Abstract
Objectives
Poor parental understanding of prescription opioid risks is associated with potentially dangerous decisions that can contribute to adverse drug events (ADE) in children and adolescents. This study examined whether an interactive Scenario-Tailored Opioid Messaging Program (STOMP™) would 1) enhance opioid risk perceptions and 2) improve the safety of parents’ decision-making.
Methods
546 parents were randomized to receive the STOMP™ versus Control information and 516 completed the program. A baseline survey assessed parents’ opioid risk knowledge, perceptions and preferences for pain relief vs. risk avoidance (PR Pref). Parents then made hypothetical decisions to give or withhold a prescribed opioid for High Risk (excessive sedation) and Low Risk (no ADE) scenarios. The STOMP™ provided immediate feedback with specific risk and guidance information; the Control condition provided general information. We reassessed knowledge, perceptions and decision-making up to three days thereafter.
Results
Following the intervention, the STOMP™ group became more risk avoidant (PR Pref mean difference −1.27 [95% Confidence Interval −0.8 to −1.75], p<0.001) and gained higher perceptions of the critical risk, excessive sedation (+0.56 [0.27 to 0.85], p<0.001). STOMP™ parents were less likely than Controls to give a prescribed opioid in the High Risk situation (Odds Ratio −0.14 [−0.24, −0.05]; p=0.006) but similarly likely to give an opioid for the No ADE situation (p=0.192).
Discussion
The STOMP™ intervention enhanced risk perceptions, shifted preferences toward opioid risk avoidance, and led to better decisions regarding when to give or withhold an opioid for pain management. Scenario-tailored feedback may be an effective method to improve pain management while minimizing opioid risks.
Keywords: Analgesic Knowledge, Opioid Risk, Opioid Adverse Events, Prevention, Children and Adolescents
Introduction
The current pain and opioid epidemics have become largely inseparable public health concerns that jointly affect millions of lives and cost billions of dollars.1,2 Indeed, the marked increase in opioid prescribing over recent years3–7 has coincided with an increased prevalence of pain and the emphasis on pain management across age groups.2 Perhaps most worrisome is that these trends have paralleled the rates of opioid-related adverse drug events (ADE) and overdose deaths observed across the United States.8–11
Importantly, children and adolescents are among the most vulnerable to poor pain outcomes with high rates of reported pain12, attempted self-management13 and opioid-related ADE admissions and injuries.14 More than three million opioid prescriptions are dispensed annually to manage pain in children and adolescents.6,7,15 The rate of serious opioid-related ADEs in children and adolescents has soared in parallel with the increased prescribing rates.14,16,17 More than 21,000 emergency room admissions18,19 and hundreds of accidental overdose deaths occur among our youth annually.17 It was recently estimated that on an average day, 237 adolescents visit an emergency department for a prescription analgesic-related event.20 Importantly, personal analgesic prescriptions were recently found to be responsible for the vast majority (90%) of unintentional opioid toxicity events in this age group,21 and legitimate prescribed use has been associated with later misuse among adolescents.22 It is imperative, therefore, that providers better address the known risks associated with opioid prescribing in children.
Widespread parental uncertainty and lack of knowledge regarding pain management and prescription opioid risks may contribute largely to the risk for ADEs in children and adolescents. On one hand, parents who are uncertain about analgesic effects may ignore pain signals and undertreat their child’s pain.23 Conversely, 1 in 10 parents has admitted to giving more than the prescribed analgesic doses24,25 and some report continuing to give opioids when signs of opioid toxicity are present (i.e., unrecognized excessive sedation).26 We recently demonstrated that risky opioid decisions were largely associated with parents’ strong preferences to relieve their children’s pain.27 However, reports that parents have failed to recognize and respond to signs of toxicity that were present prior to their child’s accidental opioid-related deaths and neurologic injury28–30 emphasize the need for improved knowledge to prevent opioid misuse and opioid-related morbidity and mortality in children.
The need to improve the way analgesic information is provided at the time of prescribing is critical in order to improve the safe use of opioid analgesics in the home setting. With this in mind, we developed and tested an interactive, Scenario-Tailored Opioid Messaging Program (STOMP™) to enhance parental analgesic knowledge and analgesic decision competency. The STOMP™ prototype presented descriptive and video-enhanced risk scenarios (e.g., child after surgery with excessive sedation) combined with interactive parent decision exercises and tailored feedback. We hypothesized that, compared to parents who received routine pain management and opioid education, those who completed the STOMP™ prototype would demonstrate 1) enhanced recognition and perceived riskiness of critical opioid ADEs and; 2) improved decisions to withhold opioids in a high risk situation (i.e., excessive sedation).
Materials and Methods
Intervention
Similar to our previous studies regarding opioid decision-making,26,27,31 we incorporated clinically relevant scenarios depicting a child with similar levels of pain either with or without signs of common low-risk or rarer high-risk ADEs. Each module included the scenario description with simple instructions regarding a hypothetical and commonly prescribed opioid (i.e., oxycodone) and the commonly used alternative non-prescription analgesic, acetaminophen. Parents were asked to consider each scenario and make intentional, analgesic administration decisions. Each decision was followed with immediate scenario-tailored feedback explaining the risk shown together with guidance about what to do to reduce the risk and manage pain in a scenario like the one depicted. The first scenario (High Risk) described a child with reported pain but who exhibited clear signs of opioid toxicity i.e. excessive sedation. This scenario was accompanied with a video showing a mom trying to awaken an over-sedated child in order to give the next prescribed dose of oxycodone. The next scenario (Low Risk) showed a child who reported the same level of pain but no other adverse symptoms since the prior dose. A third scenario (Common Risk) depicted a child with the same degree of pain but who also had the common ADE, nausea and vomiting.
