Abstract
Background
To elucidate mechanisms related to remission in winter seasonal affective disorder (SAD), we explored the course of individual depressive symptom offset across two distinct treatment modalities that show comparable outcomes at treatment endpoint: cognitive-behavioral therapy for SAD (CBT-SAD) and light therapy (LT).
Method
177 adults with SAD in a depressive episode were randomized to 6-weeks of CBT-SAD (n=88) or LT (n=89). Symptoms were assessed via the 29-item Structured Interview Guide for the Hamilton Rating Scale for Depression-SAD Version (SIGH-SAD) at pre-treatment and weekly during treatment. Survival analyses were conducted for the 17 SIGH-SAD items endorsed by more than 40 participants at pre-treatment. Within each of the included symptoms, data from participants who endorsed the symptom at pre-treatment and who had 3 or fewer weeks missing were included.
Results
For most (13/17; 76%) symptoms, CBT-SAD and LT did not differ in time to remission. However, for 4 symptoms (early insomnia, psychic anxiety, hypersomnia, and social withdrawal), LT led to symptom remission more quickly than CBT-SAD.
Conclusions
Symptom remission progressed comparably across CBT-SAD and LT for most symptoms. Despite the fact that the two treatments led to similar remission rates and improvements at treatment endpoint, for early insomnia, psychic anxiety, hypersomnia, and social withdrawal, LT led to symptom remission faster than CBT-SAD. These results suggest different mechanisms and pathways to the same therapeutic end. Speedier remission of early insomnia and hypersomnia is consistent with the theory that SAD is related to a pathological circadian phase-shift that can be corrected with LT.
Keywords: Cognitive-behavioral therapy, light therapy, seasonal affective disorder, depressive symptom course, remission
Introduction
Winter seasonal affective disorder (SAD; Rosenthal et al., 1984) is a recurrent Major Depressive Disorder (MDD) that has a regular annual onset in the fall/winter, offset in the spring, and remission through the summer until the following fall/winter. In contrast to unipolar MDD; sleep, appetite, and weight are most often reported as increased rather than decreased (referred to as “reverse vegetative” or “atypical” symptoms) and fatigue is a prominent symptom (Rosenthal et al., 1984). The sex difference in prevalence favoring females is even more discrepant in SAD than the 2:1 ratio for unipolar depression (Magnusson, 2000). The onset of depressive episodes in SAD is associated with declining photoperiod (Young, Meaden, Fogg, Cherin, & Eastman, 1997). SAD symptom severity also has been associated with cognitive vulnerability to depression constructs such as rumination (Rohan, Sigmon, & Dorhofer, 2003; Whitcomb-Smith et al., 2014; Young, Reardon, & Azam, 2008), dysfunctional attitudes (Golden, Dalgleish, & Spinks, 2006; Hodges & Marks, 1998), and negative attributional style (Enggasser & Young, 2007; Levitan, Rector, & Bagby, 1998).
Symptom transition times of onset (from asymptomatic to symptomatic) and offset (from symptomatic to asymptomatic) are valuable to study because they have the potential to provide information on mechanisms and processes that contribute to the rise and fall of symptomatology and to test theories of etiology (Iacoviello, Alloy, Abramson, & Choi, 2010; Stassen, Delini-Stula, & Angst, 1993; Young & Grabler, 1985). For example, Iacoviello et al. (2013) found that the patterns of onsets and offsets of individual symptoms of hopelessness depression were consistent with those proposed by the theory of a hopelessness depression subtype. For SAD, the observation of different temporal patterns of onset for different types of symptoms (Young, Watel, Lahmeyer, & Eastman, 1991) led to the dual vulnerability model of SAD in which psychological responses to environmentally triggered vegetative symptoms (e.g., sleep disturbance, fatigue, appetite changes) lead to the cognitive and affective symptoms that, in combination, constitute the full depressive syndrome. Tests of the dual vulnerability model have been supportive (e.g., Enggasser & Young, 2007; Whitcomb-Smith et al., 2014).
However, the offset of symptoms in SAD has not yet been studied. SAD offset in response to treatment is particularly interesting because empirically-supported treatments for SAD, at least in theory, may have different mechanisms. Bright light therapy (LT) has a long history of supportive research (see meta-analyses by Golden et al., 2005; Mårtensson, Pettersson, Berglund, & Ekselius, 2015). LT’s presumed therapeutic mechanism is through alterations in circadian rhythms (Lewy, Sack, Singer, & White, 1987), although effects in the retina (Roecklein et al., 2013) may also play a role. More recently, trials comparing cognitive-behavioral therapy tailored to SAD (CBT-SAD) to LT found no differences between the two in depression improvements or remission rates at post-treatment (Rohan et al., 2007; Rohan, Mahon et al., 2015). For example, in the parent trial, post-treatment remission rates were 47.6% in CBT-SAD and 47.2% in LT (Rohan, Mahon et al., 2015). CBT-SAD includes behavioral activation aimed at decreasing avoidance and increasing engagement in pleasurable activities during the winter and cognitive therapy aimed at restructuring depressive thoughts, including SAD-specific negative cognitions about the seasons, light availability, and weather (Rohan, 2008; LT and CBT-SAD as employed in this study are described in detail in the Methods section). CBT-SAD’s presumed antidepressant mechanism is through offsetting an underlying cognitive vulnerability to depression (Rohan, Roecklein, & Haaga, 2009). Although CBT-SAD and LT are very different treatments in terms of protocol and theoretical basis that lead to similar overall acute treatment responses, they could lead to different time courses of individual symptoms across the period of treatment. Thus, there could be different mechanisms and pathways to the same therapeutic end.
The present study examined the remission of individual symptoms in SAD across six weeks of a parent randomized clinical trial and, in particular, compared the time to remission of each symptom between participants receiving LT and those receiving CBT-SAD. Survival analysis was used to provide information about the distribution of remissions across the weeks of treatment and the risk (hazard) of remission in each week among those for whom the symptom had not yet remitted. Comparison of these results between treatments provides information about the differential longitudinal therapeutic effects of LT and CBT-SAD on the symptom level, as well as clues about the nature of the therapeutic mechanisms underlying the two treatments.
We have chosen not to make specific hypotheses about differential time courses of symptom remission according to the treatment received. There is very little theoretical or empirical literature on patterns of remission by symptom. Some ideas have been put forward that early onset core symptoms would be among the first, or among the last, to remit (see Iacoviello et al., 2010, p. 460). However, of greatest concern is that no work on this issue has considered whether remissions are spontaneous or treatment-induced, and, if treatment-induced, whether different treatments might induce different time courses in the remissions of particular symptoms. Given this situation, there was little basis for making a priori hypotheses and we took an exploratory approach.
