Abstract
Diversion of synthetic cannabinoids from the lab to drugs of abuse has become increasingly prevalent in recent years. Moreover, as earlier synthetic cannabinoids were banned, manufacturers introduced a new supply of novel compounds to serve as replacements. Hence, the chemical diversity of synthetic cannabinoid analogs has also rapidly increased. The present study examined 8 new synthetic cannabinoids: AM-1220, AM-2232, AM-2233, AM-679, EAM-2201, JWH-210, JHW-251, and MAM-2201. Each compound was assessed for binding affinity and functional activation of CB1 and CB2 receptors, and pharmacological equivalence with Δ9-tetrahydrocannabinol (THC) in THC drug discrimination. All compounds bound to and activated CB1 and CB2 receptors, although efficacy at the CB2 receptor was reduced compared to that for the CB1 receptor. Similarly, all compounds stimulated [35S]GTPγS binding through the CB1 receptor, and all compounds except AM-1220 and AM-2233 stimulated [35S]GTPγS binding through the CB2 receptor. Furthermore, these compounds, along with CP55,940, substituted for THC in THC drug discrimination. Rank order of potency in drug discrimination was correlated with CB1 receptor binding affinity. Together, these results suggest that all test compounds share the THC-like subjective effects of marijuana. Interestingly, the most potent compounds in CB1 binding in the present study were also the compounds that have been found recently in the U.S., MAM-2201, EAM-2201, JWH-210, AM-2233, and AM-1220. These results indicate that the evolution of the synthetic cannabinoid drug market may be focused toward compounds with increased potency.
Keywords: abuse liability, drug discrimination, receptor binding, synthetic cannabinoids, Δ9-tetrahydrocannabinol
1.0 Introduction
Products containing synthetic cannabinoids, commonly sold as “K2” or “spice,” have proliferated the recreational drug market over the last decade. These products are purchased online as “legal” alternatives to marijuana (Baumann et al., 2014; Ford et al., 2017; White, 2017). As earlier synthetic cannabinoids were banned, manufacturers introduced a new supply of novel compounds to serve as replacements (Seely et al., 2012; Shanks et al., 2012), producing an ever-evolving drug market for synthetic cannabinoids, in which users often ingest unknown chemical substances (Knittel et al., 2016).
Because of this evolution, newer synthetic cannabinoids have been found in the recreational drug market throughout the world. Recently, AM-2232, AM-1220, AM-679, JWH-251 were detected in powder and herbal products, and in hair and serum samples throughout Europe (Gregori et al., 2013; Hermanns-Clausen et al., 2016; Hermanns-Clausen et al., 2013; Jankovics et al., 2012; Kavanagh et al., 2013; Langer et al., 2014; Salomone et al., 2014). JWH-210 was detected in urine samples from U.S. military personnel (Wohlfarth et al., 2015), and in herbal incense products in the U.S. (Logan et al., 2012; Seely et al., 2013). More recently, MAM-2201, EAM-2201, JWH-210, AM-2233, and AM-1220 have been detected in seized products in a number of U.S. regions (NDEWS, 2016a; NDEWS, 2016b; NDEWS, 2016c; NDEWS, 2016d). Additionally, MAM-2201, AM-2233, and AM-1220 were found in seized plant products in Australia (Blakey et al., 2016), and AM-2232, MAM-2201, and EAM-2201 were detected in illegal products in Japan (Uchiyama et al., 2013).
Ingesting synthetic cannabinoids can produce several adverse consequences. A combination of MAM-2201, AM-1220, and AM-2232 led to a fatality (Zaitsu et al., 2015b), and a combination of 11 synthetic cannabinoids including, EAM-2201 and MAM-2201, led to diabetic ketoacidosis and death (Hess et al., 2015). JWH-210 produces immunosuppression, neurotoxicity in the nucleus accumbens, and cytotoxicity in brain, buccal, breast, and liver cells (Cha et al., 2015; Koller et al., 2013; Tomiyama & Funada, 2014; Yun et al., 2016). Furthermore, JWH-210 can cause central nervous system depression, cerebral seizures, and sympathomimetic toxicity (Hermanns-Clausen et al., 2016; Hermanns-Clausen et al., 2013). MAM-2201 suppresses neurotransmission, alters energy metabolism, inhibits CYP1A activity in liver, and produces cytotoxicity in forebrain (Ashino et al., 2014; Irie et al., 2015; Tauskela et al., 2016; Tomiyama & Funada, 2014; Zaitsu et al., 2015a).
Contributing to their abuse potential, many synthetic cannabinoids produce Δ9-tetrahydrocannabinol-like (THC) effects. Multiple structural classes of psychoactive cannabinoids, including tetrahydrocannabinols, bicyclic cannabinoids (e.g., CP55,940), aminoalkylindoles (e.g., WIN55,212-2), and other indole- and pyrrole-derived cannabinoids (e.g., JWH-018), bind to and activate CB1 receptors (Devane et al., 1988; Griffin et al., 1998). Furthermore, affinity and efficacy at the CB1 receptor, is often correlated with potency in producing THC-like effects in vivo (Compton et al., 1993; Pertwee, 2005; Wiley et al., 1998), including locomotor suppression, antinociception, hypothermia, catalepsy, and THC-like discriminative stimulus effects (Barrett et al., 1995; Martin et al., 1991; Vann et al., 2009). Moreover, compounds that bind to and activate CB1 receptors and produce cannabimimetic effects in animal models frequently produce “marijuana-like” intoxication in humans (Balster & Prescott, 1992).