The analgesic feedback content was developed by experts based on a review of evidence-based opioid-sparing acute pain guidelines, analgesic package inserts, and previously described educational content.31–34 The educational content in essence, reconfigured accepted analgesic use information into brief messages that immediately followed parents’ decision-making exercises. The feedback information was reviewed for accuracy and content validity by several highly experienced clinicians, each with greater than 25 years managing pediatric pain. Specifically, reviewers provided qualitative feedback and editing advice regarding each of the scenario-tailored recommended actions, the reason or description of the risks presented and “what can happen” section, as well as the recommended next steps. For example, “Giving a non-narcotic pain reliever like acetaminophen together with the prescribed oxycodone can better relieve pain so that the child can resume regular activity faster and with fewer side effects.” The final messaging content had consensus support of all expert reviewers.
The Control Group received identical scenario and made interactive decisions for each. However, the Control feedback included generic pain management information taken from our standard pain management educational pamphlet (e.g., “It is important to manage pain and the potential side effects of analgesics that can include nausea, excessive sedation, constipation.” Information for this group did not include scenario-guided recommendations and feedback. Thus, the Control Group received only routine practice educational material.
Measures
Opioid Familiarity26,27,31: This item assesses parents’ recent in-home use of or ready availability of opioids. This binary item has face validity in assessing parents’ general familiarity with and recent use of opioid analgesics either to treat their own or a family members’ pain. Opioid familiarity has been shown to have predictive validity in parents’ analgesic decision-making.31
Pain Relief Preference (PR Pref) Survey27: This survey assesses the relative importance parents place on relieving their child’s pain versus avoiding analgesic-related risks. This tool presents six risk-benefit items (e.g., “Pain relief is more important than the side effects of prescription pain drugs”) each rated from −2, strongly disagree to +2 or strongly agree. Item scores were summed to provide an overall score that ranges from −12 (strong preference for ADE risk avoidance) to +12 (strong preference to relieve child’s pain), where 0 reflects relative ambivalence (i.e., a relative desire to do both avoid risk and relieve pain). This PR Pref scale was developed based on similar tools that measure the relative importance patients place on the risks and benefits of prescribed medications.35–37 We previously demonstrated a normal distribution in a clinical sample of parents that supported the internal consistency of the instrument (Cronbach’s alpha 0.763 [95% CI 0.73, 0.83; p<0.001]) with excellent predictive validity for parents’ analgesic decision-making (F=117.11, p<0.001).27 In the present study, the PR Pref data were not normally distributed (Kolmogorov-Smirnov p<0.001) and the Cronbach’s alpha was 0.674 [95% CI 0.62 to 0.73; p<0.001]. Additionally, a factor analysis supported a two factor solution for the scale (risk avoidant vs. risk tolerant), explaining 57% of the variance in the total PR Pref score (KMO sampling adequacy >0.64; p<0.001).
Opioid ADE Understanding: This survey measures knowledge or awareness of several common, non-serious and less common but potentially serious opioid-related ADEs in a binary manner (yes/no) and the perceived risk or seriousness of each (from 0 to 5, where 5=most serious).26,27,31 We previously demonstrated that opioid ADE risk perception has predictive validity in parents’ signal recognition and in their decisions to give the prescribed opioid.26,31 Parents’ perceptions of seriousness data were skewed both in previous studies and in the present study (Kolmogorov-Smirnov p<0.001).
Procedure
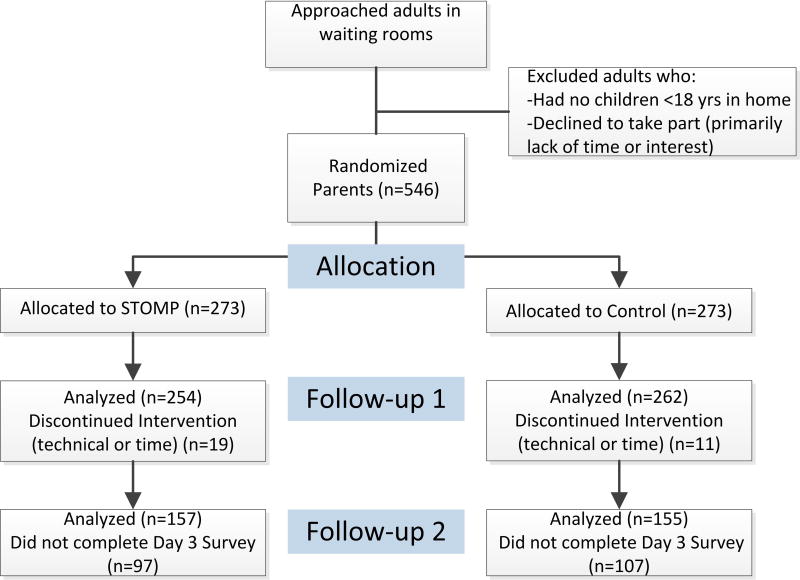
Given the educational nature of this pilot study in a non-clinical sample of volunteer adult parents, this study was deemed exempt by the Institutional Review Board at the University of Michigan with waiver of written consent (IRB-MED HUM00098971). We approached adults in surgical or general pediatric clinic waiting rooms throughout our academic, pediatric health system. These potential subjects were screened for the presence of at least one healthy child <18 years of age living in the home. We excluded parents who reported that they had a child with chronic pain or a hematologic oncologic condition. Five hundred forty-six parents who met inclusion criteria provided verbal consent to take part. These parents were then randomized by computer generated identification number to receive either the STOMP™ intervention or Control information (See Figure 1).
Figure 1.