Materials and Methods
Participants
This study constitutes a secondary analysis of data collected from a randomized clinical trial (RCT) comparing the efficacy of two distinct treatments for seasonal affective disorder (SAD; Rohan et al., 2013; 2015). All participants provided informed consent prior to enrolling in the parent RCT. The study was conducted at the University of Vermont and was approved by the institutional review board. As part of the parent RCT, participants were randomized to six weeks of cognitive-behavioral therapy for SAD (CBT-SAD) or light therapy (LT). Participants, aged 18 years or older, were recruited from the greater Burlington, VT region. Inclusion criteria consisted of (a) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM–IV–TR) criteria for Major Depression, Recurrent, with Seasonal Pattern as assessed by the Structured Clinical Interview for DSM-IV Disorders-Clinician Version (First, Spitzer, Gibbon, & Williams, 1995) and (b) symptom severity criteria of a total score of at least 20 on the Structured Interview Guide for the Hamilton Rating Scale for Depression—Seasonal Affective Disorder Version (SIGH-SAD: Williams, Link, Rosenthal, Amira, & Terman, 1992), with a minimum of 5 on the atypical scale and a minimum of 10 on the remaining items. Individuals were ineligible to participate if they (a) were receiving or planned to receive either LT or psychotherapy for depression during the same winter they intended to enroll, (b) had a history of LT or CBT-SAD, (c) had any comorbid Axis I disorder requiring immediate treatment, (d) had hypothyroidism (assessed via thyroid panel), (e) had travel arrangements through the beginning of spring (March), or (f) had suicidal ideation that would contraindicate participation.
For each symptom examined, the sample for the survival analysis included (1) those participants who exhibited the symptom at pre-treatment and (2) those participants who had no more than three weeks of missing data (see Missing Data Handling below). Applying those criteria, total sample size here was 163 (76 CBT-SAD, 87 LT). However, sample sizes for analyses of individual symptoms were smaller and varied since each symptom needed to be present at baseline to be included in the analysis (see Table 1). Most participants were female (84.0%) and non-Hispanic white (92.6%). Participants’ mean (SD) age was 45.4 (12.7) years. For detailed demographic information and baseline characteristics from the parent RCT, see Rohan, Mahon, et al., 2015.
Table 1.
Sample flow chart
| CBT-SAD | LT | |
|---|---|---|
| Parent RCT sample | 88 | 89 |
| N (% of parent RCT Sample) | N (% of parent RCT Sample) | |
| At least 3 time points (for one or more symptoms) | 76 (86.4) | 87 (97.7) |
|
| ||
| Symptom present pre-treatment [SIGH-SAD ITEM #] | N (% included sample) | N (% included sample) |
|
| ||
| Depressed Mood [H1] | 70 (92.1) | 85 (97.7) |
| Work & Activities [H2] | 73 (96.1) | 86 (98.9) |
| Social Withdrawal [A1] | 71 (93.4) | 78 (89.7) |
| Genital Symptoms [H3] | 45 (59.2) | 63 (72.4) |
| Somatic Symptoms (G.I) [H4] | 25 (32.9) | 27 (31.0) |
| Weight Gain [A2] | 33 (43.4) | 32 (36.8) |
| Appetite Increase [A3] | 40 (52.6) | 40 (46.0) |
| Increased Eating [A4] | 48 (63.2) | 53 (60.9) |
| Carbohydrate Craving/Eating [A5A] | 64 (84.2) | 72 (82.8) |
| Insomnia Early [H6] | 27 (35.5) | 36 (41.4) |
| Insomnia Middle [H7] | 49 (64.5) | 60 (69.0) |
| Hypersomnia [A6] | 39 (51.3) | 50 (57.5) |
| Fatigability [A7] | 76 (100) | 87 (100) |
| Feelings of Guilt [H10] | 56 (73.7) | 61 (70.1) |
| Anxiety Psychic [H12] | 58 (76.3) | 67 (77.0) |
| Anxiety Somatic [H13] | 64 (84.2) | 74 (85.1) |
| Hypochondriasis [H14] | 37 (48.7) | 44 (50.6) |
Treatments
CBT-SAD (Rohan, 2008) is a group therapy intervention consisting of twelve 90-minute sessions (two sessions per week) over the course of six weeks. CBT-SAD incorporates traditional components of CBT (e.g., behavioral activation, cognitive restructuring, and relapse-prevention planning) to promote effective coping with the winter season. In addition to targeting typical depressogenic thoughts, some cognitive restructuring in CBT-SAD centers on negative automatic thoughts about winter, reduced photoperiods, and cold and snowy weather conditions. Behavioral activation in CBT-SAD seeks to identify and increase pleasant activities that can be done in the winter to increase pleasure and mastery. CBT-SAD therapists included either the P.I. (K.R.) or one of two doctoral-level community therapists unaffiliated with the Rohan laboratory.
LT used standard light boxes emitting 10,000-lux of cool-white fluorescent light filtered through an ultraviolet shield (SunRay® by SunBox Company, Gaithersburg, MD). In an initial instructional session, LT participants were provided with a treatment rationale, shown how to assemble and position the light box, instructed in their starting dose (i.e., 30-min. daily upon waking), and educated about potential side effects. Adjustments to the LT dosage were made weekly using an algorithm designed to maximize treatment response, address inappropriate phase shifts, and minimize side effects. Specifically, LT participants who failed to experience a 30% or greater reduction in SIGH-SAD scores by the end of treatment week one, a 50% or greater reduction in SIGH-SAD scores by the end of treatment week two, or meet SIGH-SAD remission criteria (see below) by the end of treatment week three were instructed to increase their duration of LT by 15 minutes the next week with an upper dosage limit of two hours of light therapy. If participants experienced significant side effects, treatment was incrementally reduced to a minimum dose of 30 minutes per day. In the event of severe side effects, single day hiatuses from LT were prescribed, followed by a 50% dose reduction. An outside chronobiological psychiatrist with LT expertise reviewed all LT cases weekly and made all recommended dose adjustments per this algorithm.
Treatment integrity was measured using an adapted version of the National Institute of Mental Health’s Collaborative Study Psychotherapy Rating (see Rohan et al., 2013). Two trained clinical psychology doctoral students, blind to condition and session, rated one quarter of the CBT-SAD sessions, which were randomly selected and counterbalanced across all CBT-SAD cohorts, therapists, and session numbers (inter-rater reliability intraclass correlation coefficient = 0.76). CBT-SAD significantly differed from LT on the cognitive–behavioral and clinical management scales, indicating that CBT-SAD and LT are theoretically distinct treatments. On average, CBT-SAD participants attended most sessions (M = 9.1, SD = 3.5). Of 13 CBT-SAD participants who withdrew, seven did not attend any sessions and six attended a minimum of two and a maximum of seven sessions. LT adherence was monitored using LT diaries (Rohan, Mahon, et al., 2015).