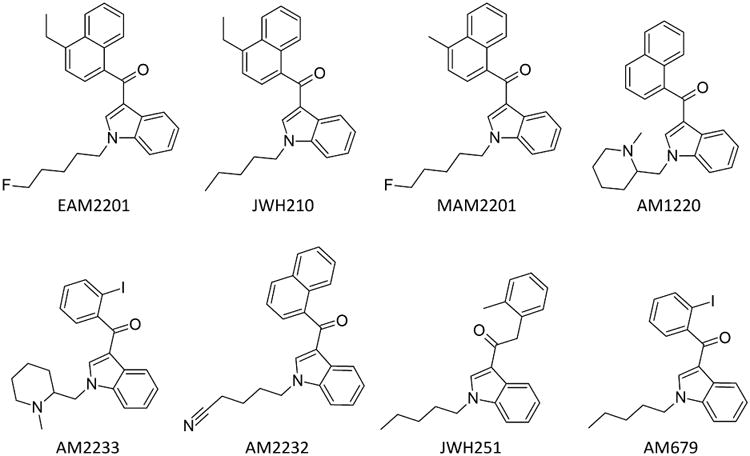
The objective of this study was to evaluate the pharmacology of eight synthetic cannabinoids, AM-1220, AM-2232, AM-2233, AM-679, EAM-2201, JWH-210, JHW-251, and MAM-2201 (Figure 1), many of which have been identified in recently confiscated products in the U.S. (NDEWS, 2016a; NDEWS, 2016b; NDEWS, 2016c; NDEWS, 2016d). Pharmacological assays were chosen based on their ability to predict cannabinoid abuse liability (Wiley & Martin, 2009). Each compound was assessed for binding affinity and functional activation of CB1 and CB2 receptors, and pharmacological equivalence with THC in THC drug discrimination.
Figure 1.
Chemical structures of test compounds.
2.0 Materials and Methods
2.1 Subjects
Singly housed male C57BL/6 inbred mice (20-25g; Jackson Laboratories, Bar Harbor, ME) were used for drug discrimination. Mice were maintained at 85-90% of free-feeding body weights by restricting daily ration of standard rodent chow. Mice were housed in temperature-controlled conditions (20–24 °C) with a 12 h standard light-dark cycle (lights on at 0600), and had ad libitum access to water in their home cages. All studies were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), the ARRIVE guidelines, and in accordance with our Institutional Animal Care and Use Committee (IACUC), and other federal and state regulations.
2.2 Drugs
THC [Δ9-tetrahydrocannabinol] and CP55,940 [(-)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol] were obtained from the National Institute on Drug Abuse (NIDA, Bethesda, MD) Drug Supply Program. AM-1220 [(R)-(1-((1-methylpiperidin-2-yl)methyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone], AM-2232 [5-(3-(1-naphthoyl)-1H-indol-1-yl)pentanenitrile], AM-2233 [1-[(N-methylpiperidin-2-yl)methyl]-3-(2-iodobenzoyl)indole], AM-679 [(2-iodophenyl)(1-pentyl-1H-indol-3-yl)methanone], EAM-2201 [(4-ethyl-1-naphthalenyl)[1-(5-fluoropentyl)-1H-indol-3-yl]-methanone], JWH-201 [4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone], JHW-251 [2-(2-methylphenyl)-1-(1-pentyl-1H-indol-3-yl)ethenone], and MAM-2201 [(1-(5-fluoropentyl)-1H-indol-3-yl)(4-methyl-1-naphthalenyl)-methanone] were provided by the Drug Enforcement Administration (DEA). For binding studies, cannabinoids were formulated in 1 mg/mL ethanol. For in vivo tests, cannabinoids were suspended in 7.8% Polysorbate 80 (VWR, Marietta, GA), and 92.2% saline (Butler Schein, Dublin, OH), and injected at a volume of 10 ml/kg.
Guanosine 5′ diphosphate, bovine serum albumin, ammonium acetate, and formic acid were purchased from Sigma Chemical (St. Louis, MO). GTPγS was purchased from Roche Diagnostics (Branchburg, NJ). [35S]GTPγS (1150-1300 Ci/mmol) and scintillation fluid (MicroScint 20) were obtained from Perkin Elmer (Waltham, MA). HPLC grade acetonitrile and water were purchased from Fisher Scientific (Fairlawn, NJ). Reference standards were obtained from Cayman Chemical (Ann Arbor, MI). The cannabinoid receptor binding assay utilized human CB1 (hCB1) and human CB2 (hCB2) membrane preparations purchased from Perkin Elmer, isolated from a HEK-293 expression system.
2.3 Equipment
Drug discrimination experiments were conducted in mouse operant chambers (Coulbourn Instruments, Whitehall, PA), housed within light- and sound-attenuating cubicles. Chambers contained two nose poke apertures, with stimulus lights located over each aperture, and a house light. A pellet dispenser delivered 20-mg food pellets (Bioserv Inc., Frenchtown, NJ) into an illuminated food cup that was centered between the two apertures. Experimental events and recording of data were controlled by a computer-based system (Coulbourn Instruments, Graphic State Software, v 3.03, Whitehall, PA).
2.4 Receptor Binding
Binding assays were conducted as previously described (e.g., see Zhang et al., 2010). Binding was initiated by adding 40 fmol of cell membrane proteins to assay tubes containing [3H]CP55,940 (ca. 130 Ci/mmol), and a sufficient quantity of buffer A (50 mM Tris•HCl, 1 mM EDTA, 3 mM MgCl2, 5 mg/mL BSA, pH 7.4) to create 0.5 mL incubation volume. In the displacement assays, the concentration of [3H]CP55,940 was 0.62 nM. Nonspecific binding was determined by including 10 μM unlabeled CP55,940. Following incubation at 30°C for 1 hr, binding was terminated by vacuum filtration through GF/C glass fiber filter plates (Packard, Meriden, CT) which were pre-soaked with 0.1% polyethyleneimine (PEI) in a 96-well sampling manifold (Millipore, Bedford, MA). Reaction vessels were washed twice with 4 mL of ice cold buffer (50 mM Tris•HCl, 1 mg/mL BSA). The filter plates were air-dried and sealed on the bottom. Liquid scintillate was added to the wells and the top sealed. After incubating the plates for a minimum of 2 hrs, the radioactivity present was determined by liquid scintillation spectrometry. Assays were done in duplicate, and results represent combined data from at least three independent experiments.
2.5 Agonist-Stimulated [35S]GTPγS Binding
G-protein coupled signal transduction ([35S]GTPγS) assays of test compounds were conducted in an incubation mixture consisting of a test compound (250 nM–1mM), GDP (20 μM), [35S]GTPγS (100 pM), and the hCB1 and hCB2 membrane preparations (0.4 pM) in 0.4 mL of assay buffer [50 mM TRIS-HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 5 mM MgCl2, 0.5% (w/v) BSA]. Nonspecific binding was determined in 100 μM unlabeled GTPγS, and basal binding was determined in the absence of drug. Duplicate samples were incubated for 1 hr at 30°C, and the bound complex was filtered from the reaction mixture and counted in a liquid scintillation counter.