Study Flow Diagram
For consistency, parents completed the baseline surveys, scenario modules (STOMP™ vs. Control) and immediate follow-up survey sequentially in one sitting using an iPad platform. A trained research assistant was present during the session to answer questions regarding use of the iPad, however, no content assistance was provided. All parents first completed the baseline survey to assess their opioid familiarity, PR Preference, and opioid ADE knowledge and risk perceptions. Immediately following this baseline assessment, parents completed the interactive scenario-decision modules and received their assigned feedback (i.e., STOMP™ vs. Control). For consistency, the modules were presented in the same order for all parents (i.e., High Risk [excessive sedation], Low Risk [no ADE], then Common Risk [nausea/vomiting]). Immediately following the scenario modules, parents rescored their ADE risk perceptions and recorded their demographic information as well as the number and ages of children in the home. Three days later, parents were sent an email with a link to the 3-day follow-up survey where they were re-assessed for PR Preferences, opioid ADE knowledge and risk perceptions. They repeated the decisional exercises (without feedback) and were then entered into a drawing to win a $100 gift card for participation.
Statistical Analysis
We used chi square tests to compare the experimental groups for differences in nominal data (e.g., sex), Mann Whitney U tests for comparing the PR Pref scores between groups, and Wilcoxon matched-pair signed rank tests to compare the change in PR Pref scores from baseline to the 3-day follow-up assessment. We used Friedman’s test for unequally distributed repeated measures to compare the perceived risk seriousness scores over time.
We used a generalized linear mixed effects model (GLMM) with Maximum Likelihood Estimations to test our first hypothesis and examine the effect of the STOMP™ intervention on parents’ perceived seriousness of the critical risk, excessive sedation while controlling for parent sex, education, PR Pref, time (i.e., immediate and 3-day), and parents’ opioid familiarity. We used a generalized mixed effects logistic model (GMLR) to test our second hypothesis and examine the effect of STOMP™ on parents’ decisions to give the prescribed opioid for the High Risk scenario controlling for parent sex, education, PR Pref, opioid familiarity and scenario. In both models, we included a random intercept for subject effects and adjusted the significance level (p<0.05) using the sequential Bonferroni adjustment for multiple comparisons.
We determined a priori that a sample of at least 133 per group would be needed to demonstrate an expected 15% difference31 between groups in the follow-up decision to give (or withhold) a prescribed opioid for the High Risk scenario (α = 0.05 [two-sided]; β = 0.20). To account for a potential loss of subjects at the 3-day follow-up survey we recruited 546 parents to take part in this study.
Results
Figure 1 depicts the recruitment process, subject allocation and analyses groups. Specifically, 262 parents in the Control Group and 254 in the STOMP™ Group completed the first assessments while a smaller number of parents completed the 3-day follow-up survey (59% of the Control and 62% of STOMP™ groups). Parents’ sex, level of education, the number of children in their home, their opioid familiarity (recent use and/or possession were similar between groups at baseline and at the 3-day follow-up (Table 1). A similar number and minority of parents in each group reported being in a medical profession involving pharmacologic training (i.e., MD, RN, or pharmacist).
Table 1.
Baseline Characteristics of the Study Groups
| Control Group n = 262 |
STOMP™ Group n=254 |
Odds Ratio (95% Confidence Limits), p value |
|
|---|---|---|---|
|
| |||
| Female Sex | 207 (79%) | 193 (77%) | 0.87 (0.57, 1.32), 0.509 |
| Number of children | |||
| 1–2 children (vs. ≥ 3) | 190 (73%) | 174 (68%) | 1.25 (0.85, 1.82), 0.288 |
| Age youngest child | 6.2 ± 5.0 | 5.7 ± 4.9 | MD −0.51 (−1.4, 0.4), 0.25 |
| Oldest child age | 10.7 ± 6.8 | 10.2 ± 6.4 | MD −0.52 (−1.7, 0.6), 0.375 |
| ≤High school graduate | 29 (11%) | 27 (11%) | |
| Some college | 98 (37%) | 74 (28%) | |
| ≥Bachelor’s degree (vs. all others) | 134 (51%) | 131 (52%) | 1.02 (0.72, 1.44), 0.930 |
| Medical professional with pharmacologic training | 21 (8%) | 23 (9%) | 1.14 (0.61, 2.11), 0.682 |
| Opioid familiarity | 109 (42%) | 105 (41%) | 0.96 (0.69, 1.37), 0.835 |
| PR Pref | −0.345 ± 3.28 | −0.357 ± 3.62 | 0.013a (−0.59, 0.61), 0.861b |
|
| |||
| Three-day Follow-up | |||
|
| |||
| Number who followed-up | 155 (59%) | 157 (62%) | 1.11 (0.78, 1.57), 0.576 |
| Female sex | 119 (77%) | 127 (81%) | 1.33 (0.77, 2.30), 0.315 |
| ≥Bachelor’s degree (vs. all others) | 87 (56%) | 90 (57%) | 1.05 (0.67, 1.64), 0.831 |
| Medical professional with pharmacology training | 16 (10%) | 17 (11%) | 1.05 (0.51, 2.17), 0.885 |
| Number of children | |||
| 1–2 children (vs. ≥ 3) | 41 (27%) | 52 (33%) | 1.37 (0.84, 2.22), 0.211 |
| Age youngest child | 6.4 ± 5.1 | 5.6 ± 4.9 | MD −0.78 (−1.9, 0.34), 0.17 |
| Oldest child age | 11.0 ± 7.0 | 10.0 ± 6.0 | MD−1.00 (−2.5, 0.45), 0.176 |
| Current analgesic use | 58 (39%) | 67 (44%) | 1.09 (0.9, 1.31), 0.391 |
| PR Pref | −0.732 ± 3.61 | −1.50 ± 3.42* | 0.77a (−0.02, 1.55), 0.051b |
PR Pref=Pain Relief Preference Score, where −12 reflects highest risk aversion, and +12 a high preference to relieve pain, and 0 reflects ambivalence;
Value is the mean difference;
Mann-Whitney U Test used to compare groups.
