Abstract
Purpose
We aimed to determine whether conventional standardized EEG features could be consolidated into a more limited number of factors and whether the derived factor scores changed during the acute period after pediatric cardiac arrest.
Methods
Children resuscitated after cardiac arrest underwent conventional continuous EEG monitoring. The EEG was scored in 12-hour epochs for up to 72-hours after return of circulation by an electroencephalographer using standardized critical care EEG terminology. We performed a polychoric factor analysis to determine whether numerous observed EEG features could be represented by a smaller number of derived factors. Linear mixed-effects regression models and heat maps evaluated whether the factor scores remained stable across epochs.
Results
We performed EEG monitoring in 89 consecutive children which yielded 453 EEG segments. We identified two factors which were not correlated. The Background Features Factor loaded with the features continuity, voltage, and frequency. The Intermittent Features Factor loaded with the features of seizures, periodic patterns, and inter-ictal discharges. Factor scores were calculated for each EEG segment. Linear mixed-effect regression results indicated that the factor scores did not change over time for the Background Features Factor (coefficient 0.18, 95%CI 0.04–0.07, p=0.52) or the Intermittent Features Factor (coefficient −0.003, 95%CI −0.02-0.01, p=0.70). However, heat maps showed that some individual subjects did experience factor score changes over time, particularly if they had medium initial factor scores.
Conclusions
Subsequent studies assessing whether EEG is informative for neurobehavioral outcomes after pediatric cardiac arrest could combine numerous EEG features into two factors, each reflecting multiple Background and Intermittent features. Further, the factor scores would be expected to remain stable during the acute period for most subjects.
Keywords: EEG, cardiac arrest, pediatric
Introduction
Among children resuscitated from cardiac arrest, clinical and resuscitation characteristics do not directly asses brain function, likely explaining why these variables are only moderately predictive of long-term outcomes.1–5 In contrast, an electroencephalogram (EEG) directly assesses brain activity and is often acquired early after cardiac arrest to identify EEG-only seizures.6,7 Furthermore, several EEG features predict short-term gross neurologic outcomes,6,8–13 and knowledge of EEG data improves prognostication accuracy by neurologists and intensivists.14 However, numerous EEG features defined by the American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology15 can be assessed, and it is unclear which features should be selected as exposure variables for prospective studies assessing EEG features as biomarkers of hypoxic-ischemic brain injury severity.
Factor analysis is a statistical method for data reduction that seeks to discover whether observed variables, some of which are correlated, can be explained by a lower number of unobserved latent variables called “factors”. If the observed variables have similar response patterns because they are associated with an unobserved underlying construct, then the derived factors could be included as the EEG exposure variables in subsequent studies assessing EEG as a biomarker of brain injury severity. As a result, this method could reduce a dataset containing a large number of potentially collinear EEG features to a smaller set of derived factors.
In this study, we aimed to determine whether: (1) standardized critical care EEG features15 could be consolidated into a more limited number of derived factors, and (2) the derived factor scores changed across 12-hour epochs during the acute period after pediatric cardiac arrest for each subject.
Methods
We included consecutively recorded EEGs from children resuscitated after cardiac arrest at the Children’s Hospital of Philadelphia who were enrolled in an Institutional Review Board approved prospective observational study of pediatric cardiac arrest. Continuous conventional full-array EEG monitoring was initiated as soon as possible after resuscitation based on an institutional pathway16 derived from recent guidelines and consensus statements.7,17,18 EEG monitoring was performed with Grass-Telefactor video EEG equipment and the international 10-20 montage with modification for neonates as needed.
A pediatric electroencephalographer blind to all clinical information evaluated the first 10-minute long EEG segment from each of up to seven epochs. The EEG was assessed at the earliest time available after resuscitation and at up to six subsequent time points relative to the time of return of circulation (12, 24, 36, 48, 60, and 72 hours). All clinical annotations in the EEG tracing were removed prior to review. Video was not available, but the electroencephalographer could adjust the montage, filters, and voltage settings. The electroencephalographer performed scoring using the American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology.15 Scoring included the categorical EEG features continuity (continuous, nearly continuous with attenuation, nearly continuous with suppression, discontinuous with attenuation, discontinuous with suppression, burst suppression, burst attenuation, and suppression), voltage (normal, low, suppressed), frequency (contains alpha, contains theta, contains delta, attenuated), seizures (present, absent), periodic patterns (present, absent), and inter-ictal epileptic discharges (present, absent). Scoring was performed using an electronic case report form in the web-based electronic data application Research Electronic Data Capture (REDCap)19 which ensured there were no missing data.
