Abstract
Although small prior studies have suggested that IgE can be low in common variable immunodeficiency (CVID), the work-up for patients with recurrent infections and suspected hypogammaglobulinemia does not include the routine measurement of serum IgE. We sought to test the hypothesis that low/undetectable serum IgE is characteristic of CVID by comparing the frequency of low/undetectable serum IgE in healthy controls and patients with CVID. We measured total serum IgE in a large multi-center cohort of patients with CVID (n=354) and compared this to large population-based cohorts of children and adults. We further compared IgE levels in patients with CVID to those with other forms of humoral immunodeficiency, and in a subset, measured levels of allergen-specific serum IgE and IgG subclasses. Lastly, we evaluated for the presence of IgE in commercially available immunoglobulin replacement products (IgRT). An undetectable serum IgE (<2 IU/ml) occurs in only 3.3% (95% CI, 1.9–5.7%) of the general population. In contrast, an undetectable IgE occurs in 75.6% (95% CI, 65.6–85.7%) of patients with CVID. Conversely, a high IgE (>180 IU/ml) is very uncommon in CVID (0.3% of patients). IgE is >2 IU/ml in 91.2% of patients with secondary hypogammaglobulinemia and thus an IgE<LLOD is suggestive of a primary humoral immunodeficiency. Allergen specific IgE is not detectable in 96.5% of patients with CVID. Sufficient quantities of IgE to change the total serum IgE are not contained in IgRT. IgG1/IgG4 ratio is increased in subjects with low IgE, regardless of whether they are controls or have CVID. These findings support the routine measurement of serum IgE in the work-up of patients with hypogammaglobulinemia.
Keywords: Common variable immunodeficiency, IgE, IgE deficiency, secondary hypogammaglobulinemia
INTRODUCTION
The differential diagnosis of a patient with recurrent sinopulmonary and cutaneous infections includes primary immunodeficiency disease (PIDD), secondary immunodeficiency, anatomic abnormalities, and underlying atopy. Common variable immunodeficiency (CVID) is the most common life-threatening PIDD, characterized by antibody deficiency, recurrent infections and complications including autoimmunity, lymphoproliferation, and hematologic malignancy [1, 2]. Current published diagnostic criteria for CVID vary slightly but include: (1) reduction in serum immunoglobulin (Ig)G by greater than 2 standard deviations below normal; (2) reduction in IgA and/or IgM; (3) abnormal specific antibody production; (4) exclusion of other defined causes of hypogammaglobulinemia; and (5) age greater than 4 years [3]. However, considerable variability exists in the interpretation of these criteria. A wide range of Ig levels exist among published CVID patients, with the majority having IgG levels at diagnosis of <450 mg/dl [4] and low or absent serum IgA but with IgM levels more variable [5, 6, 4]. Further complicating matters, the lower limit of normal for each Ig isotype varies by reference laboratory. The definition of a normal specific antibody response to vaccines, particularly the pneumococcal polysaccharide vaccine, is still widely debated and no evidence-based international consensus exists on this issue [1]. There is a need for less ambiguous, rapid, low-cost methods to distinguish CVID from milder forms of humoral PIDD or secondary hypogammaglobulinemia (e.g. due to immunosuppressant use or ongoing protein loss in the lymphatics, urine, or stool). This would prevent unnecessary delays in diagnosis and in the institution of Ig replacement therapy (IgRT), which when initiated early in the disease process has been shown to significantly reduce morbidity and mortality in CVID [7, 5]. This would also have the potential to reduce the overuse of IgRT, which is a finite and expensive resource for which demand continues to rise and therefore requires careful stewardship [8].
Total serum IgE level is not currently routinely measured as part of the work-up for patients with recurrent infections and serum IgE is not considered in establishing the diagnosis of CVID. This has been based on IgE concentrations in non-immunodeficient subjects being highly variable, due to the presence of atopic disease such as allergic rhinitis, asthma, food allergy and atopic dermatitis as well as other conditions including parasitic disease. However, prior studies of patients with CVID have demonstrated that the majority have a low serum IgE [9–16]. It is important to note that these previous studies were performed on small cohorts and nearly one third of the data were generated prior to the introduction of the ImmunoCAP solid phase sandwich immunoassay in 1989, which has greater sensitivity and validity than previously utilized tests. To test the hypothesis that serum IgE is a sensitive and specific marker of CVID, we measured the levels of total serum IgE in a large multi-center cohort of patients with CVID and compared these levels to those in large population-based cohorts of children and young adults. We further compared IgE levels in patients with CVID to those with other forms of humoral immunodeficiency, and in a subset, measured levels of allergen-specific serum IgE and IgG subclasses. Lastly, we evaluated for the presence of IgE in commercially available forms of IgRT.
METHODS
Patient cohorts
All human studies were approved by the relevant Institutional Review Board (IRB) (University of Virginia (UVA) IRB 13298, 19688, 18099, and 15596; Children’s Hospital of Wisconsin IRB 99682; Mount Sinai School of Medicine IRB; the regional ethics committee of Umea University; the National Center for Health Statistics IRB). Informed consent was provided by all human subjects.
