Abstract
Aim
To date, there is no consensus on which set of variables should be used to identify older persons at risk of disability in activities of daily living. The present study aimed to: (i) evaluate how different defocits cluster in a population of community-dwelling older persons; and (ii) investigate whether the discriminative capacity of physical performance measures towards the development of disability might be improved by adding psychological, social and environmental indicators.
Methods
Data are from 709 non-disabled older persons participating in the “Invecchiare in Chianti” study. We carried out a cluster analysis of 12 deficits in multiple functional domains, selected from the available frailty assessment instruments. Then, participants were assigned to a group, based on the obtained clusters of variables. For each group, we measured the prognostic capacity and the predictive ability for 6-year disability.
Results
The analysis showed a “physical” cluster (including weight loss, reduced grip strength/gait speed/physical activity, impaired balance, environmental barriers) and a “psychosocial” cluster (e.g. living alone, depression, low income). Thus, participants were classified into four groups according to the presence of a physical and/or psychosocial cluster. Compared with the “fit” group, the relative risks of becoming disabled in the “physical,” “psychosocial” and “mixed” deficit groups were 2.23 (95% CI 0.71–7.00), 1.52 (95% CI 0.62–3.75) and 6.37 (95% CI 2.83–14.33), respectively. The positive and negative predictive values for the “physical,” “psychosocial” and “mixed” deficit groups were, respectively, 9% and 87%, 6% and 83%, and 27% and 94%.
Conclusions
As expected, physical and psychosocial deficits cluster predominantly into different groups. Even when both are considered simultaneously, the ability to predict incident disability is still insufficient.
Keywords: cluster analysis, community-dwelling older persons, disability, frailty, predictive value
Introduction
Frailty is a clinical condition characterized by reduced capacities in multiple physiological systems, determining a state of increased vulnerability to stressors and susceptibility to adverse health outcomes (e.g. functional decline, falls, hospitalization, death).1 The concept of frailty has gained importance over the past decade because of the population aging, and the pressing need to prevent late-life disability and its burdening consequences. Early interventions might be beneficial for both improving the quality of life of older adults and reducing the costs of care.1,2
Several operational definitions of frailty have been proposed.3,4 Each instrument is based on the evaluation of a combination of different deficit indicators that, if present, poses the individual at risk of adverse outcomes. Some authors recognized frailty mainly as a biological syndrome, proposing physical signs and/or symptoms as diagnostic criteria. In this category falls the well-known frailty phenotype (FP), which is based on the evaluation of five criteria focused on the physical domain (i.e. mobility, strength, nutritional status, energy, physical activity).5 The FP is widely used for research purposes and is suitable in clinics, as it is constituted by easily accessible measures that have been associated with negative outcomes in older persons (including incident disability, death and hospitalization).5,6
Other researchers, instead, have proposed the addition of several domains to the physical one in order to better capture the older adults at risk of adverse outcomes, in line with the view of frailty as a multidimensional condition. For instance, Gobbens et al. underline that the syndrome is characterized by losses in one or more domains of human functioning (physical, psychological, social), which is caused by the influence of a range of variables and increases the risk of adverse outcomes.7 This broader approach implies that a narrow definition of frailty (only focused on physical indicators) might provide only a partial evaluation of the individual’s risk profile, potentially affecting the quality of care.8 Indeed, successful aging is not only dependent on the physical domain, but also on the mood status, the integrity of a social network, environment and housing conditions. Based on these assumptions, multiple operational definitions of frailty (e.g. the Tilburg Frailty Indicator, the Groningen Frailty Indicator, the Comprehensive Frailty Assessment Instrument) have been developed, expanding the assessment of frailty to the physical, psychological, environmental and social domains. A relevant issue regarding these frailty assessment instruments regards their ability to predict adverse outcomes: although it is better for the multidimensional questionnaires than for the FP, it seems to be still suboptimal.9–12 Furthermore, to date, most research on frailty assessment instruments has reported relative measures of association with disability, where few data are available about the prognostic performance of the scales. A strong statistical association between a marker and an outcome does not necessarily imply that the marker can effectively identify people who will develop the outcome. For these reasons, the set of factors that actually contribute to the identification of frail older adults at risk of disability is still unknown.