We performed statistical analyses using Stata version 12 (College Station, Texas). We used standard descriptive statistics to summarize the data as medians with interquartile ranges (IQR) or counts (with percentages) as appropriate. We performed a polychoric factor analysis20,21 since the observed EEG variables were all categorical. We derived the factors from the EEG segments and performed an oblique rotation to allow the factors to be correlated. To assess whether the factors changed over time for the overall cohort, we performed linear mixed-effects regression for each of the factors. This method takes into account the within-subject correlation due to repeated measures by including subject-specific random effects. We created heat maps to visualize the factor scores over time for each individual subject.
We performed two sensitivity analyses. First, to determine whether different factors would have been created if we included only individual timepoints rather than all timepoints for each patient, we repeated the factor analysis described above for the initial, 12-hour, 24-hour, and 36-hour timepoints. At each of these timepoints each subject contributed only one set of observed EEG values. Second, to determine whether different factors would have been created if we had used the scoring from different pediatric electroencephalographers, we repeated the factor analysis for the 12-hour timepoint with the EEG features assessed by three additional pediatric electroencephalographers.
Results
The study included 89 subjects who experienced cardiac arrests between August 2012 and April 2016. Table 1 provides the demographic and clinical data from this cohort. The median patient age was 2.1 years (IQR 0.27, 9.1 years). The median duration of cardiopulmonary resuscitation was 10 minutes (IQR 4, 20 minutes), and 58 (65%) subjects had in-hospital cardiac arrests. The initial EEG was recorded a median of 7.0 hours (IQR 4.4, 11.4 hours) after return of circulation.
Table 1.
Subject characteristics (N=89)
| Clinical Variable | N (%) or Median [IQR] |
|---|---|
| Age at Arrest (years) | 2.1 [0.27, 9.1] |
|
| |
| Sex: Male | 56 (62%) |
|
| |
| Race | |
| White | 46 (52%) |
| Black | 19 (21%) |
| Other | 24 (27%) |
|
| |
| Hispanic | 14 (16%) |
|
| |
| Pre-arrest Pediatric Cerebral Performance Category Score 1 = Normal 2 = Mild Disability 3 = Moderate Disability 4 = Severe Disability 5 = Coma or Vegetative State |
58 (65%) 12 (13%) 8 (9%) 9 (10%) 2 (2%) |
|
| |
| Cardiac Arrest Location In-Hospital Out-of-Hospital |
58 (65%) 31 (35%) |
|
| |
| Cardiopulmonary Resuscitation duration (minutes) (n=70) | 10 [4, 20] |
|
| |
| Induced Hypothermia | 10 (11%) |
|
| |
| Benzodiazepine Infusion | 69 (79%) |
|
| |
| Time from Cardiac Arrest to Electroencephalogram Initiation (hours) | 7 [4.4, 11.4] |
|
| |
| Pre-arrest Pediatric Cerebral Performance Category Score 1 = Normal 2 = Mild Disability 3 = Moderate Disability 4 = Severe Disability 5 = Coma or Vegetative State |
16 (18%) 16 (18%) 11 (12%) 16 (18%) 30 (34%) |
The 89 subjects each had multiple EEG assessments at successive timepoints, yielding a total of 453 EEG segments. We identified correlations among the observed EEG features of continuity, voltage, and frequency (0.64-0.76), thereby suggesting a first construct we called the Background Features Factor. Similarly, we identified correlations among the observed EEG features of seizures, periodic patterns, and inter-ictal epileptiform discharges (0.21-0.29), thereby suggesting a second construct we called the Intermittent Features Factor.
Since the observed EEG features were categorical, we performed a polychoric factor analysis to determine whether the observed EEG features could be represented by single constructs (i.e. factors). Two factors had eigenvalues of > 1 (Background Features Factor = 2.6 and Intermittent Features Factor = 1.6), and these two factors were retained given Kaiser’s criterion. The Background Features Factor explained 63% of the variance of the data, and the Intermittent Features Factor explained 39% of the variance of the data. The resulting factors were not correlated (correlation coefficient = −0.02). A third factor had an eigenvalue of 0.23 and only explained 5% of the variance of the data, so it was not studied further. We identified observed EEG features with loading factors of >0.4 for each factor. The Background Features Factor loaded with the EEG features continuity (0.84), voltage (0.94), and frequency (0.88) (Figure 1). The Intermittent Features Factor loaded with the EEG features seizures (0.68), periodic patterns (0.79), and inter-ictal epileptiform discharges (0.70) (Figure 1). Each of the variables had a uniqueness of <0.6 indicating that each variable was reasonably explained by the factors.