The Obstructive Lung Disease in Northern Sweden (OLIN) cohort is a population-based cohort of 3430 school children from which a representative sample of 964 participants participated in a blood draw in 2007 (age 19 years)[17]. The National Health and Nutrition Examination Survey (NHANES) 2005–2006 survey included measurement of total serum IgE in participants age 6 years and older [18].
An “internal cohort” of 181 patients with CVID were identified by immunologists at the Icahn School of Medicine at Mount Sinai (CCR), the Medical College of Wisconsin (JMR, JV), and UVA (LB, MGL, TPM). The diagnosis of CVID was established in these patients using currently accepted diagnostic criteria [5, 3], and serum IgE levels were not used as part of this diagnosis. All sera were analyzed for total IgE levels and allergen specific IgE was measured in the subset of patients with a total serum IgE >0.5 IU/ml. Serum IgG subclasses were measured by a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory in the subset of patients for whom sufficient quantities of serum were available.
A retrospective chart review was performed on outpatients aged 12 years or older seen in the UVA Asthma, Allergy & Immunology Clinic between 7/1/2013 and 6/30/2016, with a visit ICD-9 or ICD-10 diagnosis code corresponding to CVID, X-linked agammaglobulinemia (XLA), or secondary hypogammaglobulinemia (excluding hematologic malignancy) and on whom a serum IgE had been measured on or after the date of their diagnosis of immunodeficiency. IgG subclasses prior to the initiation of IVIG were also recorded when available. The United States Immunodeficiency Network (USIDNET) maintains a registry of PIDD patients in the United States and Canada. When queried in March 2017, the USIDNET registry included 307 patients with a diagnosis of CVID on whom a serum IgE level was available. This patient list was pared to include 173 patients from centers not included in the initial cohort, age 12 years or older (to control for the age-related increase in serum Igs), with either a serum IgA or IgM below the normal range. Rates of physician-reported food allergy, drug allergy, environmental allergy/allergic rhinitis, asthma, eczema, enteropathy, lung disease, lymphoproliferation, granulomatous disease, malignancy and autoimmunity were recorded.
IgE measurements
Total and specific IgE antibody levels were measured using the ImmunoCAP 250 instrument per manufacturer’s instructions (Phadia Thermo-Fisher, Portage, MI). Results were expressed as international units (IU) per ml, with 1 IU=1 kU=2.4 ng IgE. The assay lower limit of detection (LLOD) was 2.00 IU/mL total IgE. Allergen specific IgE was measured to d2 Dermatophagoides farinae, e1 cat dander, i6 cockroach, tx1 tree mix, gx2 grass mix, and fx5 food mix.
In a subset of 9 patients, serum total IgE was measured immediately prior to and immediately following completion of intravenous (IV) Ig infusion. Total IgE levels in commercial Ig preparations were measured using ImmunoCAP as well as an enzyme linked immunosorbent assay (ELISA) per manufacturer’s instructions (Abcam, Cambridge MA). The assay LLOD was 0.27 IU/ml total IgE.
Statistical analysis
To account for oversampling, complex sampling methods, and non-response, weights and survey strata provided with the surveys were used for the NHANES analysis. Weighted prevalence estimates of undetectable and low IgE were calculated using a random effects model using the meta package in R [19]. For the meta-analysis, data from prior to 1989 were also excluded due to differences in assay technique. Between-group comparisons of categorical variables were conducted by Fisher’s exact test.
RESULTS
Frequency of low IgE in the general population
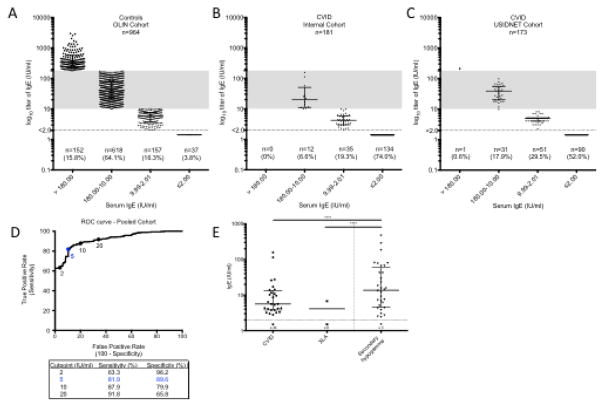
Using data from 964 Swedish students enrolled in the OLIN population-based cohort, the frequency of a total serum IgE <2.00 IU/ml (below the LLOD of the assay) was 3.8% (n=37) and the frequency of IgE <10.00 IU/ml (below the lower limit of normal (LLN) as defined by the UVA clinical laboratory) was 20.1% (n=194) (Fig 1A). Since the OLIN cohort includes participants living north of the Arctic Circle, in a cool dry climate less hospitable to common indoor allergens [17], it is plausible that there is an enhanced frequency of subjects with lower total IgE in this cohort. Therefore, to validate these findings in a North American cohort, we also queried the NHANES 2005–2006 database [20, 18], which includes total serum IgE data from a representative sample of individuals >6 years of age in the United States general population. The frequency of total serum IgE below the LLOD in NHANES 2005–2006 was 2.4% (95% CI 1.8–3.3). Based on a random effect meta-analysis with the results of other published large population-based cohorts (Table 1 and Supplemental Table S1), the prevalence of IgE below the LLOD is estimated to be 3.3% (95% CI, 1.9–5.7%).