In the present study, we evaluated how several deficits conceptually belonging to different domains (physical, psychological, social, environmental) actually cluster in a cohort of community-dwelling older persons. We also investigated whether a multidimensional approach might increase the prognostic and discriminative capacity of physical performance measures towards incident disability.
Methods
Data source
We used data from the “Invecchiare in Chianti” study, a representative population-based study, which was designed to investigate the factors contributing to the decline of mobility in older persons.13 The participants in the study were randomly selected from the populations of two town areas in the Chianti region: Greve in Chianti and Bagno a Ripoli (Tuscany, Italy). The study protocol was approved by the Italian National Institute of Research and Care on Aging ethical committee. The eligible participants were interviewed at their homes by trained study researchers using a structured questionnaire aimed at investigating their health status, physical and cognitive performance, and other factors possibly related to loss of independence in late life. The interview was followed by a physical examination at the study clinic. Comorbid diseases were ascertained examining clinical history and medical records. The first wave of the study started in 1998 and participants were followed up with evaluations every 3 years.
Definition of deficits
Deficit indicators were selected based on a literature search and were adapted according to the data collected in the “Invecchiare in Chianti” database. Among the physical deficits, we included the modified Fried’s criteria5 and impaired balance,14,15 which were defined as follows. Unintentional weight loss was defined as a reduction in weight >4.5 kg in the past 12 months. Exhaustion was described as a feeling of requiring an effort to do everything, and was considered present if the participant reported it for >3 or 4 days in the past week. Reduced physical activity was defined as having carried out <2–4 h of light exercise per week. Walking speed was evaluated over a 15-feet course with the patient taking two walks at their usual pace. The mean of the two walks was considered, and those with a walking speed below the lowest sex- and height-specific quintiles were considered slow walkers. Using a hand-held dynamometer, we measured grip strength of the dominant limb. The average of two measurements was used, and those with results lower than the sex- and body mass index-specific quintiles were considered as having poor muscle strength. Balance was measured following the design of the Short Physical Performance Battery.16 The balance test is composed of three sub-tests (i.e. side-by-side stand, semi-tandem and tandem) to obtain a score from 0 (worst performers) to 4 (best performers). Impaired balance was defined as a summary score of 0 or 1.
According to literature evidence, psychological deficits selected for the study were cognitive impairment,17,18 depressive mood17 and poor coping ability.19 A Mini-Mental State Examination score <24 (corrected for education and age) defined cognitive impairment.20 The Center for Epidemiological Studies Depression scale (20-item version) was used to measure depressive symptoms.21 A Center for Epidemiological Studies Depression scale cut-point of 16 was used to identify individuals at risk for clinical depression. Poor coping capacity was considered present if the participant had not tried to solve a difficult situation that occurred in the previous 6 months or agreed with one of the two statements “I have no control over what is happening around me” or “I am unable to solve any of my problems.”
Finally, social and environmental deficits were also taken into account.5,17,19,22,23 Living alone was defined as the absence of any cohabitant. Lack of social support was considered present if participant’s relatives or acquaintances were seldom or never available. This indicator was eventually excluded from the analysis, as it was present only in 18 participants (2.5% of the population), and 13 of them were also living alone. Low income was defined as reported financial problems. Architectural barriers were considered present when the participant reported difficulty in using one or more rooms in the house and/or moving from/to the house because of the presence of indoor and/or outdoor physical obstacles.
Performance measures and disability
The widely used Katz Index of Independence in Activities of Daily Living (ADL; including the capacity of independent dressing, moving in and out of bed, using the toilet, washing, eating, and controlling urine and fecal continence) was used to measure the functional status.24 Incident disability was defined as the loss of at least one ADL occurring during the study follow up.