Figure 1.
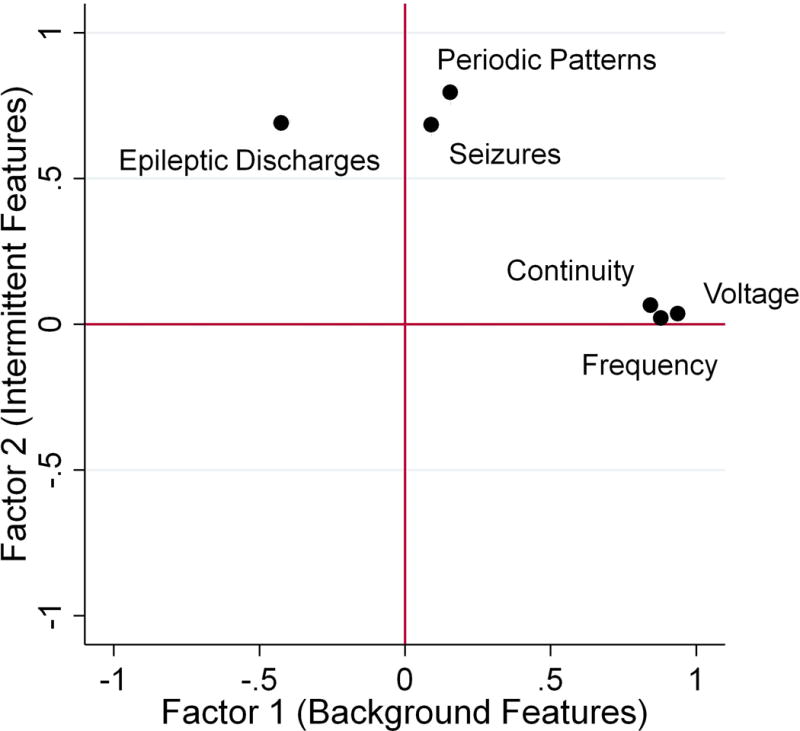
Factor loadings plot indicates that the Background Features Factor contains tightly clustered observed EEG features of continuity, voltage, and frequency while the Intermittent Features Factor contains the slightly less clustered observed EEG features of seizures, periodic patterns, and inter-ictal epileptiform discharges.
The first sensitivity analysis in which EEG segments were evaluated by individual timepoints found that the same two factors emerged with similar eigenvalues, loading factors, and uniqueness assessments as the primary analysis that included all timepoints. For example, for the initial EEG timepoint which included EEG assessments from 88 subjects, the eigenvalues were 2.7 for the Background Features Factor and 2.1 for the Intermittent Features Factor. The Background Features Factor loaded with continuity (0.90), voltage (0.89), and frequency (0.86) while the Intermittent Features Factor loaded with seizures (0.87), periodic patterns (0.87), and inter-ictal epileptiform discharges (0.69). Each of the variables had a uniqueness of <0.6. Similarly, the second sensitivity analysis of four electroencephalographers for 72 records at the 12-hour timepoint resulted in the same two factors with similar eigenvalues, loading factors, and uniqueness assessments as the primary the primary analysis that included all timepoints.
Using the primary factor analysis from all 453 EEG assessments, we derived the two factor scores for each EEG segment. Each of the factor scores could be calculated as the sum over each EEG feature score multiplying by the corresponding scoring coefficient. The scoring coefficients provided in Table 2 indicate how each factor score was calculated for a given subject. The EEG features are the F functions. The loading factors derived from the analysis are the L constants. Using the F and L values, one can calculate factor score S for an epoch of EEG in which the size of each loading factor for each EEG feature measures how much that specific feature is related to S. Within this framework, the equation to calculate each of the factor scores for a segment of EEG is represented by the equation S = FcontinuityLcontinuity + FvoltageLvoltage + FfrequencyLfrequency + FseizuresLseizures + FperiodicLperiodic + FiedLied.
Table 2.