Measures
Structured Interview Guide for the Hamilton Rating Scale for Depression–Seasonal Affective Disorder version (SIGH-SAD)
The SIGH-SAD is a 29-item clinical interview that expands the 21-item Hamilton Rating Scale for Depression (HAM-D) to include eight items assessing “atypical symptoms” of depression that are common in SAD. Total SIGH-SAD scores can range from 0 to 90. Symptom severity was assessed via blind interviewer at pre-treatment, weekly during treatment (treatment weeks 1–5) and upon completing treatment (post-treatment). Rater’s made scoring decisions based on clearly defined item scoring rules (Rohan, Rough et al., 2016). In the parent trial, episode remission was defined as a pre- to post-treatment total SIGH-SAD score reduction of at least 50% in addition to a 21-item HAM-D score ≤ 7 and an 8-item atypical score ≤ 7. Episode remission status also could be obtained by a 21-item HAM-D score ≤ 2 and an 8-item atypical score ≤ 10. All SIGH-SAD interviews were audio recorded and independently rated by a second blinded rater. Discrepancies between raters were resolved using the procedures outlined in Rohan, Rough et al. (2016). Intraclass correlations for inter-rater reliability ranged between 0.92 and 0.97 for pre-treatment, treatment weeks 1–5, and post-treatment (see Rohan, Rough et al., 2016).
Symptom Remission
In the present study, our outcome of interest was the time to sustained remission of each individual symptom (i.e., SIGH-SAD item) during the 6-weeks treatment phase. For a symptom to be included in these secondary analyses, 41 or more participants had to exhibit the symptom at pre-treatment. Any symptom endorsed by 40 or fewer participants was not examined because it was judged to be insufficiently common to allow reliable estimates in a survival analysis. Based on this criterion, 17 of the 29 SIGH-SAD symptoms were studied (Table 1). A symptom was considered remitted if the score of the corresponding SIGH-SAD item was zero at a particular week during treatment (remission week) and the symptom remained at zero through the end of treatment. For example, [2, 2, 0, 0, 0, 0] would be a remission at week three, [2, 2, 0, 1, 0, 0] would be a remission at week five, and [2, 2, 0, 1, 0, 1] would be failure to reach remission. For the purpose of this study and the survival analyses, we were only concerned with whether a symptom was absent (0) or present (> 0) each week. Therefore, following the handling of missing data, all symptom severities greater than zero were recoded to one.
Missing Data Handling
Missing data were considered separately for each symptom and each participant. Only cases that had three or fewer missed weeks were included in the survival analysis sample. In cases where a participant was missing data from one or more time points that were preceded and followed by data being present, we imputed the missing values by using the mean of the participant’s immediately preceding and succeeding non-missing values (Engels, 2003). For example, if a participant’s symptom severity data was [2, 2, 0, _, 1, 0], the missing value would be equal to 0.5 (the mean of the preceding 0 and subsequent 1). Means < 0.5 were recoded as 0 (symptom absent); means ≥ 0.5 were recoded as 1 (symptom present).
For cases with data at the final (post-treatment) timepoint, but with data missing in the immediately preceding two or three consecutive time points, the last preceding observation was carried forward. For example, for the data [2, 2, 1, _, _, 0], the missing values were replaced with 1s (Engels, 2003). Cases with data missing at the final, post-treatment timepoint were considered as right censored at the last timepoint at which there was data present. For example, [2, 1, 2, 3, _ _, _) was considered censored at week 4. As noted above, following these procedures all symptom scores consisted of either 0s (absent), 1s (present), or missing through the end of the trial.
Data Analyses
Data pre-processing, statistical analyses and visualizations were completed using R version 3.2.3 (R Core Team, 2015) with the following packages: plyr (Wickham, 2011), dplyr (Wickham & Francois, 2015), ggfortify (Horikoshi & Tang, 2015), ggplot2 (Wickham, 2009), zoo (Zeileis & Grothendieck, 2005), and survival (Therneau, 2015). All t-tests and repeated measures ANOVAs were completed using SPSS v.24 (IBM Corp., 2016). Independent samples t-tests were conducted to assess differences in symptom severity at pre-treatment. Mixed repeated measures Analysis of Variance (ANOVA) was used to assess treatment group differences in changes in symptoms severity.
Survival analyses were conducted to assess the time to symptom remission for each treatment condition (Singer & Willett, 2003). Because SIGH-SAD data were collected each treatment week, weeks were the unit of time. Participants who did not experience remission during the 6-week study period were considered censored since they may not have been observed long enough to capture a remission. Our survival analyses yielded Kaplan-Meier survival probability estimates, which show the distribution of remissions over time as a proportion of the total sample. In this application, survival indicates “survival of the symptom,” such that the lower the survival probability, the greater the rate of remission. Differences between CBT-SAD and LT Kaplan-Meier survival probability estimates for each symptom were tested using a log-rank test, which compares the survival functions as whole. Hazard rates are the probabilities of remitting in a time period in those not having the symptom at the beginning of the period (i.e., not yet remitted). Thus, hazards represent the “pressure to remit” at a given point in time. Both survival and hazard functions were examined graphically to determine which symptoms had the most similar and most divergent patterns of remission between CBT-SAD and LT.
Results
Treatment Group Differences in Overall Symptom Severity Change
Time (pre-, post-treatment) by treatment (CBT-SAD, LT) mixed ANOVAs for each symptom (Table 2) resulted in only one marginally significant interaction effect (anxiety–somatic, p =.05) However, the groups’ mean scores on this symptom did not differ significantly at post-treatment (CBT-SAD: M =.66; LT: M =.76; SE=.16, Fisher Least Significant Difference, ns). Thus, the absence of significant time and treatment group effects suggests that pre-post treatment changes in the severity of each symptom were similar in the two treatment conditions. Furthermore, for every symptom, there was a significant main effect of time, indicating that, across both treatments, symptom severity decreased over the 6 weeks.
Table 2.
