Abstract
Background
Associations of insulin resistance and hyperglycemia with a panel of liver enzymes have not been well-studied in a young, heterogeneous Hispanic/Latino population. We aimed to assess the associations of insulin resistance and glycemia with nonalcoholic fatty liver disease (NAFLD), as measured by liver enzymes and the pediatric NAFLD fibrosis index (PNFI), and whether these associations are modified by body mass index and mediated by inflammation or endothelial dysfunction.
Methods
We conducted a cross-sectional study of 1,317 boys and girls aged 8–16 years from the Hispanic Community Children’s Health Study/Study of Latino Youth. We used Poisson regression to assess the associations of fasting glucose, HbA1c, and HOMA-IR with elevated ALT (>25 U/L in boys, >22 U/L in girls), AST (≥37 U/L), GGT (≥17 U/L), and PNFI (≥9; a function of age, waist circumference, and triglyceride level).
Results
HOMA-IR was associated with elevated ALT, AST, GGT, and PNFI (prevalence ratios [95% CIs] for each 1-unit increase in the natural log of HOMA-IR: 1.99 [1.40, 2.81], 2.15 [1.12, 4.12], 1.70 [1.26, 2.30], and 1.98 [1.43, 2.74], respectively). Associations were observed in overweight/obese children, but not in normal weight children (p-interaction=0.04 for AST and p-interaction=0.07 for GGT). After further adjustment for adiponectin, high-sensitivity C-reactive protein, e-selectin, and PAI-1, associations of HOMA-IR with liver enzymes and PNFI were attenuated, but remained statistically significant for AST and PNFI.
Conclusion
Insulin resistance was associated with NAFLD in overweight/obese Hispanic/Latino youth, and this association may be partially mediated by inflammation and endothelial dysfunction.
Keywords: NAFLD, insulin resistance, adolescents, glycemia, Hispanic
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of pediatric liver disease in the United States.1,2 It is characterized by fat accumulation in the liver that can progress to liver inflammation (nonalcoholic steatohepatitis [NASH]) and then fibrosis.1 Biopsy is the gold standard for identifying and staging NAFLD, but is an invasive procedure and an impractical population-level screening test. It is therefore only selectively used in adults and is even more limited in use among children. Whereas ultrasound and other scanning methods (e.g., transient elastography) are often used in evaluating NAFLD, liver enzymes (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and gamma-glutamyl transpeptidase [GGT]) are also used clinically and in research studies as noninvasive surrogate markers of liver injury and likelihood of NAFLD and fibrosis, along with various noninvasive indices of liver fibrosis such as the pediatric NAFLD fibrosis index (PNFI), which is calculated using clinical markers (age, waist circumference, and triglyceride levels).3,4
In adults, higher levels of liver enzymes have been associated cross-sectionally and prospectively with metabolic syndrome, insulin resistance, hyperglycemia, and diabetes.5–12 Similar associations have been observed in young children and adolescents.13–19 As suggested by these studies, NAFLD could interfere with the insulin signaling pathway and lead to insulin resistance.20 However, the relationship between hyperglycemia/insulin resistance and NAFLD may be bidirectional or even circular.21 It is possible that hyperglycemia and insulin resistance could lead to liver injury through various pathways, including increased inflammation and endothelial dysfunction.22,23 Whereas both insulin resistance and NAFLD are clearly influenced by obesity, the mechanisms linking insulin resistance to NAFLD, and vice versa, have not been fully elucidated.
Insulin resistance and NAFLD both have particularly high prevalence among Hispanics/Latinos and among obese males in general.24,25 Whereas these studies have predominantly included Hispanic/Latino youth and adults of Mexican heritage, data on Hispanics/Latinos of other backgrounds are lacking. In fact, recent data in adults have shown that the prevalence of diabetes and NAFLD varies by Hispanic/Latino background.26,27 Associations of insulin resistance and in particular, hyperglycemia, with a panel of liver enzymes have not been well-studied in a young, heterogeneous Hispanic/Latino population. Given the high prevalence and increasingly early onset of obesity and glucose dysregulation in Hispanic/Latino youth,28 this is an especially important population in which to investigate these relationships. Therefore, we aimed to assess the associations of insulin resistance and glycemia with liver enzymes and PNFI in Hispanic/Latino children and adolescents; and whether these associations are modified by age, sex, or body mass index (BMI), and/or mediated by biomarkers of inflammation and endothelial dysfunction. These objectives were addressed using the diverse Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth) population aged 8–16 years from various Hispanic/Latino backgrounds.