2.6 Drug Discrimination
Adult male mice (n=8) were trained to discriminate THC using previously described procedures (Vann et al., 2009). Prior to this investigation, mice were trained to respond on one of two nose poke apertures following i.p. administration of 5.6 mg/kg THC, and to respond on the other aperture following i.p. vehicle injection (Wiley et al., 2015). Responding was reinforced on a fixed ratio 10 (FR10) schedule, under which 10 consecutive responses on the correct (injection-appropriate) aperture was reinforced with a food pellet. Responses on the incorrect aperture reset the ratio requirement on the correct aperture. Daily injections were administered on a double alternation sequence of THC and vehicle (e.g., drug, drug, vehicle, vehicle). Daily 15 min training sessions were conducted Monday-Friday until the mice consistently met three criteria: (1) the first completed FR10 was on the correct aperture, (2) ≥ 80% of the total responding occurred on the correct aperture, and (3) response rate was ≥ 0.17 responses/s. After the criteria were met, acquisition of the discrimination was established and substitution testing began.
Stimulus substitution tests were conducted up to twice a week during 15 min test sessions, with maintenance of training on intervening days. During test sessions, 10 consecutive responses on either aperture delivered reinforcement. To be tested, mice had to meet the 3 acquisition criteria on the preceding day and during the previous training session with the alternate training compound (training drug or vehicle). A dose-effect curve was determined with THC. Prior to the present study, mice were tested with other synthetic cannabinoids, as reported previously (Wiley et al., 2015). For the present study, the dose-effect curve for THC was redetermined, and dose-effect curves were obtained for JWH-210, EAM-2201, AM-1220, JHW-251, AM-2233, MAM-2201, AM-679, AM-2232, and CP55,940, in that order.
2.7 Binding Data Analysis
Specific binding was calculated as [(total binding - nonspecific binding)/(total basal binding - nonspecific binding)]. Non-specific binding was subtracted from each sample. Data were plotted and analyzed with GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Displacement data were analyzed by unweighted nonlinear regression of receptor binding data. For displacement studies, curve-fitting and Ki calculation were made with GraphPad Prism, which fits the data to one- and two-site models, and compares the two fits statistically.
Ki values were estimated from IC50 values using the Cheng-Prusoff equation. Data for [35S]GTPγS binding experiments are reported as mean and standard error of at least three replicates. Net stimulated [35S]GTPγS binding was defined as agonist-stimulated minus basal [35S]GTPγS binding, and percent stimulation was defined as (net-stimulated/basal [35S]GTPγS binding) ×100. Nonlinear iterative regression analyses of agonist concentration-effect curves were performed with GraphPad Prism. Significance was defined as p < 0.05.
2.8 Drug Discrimination Data Analysis
During the study, some mice were removed from the study due to non-drug-related health problems. Eight mice completed testing of THC, JWH-210, EAM-2201, AM-1220, and JWH-251; 7 mice completed testing of AM-2233 and MAM-2201; 6 mice completed testing of AM-679 and AM-2232; 5 mice completed testing of CP55,940. For each session, percentage of responses on the drug-associated aperture and response rate (responses/s) were calculated. Full substitution was defined as ≥ 80% responding on the drug-associated aperture (Vann et al., 2009). ED50 values were calculated on the linear part of the drug aperture selection dose-response curve for each drug using least squares linear regression analysis, followed by calculation of 95% confidence intervals (CI). Since mice that responded fewer than 10 times during a test session did not earn a reinforcer, their data were excluded from analysis of drug aperture selection, but their response rate data were included. Response-rate data were analyzed using repeated-measures ANOVA across dose. A mean substitution procedure was used to maintain equal n's across doses within drug in the case of missing data (i.e., one mouse was tested with all doses of JWH-251 except 3 mg/kg; one mouse was tested with all doses of CP55,940 except 0.3 mg/kg). Significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means.
Pearson product-moment coefficients were computed to assess the relationship between in vitro affinity, efficacy, and drug discrimination ED50 values. Pair-wise removal of missing values was used.
3.0 Results
3.1 Cannabinoid Receptor Binding and Agonist-Stimulated [35S]GTPγS Binding
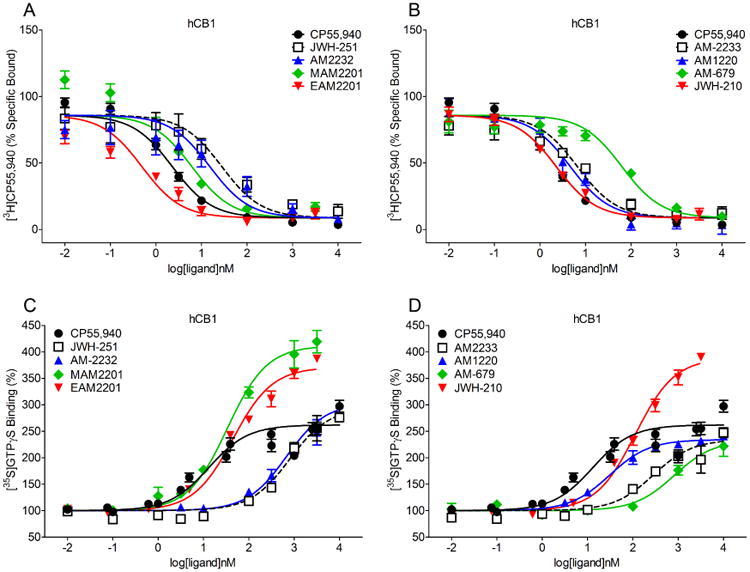
All test compounds displaced [3H]CP55,940 at the CB1 receptor binding site (Figure 2, panels A and B), with affinities ranging from 0.41 nM for EAM-2201 to 39.6 nM for AM-679 (Table 1). All test compounds also stimulated [35S]GTPγS turnover in hCB1 transfected cell membranes (Figure 2, panels C and D). The high affinity positive control CP55,940 was the most potent compound at the CB1 receptor binding site, followed by MAM-2201, AM-1220, and EAM-2201 (Table 1). However, MAM-2201, JWH-210, EAM-2201, JWH-251, and AM-2232 exhibited enhanced efficacy at CB1 compared to CP55,940 (Table 1). The compounds with the lowest affinities for the CB1 receptor, AM2322, JWH-251, and AM-679, also had the lowest potencies in the [35S]GTPγS assay, albeit even these compounds were able to increase [35S]GTPγS binding over basal levels.