Fig. 1.
Serum IgE in controls and hypogammaglobulinemia. Serum total IgE levels were measured in A) 964 controls from the OLIN population-based cohort in Northern Sweden; B) an internal cohort of 181 patients with CVID; and C) a multi-center USIDNET cohort of 173 patients with CVID. Shaded region represents normal range for IgE based on UVA lab. D) ROC curve characteristics based on pooled internal and USIDNET cohort (n=354). E) Serum IgE levels in patients with primary hypogammaglobulinemia (CVID, n=62 or XLA, n=9) compared to those with secondary hypogammaglobulinemia (n=34). Horizontal line is drawn at median and error bars show interquartile range (IQR). **** p<0.0001 using Kruskal-Wallis test with Dunn’s correction for multiple comparisons
Table 1.
Prevalence of IgE levels < LLOD in the general population
| Study | Cohort; n | IgE <LLOD (2–2.5 IU/ml) |
|---|---|---|
| Smith, 1997 [30] | Adults; n=420 | 44 |
| Levin, 2006 [38] | Pregnant adults; n=626 | 21 |
| Unsworth, 2011 [15] | Adults and children; n=2622 | 85 |
| NHANES 2005–2006 [20, 18] | US children and adults; n=8336 | 200 |
| Magen, 2014 [36] | Israeli adults and children; n=18,487 | 226 |
| Perzanowski, 2016 [17] | Swedish adults; n=964 | 37 |
| Total | N=31,455 | N=613 |
Frequency of low IgE in CVID
We next measured total serum IgE levels in our internal cohort of 181 patients with CVID. The frequency of IgE below the LLOD in was 74.0% (n=134), and the frequency of IgE below the LLN was 93.4% (n=169) (Fig 1B). In order to validate these findings, we next queried the USIDNET database. In this cohort (n=173), the frequency of IgE below the LLOD was 52.0% (n=90), and the frequency of IgE below the LLN was 81.5% (n=141) (Fig 1C), mirroring the findings of our original cohort albeit with somewhat reduced sensitivity. Taken together as a pooled cohort of patients with CVID (n=354) compared to controls from the Olin cohort (n=964), the sensitivity of an IgE below the LLOD for the diagnosis of CVID is 63.3% and the specificity is 96.2% (Fig 1D). Based on a random effect meta-analysis with the results of other published large population-based cohorts (Table 2 and Supplemental Table S2), the prevalence of IgE below the LLOD is estimated to be 75.6% (95% CI, 65.6 – 85.7%). The receiving operator characteristic (ROC) curve based on the pooled cohort identified a serum IgE cutoff of 5 IU/ml as having the most favorable sensitivity (81.9%) and specificity (89.6%).
Table 2.
Prevalence of IgE levels < LLOD in patients with CVID
| Study | Cohort; n | IgE < LLOD (2–4 IU/ml) | Low IgE (Cutoff used) |
|---|---|---|---|
| Stites, 1972 [9]a | Adults; n=12 | Not reported | 12 (<7 IU/ml) |
| Waldmann, 1972 [10]a | Adults and children; n=18 | 18 | 18 |
| McLaughlan, 1974 [11]a | Adults; n=22 | 3 | 21 (<20 IU/ml) |
| Iio, 1977a | Adult; n=36 | Not reported | 28 (<6 IU/ml) |
| Agondi et al, 2010 [14] | Adults; n=62 | 42 | 60 (<16 IU/ml) |
| Unsworth, 2011 [15] | Adults and children; n=19 | 17 | 19 (<20 IU/ml) |
| Agondi et al, 2013 [13] | Adults; n=72 | 62 | Not reported |
| Hartman et al, 2017 [16] | Adults; n=12 | 12 | 12 |
| Internal Cohort | Adults and children, n=181 | 134 | 169 (<10 IU/ml) |
| USIDNET | Adults and children; n=173 | 90 | 141 (<10 IU/ml) |
| Total | N=607 | 378 of 559 | 480 of 535 |
Not included in meta-analysis since total IgE measured prior to introduction of ImmunoCAP assay
Frequency of low IgE in secondary hypogammaglobulinemia
We performed a retrospective chart review of outpatients aged 12 years or older seen in the UVA Asthma, Allergy & Immunology Clinic with a diagnosis of CVID, XLA, or acquired (secondary) hypogammaglobulinemia. Similar to patients with CVID, 88.9% of patients with XLA (n=9) had a serum IgE <LLOD (Fig 1E). In comparison, of 34 patients with secondary hypogammaglobulinemia due to medications (including rituximab and systemic corticosteroids), or due to ongoing protein loss (stool, lymphatic or urinary), only 3 (8.8%) had an IgE <LLOD (p<0.0001) (Supplemental Fig S3). Thus, an IgE<LLOD is suggestive of a primary humoral immunodeficiency and not typical for secondary hypogammaglobulinemia.