PR preference scores were similar between groups at baseline with most parents scoring around “0” (i.e., ambivalent about the risks and benefits) (Table 1). Parents in the STOMP™ group became slightly more analgesic risk avoidant after the intervention with a PR Pref score that was on average −1.27 lower on the −12 to +12 scale than baseline (95% CI −0.8 to −1.75, p<0.001) while those in the control group had no significant change from baseline in this measure (−0.189 [−0.27 to 0.65]; p=0.418).
Opioid ADE Risk Understanding
Parents’ awareness of possible opioid-related ADEs was similar between groups at baseline (Table 2; p>0.05 for all comparisons). Parents in both groups gained an improved awareness of all ADEs at the 3-day follow-up (Table 2; p < 0.009 compared to baseline). Perceived seriousness of ADEs was also similar between groups at baseline (p ≥ 0.155). However, parents in the STOMP™ group scored the seriousness of excessive sedation higher than Controls immediately after the intervention and at the 3-day follow-up while perceptions of other common ADEs remained similar between groups (Table 2).
Table 2.
Knowledge and Perceived Seriousness of Opioid-related Adverse Effects Before Scenarios/feedback, Immediately Afterward, and at Follow-Up
| Baseline Knowledgea & Perceptionb |
Immediate Post- Scenario Perceptionb |
Three-Day Follow-up Knowledgea & Perceptionb |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | STOMP™ | Control | STOMP™ | Control | STOMP™ | |
| Constipation | 78% | 76% | - | - | 91% | 93% |
| 3.05±1.39 | 2.85±1.53 | 3.08±1.28 | 2.98±1.37 | 2.82±1.2 | 3.08±1.13 | |
| 0.20 (−0.06, 0.46), 0.184 | 0.10 (−0.13, 0.33), 0.457 | −0.26 (−0.52, 0.01), 0.065 | ||||
|
| ||||||
| Nausea vomiting | 71% | 75% | - | - | 85% | 88% |
| 2.75±1.35 | 2.69±1.54 | 3.21±1.34 | 3.28±1.37 | 2.7±1.25 | 2.8±1.23 | |
| 0.06 (−0.19, 0.31), 0.635 | 0.12 (−0.31, 0.16), 0.433 | −0.10 (−0.38, 0.19), 0.507 | ||||
|
| ||||||
| Excessive sedation | 79% | 79% | - | - | 92% | 93% |
| 3.5±1.38 | 3.35±1.54 | 4.01±1.28 | 4.4±1.12 | 3.35±1.35 | 3.91±1.21 | |
| 0.16 (−0.10, 0.41), 0.397 | 0.39 (0.18, 0.60), <0.001 | 0.56 (0.27, 0.85), <0.001 | ||||
|
| ||||||
| Addiction | 78% | 76% | - | - | 91% | 93% |
| 4.44±1.29 | 4.2±1.58 | 4.53±1.16 | 4.5±1.18 | 4.51±1.04 | 4.57±0.97 | |
| 0.24 (−0.01, 0.50), 0.155 | 0.04 (−0.17, 0.24), 0.571 | −0.06 (−0.29, 0.17), 0.625 | ||||
Knowledge is presented as the percentage of those who recognized the symptom as a possible opioid-related adverse effect (measured only at baseline and 3 days); the change in knowledge from baseline to day 3 was compared using the McNemar Test and was significant for all adverse effects (p<0.009).
Perceived seriousness of each adverse effect rated from 0=not at all serious to 5=extremely serious. Data are presented as mean ± standard deviation followed by Mean Difference [95% confidence limits], p Values for Control vs. STOMP™ group comparisons, using non-parametric Kruskal-Wallis tests.
We used a linear mixed model to test the effect of the STOMP™ intervention on parents’ perceived seriousness of excessive sedation while controlling for parent sex, education, baseline PR Pref and opioid familiarity, as well as time (immediate and 3-day). Table 3 shows that there were significant effects of sex, PR Pref, Time, and STOMP™ assignment, but not parent education or opioid familiarity. Our analyses supported our first hypothesis, showing that when controlled for other factors, perceived seriousness of excessive sedation was similar at baseline (adj. p=0.67) but higher for the STOMP™ group immediately after the intervention (mean difference 0.39 [95% CI 0.18, 0.60]; p=0.002) and at the 3-day Follow-up. (mean difference 0.59 [95% CI 0.27, 0.85]; p<0.001) (see also Figure 2).
Table 3.
Effect of STOMP™ Intervention on Parents’ Perceived Seriousness of Excessive Sedation: Results of Generalized Linear Mixed Model (all variables in model shown)
| F statistic (df) |
β (95% Confidence Interval) |
Adj. p valuea |
|
|---|---|---|---|
| Parent female sex (vs male) | 25.02(1) | 0.44 (0.27 to 0.61) | <0.001 |
| Parent education | 0.84 (1) | 0.07 (−0.08 to 0.21) | 0.359 |
| PR Preference | 62.35 (1) | −0.08 (−0.10 to −0.06) | <0.001 |
| Opioid familiarity | 2.5 (1) | −0.12 (−0.26 to 0.03) | 0.114 |
| STOMP™ (vs Control) | 13.21 (1) | 0.27 (0.12 to 0.41) | <0.001 |
| STOMP™ (vs. Control) by Time | 27.55 (4) | <0.001 | |
| Baseline | 1.54 (1) | −0.14 (−0.37 to 0.084) | 0.216 |
| Immediate post- intervention | 10.81 (1) | 0.38 (0.15 to 0.61) | 0.001 |
| 3-day Follow-up | 14.42 (1) | 0.57 (0.28 to 0.86) | <0.001 |
Sequential Bonferroni was applied for multiple comparisons.
Figure 2.