Figure 2.
Displacement of [3H]CP55,940 (panels A-B) and stimulation of [35S]GTPγS turnover (panels C-D) at the hCB1 receptor (± SEM).
Table 1.
Binding affinity, potency and efficacy for stimulation of [35S]GTPγS turnover at hCB1 and hCB2 receptors, and potencies for substitution in THC discrimination.
| Compound | CB1 Kia | [35S]GTPγS Turnoverb | CB2 Kia | [35S]GTPγS Turnoverb | ED50 (μmol/kg)e | ||
|---|---|---|---|---|---|---|---|
| CB1 EC50c | CB1 Emaxd | CB2 EC50c | CB2 Emaxd | ||||
| CP55,940 (376.6) | 1.15 (0.13) 21 | 16.9 (3.8) 13 | 163.3 (8.0) 13 | 0.55 (0.05) 16 | 9.07 (4.94) 15 | 55.1 (4.1) 15 | 0.07 (0.04-0.14) |
| THC (314) | 40.7f (1.70) | 77.5g (30.4) | 236g (12) | 36.4f (10.0) | 12.3g (6.8) | 139g (13) | 4.96 (3.41-7.21) |
| EAM-2201 (387.5) | 0.41 (0.04) 5 | 46.8 (5.0) 4 | 270.3 (5.1) 4 | 0.38 (0.05) 3 | 91.9 (65.1) 4 | 40.2 (11.1) 4 | 0.04 (0.04-0.05) |
| JWH-210 (369.5) | 1.43 (0.39) 4 | 116.1 (8.2) 4 | 287.4 (8.6) 4 | 0.94 (0.19) 3 | 51.0 (15.5) 4 | 43.6 (6.5) 4 | 0.33 (0.23-0.46) |
| MAM-2201 (373.5) | 1.86 (0.29) 4 | 36.1 (4.7) 3 | 310.2 (20.9) 3 | 0.59 (0.12) 3 | 1.93 (0.86) 4 | 23.2 (8.2) 4 | 0.11 (0.08-0.13) |
| AM-1220 (382.5) | 2.40 (0.30) 4 | 44.4 (12.4) 3 | 131.5 (2.9) 3 | 0.26 (0.04) 4 | Not determinable | Not determinable | 0.26 (0.18-0.38) |
| AM-2233 (458.3) | 6.63 (0.78) 5 | 213.3 (19.8) 3 | 143.6 (14.6) 3 | 0.48 (0.06) 3 | 715.1 (663.9) 2 | 21.0 (3.4) 2 | 0.42 (0.27-0.64) |
| AM-2232 (352.4) | 14.5 (7.1) 4 | 833.2 (57.6) 3 | 203.5 (8.1) 3 | 11.2 (1.2) 3 | 406.4 (323.2) 2 | 31.3 (10.4) 2 | 0.54 (0.32-0.92) |
| JWH-251 (319.4) | 16.2 (3.2) 4 | 918.4 (165.2) 3 | 227.6 (31.6) 3 | 15.1 (3.3) 3 | 1518.1 (1450.9) 2 | 18.3 3.1 2 | 4.07 (3.11-5.31) |
| AM-679 (417.3) | 39.6 (7.3) 4 | 880.6 (170.0) 3 | 131.7 (19.5) 3 | 2.10 (0.66) 3 | 19.0 (9.2) 4 | 31.1 (8.6) 4 | 5.80 (4.30-7.84) |
Values represent Kis (± SEM) in nM for [3H]CP55,940 displacement at specified receptor.
For each measure, n is shown below the SEM.
Values represent EC50s (± SEM) in nM for [35S]GTPγS binding at specified receptor.
Values represent % maximal increase (± SEM) for [35S]GTPγS binding over basal at specified receptor.
ED50s (± 95% confidence limits) are expressed in μmol/kg. Molecular weights are provided in parentheses below the compound names.
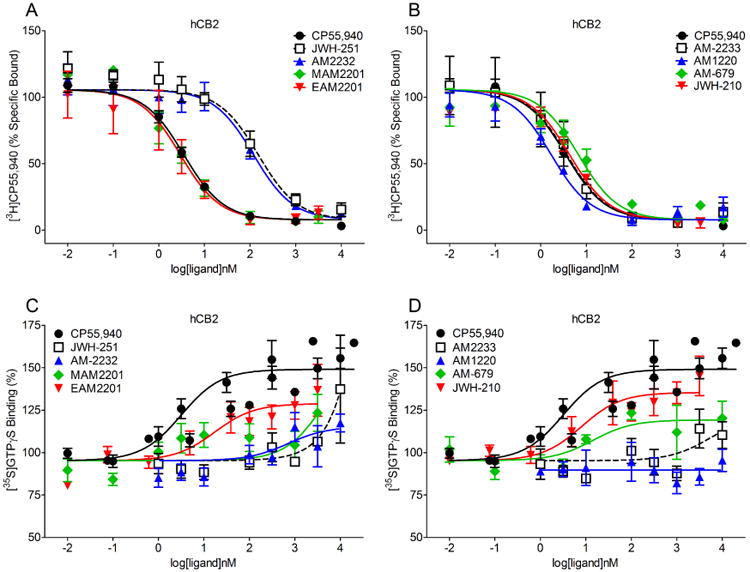
Similar to their effects at the CB1 receptor, all test compounds displaced [3H]CP55,940 at the CB2 receptor binding site (Figure 3, panels A and B), with affinities that were similar to or greater than those obtained at the CB1 receptor (Table 1). In contrast with their high affinities for the CB2 receptor, all test compounds and CP55,940 showed reduced efficacy at CB2 compared to CB1 (Figure 3, panels C and D). Maximal CB2 efficacy was observed with CP55,940, and slightly lower efficacies were observed for JWH-210 and EAM-2201. However, potencies for stimulating [35S]GTPγS turnover at the CB2 receptor varied greatly across the compounds (Table 1). Whereas MAM-2201 was approximately 4-fold more potent than CP55,940, other compounds (including several high affinity hCB2 ligands, such as AM-1220) failed to fully stimulate the CB2 receptor at high concentrations, such that potency and efficacy could only be extrapolated, or were not determinable.
Figure 3.