Main and interaction effects from repeated measures ANOVA
| Time | Tx Grp | Time * Tx Grp | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Symptom | F | dfTime,, dfError | p | F | dfTxGrp,, dfError | p | F | dfTime, dfError | p |
| Depressed Mood | 301.35 | 1, 153 | <0.001 | 1.41 | 1, 153 | 0.237 | 0.20 | 1, 153 | 0.659 |
| Work & Activities | 287.15 | 1, 157 | <0.001 | 1.16 | 1, 157 | 0.283 | 0.07 | 1, 157 | 0.795 |
| Social Withdrawal | 256.89 | 1, 147 | <0.001 | 4.84 | 1, 147 | 0.029 | 0.37 | 1, 147 | 0.541 |
| Genital Symptoms | 248.13 | 1, 106 | <0.001 | 0.96 | 1, 106 | 0.329 | 0.14 | 1, 106 | 0.709 |
| Somatic Symptoms (G.I) | 191.92 | 1, 50 | <0.001 | 2.01 | 1, 50 | 0.162 | 0.001 | 1, 50 | 0.970 |
| Weight Gain | 284.12 | 1, 63 | <0.001 | 0.40 | 1, 63 | 0.531 | 1.84 | 1, 63 | 0.180 |
| Appetite Increase | 145.20 | 1, 78 | <0.001 | 0.32 | 1, 78 | 0.576 | 0.37 | 1, 78 | 0.542 |
| Increased Eating | 198.78 | 1, 99 | <0.001 | 1.67 | 1, 99 | 0.200 | 0.12 | 1, 99 | 0.735 |
| Carbohydrate Craving/Eating | 141.55 | 1, 134 | <0.001 | 2.35 | 1, 134 | 0.128 | 0.12 | 1, 134 | 0.728 |
| Insomnia Early | 107.86 | 1, 61 | <0.001 | 4.08 | 1, 61 | 0.048 | 2.70 | 1, 61 | 0.106 |
| Insomnia Middle | 106.49 | 1, 107 | <0.001 | 0.24 | 1, 107 | 0.625 | 1.59 | 1, 107 | 0.209 |
| Hypersomnia | 121.31 | 1, 87 | <0.001 | 2.23 | 1, 87 | 0.139 | 0.27 | 1, 87 | 0.607 |
| Fatigability | 282.20 | 1, 161 | <0.001 | 0.13 | 1, 161 | 0.721 | 0.04 | 1, 161 | 0.838 |
| Feelings of Guilt | 270.41 | 1, 115 | <0.001 | 6.87 | 1, 115 | 0.010 | 1.48 | 1, 115 | 0.227 |
| Anxiety Psychic | 211.00 | 1, 123 | <0.001 | 7.12 | 1, 123 | 0.009 | 0.73 | 1, 123 | 0.393 |
| Anxiety Somatic | 145.69 | 1, 136 | <0.001 | 0.38 | 1, 136 | 0.540 | 3.90 | 1, 136 | 0.050 |
| Hypochondriasis | 126.49 | 1, 79 | <0.001 | 0.75 | 1, 79 | 0.390 | 0.01 | 1, 79 | 0.979 |
Note: Tx Grp = Treatment group.
Given the results above, we also examined whether the severity of each symptom at pre-treatment differed by treatment, corroborating the effectiveness of the random assignment. Independent samples t-tests (Table 3) revealed only two symptoms that differed significantly by treatment group at pre-treatment, feelings of guilt (with LT > CBT-SAD) and anxiety–somatic (with CBT-SAD > LT). Thus, the two treatment groups started the study with comparable severities on nearly all the symptoms studied.
Table 3.
Results of independent samples t-test at pre-treatment
| CBT-SAD | LT | |||||
|---|---|---|---|---|---|---|
| Symptom | M (SD) | M (SD) | t | df | 95% C.I. | p |
| Depressed Mood | 1.83 (0.68) | 1.76 (0.70) | 0.57 | 153 | −0.16, 0.28 | 0.568 |
| Work & Activities | 2.84 (0.41) | 2.74 (0.54) | 1.19 | 157 | −0.06, 0.24 | 0.235 |
| Social Withdrawal | 2.34 (1.04) | 2.15 (1.02) | 1.09 | 147 | −0.15, 0.52 | 0.278 |
| Genital Symptoms | 1.53 (0.51) | 1.48 (0.50) | 0.58 | 106 | −0.14, 0.25 | 0.562 |
| Somatic Symptoms (G.I) | 1.20 (0.41) | 1.33 (0.48) | −1.07 | 50 | −0.38, 0.12 | 0.288 |
| Weight Gain | 1.61 (0.50) | 1.66 (0.48) | −0.41 | 63 | −0.29, 0.19 | 0.681 |
| Appetite Increase | 2.08 (0.73) | 2.08 (0.86) | <0.001 | 78 | −0.36,0.36 | 1.000 |
| Increased Eating | 2.02 (0.70) | 1.91 (0.71) | 0.82 | 99 | −0.16, 0.40 | 0.416 |
| Carbohydrate Craving/Eating | 1.70 (0.77) | 1.58 (0.69) | 0.96 | 134 | −0.13, 0.37 | 0.339 |
| Insomnia Early | 1.50 (0.51) | 1.44 (0.50) | 0.44 | 62 | −0.20, 0.31 | 0.665 |
| Insomnia Middle | 1.57 (0.50) | 1.52 (0.50) | 0.57 | 107 | −0.14, 0.25 | 0.572 |
| Hypersomnia | 2.10 (1.10) | 1.92 (1.05) | 0.80 | 87 | −0.27, 0.64 | 0.426 |
| Fatigability | 3.05 (0.71) | 3.03 (0.81) | 0.15 | 161 | −0.22, 0.26 | 0.880 |
| Feelings of Guilt | 1.23 (0.50) | 1.54 (0.62) | −2.94 | 115 | −0.52, −0.10 | <0.01 |
| Anxiety Psychic | 1.33 (0.57) | 1.21 (0.41) | 1.34 | 123 | −0.06, 0.29 | 0.182 |
| Anxiety Somatic | 1.86 (0.77) | 1.62 (0.64) | 1.98 | 136 | <−0.01, 0.48 | <0.05 |
| Hypochondriasis | 1.89 (0.97) | 1.75 (0.89) | 0.69 | 79 | −0.27, 0.55 | 0.494 |
Note: Pre-Tx = Pre-treatment; M= Mean; SD = Standard Deviation.
Time to Symptom Remission
Survival analyses provided patterns across treatment weeks of (a) survival, i.e., the probability of the symptom continuing unremitted (“surviving”) and (b) hazards, i.e., the probability of remitting among those individuals who had not yet remitted. Figures 1–4 show the survival and hazard graphs for eight representative symptoms. Survival functions (Figures 1, 3) indicated that, regardless of treatment modality, the likelihood of remission for each of the 17 symptoms steadily increased as treatment progressed. This pattern is unsurprising given that both treatments resulted in significant and comparable episode remission rates and changes in overall symptom severity (see Rohan et al., 2015). The hazard functions of each of the 17 symptoms (Figures 2, 4) most frequently increased over time, suggesting that the pressure for each symptom to remit generally increased over time. However, in the sixth and last week of treatment risk of remission was particularly elevated in both treatment groups. This suggests that for both LT and CBT-SAD, there may be particular mechanisms contributing to symptom remission later on in treatment and, therefore, particular benefits of completing the entire course of treatment.
Figure 1.
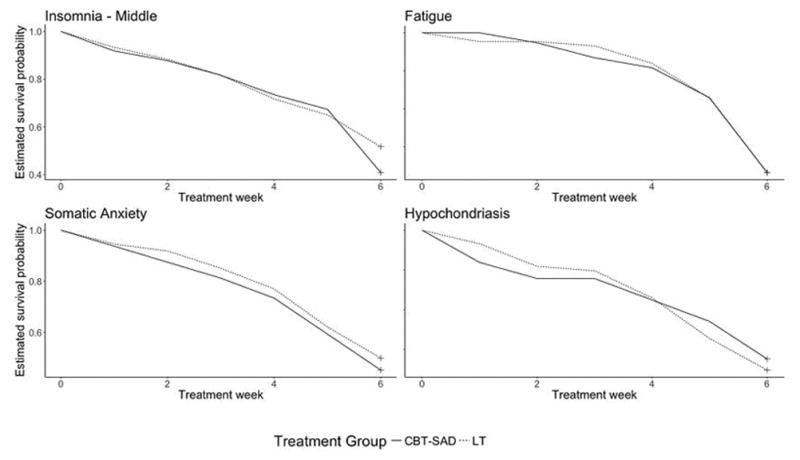
Estimated Survival Probability Curves Showing Similarities between CBT-SAD and LT
Figure 4.