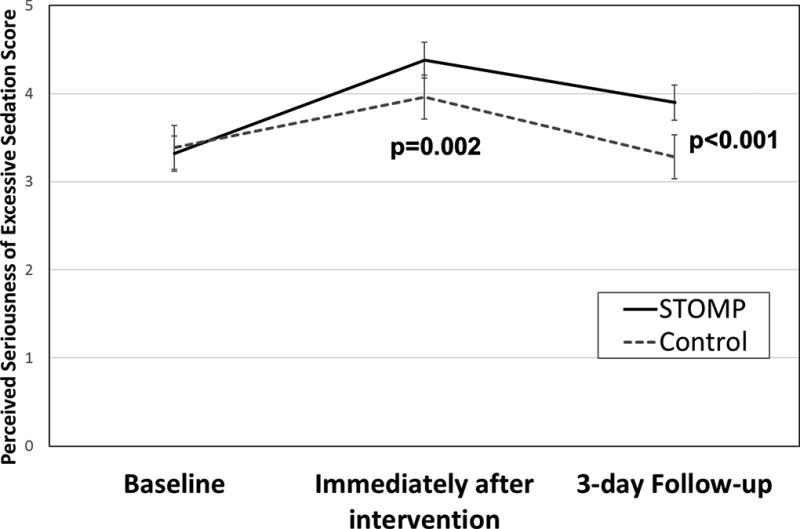
Estimated Marginal Means for Parents' Perceived Seriousness of Excessive Sedation, fixed at the mean Pain Relief Preference Score (−0.36).
Analgesic Decision Competency
Figure 3 shows the percentage of parents’ who made the hypothetical decision to give an opioid at baseline and at the 3-day follow-up for each of the scenarios. There were no differences between groups at baseline or at follow-up in the decisions to treat the child with nausea/vomiting. Compared to baseline decisions, parents in the STOMP™ group were more likely to give the prescribed opioid for the Low Risk (no ADE) scenario at follow-up (p<0.001) and less likely to do so for the High Risk, excessive sedation scenario (p=0.01). Parents in the Control group were also more likely to give the opioid for the no ADE at follow-up (p=0.025) but were no different in their decisions to treat the excessively sedated child (p=1.00). Though similar at baseline, parents in the STOMP™ group were significantly less likely than Controls at the 3-day follow-up to give the opioid to the child with excessive sedation.
Figure 3.
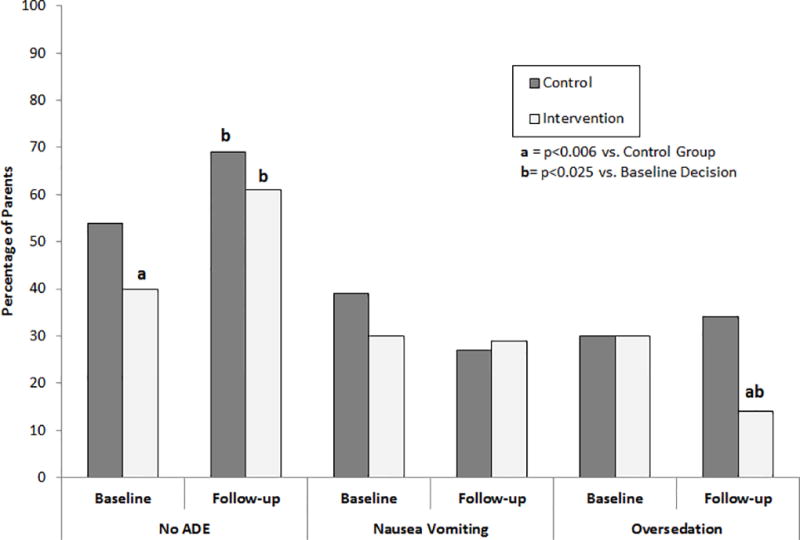
Percentage of parents’ who made the hypothetical decision to give an opioid at baseline and at the 3-day follow-up for each of the scenarios
We used a logistic mixed effect model to examine the effect of the intervention on the 3-day follow-up decision to give the prescribed opioid, controlling for the effect of parent sex, education, PR Pref, opioid familiarity and scenario. This model revealed main effects of PR Pref, intervention and intervention by scenario, but not for parent sex, education or opioid familiarity (Table 4). Parents who had received the STOMP™ feedback were significantly less likely than Controls to give an opioid in response to the high risk scenario (OR −0.14 [95% CI −0.24, −0.04], adj. p=0.006), but were equally as likely as the control parents to give the prescribed opioid in the low risk situations (p=0.192 for the no ADE scenario and p=0.456 for the nausea/vomiting scenario).
Table 4.
Effect of STOMP™ on Parents’ Decision to Give a Prescribed Opioid: Results of Generalize Mixed Logistic Regression Model (all variables in model shown)
| F Statistic (df) |
β (95% CI) | Adj. p Valuea |
|
|---|---|---|---|
| Parent female sex (vs. male) | 1.59 (1) | −0.25 (−0.65 to 0.14) | 0.208 |
| Education (≥ bachelors vs. other) | 0.74 (1) | 0.14 (−0.18 to 0.47) | 0.389 |
| PR Pref | 20.69 (1) | 0.11 (0.06 to 0.16) | <0.001 |
| Opioid Familiarity | 0.06 | 0.04 (−0.29 to 0.37) | 0.806 |
| STOMP™ (vs. Control) | 8.19 (1) | −0.11 (−0.19 to −0.03) | 0.004 |
| STOMP™ (vs. Control) by Scenario | 27.098 (4) | <0.001 | |
| No Adverse Event Present | 1.71 (1) | −0.08 (−0.19 to 0.04) | 0.192 |
| Excessive Sedation | 7.50 (1) | −0.14 (−0.24 to −0.04) | 0.006 |
| Nausea Vomiting | 0.56 (1) | −0.04 (−0.16 to 0.07) | 0.456 |
The sequential Bonferroni was applied for multiple comparisons.