Displacement of [3H]CP55,940 (panels A-B) and stimulation of [35S]GTPγS turnover (panels C-D) at the hCB2 receptor (± SEM).
3.2 Drug Discrimination
Mice trained to discriminate THC from vehicle showed full, dose-dependent substitution for THC, and full substitution at the 5.6 mg/kg THC training dose (Figure 4, panels A-B). All synthetic cannabinoids also fully substituted for THC, and all showed dose-dependent substitution except EAM-2201, which produced a bitonic function with substitution at the middle dose (Figure 4, panels A-B). Rank order of potency for substitution was EAM-2201 > CP55,940 > MAM-2201 >AM-1220 > JWH-210 > AM-2232 > AM-2233 > JWH-251 > THC > AM-679, which was similar to the rank order potency for CB1 binding affinity (Table 1). During control tests of vehicle and 5.6 mg/kg THC, mice responded predominantly on the vehicle- and THC-associated apertures, respectively, across the course of the study (Figure 4, panels A-B).
Figure 4.
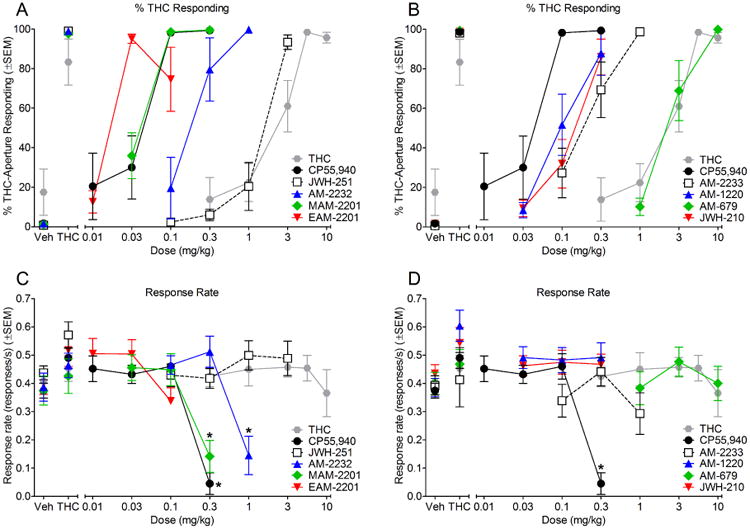
Effects of cannabinoids on percentage of responses on the THC-associated aperture (panels A-B) and response rate (panels C-D). Points above Veh and THC show data for tests of vehicle and 5.6 mg/kg THC, respectively, conducted before each dose-effect determination. All doses of THC and JWH-251 (n=7-8); all doses of JWH-210, EAM-2201, and AM-1220 (n=8); all doses of AM-2233 (n=6-7); all doses of MAM-2201 (n=5-7); all doses of AM-679 (n=6); 0.1-0.3 mg/kg AM-2232 (n=6), 1.0 mg/kg AM-2232 (n=3 for % THC responding and n=6 for response rate); 0.01-0.1 mg/kg CP55,940 (n=5), 0.3 mg/kg CP55,940 (n=1 for % THC responding and n=4 for response rate). Asterisks (*) indicate significant differences (p<0.05) compared to vehicle.
All synthetic cannabinoids substituted for THC at a minimum of one dose that did not significantly reduce response rate. Although full substitution also occurred at 0.3 mg/kg MAM-2201, 1.0 mg/kg AM-2232, and 0.3 mg/kg CP55,940, these effects were accompanied by reductions in response rates [MAM-2201: F(3,18)=17.06, p<0.05; AM-2232: F(3,15)=10.19, p<0.05; CP55,940: F(4,16)=28.30, p<0.05] (Figure 4, panels C-D). There was also a significant effect of dose on response rate for EAM-2201 [F(3,21)=7.48, p<0.05], but no doses produced significant differences from vehicle. THC did not alter response rates compared to vehicle across the dose range tested (Figure 4, panels C-D).
Pearson product-moment coefficients were computed between each in vitro dependent variable and drug discrimination ED50 values to demonstrate which in vitro measure was most highly associated with in vivo subjective effects. There was a significant positive correlation between CB1 receptor binding affinity and drug discrimination ED50 values [r(18)=0.93, p < 0.05]. No other in vitro measure was significantly correlated with drug discrimination ED50 values.
4.0 Discussion
All test compounds bound to the CB1 receptor with nanomolar affinity, and activated G-proteins, similar to CP55,940 (present study) and other psychoactive cannabinoids, including phytocannabinoids (Thomas et al., 1998), aminoalkylindoles (Compton et al., 1992a), and bicyclic cannabinoids (Compton et al., 1992b). All eight test compounds also bound to the CB2 receptor with nanomolar affinity, although efficacy at the CB2 receptor was reduced compared to the CB1 receptor, which was also true for the positive control CP55,940. These results are in accordance with previous studies which found that EAM-2201, MAM-2201, JWH-210, and JWH-251 are potent and effective agonists at the CB1 receptor (Cannaert et al., 2016; Cha et al., 2014; Cha et al., 2015; Costain et al., 2016; Gatch & Forster, 2016; Huffman et al., 2005), and MAM-2201, JWH-210, and EAM-2201 also activate the CB2 receptor (Cannaert et al., 2016). While the present study found that JWH-251 had an affinity of 15 ± 3 nM for the hCB2 receptor, a previous study using similar methods reported a lower CB1 affinity of 146 ± 36 nM (Huffman et al., 2005). The reason for this discrepancy is unknown, however, the estimates for the CB1 affinity of JWH-251 were less dissimilar between the two laboratories (16 ± 3 nM vs 29 ± 3 nM), despite having been evaluated in CB1 receptors derived from different species (rat in previous study and human in current study).
Interestingly, AM-1220 and AM-2233 showed high affinity for the CB2 receptor, but did not stimulate [35S]GTPγS binding through the CB2 receptor. AM-1220 and AM-2233 are racemic mixtures, and their profile of affinity and efficacy suggests that the (piperidin-2-yl)methyl side chain may permit ligand binding, but favors or promotes conformations of the CB2 receptor that do not promote G-protein activation. The racemic nature of AM-2233 also affects binding affinity, with most of AM-2233's CB1 receptor affinity originating from the (R)-enantiomer (Deng et al., 2005). Given these findings, it is possible that AM-1220 and AM-2233 can act as CB1 receptor agonists and CB2 receptor antagonists, depending upon the tone of the endocannabinoid signaling system.