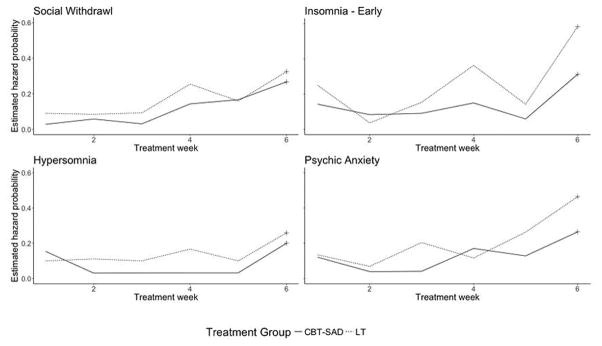
Estimated Hazard Probability Curves Showing Differences between CBT-SAD and LT
Figure 3.
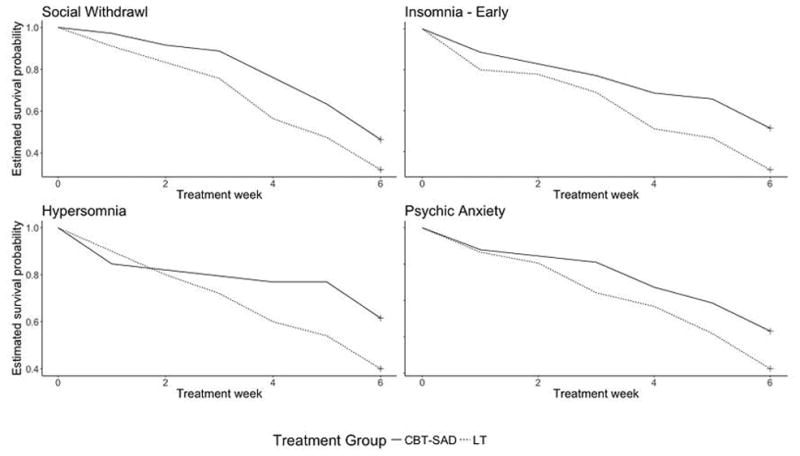
Estimated Survival Probability Curves Showing Differences between CBT-SAD and LT
Figure 2.
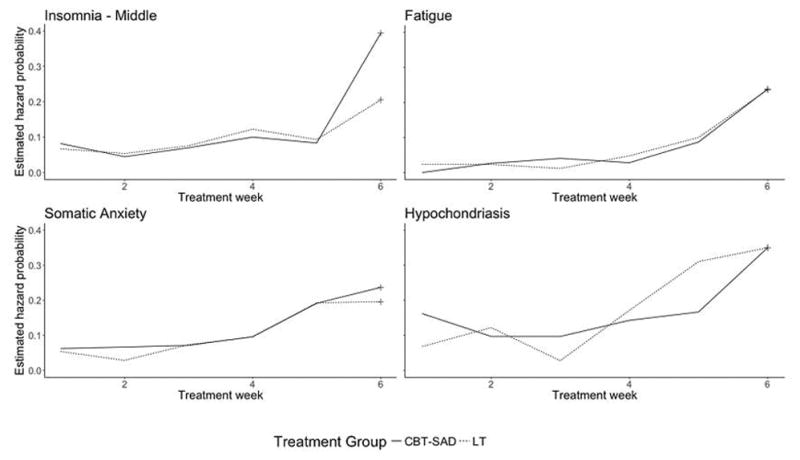
Estimated Hazard Probability Curves Showing Similarities between CBT-SAD and LT
Of the 17 symptoms studied, 13 failed to show a statistically significant difference between CBT-SAD and LT in the pattern of remission (Table 4). Figures 1 and 2 show the graphical results for four representative such symptoms: middle insomnia, fatigue, somatic anxiety, and hypochondriasis. Across treatments, the symptom remission rates are very similar (Figure 1) and hazard functions (Figure 2) do not show substantially different patterns.
Table 4.
Log-rank test results between groups for estimated survival curves
| CBT | LT | χ2 | df | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | n | O | E | (O-E)2/E | (O-E)2/V | n | O | E | (O-E)^2/E | (O-E)^2/V | |||
| Depressed Mood | 70 | 41 | 48.4 | 1.14 | 2.89 | 85 | 57 | 49.6 | 1.11 | 2.89 | 2.9 | 1 | 0.09 |
| Work & Activities | 73 | 45 | 52.3 | 1.03 | 2.56 | 86 | 60 | 52.7 | 1.02 | 2.56 | 2.6 | 1 | 0.11 |
| Social Withdrawal | 71 | 38 | 47.6 | 1.94 | 4.9 | 78 | 53 | 43.4 | 2.12 | 4.90 | 4.9 | 1 | <0.05 |
| Genital Symptoms | 45 | 27 | 28.5 | 0.78 | 0.16 | 63 | 41 | 39.5 | 0.06 | 0.16 | 0.2 | 1 | 0.69 |
| Somatic Symptoms (G.I) | 25 | 22 | 19.0 | 0.47 | 1.11 | 27 | 21 | 24 | 0.37 | 1.11 | 1.1 | 1 | 0.29 |
| Weight Gain | 33 | 26 | 28.3 | 0.18 | 512 | 32 | 30 | 27.7 | 0.19 | 0.51 | 0.5 | 1 | 0.47 |
| Appetite Increase | 40 | 22 | 24.8 | 0.32 | 0.77 | 40 | 25 | 22.2 | 0.35 | 0.77 | 0.8 | 1 | 0.38 |
| Increased Eating | 48 | 25 | 28.2 | 0.36 | 0.85 | 53 | 31 | 27.8 | 0.37 | 0.85 | 0.8 | 1 | 0.36 |
| Carbohydrate Craving/Eating | 64 | 23 | 26.7 | 0.52 | 1.13 | 72 | 32 | 28.3 | 0.50 | 1.13 | 1.1 | 1 | 0.29 |
| Insomnia Early | 28 | 17 | 23.6 | 1.84 | 4.75 | 36 | 31 | 24.4 | 1.78 | 4.75 | 4.7 | 1 | <0.05 |
| Insomnia Middle | 49 | 29 | 26.3 | 0.28 | 0.61 | 60 | 29 | 31.7 | 0.23 | 0.61 | 0.6 | 1 | 0.43 |
| Hypersomnia | 39 | 15 | 21.2 | 1.79 | 3.88 | 50 | 30 | 23.8 | 1.59 | 3.88 | 3.9 | 1 | <0.05 |
| Fatigability | 76 | 28 | 27.9 | <0.01 | <0.01 | 87 | 32 | 32.1 | <0.01 | <0.01 | <0.1 | 1 | 0.98 |
| Feelings of Guilt | 56 | 47 | 47.7 | 0.01 | 0.03 | 61 | 45 | 44.3 | 0.01 | 0.03 | <0.1 | 1 | 0.858 |
| Anxiety Psychic | 58 | 33 | 42.9 | 2.27 | 5.65 | 67 | 52 | 42.1 | 2.31 | 5.65 | 5.7 | 1 | <0.05 |
| Anxiety Somatic | 64 | 35 | 32.7 | 0.16 | 0.34 | 74 | 37 | 39.3 | 0.13 | 0.34 | 0.3 | 1 | 0.56 |
| Hypochondriasis | 37 | 24 | 25.3 | 0.07 | 0.16 | 44 | 31 | 29.7 | 0.06 | 0.16 | 0.2 | 1 | 0.69 |
Note: n = number of participants included in analysis; O = Observed; E = Expected, V = Variance
Of the 17 symptoms, four had statistically significantly different patterns of remission between CBT-SAD and LT (Table 4): social withdrawal, early insomnia, hypersomnia, and psychic anxiety. Relative to participants receiving CBT-SAD, remission of these four symptoms occurred more frequently and more rapidly in participants who received LT (Figure 3). This might be expected for the sleep-related symptoms, early insomnia and hypersomnia, since LT is known to affect circadian rhythms and is used as a treatment for sleep disturbances (Lewy, Lefler, Emens, & Bauer, 2006; Lewy, Sack, Miller, & Hoban, 1987). More unexpected, however, are the differences between treatments in remission of social withdrawal and psychic anxiety.