Sample selection
From the original study population (n = 1308), we selected 1026 participants aged ≥65 years. Then, participants with missing values for any deficit measures (n = 226) and those with no follow-up information (n = 67) were excluded, identifying a cohort of 733 individuals. The 67 participants lost during the follow up were older, were more likely to be frail (52% had reduced gait speed vs 20% of the remaining cohort) and 53 of them died before the 3-year follow-up visit. As the aim of the present study was to examine the factors contributing to incident ADL disability, we excluded a small sample of 24 individuals with impairment in one or more ADL at baseline, leaving the analytic sample to 709 individuals. Disabled individuals at baseline had a mean of 1.8 impaired ADL and had similar characteristics compared with the selected cohort, with the exception of older age (mean age 77.8 vs 73.4 years).
Statistical analysis
Descriptive statistics were used to present demographic characteristics of the study population and the prevalence of deficits. We carried out a hierarchical cluster analysis to group the deficit indicators without any a priori hypothesis. Cluster analysis is a multivariate analysis that attempts to form “clusters” of objects that are “similar” to each other but differ among clusters. On the basis of clusters of variables, we then divided the study population into groups. We therefore examined the prognostic capacity of each group by calculating relative risks for incident disability. The discriminative capacity was evaluated calculating the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the same outcome.
Statistical analyses were carried out using R 3.1 for Linux (R Foundation for Statistical Computing, Vienna, Austria).
Results
The population included 709 older persons (mean age 73.4 years, SD 6.5, 56.3% women). The general characteristics of the population are reported in Table 1. At baseline, more than half of the participants suffered from hypertension, approximately one-third had osteoarthritis and chronic renal failure, 20% of the population was diagnosed with heart failure, while the prevalence of diabetes mellitus, peripheral artery disease and chronic obstructive pulmonary disease was approximately 10%. Compared with the fit group, participants who became disabled at follow up were more likely to be female and had a higher prevalence of chronic illnesses, with the exception of diabetes mellitus and chronic obstructive pulmonary disease, whereas cancer was more prevalent among non-disabled participants.
Table 1.
Characteristics of the population at baseline and of participants disabled and non-disabled† at 6-year follow up
| All | Non-disabled at follow up | Disabled at follow up | |
|---|---|---|---|
| n | 709 | 617 | 92 |
| Age, mean (SD) | 73.4 (6.5) | 73.4 (6.5) | 73.4 (6.5) |
| Sex, female (%) | 56.3 | 54.9 | 65.2 |
| Hypertension (%) | 61.5 | 60 | 71.7 |
| Heart failure (%) | 21.9 | 20.3 | 32.6 |
| Ischemic heart disease (%) | 9.4 | 8.9 | 13 |
| Cerebrovascular disease (%) | 3.8 | 3.1 | 8.7 |
| Diabetes mellitus (%) | 12.1 | 12 | 13 |
| Chronic obstructive pulmonary disease (%) | 10.3 | 10.2 | 10.9 |
| Chronic renal failure, stages III–V‡ (%) | 33.8 | 28.8 | 67.8 |
| Parkinson’s disease (%) | 2 | 1.1 | 7.6 |
| Peripheral artery disease (%) | 15.9 | 14.1 | 28.3 |
| Hip or knee osteoarthritis (%) | 30.7 | 28.7 | 44.6 |
| Dementia (%) | 1.4 | 1.1 | 3.3 |
| Cancer (%) | 5.6 | 6.2 | 2.2 |
Disability: loss of at least one Katz Index of Independence in Activities of Daily Living at follow up.
Creatinine clearance was estimated by the Cockcroft–Gault equation.