All test compounds substituted fully for THC in drug discrimination. Only two of the test compounds were previously examined for in vivo pharmacological effects. In accordance with the present study, JWH-210 decreased locomotor activity and balance, and produced conditioned place preference (Cha et al., 2014; Gatch & Forster, 2016), and both JWH-210 and AM-2233 substituted for THC in drug discrimination in rats (Gatch & Forster, 2016; Jarbe et al., 2011; Wiley et al., 2014). Furthermore, results of the present study are consistent with research showing that other synthetic cannabinoids, including JWH-018, JWH-073, JWH-200, JWH-203, JWH-250, AM-2201, CP 47,497-C8-homolog (Gatch & Forster, 2014), ADBICA, ADB-PINACA, THJ-2201, RCS-4, JWH-122, JWH-210 (Gatch & Forster, 2016), XLR-11, UR-144 (Wiley et al., 2013), AB-CHMINACA, AB-PINACA, and FUBIMINA (Wiley et al., 2015), also substituted for THC in drug discrimination.
The THC discrimination assay is a pharmacologically selective animal model that predicts marijuana-like subjective effects of novel compounds in humans (Balster & Prescott, 1992; Jarbe & Gifford, 2014). Its pharmacological selectivity has been empirically demonstrated in mice (McMahon et al., 2008; Vann et al., 2009), rats (Barrett et al., 1995), nonhuman primates (Wiley et al., 1995), and humans (Lile et al., 2009). Results of the present study indicate that all test compounds likely share the THC-like subjective effects that humans have reported when using other synthetic cannabinoids (Gunderson et al., 2012). Additionally, rank order potency of substitution for THC in drug discrimination was positively correlated with affinity for the CB1 receptor in the present study. These results suggest that the discriminative stimulus effects of the test compounds are CB1 receptor-mediated. Future studies could confirm this by examining if the CB1 receptor antagonist rimonabant blocks these discriminative stimulus effects. The CB1 receptor affinity of these synthetic cannabinoids correlated with their potency in the drug discrimination assay; however, this was not observed with CB2 receptor affinity. These findings are consistent with previous observations that the discriminative stimulus effects of these synthetic cannabinoids are CB1 receptor dependent, as verified by the CB1R-selective inverse agonist/antagonist rimonabant eliciting rightward shifts in the dose-response curve, and pA2 and pKB analyses for quantitative confirmation of CB1R mediation (Grim et al., 2016; Grim et al., 2017; Hruba & McMahon, 2017).
The present study is the first to examine the in vivo pharmacology of AM-1220, AM-2232, AM-679, EAM-2201, JHW-251, and MAM-2201. Demonstrating rewarding or reinforcing effects of THC and other cannabinoids using common preclinical measures of abuse liability (e.g., self-administration, conditioned place preference, and intra-cranial self-stimulation) is particularly challenging, and results have been inconsistent (Chaperon et al., 1998; Gardner et al., 1988; Lefever et al., 2014; Valjent & Maldonado, 2000; Vlachou et al., 2007). Hence, drug discrimination is particularly useful for measuring abuse liability of cannabinoids. Like those before them, these synthetic cannabinoids produced THC-like effects, suggesting high abuse liability. The potential for abuse may be particularly high for EAM-2201 and MAM-2201 because they are some of the most potent synthetic cannabinoids that have been found on the drug market to date (Cannaert et al., 2016). Furthermore, EAM-2201 and MAM-2201 have been found recently in the U.S. and Australia (Blakey et al., 2016; NDEWS, 2016a; NDEWS, 2016b; NDEWS, 2016d), both of which have not yet formally controlled these compounds, albeit controlling specific compounds is not necessarily the best solution because it would likely engender a new generation of synthetic cannabinoids on the drug market (Knittel et al., 2016).
5.0 Conclusion
In summary, all test compounds produced THC-like effects in cannabinoid receptor binding and efficacy, and in THC drug discrimination, with few departures from effects of the positive control compounds CP55,940 and THC. The primary difference between compounds was potency. All synthetic cannabinoids tested here were more potent than THC in CB1 and CB2 binding and drug discrimination, similar to many other synthetic cannabinoids (for a review see Jarbe & Raghav, 2017; Wiley et al., 2017), with the exception being that AM-679 was slightly less potent than THC in drug discrimination. Together, these results suggest that all test compounds would share the THC-like subjective effects of marijuana. These effects are consistent with the detection of these products in several countries (Blakey et al., 2016; Gregori et al., 2013; Hermanns-Clausen et al., 2016; Hermanns-Clausen et al., 2013; Jankovics et al., 2012; Kavanagh et al., 2013; Langer et al., 2014; Salomone et al., 2014; Uchiyama et al., 2013), including detection in recently seized products in the U.S. (NDEWS, 2016a; NDEWS, 2016b; NDEWS, 2016c; NDEWS, 2016d). Interestingly, the most potent compounds in CB1 binding in the present study were also the compounds that have been found recently in the U.S., MAM-2201, EAM-2201, JWH-210, AM-2233, and AM-1220, suggesting that the evolution of the synthetic cannabinoid drug market may be focused toward more potent compounds.
The present study examined 8 new synthetic cannabinoids for pharmacological effects.
All compounds bound to and activated CB1 and CB2 receptors.
These compounds substituted for THC in THC drug discrimination.
Potency in drug discrimination was correlated with CB1 receptor binding affinity.
The drug market is trending toward synthetic cannabinoids with increased potency.
Acknowledgments
The authors thank Nikita Pulley and Michael Wallgren for technical assistance. Research was generously supported by National Institute on Drug Abuse grants DA-003672 and DA-040460, and a National Institute of Justice/Drug Enforcement Administration contract DJD-14-HQ-G-0170.