Materials and Methods
Study population
There were 1,466 boys and girls, aged 8–16 years, recruited into SOL Youth (described previously29) from four US cities.30 Our analytic sample included 1,317 participants. See exclusions in the Online Supplement. The study was approved by institutional review boards at each site. Written informed consent was obtained from parents/legal guardians. Assent was obtained from children.
Laboratory measurements
Morning blood specimens were collected from fasting participants. Measurements included ALT, AST, GGT, fasting glucose, HbA1c, insulin, adiponectin, plasminogen activator inhibitor (PAI)-1, e-selectin, high-sensitivity C-reactive protein (hs-CRP), HDL-c, triglycerides, cholesterol, LDL-c (calculated using the Fridewald equation). We calculated HOMA-IR as [glucose (in mg/dL)*[insulin (in pmol/L)]/6]/405.31 See Online Supplement for more details.
Self-reported covariates
The following were reported by the child/adolescent and/or the parent: age, sex, Hispanic/Latino background, household income, parental education attainment, alcohol use in the past 30 days, having experienced menarche (girls), and stage of facial hair growth (boys).32 We created a dichotomous variable for pubertal (having reached menarche for girls and having at least started growing facial hair for boys) or pre-pubertal status.
Measured covariates
Centers for Disease Control age- and sex-specific BMI percentiles,33 and NHLBI age-, sex-, and height-specific diastolic and systolic blood pressure percentiles were calculated.34 Elevated waist circumference was defined as having an age- and sex-specific waist circumference ≥90th percentile.35 We report the number of minutes of moderate/vigorous activity (≥441 counts/15 seconds) per day from accelerometers.36 See Online Supplement for additional details.
Variable definitions
We defined elevated biomarker levels as follows: ALT >25 U/L in boys and >22 U/L in girls;37 AST ≥37 U/L; GGT ≥17 U/L; PNFI ≥9 (since suggestive of hepatic fibrosis)4; HOMA-IR ≥2.6 (insulin resistance);38 and hyperglycemia as either fasting glucose ≥100 mg/dL or HbA1c ≥5.7%.39 We calculated the PNFI as (1/(1+(exp(−lp))))*10, where lp= (−6.539*(ln(age))) + (0.207*waist) + (1.957*(ln(tg))) − 10.074.4 BMI was categorized as underweight/normal weight (<85th percentile), overweight (85th to <95th percentile), or obese (≥95th percentile).
Statistical analyses
We log-transformed variables that were not normally distributed. We used Wald tests to compare characteristics by level (normal versus elevated) of ALT, AST, GGT, and PNFI. We compared the percentage of participants who had elevated levels of ALT, AST, GGT, PNFI, fasting glucose, HbA1c, or HOMA-IR across categories of age, sex, BMI, Mexican background, and Hispanic/Latino background using a Wald test of joint significance from logistic regression. Supplemental analyses were stratified by sex, pubertal status, and BMI category.
We used Poisson regression with robust variance to assess the association of HOMA-IR, fasting glucose, and HbA1c (as continuous variables) with elevated ALT, AST, GGT, and PNFI. We sequentially adjusted for covariates listed previously. Models for PNFI were not adjusted for age, waist circumference, or triglyceride level since PNFI is a function of those variables. A sensitivity analysis used linear regression with ALT, AST, GGT, and PNFI as continuous variables.
We tested for the interaction of age, sex, Mexican background and BMI (normal weight vs overweight/obese) with HOMA-IR, glucose, and HbA1c using Wald tests. We observed evidence of statistically significant interactions of BMI with HOMA-IR and therefore present the associations of HOMA-IR with ALT, AST, GGT, and PNFI stratified by BMI category. These stratified analyses were not adjusted for BMI percentile or elevated waist circumference, since only one participant in the normal weight category had elevated waist circumference.
Subsequent analyses examined potential mediators of the association between liver function measures and study endpoints by sequentially adjusting for adiponectin, ln of hs-CRP, e-selectin, and ln of PAI-1. See additional details in the Online Supplement. A sensitivity analysis excluded 59 participants who reported alcohol use in the past 30 days.