In Table 2 we reported the prevalence of the selected deficits. According to the FP, 5.9% of the baseline sample was frail: this prevalence increased to 19.6% among people who became disabled at follow up. Among Fried et al.’s criteria, weight loss had a low prevalence (4.9%), which was not higher among people who lost one or more ADL at follow up. Similarly, there was no statistical significant difference between the groups for reported exhaustion. As expected, all the remaining deficits had a higher prevalence among people who developed disability at follow up with the exception of low income and living alone. More that 40% of the entire cohort reported to have coping problems, nearly 30% had cognitive decline and 20% lived alone.
Table 2.
Prevalence of deficits in the baseline population, and among the disabled and non-disabled participants at 6-year follow up†
| All | Non-disabled at follow up | Disabled at follow up | P-value | |
|---|---|---|---|---|
| Weight loss | 4.9 | 5 | 4.3 | 0.983 |
| Exhaustion | 16.8 | 16 | 21.7 | 0.225 |
| Low physical activity | 13.5 | 10 | 37 | <0.01 |
| Low walking speed | 17.5 | 12.8 | 48.9 | <0.01 |
| Low grip strength | 17.2 | 14.7 | 33.7 | <0.01 |
| Frailty (≥3 FP criteria) | 5.9 | 3.9 | 19.6 | <0.01 |
| Impaired balance | 6.5 | 3.2 | 28.3 | <0.01 |
| Cognitive decline | 27.5 | 25.9 | 38 | 0.021 |
| Depression | 31 | 28 | 51.1 | <0.001 |
| Living alone | 18.2 | 17.2 | 25 | 0.095 |
| Environmental barriers | 8.2 | 5.8 | 23.9 | <0.01 |
| Coping problems | 42 | 39.2 | 60.9 | <0.01 |
| Low income | 9.3 | 9.1 | 10.9 | 0.719 |
Data are expressed as percentages.
Disability: loss of at least one Katz Index of Independence in Activities of Daily Living at follow up. FP, frailty phenotype.
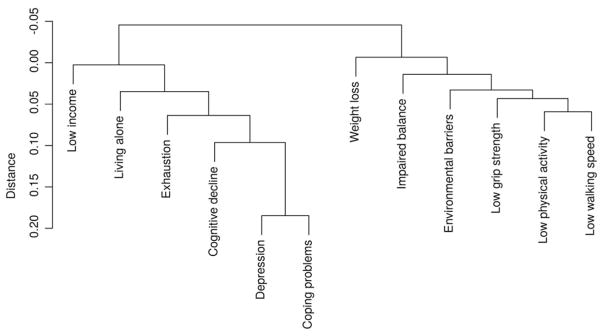
As shown in Figure 1, the cluster analysis of the deficits revealed two clusters. The “physical” cluster combined physical and environmental deficits: weight loss, poor grip strength, slow gait speed, low physical activity, presence of architectural barriers and impaired balance; the “psychosocial” cluster included six deficits: depressive symptoms, poor coping capacity, cognitive impairment, exhaustion, living alone and financial difficulties. As expected, in the “physical” cluster, there is a close link among all the variables, in particular grip strength, physical activity and walking speed; in the “psychosocial” cluster, depression and coping problems are closer to cognitive decline than to other deficits (Fig. 1). Once the results of the cluster analysis were obtained, four groups of participants were then identified: (i) the “fit” group, including individuals with no deficit (n = 142); (ii) the “physical deficit” group, individuals with at least one physical deficit indicator (n = 53); (iii) the “psychosocial deficit” group, including individuals with at least one psychosocial deficit (n = 280); and (iv) the “mixed deficit” group, consisting of individuals with deficits included in both clusters (n = 234).
Figure 1.
Dendrogram resulting from cluster analysis testing the distribution of deficit indicators in the population.
Over the 6 years of follow up, 92 participants reported incident ADL disability. Compared with the “fit” group, the relative risks of becoming disabled in the “physical,” “psychosocial” and “mixed” deficit groups were 2.23 (95% CI 0.71–7.00), 1.52 (95% CI 0.62–3.75) and 6.37 (95% CI 2.83–14.33), respectively.