Discussion
Knowing when it is safe to give a prescribed opioid and when to stop or withhold these potent analgesics is imperative to ensure the safety of children who are prescribed these agents for pain management. Failing to stop giving prescribed opioids when excessive sedation (i.e., first sign of toxicity) is present can lead to accidental opioid-related death and neurologic injury in children. Previously, we demonstrated that parents with lower ADE risk perceptions failed to recognize the importance of excessive sedation and were more likely to continue giving opioids to a child with well-described signs of opioid toxicity (i.e., excessive sedation).26,31 Based on those findings, we designed the STOMP™ intervention to incorporate descriptive and video-enhanced scenarios, interactive decision exercises and specific risk-based feedback and guidance with the intent of improving participants’ situational awareness and decision-making competency.
The results of this experimental pilot study show that compared to parents who received only general or routine risk information, parents who received the STOMP™ intervention exhibited enhanced perception of excessive sedation risks and made less risky and more appropriate opioid decisions. Our findings also show that we were able to enhance the perceived riskiness of excessive sedation without affecting the perception of other opioid-related ADE risks like constipation or nausea and vomiting. Additionally, our directed feedback decreased the proportion of parents who would give a prescribed opioid to a child with excessive sedation, while increasing the number who would give the analgesic in the absence of other symptoms. In this manner, our guided feedback improved both safe and effective opioid decisions. Of interest was that parents in the Control Group who had received generic or routine risk information gained a higher awareness of opioid-related ADEs but had no change in their risk perceptions. However, similar to our previous findings,31 awareness of ADEs alone was insufficient to enhance safe and effective decision-making as evidenced by no change in the decisions of parents in the Control or routine information group. Rather, STOMP feedback enhanced the perceived importance of specific ADE risks and led to more appropriate opioid decisions. Since children’s analgesic responses may vary widely, our data have important clinical implications. Scenario-tailored risk information combined with guidance may help parents to develop the skills needed to recognize critical symptoms and to take the correct actions when potentially serious symptoms arise.
Of interest, we found that parental sex had a strong independent effect on perceived seriousness of excessive sedation but not on opioid decision-making. We previously reported that strong parental preferences to relieve pain dampened the effect of knowledge on decision making.27 We also found that mothers exhibited a higher preference to relieve their child’s pain and reported lower treatment thresholds than fathers. These findings may help to explain our somewhat conflicting findings that while mothers exhibited greater risk perception they made similar opioid decisions as fathers. That we did not seek to examine the effects of race or child’s age on parental decisions warrants further study in order to better elucidate how other family characteristics interact with knowledge and preferences to influence the differing clinical decisions of mothers and fathers.
Previous studies have attempted to improve parents’ ability to manage pain in their children primarily by providing generic informational pamphlets or specific prescription opioid administration instructions (e.g., give around-the-clock).38–42 In one of these, parents who were randomized to pain management instruction sheets were found to give more opioids and report lower pain scores for their children compared to parents who received routine information.38,42 Other studies have suggested the need to diminish parents’ analgesic risk perceptions in order to enhance their use of prescribed opioids.23,43 A major limitation of these studies was their universal emphasis on getting parents to give more opioids and their lack of attention to opioid-related ADEs. Indeed, one of these studies reported a significantly higher rate of daytime sedation in children whose parents had been instructed to give the prescribed opioid around-the-clock after tonsillectomy, presumably without instruction on the critical importance of this sign of toxicity.42 Given that children who undergo surgery – particularly tonsillectomy – are at particular risk for opioid toxicity and apnea,44–46 parental understanding of opioid risks is essential for safe use. In contrast to these studies, we addressed both appropriate and inappropriate analgesic use by tailoring education to specific situations. Our finding that scenario-tailoring led to better overall decision competency is encouraging that specific risk feedback combined with guidance about what to do is better than typical or routine analgesic information. From this pilot, it is unclear whether the effects of the STOMP intervention last longer than three days. Future studies should address whether a single dose of information is sufficient to maintain ADE understanding over an entire course of analgesic use or need.
Improving parental opioid risk knowledge and analgesic competency may also be an important measure to minimize adolescent misuse. Approximately 1 in 4 older adolescents have reported using opioids either medically or non-medically, and 80% of those who reported misuse had done so with their own previously prescribed drug.47 Misuse and self-treatment with prescription drugs has been, in part, attributed to perceptions that prescribed drugs are safe.48 Such attitudes and analgesic knowledge are influenced primarily by parents who are considered role-models for both appropriate analgesic use and inappropriate or misuse.49,50 A majority of children who self-treat their pain with analgesics exhibit poor knowledge about these drugs and most had obtained their information from a parent.51 Improving parental opioid risk understanding may, therefore, improve adolescent’s understanding as well.
Limitations
The ability to generalize our findings are somewhat limited by the sample that included volunteer parents in a tertiary care clinic setting whose children were largely healthy at the time of the survey and intervention. Given that opioid familiarity (recent or current use or presence in the home) did not significantly impact our findings suggests that the STOMP™ will have a similar effect for parents of children being treated with a prescribed opioid for a painful condition or following a painful procedure. The strong effect of parents’ preference to relieve their children‘s pain cannot be overlooked, as we previously found that this preference can diminish the effect of risk knowledge on safe decision-making.27 The finding that the STOMP™ intervention succeeded in both shifting parental preferences, enhancing risk perceptions and improving decisions is encouraging. A potential effect of scenario ordering on parental decision making cannot be dismissed given that all parents in both groups received the same scenarios in the same order with the highest risk scenario first. A carry-over effect could have dampened the number of parents who chose to give an opioid to the low risk (i.e., no ADE scenario). Further testing in a sample whose children are prescribed an opioid for a painful condition/event is warranted to see if these results hold steadfast. It remains unknown whether additional risk messages such as the risk of diversion or misuse with important disposal informational feedback will have a similar and positive effect on parental behaviors. However, our STOMP™ modular design is designed to be adaptable to varying types and numbers of risk messages that can be conveyed to a variety of parents or patients over time.