EEG Feature Score and Loading Factors.
| EEG Feature | EEG Feature Score | Background Features Factor Loading Factor | Intermittent Features Factor Loading Factor |
|---|---|---|---|
| Continuity | 1 Continuous 2 Nearly Continuous with Attenuation 3 Nearly Continuous with Suppression 4 Discontinuous with Attenuation 5 Discontinuous with Suppression 6 Burst-Suppression 7 Burst-Attenuation 8 Suppression |
0.1998 | 0.0613 |
| Voltage | 0 Normal 1 Low 2 Suppressed |
0.5961 | −0.0037 |
| Frequency | 0 Contains Alpha 1 Contains Theta 2 Contains Delta 3 Attenuated |
0.2344 | −0.0125 |
| Seizures | 0 Absent 1 Present |
0.0244 | 0.2076 |
| Periodic Patterns | 0 Absent 1 Present |
−0.0401 | 0.4790 |
| Inter-ictal Epileptiform Discharges | 0 Absent 1 Present |
−0.0033 | 0.4059 |
The linear mixed-effect regression results indicated that the EEG did not change over time for the Background Features Factor (coefficient 0.18, 95% CI 0.04 – 0.07, p=0.52) or the Intermittent Features Factor (coefficient −0.003, 95% CI −0.02-0.01, p=0.70). Figure 2 provides heat maps for each factor to assess for changes in factor scores for individual subjects over time. The plots indicated that while the linear mixed-effects regression identified no statistically significant change over time for the cohort, some subjects did change factor scores over time, particularly if they had medium initial factor values for the Background Features Factor (score of 1.5 to 3) or the Intermittent Features Factor (score of 0.3 to 0.5).
Figure 2.
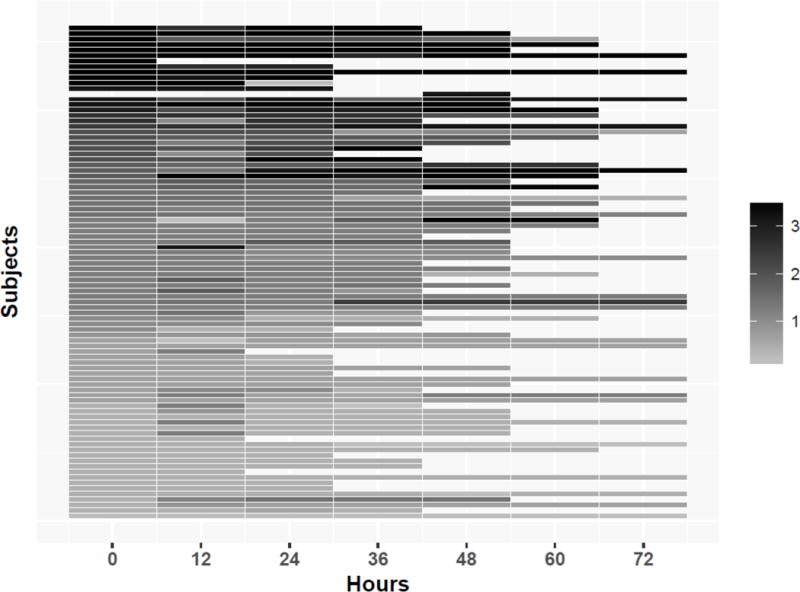
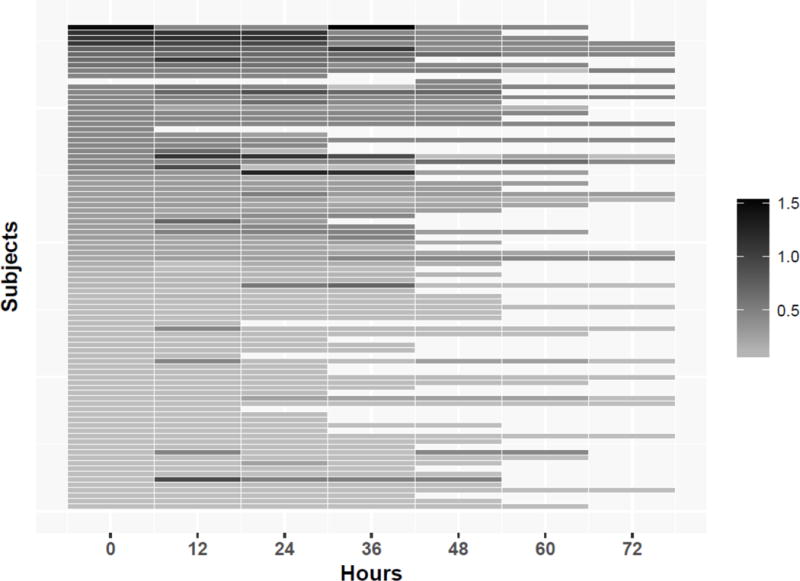
Heatmap of the factor score for each subject over time for the (a) Background Features Factor and (b) Intermittent Features Factor.