The predictive capacity of this classification is summarized in Table 3. The probability that a participant in the “physical deficit” group would develop a disability was 9.4% (PPV), whereas the probability of no ADL disability for a participant in the “fit” group was 86.7% (NPV). The corresponding values for the “psychosocial deficit” and “mixed deficit” groups were PPV 6.4% and NPV 82.7%, and PPV 26.9% and NPV 93.4%, respectively (Table 3). Similar results were found when analyzing the predictive value of the classical FP.
Table 3.
Predictive capacity of the three groups of deficit indicators for incident disability†
| Groups | Sensitivity | Specificity | Positive predictive value | Negative predictive value | PLR | NLR |
|---|---|---|---|---|---|---|
| Fit (n = 142) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Physical deficit (n = 53) | 0.054 | 0.922 | 0.094 | 0.867 | 0.698 | 1.03 |
| Psychosocial deficit (n = 280) | 0.196 | 0.575 | 0.064 | 0.827 | 0.461 | 1.398 |
| Mixed deficit (n = 234) | 0.685 | 0.722 | 0.269 | 0.939 | 2.471 | 0.436 |
Loss of at least one activity of daily living at follow up. NLR, negative likelihood ratio; PLR, positive likelihood ratio.
Discussion
In the present study, we analyzed how several deficits pertaining to physical, psychological, social and environmental domains cluster in a representative population of community-dwelling older persons. We obtained two clusters of deficits: one composed of measures regarding the physical domain and the other gathering psychological, social and environmental measures. When deficits fibelonging to both clusters were present, the risk of developing disability was greater.
The present findings support the theory that both physical and psychosocial deficits might contribute to the definition of frailty.4,7,25,26 Frailty is a dynamic condition exposing older persons to an increased risk of disability and adverse outcomes. An approach focused on the physical issues and underestimating the multidomain complexity of the individual might insufficiently capture the frailty condition, misclassifying the individuals potentially amenable to ad hoc preventive interventions. Indeed, psychological, social and environmental factors are all associated with dependency in later life; therefore, deficit in these domains could contribute to defining the frailty syndrome.5,17,19,22,23,27
The present results show that the abnormalities in the physical domain are not significantly associated with an increased risk of incident disability. This finding might seem in contrast with available literature reporting physical impairment (especially slow gait speed) as strongly and independently associated with adverse outcomes.28,29 However, it must be noted that the strength of the association reported in the present study is similar to that reported in the literature. It is likely the small number of participants included in the “physical deficit” group (n = 53) and the low incidence of outcome in the sample (13%) might have limited the statistical power of the analysis.
Interestingly, four of the Fried’s criteria clustered in the “physical” deficit group, whereas the “exhaustion” criterion was part of the psychosocial cluster. This might be (at least partly) due to the fact that the exhaustion criterion was derived by questions retrieved from a scale measuring depressive symptoms (i.e. Center for Epidemiological Studies Depression scale), thus a domain different from the physical one.
Although the FP is able to explore mainly the physical domain of frailty, a recent study reported that, in a cohort of 8684 community-dwelling older people, the FP is associated with worse scores in social (e.g. social network type), psychological (e.g. psychological distress, mastery) and physical (e.g. chronic diseases) domains.30 These data are consistent with the present finding that the FP includes markers clustering into two different groups.
The present data confirm that the predictive ability of commonly used deficit indicators remains relatively poor. The probability of becoming disabled among people identified at risk according to the presence of physical and/or psychosocial deficits is relatively low, and none of the markers can singularly be used as a screening test for this syndrome. In fact, the PPV was higher when physical and psychosocial deficit indicators were simultaneously considered (PPV 27%), but still far from providing a sufficient detection. This finding is consistent with previous literature showing that commonly used frailty assessment instruments are not particularly sensitive at identifying older persons at risk of negative health-related events.9,10 The available instruments might be rather used for detecting fit individuals, due to the high specificity. Improving the predictive validity of current instruments is thus necessary in order to plan effective interventions aimed at preserving independent function in later life.