Summary
In summary, the current study suggests that it is possible with an interactive, scenario-tailored risk messaging to enhance opioid risk knowledge, change parental preferences, and improve their safe and effective decision-making competency. It will remain important to examine how such analgesic education will affect pain outcomes for children and adolescents who are prescribed opioids for a painful condition or procedure. Until further research is available, findings here could be used to guide specific and important opioid safety messages for parent education.
Supplementary Material
Acknowledgments
Funding: The work of Dr. Sean Esteban McCabe was supported by research grants R01DA031160, R01DA036541 and R01CA203809.
Contributor Information
Terri Voepel-Lewis, Department of Anesthesiology, University of Michigan, Ann Arbor MI 48109-4245.
Brian J. Zikmund-Fisher, Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor MI 48109-4245.
Carol J. Boyd, Institute for Research on Women and Gender, University of Michigan, Ann Arbor MI 48109-4245.
Philip T. Veliz, Institute for Research on Women and Gender, University of Michigan, Ann Arbor MI 48109-4245.
Sean Esteban McCabe, Institute for Research on Women and Gender, University of Michigan, Ann Arbor MI 48109-4245.
Monica Weber, Department of Anesthesiology, University of Michigan, Ann Arbor MI 48109-4245.
Alan R. Tait, Anesthesiology, University of Michigan, Ann Arbor MI 48109-4245.
References
- 1.Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 2.Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington DC: Institute of Medicine; Jun, 2011. Committee on Advancing Pain Research Care, and Education. [Google Scholar]
- 3.Paulozzi LJ, Strickler GK, Kreiner PW, et al. Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill Summ. 2015;64:1–14. doi: 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Injury Prevention and Control. Vital Signs: Opioid Painkiller Prescribing. Atlanta: Centers for Disease Control; Jul, 2014. [Google Scholar]
- 5.Levy B, Paulozzi L, Mack KA, et al. Trends in Opioid Analgesic-Prescribing Rates by Specialty, U.S., 2007–2012. Am J Prev Med. 2015;49:409–413. doi: 10.1016/j.amepre.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortuna RJ, Robbins BW, Caiola E, et al. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics. 2010;126:1108–1116. doi: 10.1542/peds.2010-0791. [DOI] [PubMed] [Google Scholar]
- 7.Groenewald CB, Rabbitts JA, Gebert JT, et al. Trends in opioid prescriptions among children and adolescents in the United States: a nationally representative study from 1996 to 2012. Pain. 2016;157:1021–1027. doi: 10.1097/j.pain.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulozzi LJ. Vital Signs: Overdoses of Prescription Opioid Pain Relievers - United States 1999–2008. Atlanta: Centers for Disease Control; 2011. [PubMed] [Google Scholar]
- 9.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug and Alcohol Dependence. 2013;131:263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dart RC, Surratt HL, Cicero TJ, et al. Trends in Opioid Analgesic Abuse and Mortality in the United States. New England Journal of Medicine. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JE, Campagna E, Dart RC. The underrecognized toll of prescription opioid abuse on young children. Ann emerg medicine. 2009;53:419–424. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 12.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Fouladbakhsh JM, Vallerand AH, Jenuwine ES. Self-treatment of pain among adolescents in an urban community. Pain Manag Nurs. 2012;13:80–93. doi: 10.1016/j.pmn.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012;160:265–270. e261. doi: 10.1016/j.jpeds.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. Jama. 2011;305:1299–1301. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burghardt LC, Ayers JW, Brownstein JS, et al. Adult prescription drug use and pediatric medication exposures and poisonings. Pediatrics. 2013;132:18–27. doi: 10.1542/peds.2012-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrist J, Ballesteros M. Vital signs: Unintentional injury deaths among persons aged 0–19 years -United States, 2000–2009. Atlanta: Centers for Disease Control; Apr 16, 2012. [Google Scholar]
- 18.Cohen AL, Budnitz DS, Weidenbach KN, et al. National surveillance of emergency department visits for outpatient adverse drug events in children and adolescents. J Pediatr. 2008;152:416–421. doi: 10.1016/j.jpeds.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Tadros A, Layman SM, Davis SM, et al. Emergency department visits by pediatric patients for poisoning by prescription opioids. Am J Drug Alcohol Abuse. 2016;42:550–555. doi: 10.1080/00952990.2016.1194851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Substance Abuse and Mental Health Services AdministrationS. The CBHSQ Report. Rockville, MD: Center for Behavioral Health Statistics and Quality; Jun 10, 2014. [Google Scholar]
- 21.Chung CP, Callahan ST, Cooper WO, et al. Development of an algorithm to identify serious opioid toxicity in children. BMC Res Notes. 2015;8:293. doi: 10.1186/s13104-015-1185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miech R, Johnston L, O'Malley PM, et al. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics. 2015;136:e1169–1177. doi: 10.1542/peds.2015-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rony RY, Fortier MA, Chorney JM, et al. Parental postoperative pain management: attitudes, assessment, and management. Pediatrics. 2010;125:e1372–1378. doi: 10.1542/peds.2009-2632. [DOI] [PubMed] [Google Scholar]
- 24.Hamers JP, Abu-Saad HH. Children's pain at home following (adeno) tonsillectomy. Eur J Pain. 2002;6:213–219. doi: 10.1053/eujp.2001.0326. [DOI] [PubMed] [Google Scholar]
- 25.Unsworth V, Franck LS, Choonara I. Parental assessment and management of children's postoperative pain: a randomized clinical trial. J Child Health Care. 2007;11:186–194. doi: 10.1177/1367493507079558. [DOI] [PubMed] [Google Scholar]