Examination of hazard functions (Figure 4) may provide clues to the nature of processes underlying these remission (survival) differences because hazards represent the unique risk associated with a particular period of time (Singer & Willett, 2003, p. 341). However, hazard estimates are often (as here) more variable than survival estimates because they depend in part on the number of participants remaining at risk in each time period (Singer & Willett, 2003, p. 349). In addition, as noted earlier, the final treatment week may include special remission processes for both groups, and so hazard function patterns might most clearly be interpreted from weeks one through five. It also is useful to remember in discerning patterns that the points in the hazard graphs are empirical values that include “error” around an underlying pattern – like the scatter of points around a regression line.
Overall, for all four symptoms at all weeks, the risk of remission was nearly always either close to equal or, more often, greater for LT compared to CBT-SAD. These higher risks of remission in LT contribute to the more rapid remissions seen in survival functions (Figure 3). For early insomnia and psychic anxiety, LT showed a generally increasing pressure to remit across time not seen with CBT-SAD. For hypersomnia, the pressure to remit is constant for both treatments, but greater for LT. For social withdrawal, the patterns for the two treatments were also parallel, constant over the first three weeks and increasing thereafter, with the pressure to remit greater for LT.
Discussion
For the large majority of symptoms included in this study (13/17; 76%), time to remission did not differ based on whether participants received CBT-SAD or LT. The fact that most the symptoms remitted comparably across time in the two treatments may account for the overall comparable episode remission rates (47.6% in CBT-SAD and 47.2% in LT), which were based on SIGH-SAD total scores (Rohan, Mahon, et al., 2015). On an individual symptom level, most depressive symptoms, typical and atypical, remitted comparably in treatments that presumably operate on distinct mechanisms. This finding is consistent with that of Stassen, Delini-Stula, and Angst (1993), who found using survival analysis that the time course of improvement in depression was no different between patients who responded to antidepressant medication and those who responded to placebo. Following the interpretation of those authors, it may be that there are common recovery mechanisms in SAD that can be triggered by either LT or CBT-SAD and that once those recovery mechanisms are triggered the course of recovery is essentially the same regardless of the treatment modality.
However, for four symptoms, LT led to more rapid remission than did CBT-SAD: early insomnia, hypersomnia, psychic anxiety, and social withdrawal. The observation that LT led to faster remission of two sleep symptoms is consistent with sleep having a circadian regulation component (Cirelli, 2009) and with the theory that SAD is related to a pathological circadian phase-shift that can be corrected with LT (Lewy et al., 2006; Lewy, Sack, Miller et al., 1987). Earlier remission of insomnia and hypersomnia with LT suggests that those in CBT-SAD with these particular symptoms may benefit from a supplementary course of chronobiological treatment in order to remit these particular symptoms more quickly. CBT-SAD is hypothesized to improve SAD by modifying psychological phenomena such as dysfunctional attitudes, maladaptive seasonal beliefs, rumination, and low response-contingent positive reinforcement in the winter, which are correlates of SAD (Hodges & Marks, 1998; Levitan et al., 1998; Rohan et al., 2003; Young & Azam, 2003). Given its focus on maladaptive cognitions and pleasurable activities, CBT-SAD may take longer than LT to reduce particular symptoms, like hypersomnia and early insomnia.
Why remission of social withdrawal and psychic anxiety would be faster with LT is less clear. Unfortunately, the SIGH-SAD does not contain any items that appear to capture negative cognitive styles (i.e., a tendency towards negative inferences and dysfunctional attitudes; see Alloy et al., 2000), which might be better candidates for earlier remission in CBT-SAD than in LT. For all four of these symptoms, it is useful to consider not only the time course of the symptom’s remission but also the time course of the treatments themselves. Aside from minor dose adjustments, LT is relatively homogenous across time. The generally rising hazard functions for all symptoms with LT suggest that the impact of LT accumulates with continuing treatment, increasing the pressure to remit. In contrast, CBT-SAD is not homogenous across time, but intentionally unfolds with various components scheduled across sessions according to a fixed protocol (Rohan, 2008): psychoeducation on the treatment rationale, behavioral activation, cognitive restructuring of automatic thoughts, core belief modification, and recurrence prevention. In addition, tailoring treatment to individuals in group therapy is different than individual dosage adjustment based on response and side effects. Thus, the time course of symptom remissions may differ between treatments because the time courses of the treatments differ. Despite these considerations, it is important to note that such treatment differences in symptom remission were the exception and occurred for only 4 of 17 symptoms.
Regardless of the exact connection between patterns of acute symptom remission and the mechanisms driving symptom change, a contribution of this study is a dissection of patterns of individual depressive symptom remission. Understanding the course of symptom remission may be one way for clinicians (and patients) to understand what response to expect from treatment. If the clinician is interested in specifically targeting sleep symptoms of SAD more rapidly, he/she may wish to pursue LT rather than CBT-SAD, or possibly augment CBT-SAD with LT. The treating clinician practicing CBT-SAD should recognize that the sleep-related symptoms may improve later in the course of treatment and should consider problem-solving ways to increase social activities during treatment if they are an important source of positive reinforcement for the patient. However, these results and the primary efficacy results in the parent study (Rohan et al., 2015) highlight that regardless of the assumed mechanism of action, cognitive or chronobiological, the substantial majority of symptoms and overall symptom severity are comparably reduced across 6-weeks of CBT-SAD and LT. To the treating clinician, the practical implication is that, overall, both treatments end up in the same place after six weeks of treatment, but the time course to arrive there is faster in LT than in CBT-SAD for certain symptoms.