IgG subclasses and other Ig isotypes in CVID
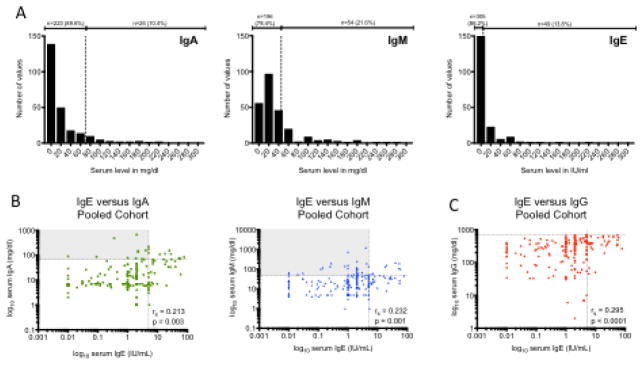
We next compared the performance of IgE for diagnosis of CVID with that of other Ig subtypes including IgA and IgM. We found that the frequency of IgE <5 IU/ml (86.2%) in patients with CVID was similar to that of IgA <LLN (89.6%) and higher than that of IgM <LLN (78.4%) (Fig 2A). There is significant correlation between serum IgE and serum IgA and IgM, as assessed using Spearman rank-correlation coefficient (rho) (Fig 2B). Of note, there is a subset of patients with either a normal IgA or a normal IgM (but who have a low IgM or IgA, respectively, and thus fulfill the diagnostic criteria for CVID) who have low serum IgE (upper left quadrants, Fig 2B). There is also a significant correlation between serum IgE with serum IgG, suggesting that lower IgE occurs in patients with more severe hypogammaglobulinemia (Fig 2C).
Fig. 2.
IgE compared to other serum Ig isotypes in patients with CVID. A) Distribution of serum IgA, IgM and IgE in CVID. Vertical dotted line represents LLN. B) Correlation of serum IgE with serum IgA and IgM. C) Correlation of serum IgE with serum IgG. Horizontal dotted lines represent LLN; vertical dotted lines represent IgE cutoff of 5 IU/ml. Rs = Spearman r
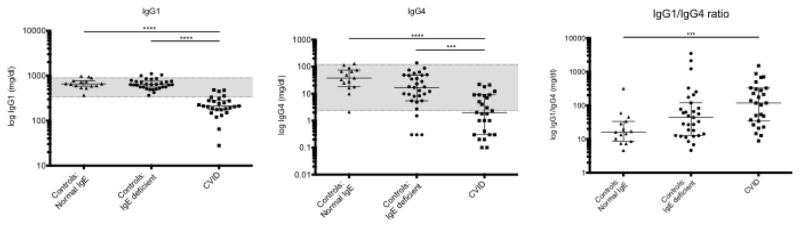
The generation of IgE has been proposed to occur either via direct isotype switch from IgM or via indirect class switch via an IgG1 or IgG4 intermediate [21]. We assessed the relative concentration of serum IgG1 and IgG4 in healthy controls with IgE ≥LLOD (n=15); healthy controls with IgE <LLOD (n=31); and subjects with CVID on whom serum IgG subclasses were available prior to the initiation of IVIG (n=27). As expected, there was a significant decrease in IgG1 and IgG4 in subjects with CVID compared to healthy controls (Fig 3, left and middle). Interestingly, there was a marked increase in the ratio of IgG1 to IgG4 in those with low IgE (both controls and those with CVID), suggestive of a relative decrease in IgG4 concentrations in patients with low IgE (Fig 3, right).
Fig. 3.
