sites during consolidative therapy (Table 4). The number of metastatic sites included in these series ranges from 4 to 27, with local control rates ranging from 74% to 94% (2-5, 14). In the study of Bradfield et al (4), all initial sites of metastatic disease were irradiated after stem cell transplantation, regardless of the response to induction therapy. Among the 17 patients who received RT, there were no local failures at irradiated sites. However, there was a significant amount of myelosuppression despite post-RT stem cell infusion. Specifically, grade 4 platelet and red blood cell toxicity occurred in 9 patients, and grade 4 neutropenia developed in 3 patients, attributed to bone marrow suppression from the large extent of bony irradiation. Polishchuk et al (5) sought to evaluate the site of first metastatic relapse among patients with high-risk neuro-blastoma. In their cohort, 82.4% of the 159 sites of first metastatic relapse overlapped anatomically with 525 sites present at diagnosis. Only 3 of 19 sites (15.8%) that were irradiated had recurrence versus 128 of 506 unirradiated sites (25.3%). This study concluded that bone relapse tends to occur at anatomic sites of initial disease at diagnosis and that relapses are less common in the subset of metastatic sites that are irradiated. Other small series have confirmed that patients are most likely to undergo relapse at non-irradiated metastatic sites of disease in complete response after induction chemotherapy (14).
Table 4. Single-institution experiences evaluating local control at irradiated metastatic sites.
Similarly, the site of first relapse was at an untreated, pre-existing disease site in 65% of our patients. Our results further confirm that the most common site of first relapse is at a previously involved metastatic site that completely resolves with chemotherapy and is subsequently not irradiated. However, when it was feasible to deliver RT to sites that cleared with induction chemotherapy, we found that RT was >90% effective in providing disease control in our cohort. Further supporting the importance of distant disease control as a part of consolidative therapy, survival was better in patients without local relapse at an irradiated site. Thus, for patients who present with a limited metastatic burden (ie, oligometastatic), RT to all sites of initial metastatic involvement, even those that clear with systemic therapy, may play a significant role in long-term disease control.
Unfortunately, though, >80% of the patients in our cohort who underwent relapse at a pre-existing disease site presented with diffuse osseous metastases at diagnosis. It is not feasible to irradiate all initial sites of metastases given bone marrow tolerance in the acute setting and the potential late consequences of multisite irradiation. The maximum number of sites that can and should be irradiated as a part of consolidative therapy remains unknown, especially in the setting of advancements in immunotherapy, MIBG therapy, and vaccine therapy (15-17). For the majority of patients who present with diffuse metastases, systemic therapy is likely the more effective treatment for widespread disease control. The role of MIBG therapy in the upfront setting for patients with diffuse bone metastases is also an area of current investigation.
Limitations of this analysis include the retrospective design, which confounds dose analysis, and dependence on available imaging for the detection of metastatic disease both at diagnosis and at subsequent follow-up visits after RT. In addition, at MSK, we use a hyperfractionated approach when treating children with high-risk neuroblastoma, in part to allow for a quicker return to immuno-therapy. Although this is not routinely done elsewhere, we do not believe there would be a vast difference in terms of biological effectiveness between once-daily and twice-daily treatments of metastatic sites.
In summary, distant failure remains the dominant form of relapse and is an ongoing challenge in improving the cure rates for patients with high-risk neuroblastoma. Local control of nonpersistent sites of metastases that respond completely to chemotherapy is similar to local control at the primary site after gross total resection. We recommend consideration of consolidative RT to nonpersistent sites of oligometastatic disease, as well as nonpersistent sites that are deemed at high risk of relapse, such as those in the skull. Higher doses of RT may be needed for control of metastatic sites that persist after chemotherapy, as is likely true for control of gross residual tumor at the primary site as well. Whether irradiation of all measurable metastases that respond completely to induction therapy is warranted remains an area of active investigation and will ultimately depend on finding the balance between optimal disease control and late toxicity.
Summary.
Although radiation therapy to persistent sites of metastatic disease is recommended as part of consolidative therapy for high-risk neuroblastoma, there are few data evaluating the value of this approach in achieving long-term control. Our results show that irradiation of metastatic sites of disease is effective in achieving local control and that response to induction chemotherapy is a significant prognostic factor for control at irradiated sites.
Acknowledgments
Funding provided by National Institutes of Health grant P30 CA 008748.
Footnotes
Conflict of interest: none.
References
- 1.Casey DL, Kushner BH, Cheung NK, et al. Local control with 21-Gy radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2016;96:393–400. doi: 10.1016/j.ijrobp.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 3.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–1554. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 4.Bradfield SM, Douglas JG, Hawkins DS, et al. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer. 2004;100:1268–1275. doi: 10.1002/cncr.20091. [DOI] [PubMed] [Google Scholar]
- 5.Polishchuk AL, Li R, Hill-Kayser C, et al. Likelihood of bone recurrence in prior sites of metastasis in patients with high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2014;89:839–845. doi: 10.1016/j.ijrobp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Kushner BH, Kramer K, LaQuaglia MP, et al. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–4892. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 7.Wolden SL, Barker CA, Kushner BH, et al. Brain-sparing radiotherapy for neuroblastoma skull metastases. Pediatr Blood Cancer. 2008;50:1163–1168. doi: 10.1002/pbc.21384. [DOI] [PubMed] [Google Scholar]
- 8.Kushner BH, Cheung NK, Barker CA, et al. Hyperfractionated low-dose (21 Gy) radiotherapy for cranial skeletal metastases in patients with high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;75:1181–1186. doi: 10.1016/j.ijrobp.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibley GS, Mundt AJ, Goldman S, et al. Patterns of failure following total body irradiation and bone marrow transplantation with or without a radiotherapy boost for advanced neuroblastoma. Int J Radiat Oncol Biol Phys. 1995;32:1127–1135. doi: 10.1016/0360-3016(95)00011-m. [DOI] [PubMed] [Google Scholar]
- 10.Sangthawan D, DesRosiers PM, Randall ME, et al. Relapse in the skull after myeloablative therapy for high-risk neuroblastoma. Pediatr Hematol Oncol. 2003;20:23–30. [PubMed] [Google Scholar]
- 11.La Quaglia MP, Kushner BH, Su W, et al. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004;39:412–417. doi: 10.1016/j.jpedsurg.2003.11.028. discussion 412-417. [DOI] [PubMed] [Google Scholar]
- 12.Simon T, Hero B, Bongartz R, et al. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children >1 year with residual local disease. Strahlenther Onkol. 2006;182:389–394. doi: 10.1007/s00066-006-1498-8. [DOI] [PubMed] [Google Scholar]
- 13.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: A Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 14.Mazloom A, Louis CU, Nuchtern J, et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2014;90:858–862. doi: 10.1016/j.ijrobp.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung NK, Burch L, Kushner BH, et al. Monoclonal antibody 3F8 can effect durable remissions in neuroblastoma patients refractory to chemotherapy: A phase II trial. Prog Clin Biol Res. 1991;366:395–400. [PubMed] [Google Scholar]
- 16.Cheung NK, Miraldi FD. Iodine 131 labeled GD2 monoclonal antibody in the diagnosis and therapy of human neuroblastoma. Prog Clin Biol Res. 1988;271:595–604. [PubMed] [Google Scholar]
- 17.Matthay KK, DeSantes K, Hasegawa B, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]