Discussion
These data indicate that standardized assessments of observed EEG features from children resuscitated from cardiac arrest can be reduced to two derived factors reflecting Background Features (continuity, voltage, frequency) and Intermittent Features (seizures, periodic patterns, and inter-ictal epileptiform discharges). There were no statistically significant changes in either factor score over time for the full cohort. However, subject level data indicated that some subjects did have factor score changes over time, particularly for middle values for both factors. The stability of the factor scores over time for most subjects indicates that future studies assessing EEG as a biomarker of brain injury might rely on EEG assessed over a broad time window since few subjects would experience changes in their factor scores over time. However, for neuroprognostication in individual patients, repeated EEG assessments over time may be required, particularly for patients with middle scores of the Background Features Factor since these can improve or worsen, potentially leading to overly favorable or unfavorable predictions based on an initial EEG assessment.
We have previously shown that that assessments of the Overall Background Category by four pediatric electroencephalographers had nearly-perfect interrater agreement,22 a finding consistent with prior literature indicating that there is higher reproducibility for broader interpretive categories than more narrow EEG features in both children and adults.23–33 The Overall Background Category is a categorical variable that scores the EEG background as normal, slow-disorganized, discontinuous or burst-suppression, or attenuated-featureless. It has been used in proposed pediatric EEG prognostication systems.6,8–13 However, this variable requires an electroencephalographer to combine visual assessments of continuity, voltage, and frequency to score the EEG. In contrast, combining these correlated observed EEG features (continuity, voltage, and frequency) into a standardized factor has several advantages for future studies. Future studies assessing EEG as a biomarker of early brain injury severity would not need to arbitrarily select between the numerous correlated EEG features as primary exposure variables. Instead, these studies could assess the Background Features Factor as the primary exposure variable. This single EEG variable would contain information about three EEG characteristics (continuity, voltage, frequency) in an objective and reproducible manner. This approach would require fewer comparisons than if each EEG feature were assessed independently, thereby reducing the multiplicity problem that would occur if each EEG feature were assessed separately. Thus, an appropriately powered study could be performed with fewer subjects, which is important given pediatric cardiac arrest is relatively uncommon. Additionally, in the future, the EEG features used to derive the Background Features Factor might be assessed quantitatively. This would allow for objective assessment of brain function in critical care settings as real-time measures of brain function without the need for continual electroencephalographer availability. Potentially, quantitatively derived Background Features Factor scores could be displayed at bedside, so intensivists could objectively stratify patients based on brain injury severity for neuroprotective interventions and track brain function over time.
This study has several strengths. First, the EEG tracings were obtained from a large cohort of consecutive children resuscitated after cardiac arrest, and therefore the EEG assessments represent the full spectrum and true prevalence of the various EEG features. Second, the reviewer could modify any settings which mimics real-world EEG reading. Third, the sensitivity analyses indicated that variations in the EEG data used to derive the factors did not substantially impact the factors we identified. This study also has limitations. First, the EEG epochs used for the main analyses were only assessed by one pediatric electroencephalographer. We performed a sensitivity analysis in which we compared four electroencephalographer scores which were available for the 12-hour timepoint and found the same factors were identified. However, this sensitivity analysis was done on a limited number of observed EEG features and might be assessed in a larger dataset in the future. Second, the Background Features Factor had higher factor loading scores and greater stability over time than the Intermittent Features Factor. This is logical since the background features would be expected to be more stable than intermittent features. Future studies might combine the background features (continuity, voltage, frequency) into one derived factor while retaining epileptiform discharges, periodic patterns, and seizures as individual EEG features. Finally, it remains to be determined whether the derived factors are associated with neurobehavioral outcomes.
Overall, these data indicate that subsequent studies assessing EEG after pediatric cardiac arrest for early brain injury stratification or neuroprognostication could combine numerous EEG features into a more limited number of derived factors, thereby reducing the dataset for analysis while also including information contained in numerous EEG features. Further, the factor scores would be expected to remain stable across 12-hour epochs during the acute period for large cohorts, potentially allowing inclusion of EEG obtained over a wide time window. However, for individual subjects, and particularly those with middle scores for the Background Features Factor, the scores could change over time, thereby indicating that repeated EEG assessments might lead to higher accuracy for brain injury severity stratification and neuroprognostication.