We used MI ESTIMATE commands in Stata version 14.0 (StataCorp, College Station, Texas, USA) to simultaneously account for the complex survey sampling design and multiple imputation. See Online Supplement.
Results
Among 1,317 participants, mean age was 12.2 years, 51% were male, and 46% were overweight or obese. Participants who were male, overweight/obese, or had elevated waist circumference were more likely to have elevated levels of liver enzymes and PNFI (Table 1). Those with elevated liver enzymes and elevated PNFI were more likely to have lipid abnormalities (higher levels of total cholesterol, LDL-c, and triglycerides, and lower levels of HDL-c), higher levels of inflammation (as measured by higher hs-CRP), lower levels of adiponectin, greater endothelial dysfunction (higher levels of PAI-1 and e-selectin), and more insulin resistance (as measured by higher HOMA-IR) (Table 1).
Table 1.
Study population characteristics by levels of liver enzymes and Pediatric NAFLD Fibrosis Index
| ALT* | AST | GGT | PNFI | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| ≤25 U/L (N=1,146) |
>25 U/L (N=171) |
<37 U/L (N=1,266) |
≥37 U/L (N=51) |
<17 U/L (N=1,042) |
≥17 U/L (N=275) |
<9 (N=1,149) |
≥9 (N=168) |
|
|
|
|
|
||||||
| Weighted Mean (SE) or % |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
Weighted Mean or % (SE) |
|
|
|
|
|
||||||
| Age, years | 12.2 (0.1) | 11.9 (0.3) | 12.2 (0.1) | 11.5 (0.4) | 12.0 (0.1) | 13.0 (0.2) | 12.2 (0.1) | 12.2 (0.2) |
| Male | 50.0% | 62.0% | 50.7% | 72.7% | 48.1% | 64.6% | 51.1% | 54.7% |
| Household income | ||||||||
| ≤$20, 000 | 53.7% | 46.4% | 53.1% | 41.9% | 52.0% | 55.4% | 51.6% | 61.9% |
| $21,000–$40,000 | 31.3% | 36.3% | 31.4% | 46.7% | 32.8% | 28.7% | 33.5% | 19.7% |
| >$40,000 | 15.0% | 17.3% | 15.5% | 11.5% | 15.2% | 15.9% | 14.9% | 18.3% |
| Parental education | ||||||||
| <High school | 37.3% | 42.1% | 37.6% | 46.5% | 36.9% | 41.7% | 37.5% | 41.3% |
| High school | 29.8% | 28.9% | 29.7% | 27.2% | 30.0% | 28.2% | 29.3% | 32.4% |
| >High school | 32.9% | 29.0% | 32.7% | 26.3% | 33.0% | 30.2% | 33.2% | 26.2% |
| Alcohol use in the past 30 days | 5.3% | 3.9% | 5.1% | 5.2% | 4.6% | 7.0% | 4.7% | 8.7% |
| Waist circumference ≥90th percentile | 9.8% | 31.0% | 11.7% | 35.2% | 8.0% | 30.0% | 3.5% | 82.8% |
| BMI category | ||||||||
| Under/Normal weight | 58.0% | 26.9% | 55.0% | 26.8% | 60.3% | 30.0% | 60.9% | 0.4% |
| Overweight | 20.4% | 14.8% | 20.1% | 6.4% | 20.2% | 17.7% | 22.0% | 1.8% |
| Obese | 21.6% | 58.3% | 24.8% | 66.8% | 19.6% | 52.3% | 17.1% | 97.8% |
| Moderate/vigorous activity, minutes per day | 36.2 (1.1) | 31.1 (2.2) | 35.6 (1.0) | 33.6 (3.8) | 36.5 (1.1) | 31.9 (1.8) | 36.7 (1.0) | 27.0 (2.3) |
| Pubertal | 59.7% | 45.0% | 58.3% | 45.1% | 56.2% | 64.3% | 58.0% | 56.9% |