A limitation of the present study might arise from the relatively small size of the whole sample (709 participants) and of each group, which could explain the non-significant association with incident disability (with the exception of the mixed deficit group). Second, the relatively low prevalence of the outcome (13%) could have affected sensitivity and PPV, which was low for incident disability among all three groups (physical, psychosocial and mixed). However, similar results (PPV 0.38) would have been reported for the “mixed” deficit group, even in a hypothetical scenario with 20% of incident disability.
In conclusion, the results of the present analysis let us identify different risk “profiles” for incident disability among community-dwelling older people, in particular a group mainly characterized by physical deficits and another identified by a psychosocial impairment. These findings are in line with previous recommendations describing frailty as a multidimensional phenomenon, thus requiring individualization of its management. Unfortunately, even when applying more holistic parameters for detecting frailty, the predictive capacity remains inadequate. Specific research should be continued in the field in order to enrich or modify the current operational definitions of frailty with the aim of improving the capacity to discriminate the older persons in the need of preventive actions. In particular, more attention should be given to latent and still unrecognized factors, such as interaction with the healthcare system, incident life events and biomarkers of frailty, that might contribute to disability and should be necessarily included in the definition of frailty.
Acknowledgments
The authors are particularly grateful to the “Invecchiare in Chianti” study members who contributed to data collection.
Footnotes
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerreta F, Eichler H-G, Rasi G. Drug policy for an aging population--the European Medicines Agency’s geriatric medicines strategy. N Engl J Med. 2012;367:1972–1974. doi: 10.1056/NEJMp1209034. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc. 2010;11:338–343. doi: 10.1016/j.jamda.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Pedone C, Costanzo L, Cesari M, Bandinelli S, Ferrucci L, Antonelli Incalzi R. Are performance measures necessary to predict loss of independence in elderly people? J Gerontol A Biol Sci Med Sci. 2016;71:84–89. doi: 10.1093/gerona/glv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pijpers E, Ferreira I, Stehouwer CDA, Nieuwenhuijzen Kruseman AC. The frailty dilemma. Review of the predictive accuracy of major frailty scores. Eur J Intern Med. 2012;23:118–123. doi: 10.1016/j.ejim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Widagdo IS, Pratt N, Russell M, Roughead EE. Predictive performance of four frailty measures in an older Australian population. Age Ageing. 2015;44:967–972. doi: 10.1093/ageing/afv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton JL, Gould RL, Daley S, et al. Psychometric properties of multicomponent tools designed to assess frailty in older adults: a systematic review. BMC Geriatr. 2016;16:55. doi: 10.1186/s12877-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 19.Raphael D, Cava M, Brown I, et al. Frailty: a public health perspective. Can J Public Health. 1995;86:224–227. [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385–401. [Google Scholar]
- 22.Woo J, Goggins W, Sham A, Ho SC. Social determinants of frailty. Gerontology. 2005;51:402–408. doi: 10.1159/000088705. [DOI] [PubMed] [Google Scholar]
- 23.Levers M-J, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56:282–291. doi: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- 24.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 25.Markle-Reid M, Browne G. Conceptualizations of frailty in relation to older adults. J Adv Nurs. 2003;44:58–68. doi: 10.1046/j.1365-2648.2003.02767.x. [DOI] [PubMed] [Google Scholar]
- 26.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 27.Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Determinants of frailty. J Am Med Dir Assoc. 2010;11:356–364. doi: 10.1016/j.jamda.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesari M, Kritchevsky SB, Penninx BWHJ, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 30.Op het Veld LPM, van Rossum E, Kempen GIJM, de Vet HCW, Hajema K, Beurskens AJHM. Fried phenotype of frailty: cross-sectional comparison of three frailty stages on various health domains. BMC Geriatr. 2015;15:77. doi: 10.1186/s12877-015-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]