Serum Ig subclasses in controls with normal IgE (n=15), controls with IgE <LLOD (n=31) and patients with CVID (n=27). Serum IgG1 (left) levels, IgG4 levels (middle) and IgG1/IgG4 ratio (right) are shown for each group. Shaded region represents normal range based on reference lab. Horizontal line is drawn at median and error bars show IQR. **** p <0.0001, ** p <0.01 using Kruskal-Wallis test with Dunn’s correction for multiple comparisons
Allergen sensitization in CVID
Given that a low/undetectable total serum IgE is a prominent feature of CVID, we sought to determine the frequency of specific allergen sensitization. In the subset of 38 subjects with CVID and a calculated IgE of 0.50 IU/ml or higher (values between 0.50 and 1.99 IU/ml are below the assay manufacturer’s specified LLOD, but can be extrapolated from the standard curve), serum was tested for presence of specific IgE against a panel of allergens including Dermatophagoides farinae, cat dander, German cockroach, tree mix, grass mix and food mix. Overall, only 4 of 113 subjects (3.5%) were found to have measurable allergen specific IgE (range 0.46–3.38 IU/ml) (data not shown), indicating that even in those subjects in whom total IgE production persisted, albeit at a low level, there was an overall inability to detect circulating antigen-specific IgE. In comparison, the prevalence of allergic sensitization in healthy subjects based on NHANES 2005–2006 (as measured by at least 1 positive sIgE to 15 inhalant allergens and 4 food allergens) was 44.6% [18]. Although the rate of self-reported and/or physician-diagnosed allergy was not available for the de-identified internal cohort, the rate of reported allergy in the USIDNET cohort was as follows: food allergy 6.4%, drug allergy 42.8%, environmental allergy/allergic rhinitis 23.1%, asthma 45.1%, and eczema 12.7%. None of these rates were significantly different in patients with IgE < LLOD compared to those with total serum IgE ≥LLOD (Supplemental Fig S4).
Frequency of non-infectious disorders in CVID
We next sought to evaluate if the presence of IgE < LLOD in patients with CVID characterizes a specific phenotype defined by an increased frequency of non-infectious. We first stratified subjects from the USIDNET cohort based on the presence of IgE < LLOD or ≥ LLOD and then compared physician-reported rates of known complications of CVID, including enteropathy, lung disease, lymphoproliferation, granulomatous disease, malignancy and autoimmunity. There was an increased incidence of enteropathy and lymphoproliferation (i.e. lymphadenopathy, splenomegaly) disease in patients with IgE < LLOD (Supplemental Figure S5). The mechanism for this is not clear but could be the subject of future investigation.
Quantification of IgE in Ig replacement products
One possible explanation for preserved IgE levels in a subset of patients with CVID on IgRT is the presence of small amounts of IgE in infused Ig product that is rapidly cleared from the serum (but remains bound to the surface of mast cells and basophils) [22, 23]. Current preparations of Ig contain largely purified pooled IgG, with published concentrations of IgA due to a theoretical concern for anaphylaxis in patients with anti-IgA antibodies [24, 25]. Previous small studies of Ig preparations performed in the 1980s demonstrated measurable IgE [26–29]. However, the amount of IgE contained in current commercial Ig preparations with modern purification methods is not known. Therefore, we tested 49 lots of Ig products using both an IgE ELISA and the ImmunoCAP assay. In all cases, the ImmunoCAP was more sensitive than the ELISA, presumably due to less interference with this assay by concentrated IgG present in the product. The serum IgE detected by ImmunoCAP ranged from 0.96 IU/ml to 23.57 IU/ml (Supplemental Table S6). To determine if this quantity of infused IgE had demonstrable effects on total serum IgE, we measured serum IgE levels immediately prior to and immediately post IVIG infusion in 9 subjects. In 6 of 9 subjects, there was no change in undetectable serum IgE levels with infusion. In all 3 subjects in whom there was measurable baseline serum IgE (range 0.29 – 7.46 IU/ml), there was in fact a slight drop in serum IgE post-infusion by a mean of 0.92 IU/ml (data not shown). We suspect that this slight decrease in measured IgE due to interference of the assay by the high concentrations of infused IgG and is likely clinically insignificant. This suggests that current Ig products are not a significant source of IgE.
DISCUSSION
The results of this study demonstrate that while a low serum IgE is often felt to merely reflect the absence of atopy, in fact a serum IgE <2 IU/ml (the LLOD) occurs uncommonly and is found in only 3.3% (95% CI, 1.9–5.7%) of the general population. In contrast, in a large, multi-center cohort of 354 patients with CVID, 63.3% of patients were found to have an IgE <2 IU/ml. When combined with previously published reports (n=607) to calculate a pooled estimate using a random effect meta-analysis, the prevalence of an undetectable IgE (<2 IU/ml) in CVID is 75.6% (95% CI, 76.6–85.7%). We would propose the routine measurement of serum IgE (in addition to serum IgG, IgA, and IgM) in the work-up of patients with recurrent infections and suspected PIDD, as a rapid, low-cost diagnostic tool. For those patients who fulfill the diagnostic criteria for CVID, the finding of a serum IgE <2 IU/ml adds to the diagnostic certainty. Conversely, clinicians should be aware that a high IgE (>180 IU/ml) is very uncommon in CVID (1 of 354 subjects, or 0.3% of the pooled cohort), and consider alternate diagnoses. These data also demonstrate that, in contrast, an IgE <2 IU/ml is not typical for secondary hypogammaglobulinemia due to immunosuppressant use or loss in the stool/urine and occurred in only 8.8% of patients. This is consistent with prior studies of patients with CVID which have shown decreased synthesis of IgE (8% of control synthetic rates) and not increased catabolism [12]. In comparison, patients with excess catabolism or loss of immunoglobulins had normal serum IgE concentrations [10].