Abbreviations
- AM-1220
(R)-(1-((1-methylpiperidin-2-yl)methyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone
- AM-2232
5-(3-(1-naphthoyl)-1H-indol-1-yl)pentanenitrile
- AM-2233
1-[(N-methylpiperidin-2-yl)methyl]-3-(2-iodobenzoyl)indole
- AM-679
(2-iodophenyl)(1-pentyl-1H-indol-3-yl)methanone
- CP55,940
(-)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- EAM-2201
(4-ethyl-1-naphthalenyl)[1-(5-fluoropentyl)-1H-indol-3-yl]-methanone
- GTP
guanosine-5′-triphosphate
- JWH-210
4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone
- JHW-251
2-(2-methylphenyl)-1-(1-pentyl-1H-indol-3-yl)ethenone
- MAM-2201
(1-(5-fluoropentyl)-1H-indol-3-yl)(4-methyl-1-naphthalenyl)-methanone
- THC
Δ9-tetrahydrocannabinol
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashino T, Hakukawa K, Itoh Y, Numazawa S. Inhibitory effect of synthetic cannabinoids on CYP1A activity in mouse liver microsomes. J Toxicol Sci. 2014;39:815–820. doi: 10.2131/jts.39.815. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Solis E, Jr, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Bath salts, spice, and related designer drugs: the science behind the headlines. J Neurosci. 2014;34:15150–15158. doi: 10.1523/JNEUROSCI.3223-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey K, Boyd S, Atkinson S, Wolf J, Slottje PM, Goodchild K, et al. Identification of the novel synthetic cannabimimetic 8-quinolinyl 4-methyl-3-(1-piperidinylsulfonyl)benzoate (QMPSB) and other designer drugs in herbal incense. Forensic Sci Int. 2016;260:40–53. doi: 10.1016/j.forsciint.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Cannaert A, Storme J, Franz F, Auwarter V, Stove CP. Detection and Activity Profiling of Synthetic Cannabinoids and Their Metabolites with a Newly Developed Bioassay. Anal Chem. 2016;88:11476–11485. doi: 10.1021/acs.analchem.6b02600. [DOI] [PubMed] [Google Scholar]
- Cha HJ, Lee KW, Song MJ, Hyeon YJ, Hwang JY, Jang CG, et al. Dependence Potential of the Synthetic Cannabinoids JWH-073, JWH-081, and JWH-210: In Vivo and In Vitro Approaches. Biomol Ther (Seoul) 2014;22:363–369. doi: 10.4062/biomolther.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HJ, Seong YH, Song MJ, Jeong HS, Shin J, Yun J, et al. Neurotoxicity of Synthetic Cannabinoids JWH-081 and JWH-210. Biomol Ther (Seoul) 2015;23:597–603. doi: 10.4062/biomolther.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992a;263:1118–1126. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: Classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992b;260:201–209. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Costain WJ, Tauskela JS, Rasquinha I, Comas T, Hewitt M, Marleau V, et al. Pharmacological characterization of emerging synthetic cannabinoids in HEK293T cells and hippocampal neurons. Eur J Pharmacol. 2016;786:234–245. doi: 10.1016/j.ejphar.2016.05.040. [DOI] [PubMed] [Google Scholar]
- Deng H, Gifford AN, Zvonok AM, Cui G, Li X, Fan P, et al. Potent cannabinergic indole analogues as radioiodinatable brain imaging agents for the CB1 cannabinoid receptor. J Med Chem. 2005;48:6386–6392. doi: 10.1021/jm050135l. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Ford BM, Tai S, Fantegrossi WE, Prather PL. Synthetic Pot: Not Your Grandfather's Marijuana. Trends Pharmacol Sci. 2017;38:257–276. doi: 10.1016/j.tips.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, et al. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. - Delta9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds. Behav Pharmacol. 2014;25:750–757. doi: 10.1097/FBP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology (Berl) 2016;233:1901–1910. doi: 10.1007/s00213-016-4237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori A, Damiano F, Bonavia M, Mileo V, Varani F, Monfreda M. Identification of two cannabimimetic compounds WIN48098 and AM679 in illegal products. Sci Justice. 2013;53:286–292. doi: 10.1016/j.scijus.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of Cannabinoid Receptor Agonists and Antagonists Using the Guanosine-5′-O-(3-[35S]thio)-triphosphate Binding Assay in Rat Cerebellar Membranes. J Pharmacol Exp Ther. 1998;285:553–560. [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Gonek MM, Wiley JL, Thomas BF, Endres GW, et al. Stratification of Cannabinoid 1 Receptor (CB1R) Agonist Efficacy: Manipulation of CB1R Density through Use of Transgenic Mice Reveals Congruence between In Vivo and In Vitro Assays. J Pharmacol Exp Ther. 2016;359:329–339. doi: 10.1124/jpet.116.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Thomas BF, Wiley JL, Endres GW, Negus SS, et al. Apparent CB1 Receptor Rimonabant Affinity Estimates: Combination with THC and Synthetic Cannabinoids in the Mouse In Vivo Triad Model. J Pharmacol Exp Ther. 2017;362:210–218. doi: 10.1124/jpet.117.240192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21:320–326. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kithinji J, Spehl M, Angerer V, Franz F, Eyer F, et al. Adverse effects after the use of JWH-210 - a case series from the EU Spice II plus project. Drug Test Anal. 2016;8:1030–1038. doi: 10.1002/dta.1936. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Hess C, Stockhausen S, Kernbach-Wighton G, Madea B. Death due to diabetic ketoacidosis: Induction by the consumption of synthetic cannabinoids? Forensic Sci Int. 2015;257:e6–11. doi: 10.1016/j.forsciint.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Hruba L, McMahon LR. Apparent Affinity Estimates and Reversal of the Effects of Synthetic Cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by Rimonabant in Rhesus Monkeys. J Pharmacol Exp Ther. 2017;362:278–286. doi: 10.1124/jpet.117.240572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Irie T, Kikura-Hanajiri R, Usami M, Uchiyama N, Goda Y, Sekino Y. MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar Purkinje cells via activation of presynaptic CB1 receptors. Neuropharmacology. 2015;95:479–491. doi: 10.1016/j.neuropharm.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Jankovics P, Varadi A, Tolgyesi L, Lohner S, Nemeth-Palotas J, Balla J. Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary. Forensic Sci Int. 2012;214:27–32. doi: 10.1016/j.forsciint.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Gifford RS. “Herbal incense”: designer drug blends as cannabimimetics and their assessment by drug discrimination and other in vivo bioassays. Life Sci. 2014;97:64–71. doi: 10.1016/j.lfs.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Raghav JG. Tripping with Synthetic Cannabinoids (“Spice”): Anecdotal and Experimental Observations in Animals and Man. Curr Top Behav Neurosci. 2017;32:263–281. doi: 10.1007/7854_2016_16. [DOI] [PubMed] [Google Scholar]
- Kavanagh P, Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Detection and tentative identification of urinary phase I metabolites of phenylacetylindole cannabimimetics JWH-203 and JWH-251, by GC-MS and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;934:102–108. doi: 10.1016/j.jchromb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Knittel JL, Holler JM, Chmiel JD, Vorce SP, Magluilo J, Levine B, et al. Analysis of Parent Synthetic Cannabinoids in Blood and Urinary Metabolites by Liquid Chromatography Tandem Mass Spectrometry. J Anal Toxicol. 2016;40:173–186. doi: 10.1093/jat/bkv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller VJ, Zlabinger GJ, Auwärter V, Fuchs S, Knasmueller S. Toxicological profiles of selected synthetic cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB1. Arch Toxicol. 2013;87:1287–1297. doi: 10.1007/s00204-013-1029-1. [DOI] [PubMed] [Google Scholar]
- Langer N, Lindigkeit R, Schiebel HM, Ernst L, Beuerle T. Identification and quantification of synthetic cannabinoids in ‘spice-like’ herbal mixtures: a snapshot of the German situation in the autumn of 2012. Drug Test Anal. 2014;6:59–71. doi: 10.1002/dta.1499. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav. 2014;118:30–35. doi: 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57:1168–1180. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington, D.C: 2011. [Google Scholar]
- NDEWS. Atlanta Metro Sentinel Community Site Drug Use Patterns and Trends, 2016. [Accessed: October 9, 2017];National Drug Early Warning System. 2016a Available from https://ndews.umd.edu/sites/ndews.umd.edu/files/u1424/atlanta_scs_drug_use_patterns_and_trends_2016.pdf.