This study has several limitations. One issue is the decision to study complete remission of a symptom, as opposed to substantial improvement or the trajectory of decreasing symptom severity. In addition to remission being of interest, this decision was based on the fact that “remitted” (0 on a SIGH-SAD item) has a relatively unequivocal meaning compared to other response anchors and that “remitted” means the same thing for all symptoms, as opposed to the variety of response options in the various SIGH-SAD items. However, complete remission is potentially a different construct than improvement or even episode remission. In fact, based our remission criteria (50% reduction in total SIGH-SAD score in addition to a HAM-D score ≤ 7 and an atypical score ≤ 7 or HAM-D score ≤ 2 and an atypical score ≤ 10), it is possible for an individual to meet typical criteria for SAD episode remission, while failing to completely remit on up to 14 different SAD symptoms. For each individual symptom to remit entirely may be atypical for most participants regardless of which treatment they received. In non-seasonal depression, residual symptoms at episode remission are commonplace and complete symptom remission is relatively rare (Tranter, O’Donovan, Chandarana, & Kennedy, 2002).
Several symptoms were not studied in the current secondary analyses because 40 or fewer participants endorsed them at pre-treatment. Among these excluded SIGH-SAD symptoms were weight loss, late insomnia, somatic symptoms, suicidal thoughts, psychomotor retardation, psychomotor agitation, depersonalization/derealization, paranoia, and obsessive/compulsive behaviors. Although these symptoms are less common and not considered core symptoms of SAD, understanding the course of less frequent symptoms may provide insights for clinicians who are working with patients with significant comorbidities or unusual SAD presentations.
In the parent trial, CBT-SAD had more durable effects than LT at follow-up two winters later (Rohan, Meyerhoff et al., 2015). Specifically, CBT-SAD participants had fewer depression recurrences (27.3% vs. 45.6%) two winters following treatment, less severe symptoms on the SIGH-SAD as well as the Beck Depression Inventory-Second Edition (BDI-II; Beck, Steer, & Brown, 1996), and a larger proportion of remissions defined by BDI-II ≤ 8 (68.3% vs. 44.5%) than LT participants. This study’s focus on the patterns of acute remission of individual SAD symptoms across theoretically distinct treatments reveals that while CBT-SAD has comparable treatment effects to LT at the episode level during acute treatment, and has clear prophylactic advantages over LT across time, for certain symptoms, CBT-SAD may operate more slowly than LT. This initial examination of the specific symptom-level data highlights that regardless of the underlying chronobiological or psychological mechanism of a particular SAD symptom, the psychological and chronobiological mechanisms may be more interrelated than previously thought. Psychological treatments such as CBT-SAD can effectively treat symptoms that are largely considered to be chronobiological in nature if given the proper amount of time for the treatment to take effect. Similarly, symptoms that might operate through psychological mechanisms can be effectively treated in the short term through chronobiological treatments. Future work examining the specific cognitive and chronobiological mechanisms of change leading to symptom-level remission is warranted.
Footnotes
Disclosure Statement: This work was supported by Grant R01MH078982 from the National Institute of Mental Health to Kelly J. Rohan. Kelly J. Rohan receives royalties from Oxford University Press for an SAD treatment manual.
References
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, … Lapkin JB. The Temple-Wisconsin Cognitive Vulnerability to Depression Project: lifetime history of axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2000;109(3):403–418. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nature Reviews Neuroscience. 2009;10(8):549–560. doi: 10.1038/nrn2683. http://doi.org/10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels J. Imputation of missing longitudinal data: a comparison of methods. Journal of Clinical Epidemiology. 2003;56(10):968–976. doi: 10.1016/s0895-4356(03)00170-7. http://doi.org/10.1016/S0895-4356(03)00170-7. [DOI] [PubMed] [Google Scholar]
- Enggasser JL, Young MA. Cognitive Vulnerability to Depression in Seasonal Affective Disorder: Predicting Mood and Cognitive Symptoms in Individuals with Seasonal Vegetative Changes. Cognitive Therapy and Research. 2007;31(1):3–21. http://doi.org/10.1007/s10608-006-9076-z. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Golden AM, Dalgleish T, Spinks H. Dysfunctional attitudes in seasonal affective disorder. Behaviour Research and Therapy. 2006;44(8):1159–1164. doi: 10.1016/j.brat.2005.09.004. http://doi.org/10.1016/j.brat.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Golden R, Gaynes B, Ekstrom R, Hamer R, Jacobsen F, Suppes T, … Nemeroff C. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. The Efficacy of Light Therapy in the Treatment of Mood Disorders: A Review and Meta-Analysis of the Evidence. 2005:162. doi: 10.1176/appi.ajp.162.4.656. http://doi.org/10.1176/appi.ajp.162.4.656. [DOI] [PubMed]
- Hodges S, Marks M. Cognitive characteristics of seasonal affective disorder: A preliminary investigation. Journal of Affective Disorders. 1998;50(1):59–64. doi: 10.1016/s0165-0327(98)00034-2. http://doi.org/10.1016/S0165-0327(98)00034-2. [DOI] [PubMed] [Google Scholar]
- Horikoshi M, Tang Y. ggfortify: Data Visualization Tools for Statistical Analysis Results (Version 0.1.0) 2015 Retrieved from https://CRAN.R-project.org/package=ggfortify.