We were unable to detect specific allergen sensitization in 96.5% of patients, with only low titer IgE detected in 3 patients. This is consistent with a general inability to detect allergen sensitization via serum and/or skin prick testing in patients with CVID, despite many patients reporting symptoms of rhinitis, wheeze, or adverse reactions to antibiotics [14, 13, 30, 16]. We hypothesize that most patients with CVID are unable to generate new antigen-specific IgE and thus are not at risk for the development of new allergic disease. Equally, however, it is possible that since traditional serologic assays are not capable of detecting IgE bound to the surface of long-lived tissue mast cells, that these assays could result in the under-diagnosis of allergic conditions in patients with CVID. This underscores the importance for clinicians of exploring alternative explanations when patients with CVID report possible allergic symptoms, and utilizing other methodologies such as provocation challenge for diagnosis when such allergic conditions are suspected. It also emphasizes that measurement of serum IgE as part of the initial work-up of patients with recurrent sinopulmonary infections may eliminate the need to pursue additional skin or serum testing to identify allergic sensitization, which can be costly and time-consuming for patients.
The mechanism underlying the high prevalence of undetectable IgE in CVID is not clear. Our data demonstrate a relative decrease in IgG4 in both patients with CVID and controls with low IgE. Low IgE often occurs along with decreased levels of IgA, IgG2 and IgG4 [15, 31, 30]. Interestingly, these are the most downstream immunoglobulin heavy chain loci on chromosome 14, with IgA1, IgG2, IgG4, IgE and IgA2 occurring in sequential order [32]. This could be suggestive of an underlying isotype switch defect in patients with CVID, as has been previously demonstrated for a small subset of patients with CVID and mutations in TACI, ICOS, CD40L and BAFFR and further seen in patients with phenotypic impairments in isotype switching of unknown genetic etiology [33, 34].
The clinical significance of a low IgG and IgE in the setting of a normal IgA and IgM is not yet established and thus whether IgE adds further sensitivity in the diagnosis of CVID is not known. Similarly, the significance of an isolated low IgE (termed “selective IgE deficiency”) is unknown and thus IgE deficiency is not currently included in the consensus classification of PIDD [35]. Interestingly, selective IgE deficiency has been associated in small cohorts with a significantly higher prevalence of sinopulmonary infections, Helicobacter pylori associated gastritis and ulcers, chronic fatigue and autoimmunity, similar to patients with CVID [36, 30, 37]. Whether selective IgE deficiency is an early marker of evolving CVID, or occurs only in patients with established CVID, is not known based on this cross-sectional study of subjects but is the subject of current investigation. If subjects who are IgE deficient do in fact have a defect in isotype switching that underlies their low IgE, it is possible that this could evolve into a more global defect affecting other isotypes, similar to what is seen with patients with selective IgA deficiency that evolve into CVID ([34]. Also of interest would be to evaluate the response to polysaccharide vaccination (either with the Salmonella typhi polysaccharide vaccine or the 23-valent pneumococcal polysaccharide vaccine) in patients with reduced IgE, either alone or in combination with a low IgG. It is our own experience, as well as the experience of others, that subjects who are incidentally found to have IgE deficiency should be screened for symptoms suggestive of immunodeficiency (i.e. recurrent infections, autoimmunity) and if such symptoms are present, other serum Igs should be measured. This has proven to be a useful strategy that can lead to the identification of patients with PIDD that would otherwise not be diagnosed [15]. Larger-scale studies of the cost-effectiveness of this approach on a system-wide basis need to be performed.
Supplementary Material
Table S1. Fixed effect and random effect meta-analysis summary for prevalence of IgE < LLOD in general population
Table S2. Fixed effect and random effect meta-analysis summary for prevalence of undetectable IgE (< LLOD) in CVID
Figure S3. Distribution of serum immunoglobulins in patients with secondary hypogammaglobulinemia (n=34)
Lines and error bars represent median +/− IQR. Shaded boxes represent normal range.
Figure S4. Rates of physician-reported allergy in the USIDNET cohort (n=173)
All comparisons between groups are non-significant using Fisher’s exact test
Figure S5. Rates of physician-reported non-infectious disorders in the USIDNET cohort (n=173)
* p < 0.05, ** p < 0.01. All other comparisons between groups are non-significant using Fisher’s exact test
Table S6. IgE content of commercial Ig preparations
Acknowledgments
This work was supported by National Institutes of Health grants: R56AI120055 (LB), U01AI123337 (LB), AI057438 (LB), AI020565 (TPM) and AI100799 (TPM) as well as the University of Virginia School of Medicine (MGL).
ABBREVIATIONS
- CLIA
Clinical Laboratory Improvement Amendments
- CVID
common variable immune deficiency
- ELISA
enzyme linked immunosorbent assay
- Ig
immunoglobulin
- IgRT
immunoglobulin replacement therapy
- IU
international units
- IVIG
intravenous immunoglobulin
- LLN
lower limit of normal
- LLOD
lower limit of detection
- NHANES
National Health and Nutrition Examination Survey
- OLIN
Obstructive Lung Disease in Northern Sweden
- PIDD
primary immune deficiency disease
- USIDNET
United States Immunodeficiency Network
- UVA
University of Virginia
- XLA
x-linked agammaglobulinemia
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
John Routes received independent contractor fees from CSL Behring. The rest of the authors declare that they have no relevant conflicts of interest.