| Systolic BP ≥90th percentile | 4.1% | 4.1% | 4.0% | 8.6% | 3.8% | 5.3% | 4.2% | 3.4% |
| Diastolic BP ≥90th percentile | 2.5% | 2.0% | 2.3% | 5.9% | 2.1% | 3.7% | 2.4% | 2.8% |
| Total cholesterol, mg/dL | 153 (1.1) | 159 (2.7) | 154 (1.1) | 155 (4.3) | 153 (1.2) | 158 (2.5) | 153 (1.1) | 166 (3.0) |
| LDL-c, mg/dL | 86 (1.0) | 90 (2.3) | 87 (0.9) | 88 (3.8) | 86 (1.0) | 91 (2.0) | 85 (0.9) | 97 (2.6) |
| HDL-c, mg/dL | 53 (0.5) | 49 (1.1) | 52 (0.4) | 47 (1.9) | 53 (0.5) | 48 (0.8) | 53 (0.5) | 42 (0.8) |
| Triglycerides, mg/dL† | 66 (49–91) | 89 (59–126) | 67 (50–93) | 100 (74–116) | 65 (48–89) | 83 (57–129) | 64 (47–86) | 124 (90–171) |
| hs-CRP, mg/L† | 0.4 (0.2–1.0) | 0.9 (0.3–2.5) | 0.4 (0.2–1.1) | 1.4 (0.6–2.9) | 0.4 (0.2–0.9) | 1.1 (0.4–2.5) | 0.4 (0.2–0.9) | 2.1 (1.0–4.1) |
| Adiponectin, ng/mL | 8060 (162) | 6640 (484) | 7935 (160) | 6283 (573) | 8308 (184) | 6215 (231) | 8211 (163) | 5281 (222) |
| PAI-1, ng/mL† | 1.8 (1.1–3.1) | 3.7 (1.9–5.7) | 1.9 (1.2–3.3) | 4.2 (1.9–6.0) | 1.8 (1.1–3.0) | 3.1 (1.5–4.7) | 1.7 (1.1–3.0) | 3.9 (2.8–6.3) |
| e-selectin, ng/mL | 47.7 (0.8) | 65.2 (2.6) | 49.2 (0.8) | 70.0 (5.4) | 48.4 (0.8) | 55.8 (1.9) | 48.1 (0.8) | 64.2 (2.9) |
| Fasting glucose, mg/dL | 91.7 (0.3) | 91.8 (0.6) | 91.6 (0.3) | 93.6 (1.0) | 91.7 (0.3) | 91.9 (0.5) | 91.6 (0.3) | 92.8 (0.7) |
| Fasting glucose ≥100 mg/dL | 10.5% | 6.4% | 9.9% | 12.5% | 10.0% | 9.9% | 9.3% | 15.8% |
| HbA1c, % | 5.2 (0.01) | 5.3 (0.02) | 5.2 (0.01) | 5.3 (0.06) | 5.2 (0.01) | 5.3 (0.03) | 5.2 (0.01) | 5.3 (0.03) |
| HbA1c ≥5.7% | 8.0% | 6.8% | 8.0% | 4.2% | 6.9% | 11.5% | 7.4% | 11.0% |
| HOMA-IR ≥2.6 | 49.5% | 74.0% | 51.8% | 75.7% | 47.7% | 71.8% | 47.2% | 95.0% |
| HOMA-IR† | 2.6 (1.7–3.9) | 4.4 (2.5–6.8) | 2.7 (1.7–4.1) | 4.7 (2.6–7.4) | 2.5 (1.7–3.8) | 4.3 (2.3–6.7) | 2.5 (1.7–3.7) | 6.3 (4.6–8.6) |
Bolded values indicate p<0.05 for comparison of characteristics in persons with normal versus elevated liver enzymes or PNFI. P-values were calculated using Wald tests.
ALT cut-points for boys were ≤25 U/L versus >25 U/L. For girls, cut-points were ≤22 U/L versus >22 U/L.
Weighted medians (25th–75th percentiles) are presented, since distributions of these variables were skewed.
Boys were more likely than girls to have elevated liver enzymes and hyperglycemia (as measured by fasting glucose and HbA1c), but less likely to have insulin resistance (47% of boys versus 59% of girls) (Table 2). Obese children were more likely to have elevated liver enzymes and PNFI, hyperglycemia, and insulin resistance (Table 2). These results for obesity were similar regardless of pubertal status (eTable 1). Participants of Mexican heritage were more likely to have elevated ALT than those of non-Mexican heritage (Table 2).
Table 2.