- Iacoviello BM, Alloy LB, Abramson LY, Choi JY. The Early Course of Depression: A Longitudinal Investigation of Prodromal Symptoms and Their Relation to the Symptomatic Course of Depressive Episodes. Journal of Abnormal Psychology. 2010;119(3):459–467. doi: 10.1037/a0020114. http://doi.org/10.1037/a0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM, Alloy LB, Abramson LY, Choi JY, Morgan JE. Patterns of Symptom Onset and Remission in Episodes of Hopelessness Depression. Depression and Anxiety. 2013;30(6):564–573. doi: 10.1002/da.22085. http://doi.org/10.1002/da.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS statistics for Mac, version 24.0. 2016. [Google Scholar]
- Levitan RD, Rector NA, Bagby RM. Negative Attributional Style in Seasonal and Nonseasonal Depression. American Journal of Psychiatry. 1998;155(3):428–430. doi: 10.1176/ajp.155.3.428. http://doi.org/10.1176/ajp.155.3.428. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Singer CM, White DM. The phase shift hypothesis for bright light’s therapeutic mechanism of action: Theoretical considerations and experimental evidence. Psychopharmacology Bulletin. 1987;23(3):349–353. [PubMed] [Google Scholar]
- Lewy A, Lefler B, Emens J, Bauer V. The circadian basis of winter depression. The Circadian Basis of Winter Depression. 2006:103. doi: 10.1073/pnas.0602425103. http://doi.org/10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed]
- Lewy A, Sack R, Miller L, Hoban T. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235(4786):352–354. doi: 10.1126/science.3798117. http://doi.org/10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Magnusson A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatrica Scandinavica. 2000;101(3):176–184. http://doi.org/10.1034/j.1600-0447.2000.101003176.x. [PubMed] [Google Scholar]
- Mårtensson B, Pettersson A, Berglund L, Ekselius L. Bright white light therapy in depression: A critical review of the evidence. Journal of Affective Disorders. 2015;182(Supplement C):1–7. doi: 10.1016/j.jad.2015.04.013. http://doi.org/10.1016/j.jad.2015.04.013. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Retrieved from https://www.R-project.org/ [Google Scholar]
- Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, … Franzen P. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Research. 2013;210(1):150–158. doi: 10.1016/j.psychres.2013.05.023. http://doi.org/10.1016/j.psychres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ. Coping with the seasons a cognitive-behavioral approach to seasonal affective disorder: therapist guide. Oxford; New York: Oxford University Press; 2008. Retrieved from http://site.ebrary.com/id/10263641. [Google Scholar]
- Rohan KJ, Evans M, Mahon JN, Sitnikov L, Ho SY, Nillni YI, … Vacek PM. Cognitive-behavioral therapy vs. light therapy for preventing winter depression recurrence: study protocol for a randomized controlled trial. Trials. 2013;14(1):1. doi: 10.1186/1745-6215-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ, Mahon JN, Evans M, Ho S-Y, Meyerhoff J, Postolache TT, Vacek PM. Randomized trial of cognitive-behavioral therapy versus light therapy for seasonal affective disorder: Acute outcomes. American Journal of Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14101293. appi.ajp.2015.14101293. http://doi.org/10.1176/appi.ajp.2015.14101293. [DOI] [PMC free article] [PubMed]
- Rohan KJ, Meyerhoff J, Ho S-Y, Evans M, Postolache TT, Vacek PM. Outcomes one and two winters following cognitive-behavioral therapy or light therapy for seasonal affective disorder. American Journal of Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15060773. appi.ajp.2015.1. http://doi.org/10.1176/appi.ajp.2015.15060773. [DOI] [PMC free article] [PubMed]
- Rohan KJ, Roecklein KA, Haaga DAF. Biological and psychological mechanisms of seasonal affective disorder: A review and integration. Current Psychiatry Reviews. 2009;5:37–47. [Google Scholar]
- Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, Barton FB. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. Journal of Consulting and Clinical Psychology. 2007;75(3):489–500. doi: 10.1037/0022-006X.75.3.489. http://doi.org/10.1037/0022-006X.75.3.489. [DOI] [PubMed] [Google Scholar]
- Rohan KJ, Rough JN, Evans M, Ho SY, Meyerhoff J, Roberts LM, Vacek PM. A protocol for the Hamilton Rating Scale for Depression: Item scoring rules, Rater training, and outcome accuracy with data on its application in a clinical trial. Journal of Affective Disorders. 2016;200:111–118. doi: 10.1016/j.jad.2016.01.051. http://doi.org/10.1016/j.jad.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan K, Sigmon S, Dorhofer D. Cognitive-behavioral factors in seasonal affective disorder. Cognitive-Behavioral Factors in Seasonal Affective Disorder. 2003:71. doi: 10.1037//0022-006x.71.1.22. http://doi.org/10.1037/0022-006X.71.1.22. [DOI] [PubMed]
- Rosenthal N, Sack D, Gillin J, Lewy A, Goodwin F, Davenport Y, … Wehr T. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Seasonal Affective Disorder. A Description of the Syndrome and Preliminary Findings with Light Therapy. 1984:41. doi: 10.1001/archpsyc.1984.01790120076010. http://doi.org/10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; USA: 2003. [Google Scholar]
- Stassen HH, Delini-Stula A, Angst J. Time course of improvement under antidepressant treatment: A survival-analytical approach. European Neuropsychopharmacology. 1993;3(2):127–135. doi: 10.1016/0924-977x(93)90264-m. http://doi.org/10.1016/0924-977X(93)90264-M. [DOI] [PubMed] [Google Scholar]
- Therneau T. A Package for Survival Analysis in S (Version 2.38) 2015 Retrieved from https://CRAN.R-project.org/package=survival.
- Tranter R, O’Donovan C, Chandarana P, Kennedy S. Prevalence and outcome of partial remission in depression. Journal of Psychiatry & Neuroscience: JPN. 2002;27(4):241–247. [PMC free article] [PubMed] [Google Scholar]
- Whitcomb-Smith S, Sigmon ST, Martinson A, Young M, Craner J, Boulard N. The Temporal Development of Mood, Cognitive, and Vegetative Symptoms in Recurrent SAD Episodes: A Test of the Dual Vulnerability Hypothesis. Cognitive Therapy and Research. 2014;38(1):43–54. http://doi.org/10.1007/s10608-013-9577-5. [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- Wickham H. The Split-Apply-Combine Strategy for Data Analysis. Journal of Statistical Software. 2011;40(1):1–29. [Google Scholar]
- Wickham H, Francois R. dplyr: A Grammar of Data Manipulation (Version 0.4.3) 2015 Retrieved from https://CRAN.R-project.org/package=dplyr.
- Williams J, Link M, Rosenthal N, Amira L, Terman M. Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder Version (SIGH-SAD) New York: New York State Psychiatric Institute; 1992. [Google Scholar]
- Young MA, Azam OA. Ruminative Response Style and the Severity of Seasonal Affective Disorder. Cognitive Therapy and Research. 2003;27(2):223–232. http://doi.org/10.1023/A:1023565427082. [Google Scholar]
- Young MA, Grabler P. Rapidity of symptom onset in depression. Psychiatry Research. 1985;16(4):309–315. doi: 10.1016/0165-1781(85)90122-2. http://doi.org/10.1016/0165-1781(85)90122-2. [DOI] [PubMed] [Google Scholar]
- Young MA, Meaden PM, Fogg LF, Cherin EA, Eastman CI. Which environmental variables are related to the onset of seasonal affective disorder? Journal of Abnormal Psychology. 1997;106(4):554–562. doi: 10.1037//0021-843x.106.4.554. http://doi.org/10.1037/0021-843X.106.4.554. [DOI] [PubMed] [Google Scholar]
- Young M, Reardon A, Azam O. Rumination and vegetative symptoms: A test of the dual vulnerability model of seasonal depression. Rumination and Vegetative Symptoms: A Test of the Dual Vulnerability Model of Seasonal Depression. 2008:32. http://doi.org/10.1007/s10608-008-9184-z.
- Young M, Watel L, Lahmeyer H, Eastman C. The temporal onset of individual symptoms in winter depression: differentiating underlying mechanisms. The Temporal Onset of Individual Symptoms in Winter Depression: Differentiating Underlying Mechanisms. 1991:22. doi: 10.1016/0165-0327(91)90065-z. http://doi.org/10.1016/0165-0327(92)90069-I. [DOI] [PubMed]
- Zeileis A, Grothendieck G. zoo: S3 Infrastructure for Regular and Irregular Time Series. Journal of Statistical Software. 2005;14(6):1–27. [Google Scholar]