AUTHORSHIP CONTRIBUTIONS
Conceptualization, methodology: MGL, SCP, JWS, TAP, LB. Formal analysis: MGL, ECM, JP. Funding acquisition: MGL, LB, TAP. Investigation, resources: MGL, TVP, LJW, AJS, RF, KES, PL, CLH, DEB, JWS, JV, TAP, CCR, JMR, LB. Supervision: TAP, LB, JMR, CCR. Writing and reviewing manuscript: MGL, LB, JMR, CCR, JV, JWS, SCP, TAP.
References
- 1.Seppanen M, Aghamohammadi A, Rezaei N. Is there a need to redefine the diagnostic criteria for common variable immunodeficiency? Expert review of clinical immunology. 2014;10(1):1–5. doi: 10.1586/1744666X.2014.870478. [DOI] [PubMed] [Google Scholar]
- 2.Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmunity reviews. 2006;5(2):156–9. doi: 10.1016/j.autrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 5.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. British journal of haematology. 2009;145(6):709–27. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical immunology. 1999;92(1):34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 7.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clinical immunology. 2010;137(1):21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Orange JS, Ochs HD, Cunningham-Rundles C. Prioritization of evidence-based indications for intravenous immunoglobulin. J Clin Immunol. 2013;33(6):1033–6. doi: 10.1007/s10875-013-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stites DP, Ishizaka K, Fudenberg HH. Serum IgE concentrations in hypogammaglobulinaemia and selective IgA deficiency. Studies on patients and family members. Clinical and experimental immunology. 1972;10(3):391–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann TA, Polmar SH, Balestra ST, Jost MC, Bruce RM, Terry WD. Immunoglobulin E in immunologic deficiency diseases. II. Serum IgE concentration of patients with acquired hypogammaglobulinemia, thymoma and hypogammaglobulinemia, myotonic dystrophy, intestinal lymphangiectasia and Wiskott-Aldrich syndrome. Journal of immunology. 1972;109(2):304–10. [PubMed] [Google Scholar]
- 11.McLaughlan P, Stanworth DR, Webster AD, Asherson GL. Serum IgE in immune deficiency disorders. Clinical and experimental immunology. 1974;16(3):375–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Iio A, Strober W, Broder S, Polmar SH. The metabolism of IgE in patients with immunodeficiency states and neoplastic conditions. The Journal of clinical investigation. 1977;59(5):743–55. doi: 10.1172/JCI108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agondi RC, Barros MT, Kokron CM, Cohon A, Oliveira AK, Kalil J, et al. Can patients with common variable immunodeficiency have allergic rhinitis? American journal of rhinology & allergy. 2013;27(2):79–83. doi: 10.2500/ajra.2013.27.3855. [DOI] [PubMed] [Google Scholar]
- 14.Agondi RC, Barros MT, Rizzo LV, Kalil J, Giavina-Bianchi P. Allergic asthma in patients with common variable immunodeficiency. Allergy. 2010;65(4):510–5. doi: 10.1111/j.1398-9995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 15.Unsworth DJ, Virgo PF, Lock RJ. Immunoglobulin E deficiency: a forgotten clue pointing to possible immunodeficiency? Annals of clinical biochemistry. 2011;48(Pt 5):459–61. doi: 10.1258/acb.2011.011052. [DOI] [PubMed] [Google Scholar]
- 16.Hartman H, Schneider K, Hintermeyer M, Bausch-Jurken M, Fuleihan R, Sullivan KE, et al. Lack of Clinical Hypersensitivity to Penicillin Antibiotics in Common Variable Immunodeficiency. J Clin Immunol. 2017;37(1):22–4. doi: 10.1007/s10875-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perzanowski MS, Ronmark E, James HR, Hedman L, Schuyler AJ, Bjerg A, et al. Relevance of specific IgE antibody titer to the prevalence, severity, and persistence of asthma among 19-year-olds in northern Sweden. The Journal of allergy and clinical immunology. 2016;138(6):1582–90. doi: 10.1016/j.jaci.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salo PM, Arbes SJ, Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. The Journal of allergy and clinical immunology. 2014;134(2):350–9. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 20.Gergen PJ, Arbes SJ, Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005–2006. The Journal of allergy and clinical immunology. 2009;124(3):447–53. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Looney TJ, Lee JY, Roskin KM, Hoh RA, King J, Glanville J, et al. Human B-cell isotype switching origins of IgE. The Journal of allergy and clinical immunology. 2016;137(2):579–86e7. doi: 10.1016/j.jaci.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo S, Nakayama T, Matsuoka K, Yonekawa H, Karasuyama H. Long term maintenance of IgE-mediated memory in mast cells in the absence of detectable serum IgE. J Immunol. 