Prevalence of elevated liver enzymes, elevated PNFI, hyperglycemia, and insulin resistance by age, sex, body mass index, and Hispanic/Latino background
| ALT >25 U/L* |
AST ≥37 U/L |
GGT ≥17 U/L |
PNFI ≥9 |
Fasting glucose ≥100 mg/dL |
HbA1c ≥5.7% | HOMA-IR ≥2.6 |
|
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Weighted % (SE) |
Weighted % (SE) |
Weighted % (SE) |
Weighted % (SE) |
Weighted % (SE) |
Weighted % (SE) |
Weighted % (SE) |
|
|
|
|||||||
| Overall (N=1,317) | 12.8% | 3.4% | 20.5% | 11.4% | 10.0% | 7.8% | 52.6% |
| By age | |||||||
| 8–12 years (n=739) | 14.4% | 4.5% | 16.1% | 11.9% | 10.9% | 9.8% | 50.7% |
| 13–14 years (n=339) | 12.3% | 2.5% | 22.0% | 12.7% | 6.9% | 6.4% | 60.2% |
| 15–16 years (n=239) | 9.8% | 1.9% | 28.9% | 8.9% | 11.0% | 5.1% | 49.9% |
| By sex | |||||||
| Female (n=659) | 10.0% | 1.9% | 15.0% | 10.6% | 5.7% | 7.0% | 58.9% |
| Male (n=658) | 15.4% | 4.8% | 25.8% | 12.1% | 14.1% | 8.6% | 46.7% |
| By BMI | |||||||
| Normal weight (n=674) | 6.4% | 1.7% (0.5) | 11.4% | 0.1% | 8.7% | 6.3% | 33.5% |
| Overweight (n=275) | 9.6% | 1.1% (0.5) | 18.5% | 1.0% | 9.1% | 5.5% | 56.8% |
| Obese (n=368) | 28.3% | 8.7% (2.2) | 40.9% | 42.3% | 13.3% | 12.8% | 88.8% |
| By Hispanic/Latino Background | |||||||
| Dominican (n=150) | 5.8% | 1.8% | 16.0% | 13.0% | 4.1% | 8.8% | 49.5% |
| Puerto Rican (n=119) | 9.1% | 0% | 25.0% | 13.7% | 7.4% | 6.2% | 51.6% |
| Cuban (n=94) | 1.1% | 0% | 19.0% | 3.5% | 16.0% | 7.9% | 65.7% |
| Central American (n=100) | 15.8% | 5.0% | 33.1% | 8.2% | 13.9% | 5.8% | 58.8% |
| Mexican (n=585) | 17.2% | 3.7% | 19.7% | 11.8% | 10.5% | 6.4% | 53.9% |
| South American (n=61) | 13.0% | 11.4% | 18.0% | 10.6% | 7.9% | 9.0% | 50.5% |
| Mixed/Other (n=208) | 10.3% | 4.5% | 20.5% | 11.3% | 12.2% | 12.9% | 46.0% |
| By Mexican Background | |||||||
| Non-Mexican (n=732) | 8.9% | 3.2% | 21.3% | 11.0% | 9.6% | 9.1% | 51.6% |
| Mexican (n=585) | 17.2% | 3.7% | 19.7% | 11.8% | 10.5% | 6.4% | 53.9% |
Elevated ALT was defined as >25 U/L for boys and >22 U/L for girls.
Bolded values indicate p<0.05 for comparison of characteristics in persons with normal versus elevated liver enzymes, PNFI, hyperglycemia, or insulin resistance. P-values were calculated using Wald tests.
Higher levels of HbA1c were associated with elevated ALT and PNFI in minimally adjusted models, but associations were not statistically significant in fully adjusted models (Figure). We did not observe any associations of fasting glucose with elevated liver enzymes and PNFI. Associations of HOMA-IR with elevated liver enzymes and PNFI were statistically significant and remained so after full adjustment (Model 3) (prevalence ratios [95% CIs] were 1.99 [1.40, 2.81], 2.15 [1.12, 4.12], 1.70 [1.26, 2.30], and 1.98 [1.43, 2.74] for ALT, AST, GGT, and PNFI, respectively) (Figure). Sensitivity analyses using liver enzymes and PNFI as continuous variables in linear regression models yielded similar results (eTable 2).
Figure. Associations of insulin resistance and hyperglycemia with elevated liver enzymes and PNFI.