- NDEWS. Chicago Metro Sentinel Community Site Drug Use Patterns and Trends, 2016. [Accessed: October 9, 2017];National Drug Early Warning System. 2016b Available from https://ndews.umd.edu/sites/ndews.umd.edu/files/u1424/chicago_scs_drug_use_patterns_and_trends_2016.pdf.
- NDEWS. Denver Metro Sentinel Community Site Drug Use Patterns and Trends, 2016. [Accessed: October 9, 2017];National Drug Early Warning System. 2016c Available from https://ndews.umd.edu/sites/ndews.umd.edu/files/u1424/denver_scs_drug_use_patterns_and_trends_2016.pdf.
- NDEWS. Texas Sentinel Community Site Drug Use Patterns and Trends, 2016. [Accessed: October 9, 2017];National Drug Early Warning System. 2016d Available from https://ndews.umd.edu/sites/ndews.umd.edu/files/u1424/texas_scs_drug_use_patterns_and_trends_2016.pdf.
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Rosati O, Messina F, Pelosi A, Curini M, Petrucci V, Gertsch J, et al. One-pot heterogeneous synthesis of Delta(3)-tetrahydrocannabinol analogues and xanthenes showing differential binding to CB(1) and CB(2) receptors. Eur J Med Chem. 2014;85:77–86. doi: 10.1016/j.ejmech.2014.07.062. [DOI] [PubMed] [Google Scholar]
- Salomone A, Luciano C, Di Corcia D, Gerace E, Vincenti M. Hair analysis as a tool to evaluate the prevalence of synthetic cannabinoids in different populations of drug consumers. Drug Test Anal. 2014;6:126–134. doi: 10.1002/dta.1556. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, et al. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233:416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Tauskela JS, Comas T, Hewitt M, Aylsworth A, Zhao X, Martina M, et al. Effect of synthetic cannabinoids on spontaneous neuronal activity: Evaluation using Ca(2+) spiking and multi-electrode arrays. Eur J Pharmacol. 2016;786:148–160. doi: 10.1016/j.ejphar.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Tomiyama Ki, Funada M. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB1 receptors and apoptotic cell death. Toxicol Appl Pharmacol. 2014;274:17–23. doi: 10.1016/j.taap.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol. 2007;18:311–319. doi: 10.1097/FBP.0b013e3282186cf2. [DOI] [PubMed] [Google Scholar]
- White CM. The Pharmacologic and Clinical Effects of Illicit Synthetic Cannabinoids. J Clin Pharmacol. 2017;57:297–304. doi: 10.1002/jcph.827. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of Delta9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014;124:123–128. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Preclinical pharmacological and brain bioassay systems for CB1 cannabinoid receptors. In: Reggio PH, editor. The Cannabinoid Receptors. Humana Press; New York: 2009. pp. 329–360. [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, et al. AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Delta9-Tetrahydrocannabinol-Like Effects in Mice. J Pharmacol Exp Ther. 2015;354:328–339. doi: 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Delta9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF. Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2016_17. [DOI] [PubMed] [Google Scholar]
- Wohlfarth A, Scheidweiler KB, Castaneto M, Gandhi AS, Desrosiers NA, Klette KL, et al. Urinary prevalence, metabolite detection rates, temporal patterns and evaluation of suitable LC-MS/MS targets to document synthetic cannabinoid intake in US military urine specimens. Clin Chem Lab Med. 2015;53:423–434. doi: 10.1515/cclm-2014-0612. [DOI] [PubMed] [Google Scholar]
- Yun J, Gu SM, Lee TH, Song YJ, Seong S, Kim YH, et al. Synthetic Cannabinoid-Induced Immunosuppression Augments Cerebellar Dysfunction in Tetanus-Toxin Treated Mice. Biomol Ther (Seoul) 2016 doi: 10.4062/biomolther.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsu K, Hayashi Y, Suzuki K, Nakayama H, Hattori N, Takahara R, et al. Metabolome disruption of the rat cerebrum induced by the acute toxic effects of the synthetic cannabinoid MAM-2201. Life Sci. 2015a;137:49–55. doi: 10.1016/j.lfs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Zaitsu K, Nakayama H, Yamanaka M, Hisatsune K, Taki K, Asano T, et al. High-resolution mass spectrometric determination of the synthetic cannabinoids MAM-2201, AM-2201, AM-2232, and their metabolites in postmortem plasma and urine by LC/Q-TOFMS. Int J Legal Med. 2015b;129:1233–1245. doi: 10.1007/s00414-015-1257-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilliam A, Maitra R, Damaj MI, Tajuba JM, Seltzman HH, et al. Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J Med Chem. 2010;53:7048–7060. doi: 10.1021/jm1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]