References
- 1.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–9. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 2.Topjian AA, Clark AE, Casper TC, et al. Early lactate elevations following resuscitation from pediatric cardiac arrest are associated with increased mortality*. Pediatr Crit Care Med. 2013;14:e380–7. doi: 10.1097/PCC.0b013e3182976402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starling RM, Shekdar K, Licht D, Nadkarni VM, Berg RA, Topjian AA. Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest. Pediatr Crit Care Med. 2015;16:542–8. doi: 10.1097/PCC.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42:1518–23. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med. 2015;16:146–54. doi: 10.1097/PCC.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–40. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler SK, Topjian AA, Gutierrez-Colina AM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2011;14:37–43. doi: 10.1007/s12028-010-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav. 2015;49:238–44. doi: 10.1016/j.yebeh.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topjian AA, Sanchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest. Pediatr Crit Care Med. 2016;17:547–57. doi: 10.1097/PCC.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostendorf AP, Hartman ME, Friess SH. Early Electroencephalographic Findings Correlate With Neurologic Outcome in Children Following Cardiac Arrest. Pediatr Crit Care Med. 2016;17:667–76. doi: 10.1097/PCC.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschen MP, Topjian AA, Hammond R, Illes J, Abend NS. Neuroprognostication after pediatric cardiac arrest. Pediatr Neurol. 2014;51:663–8 e2. doi: 10.1016/j.pediatrneurol.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 16.The Children’s Hospital of Philadelphia, Critical Care Pathway for EEG Monitoring. 2016 (Accessed February 1, 2016, 2016 at http://www.chop.edu/clinical-pathway/critical-care-pathway-eeg-monitoring-clinical-pathways.)
- 17.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 18.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holgado-Tello FP, S C-M, Barbero-Garcia I, Vila-Abad E. Polychoric versus Pearson correlations in exploratory and confirmatory factor analysis of ordinal variables. Quality and Quantity. 2010;44 [Google Scholar]
- 21.How can I perform a factor analysis with categorical (or categorical and continuous) variables? Stata FAQ. (Accessed November 20, 2017, at https://stats.idre.ucla.edu/stata/faq/how-can-i-perform-a-factor-analysis-with-categorical-or-categorical-and-continuous-variables/.)
- 22.Abend NS, Massey SL, Fitzgerald M, et al. Interrater Agreement of EEG Interpretation after Pediatric Cardiac Arrest Utilizing Standardized Critical Care EEG Terminology. Journal of Clinical Neurophysiology. doi: 10.1097/WNP.0000000000000424. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroink H, Schimsheimer RJ, de Weerd AW, et al. Interobserver reliability of visual interpretation of electroencephalograms in children with newly diagnosed seizures. Dev Med Child Neurol. 2006;48:374–7. doi: 10.1017/S0012162206000806. [DOI] [PubMed] [Google Scholar]
- 24.Piccinelli P, Viri M, Zucca C, et al. Inter-rater reliability of the EEG reading in patients with childhood idiopathic epilepsy. Epilepsy Res. 2005;66:195–8. doi: 10.1016/j.eplepsyres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Azuma H, Hori S, Nakanishi M, Fujimoto S, Ichikawa N, Furukawa TA. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin Neurosci. 2003;57:485–9. doi: 10.1046/j.1440-1819.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- 26.Little SC, Raffel SC. Intra-rater reliability of EEG interpretations. J Nerv Ment Dis. 1962;135:77–81. doi: 10.1097/00005053-196207000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Williams GW, Luders HO, Brickner A, Goormastic M, Klass DW. Interobserver variability in EEG interpretation. Neurology. 1985;35:1714–9. doi: 10.1212/wnl.35.12.1714. [DOI] [PubMed] [Google Scholar]
- 28.Gerber PA, Chapman KE, Chung SS, et al. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophysiol. 2008;25:241–9. doi: 10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 29.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol. 1988;5:161–74. doi: 10.1097/00004691-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Young GB, McLachlan RS, Kreeft JH, Demelo JD. An electroencephalographic classification for coma. Can J Neurol Sci. 1997;24:320–5. doi: 10.1017/s0317167100032996. [DOI] [PubMed] [Google Scholar]
- 31.Westhall E, Rosen I, Rossetti AO, et al. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol. 2015;126:2397–404. doi: 10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Ronner HE, Ponten SC, Stam CJ, Uitdehaag BM. Inter-observer variability of the EEG diagnosis of seizures in comatose patients. Seizure. 2009;18:257–63. doi: 10.1016/j.seizure.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Wusthoff CJ, Sullivan J, Glass HC, et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia. 2017;58:429–35. doi: 10.1111/epi.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]