- 26.Voepel-Lewis T, Zikmund-Fisher B, Smith EL, et al. Opioid-related adverse drug events: do parents recognize the signals? Clin J Pain. 2015;31:198–205. doi: 10.1097/AJP.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 27.Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, et al. Parents' preferences strongly influence their decisions to withhold prescribed opioids when faced with analgesic trade-off dilemmas for children: a prospective observational study. Int J Nurs Stud. 2015;52:1343–1353. doi: 10.1016/j.ijnurstu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Kelly LE, Rieder M, van den Anker J, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343–1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 29.Madadi P, Hildebrandt D, Gong IY, et al. Fatal hydrocodone overdose in a child: pharmacogenetics and drug interactions. Pediatrics. 2010;126:e986–989. doi: 10.1542/peds.2009-1907. [DOI] [PubMed] [Google Scholar]
- 30.Coté CJ, Posner KL, Domino KB. Death or Neurologic Injury after Tonsillectomy in Children with a Focus on Obstructive Sleep Apnea: Houston, We Have a Problem! Anesth Analg. 2014;118:1276–1283. doi: 10.1213/ANE.0b013e318294fc47. [DOI] [PubMed] [Google Scholar]
- 31.Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, et al. Parents' Analgesic Trade-Off Dilemmas: How Analgesic Knowledge Influences Their Decisions to Give Opioids. Clin J Pain. 2016;32:187–195. doi: 10.1097/AJP.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 32.Manworren RC, McElligott CD, Deraska PV, et al. Efficacy of Analgesic Treatments to Manage Children's Postoperative Pain After Laparoscopic Appendectomy: Retrospective Medical Record Review. AORN J. 2016;103:317, e311–311. doi: 10.1016/j.aorn.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Gordon DB, de Leon-Casasola OA, Wu CL, et al. Research Gaps in Practice Guidelines for Acute Postoperative Pain Management in Adults: Findings From a Review of the Evidence for an American Pain Society Clinical Practice Guideline. J Pain. 2016;17:158–166. doi: 10.1016/j.jpain.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. J Psychosom Res. 2008;64:41–46. doi: 10.1016/j.jpsychores.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Johnson FR, Hauber AB. Quantifying patient benefit-risk tradeoff preferences: A brief introduction. Research Triangle Park: RTI Health Solutions; 2008. [Google Scholar]
- 37.Johnson FR, Hauber AB, Poulos CM. A brief introduction to the use of stated-choice methods to measure preferences for treatment benefits and risks. Research Triangle Park: Research Triangle Institute; 2009. [Google Scholar]
- 38.Bailey L, Sun J, Courtney M, et al. Improving postoperative tonsillectomy pain management in children – A double blinded randomised control trial of a patient analgesia information sheet. International J Pediatr Otorhinolaryn. 2015;79:732–739. doi: 10.1016/j.ijporl.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Huth MM, Broome ME, Mussatto KA, et al. A study of the effectiveness of a pain management education booklet for parents of children having cardiac surgery. Pain Manag Nurs. 2003;4:31–39. doi: 10.1053/jpmn.2003.7. [DOI] [PubMed] [Google Scholar]
- 40.Sutters KA, Holdridge-Zeuner D, Waite S, et al. A descriptive feasibility study to evaluate scheduled oral analgesic dosing at home for the management of postoperative pain in preschool children following tonsillectomy. Pain Med. 2012;13:472–483. doi: 10.1111/j.1526-4637.2011.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutters KA, Miaskowski C, Holdridge-Zeuner D, et al. A randomized clinical trial of the effectiveness of a scheduled oral analgesic dosing regimen for the management of postoperative pain in children following tonsillectomy. Pain. 2004;110:49–55. doi: 10.1016/j.pain.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Sutters KA, Miaskowski C, Holdridge-Zeuner D, et al. A randomized clinical trial of the efficacy of scheduled dosing of acetaminophen and hydrocodone for the management of postoperative pain in children after tonsillectomy. Clin J Pain. 2010;26:95–103. doi: 10.1097/AJP.0b013e3181b85f98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosales A, Fortier MA, Campos B, et al. Postoperative pain management in Latino families: parent beliefs about analgesics predict analgesic doses provided to children. Paediatr Anaesth. 2016;26:307–314. doi: 10.1111/pan.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 45.Sadhasivam S, Myer CM., 3rd Preventing opioid-related deaths in children undergoing surgery. Pain Med. 2012;13:982–983. doi: 10.1111/j.1526-4637.2012.01419.x. [DOI] [PubMed] [Google Scholar]
- 46.Subramanyam R, Chidambaran V, Ding L, et al. Anesthesia- and opioids-related malpractice claims following tonsillectomy in USA: LexisNexis claims database 1984–2012. Paediatr Anaesth. 2014;24:412–420. doi: 10.1111/pan.12342. [DOI] [PubMed] [Google Scholar]
- 47.McCabe SE, West BT, Teter CJ, et al. Medical and nonmedical use of prescription opioids among high school seniors in the United States. Arch Pediatr Adolesc Med. 2012;166:797–802. doi: 10.1001/archpediatrics.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young AM, Glover N, Havens JR. Nonmedical use of prescription medications among adolescents in the United States: a systematic review. J Adolesc Health. 2012;51:6–17. doi: 10.1016/j.jadohealth.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Holmstrom IK, Bastholm-Rahmner P, Bernsten C, et al. Swedish teenagers and over-the-counter analgesics - responsible, casual or careless use. Res Social Adm Pharm. 2014;10:408–418. doi: 10.1016/j.sapharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Skarstein S, Lagerlov P, Kvarme LG, et al. High use of over-the-counter analgesic; possible warnings of reduced quality of life in adolescents - a qualitative study. BMC Nurs. 2016;15:16. doi: 10.1186/s12912-016-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shehnaz SI, Khan N, Sreedharan J, et al. Drug knowledge of expatriate adolescents in the United Arab Emirates and their attitudes towards self-medication. Int J Adolesc Med Health. 2014;26:423–431. doi: 10.1515/ijamh-2013-0315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.