2003;170(2):775–80. doi: 10.4049/jimmunol.170.2.775. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MG, Woodfolk JA, Schuyler AJ, Stillman LC, Chapman MD, Platts-Mills TA. Half-life of IgE in serum and skin: Consequences for anti-IgE therapy in patients with allergic disease. The Journal of allergy and clinical immunology. 2017;139(2):422–8. e4. doi: 10.1016/j.jaci.2016.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rachid R, Castells M, Cunningham-Rundles C, Bonilla FA. Association of anti-IgA antibodies with adverse reactions to gamma-globulin infusion. The Journal of allergy and clinical immunology. 2011;128(1):228–30. e1. doi: 10.1016/j.jaci.2011.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurcan HM, Keskin DB, Ahmed AR. Information for healthcare providers on general features of IGIV with emphasis on differences between commercially available products. Autoimmunity reviews. 2010;9(8):553–9. doi: 10.1016/j.autrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Paganelli R, Quinti I, D’Offizi GP, Papetti C, Cabello A, Aiuti F. A study of IgE in immunoglobulin preparations for intravenous administration. I. IgE in intravenous IgG. Vox sanguinis. 1986;51(2):87–91. doi: 10.1111/j.1423-0410.1986.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 27.Tovo PA, Gabiano C, Grazia Roncarolo M, Altare F. IgE content of commercial intravenous IgG preparations. Lancet. 1984;1(8374):458. doi: 10.1016/s0140-6736(84)91797-5. [DOI] [PubMed] [Google Scholar]
- 28.Newland AC, Macey MG, Bubel M. IgE in intravenous IgG. Lancet. 1984;1(8391):1406–7. doi: 10.1016/s0140-6736(84)91896-8. [DOI] [PubMed] [Google Scholar]
- 29.Stephan W, Dichtelmuller H. IgE in immunoglobulin for intravenous use. Lancet. 1984;1(8382):912. doi: 10.1016/s0140-6736(84)91377-1. [DOI] [PubMed] [Google Scholar]
- 30.Smith JK, Krishnaswamy GH, Dykes R, Reynolds S, Berk SL. Clinical manifestations of IgE hypogammaglobulinemia. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 1997;78(3):313–8. doi: 10.1016/S1081-1206(10)63188-2. [DOI] [PubMed] [Google Scholar]
- 31.Levy Y, Nakum A, Segal N, Monselise Y, Danon YL. The association of selective IgA deficiency and IgE hypogammaglobulinemia. Allergy. 2005;60(6):836–8. doi: 10.1111/j.1398-9995.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan JG, Rabbitts TH. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982;300(5894):709–13. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- 33.Aan de Kerk DJ, Jansen MH, Jolles S, Warnatz K, Seneviratne SL, Ten Berge IJ, et al. Phenotypic and Functional Comparison of Class Switch Recombination Deficiencies with a Subgroup of Common Variable Immunodeficiencies. Journal of clinical immunology. 2016;36(7):656–66. doi: 10.1007/s10875-016-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castigli E, Geha RS. Molecular basis of common variable immunodeficiency. J Allergy Clin Immunol. 2006;117(4):740–6. doi: 10.1016/j.jaci.2006.01.038. quiz 7. [DOI] [PubMed] [Google Scholar]
- 35.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magen E, Schlesinger M, David M, Ben-Zion I, Vardy D. Selective IgE deficiency, immune dysregulation, and autoimmunity. Allergy and asthma proceedings: the official journal of regional and state allergy societies. 2014;35(2):e27–33. doi: 10.2500/aap.2014.35.3734. [DOI] [PubMed] [Google Scholar]
- 37.Magen E, Schlesinger M, Ben-Zion I, Vardy D. Helicobacter pylori infection in patients with selective immunoglobulin E deficiency. World J Gastroenterol. 2015;21(1):240–5. doi: 10.3748/wjg.v21.i1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin TA, Ownby DR, Smith PH, Peterson EL, Williams LK, Ford J, et al. Relationship between extremely low total serum IgE levels and rhinosinusitis. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2006;97(5):650–2. doi: 10.1016/S1081-1206(10)61095-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fixed effect and random effect meta-analysis summary for prevalence of IgE < LLOD in general population
Table S2. Fixed effect and random effect meta-analysis summary for prevalence of undetectable IgE (< LLOD) in CVID
Figure S3. Distribution of serum immunoglobulins in patients with secondary hypogammaglobulinemia (n=34)
Lines and error bars represent median +/− IQR. Shaded boxes represent normal range.
Figure S4. Rates of physician-reported allergy in the USIDNET cohort (n=173)
All comparisons between groups are non-significant using Fisher’s exact test
Figure S5. Rates of physician-reported non-infectious disorders in the USIDNET cohort (n=173)
* p < 0.05, ** p < 0.01. All other comparisons between groups are non-significant using Fisher’s exact test
Table S6. IgE content of commercial Ig preparations