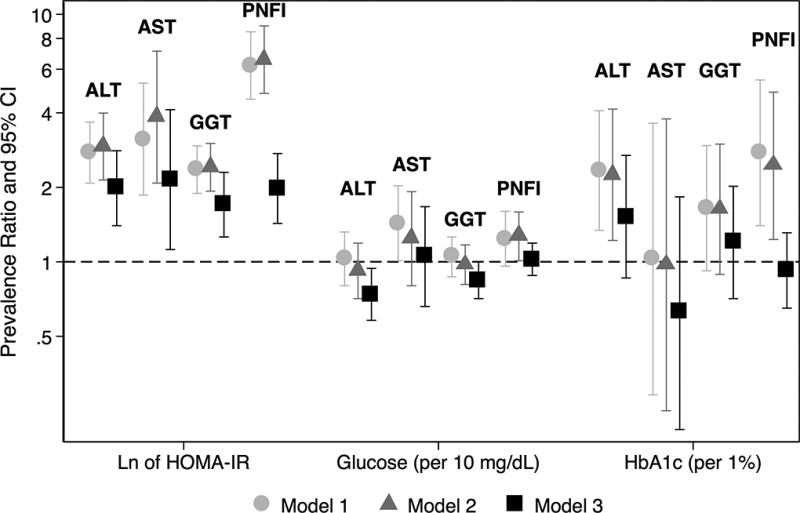
Elevated liver enzymes were defined as ALT >25 U/L for boys and >22 U/L for girls, AST ≥37 U/L, GGT ≥17 U/L. Elevated PNFI was defined as ≥9. Prevalence ratios were obtained using Poisson regression models with robust variance. We specified the models as follows: Model 1: Unadjusted; Model 2 (Sociodemographics): Model 1 + age, sex, Mexican background, field center, household income, and parental education; Model 3 (traditional risk factors): Model 2 + moderate/vigorous activity, elevated waist circumference, BMI percentile, systolic blood pressure percentile, LDL-c, HDL-c, ln of triglycerides, and pubertal status. Models for PNFI were not adjusted for age, waist circumference, or triglycerides, since PNFI is calculated using these variables.
We observed statistically significant interactions between BMI (overweight/obese versus under/normal weight) and HOMA-IR in association with elevated liver enzymes (P=0.04 for elevated AST and P=0.07 for elevated GGT). In multivariable models that were stratified by BMI category, we found no statistically significant association of HOMA-IR with elevated liver enzymes in under/normal weight children. However, we observed consistent statistically significant associations in those who were overweight or obese (prevalence ratios [95% CIs] were 2.40 [1.61, 3.58], 4.50 [2.14, 9.48], 1.75 [1.34, 2.29], and 3.12 [2.39, 4.05] for elevated ALT, AST, GGT, and PNFI, respectively) (Table 3). Results were attenuated after adjustment for adiponectin, but remained statistically significant. Additional sequential adjustment for ln of hs-CRP, e-selectin, and ln of PAI-1 continued to weaken the observed association between HOMA-IR and liver enzymes/PNFI in overweight or obese children. After full adjustment, associations for elevated ALT and GGT were no longer statistically significant (PRs [95% CIs] were 1.20 [0.74, 1.96] and 1.26 [0.91, 1.73], respectively) (Table 3). In a sensitivity analysis that restricted to participants who did not report alcohol consumption in the previous 30 days, results were similar, although magnitudes of association were slightly attenuated (eTable 3).
Table 3.
Associations of insulin resistance with elevated liver enzymes and PNFI, adjusted for inflammation and endothelial dysfunction biomarkers and stratified by BMI
| High ALT | High AST | High GGT | High PNFI* | |
|---|---|---|---|---|
|
|
|
|
||
| Prevalence Ratio (95%CI) |
Prevalence Ratio (95%CI) |
Prevalence Ratio (95%CI) |
Prevalence Ratio (95%CI) |
|
|
|
|
|
||
| Ln of HOMA-IR | ||||
| Under/normal weight (N=674) | ||||
| Base model† | 1.57 (0.87, 2.84) | 0.82 (0.28, 2.41) | 1.53 (0.92, 2.56) | -- |
| Additionally adjusted for adiponectin | 1.58 (0.88, 2.84) | 0.84 (0.28, 2.53) | 1.51 (0.91, 2.52) | -- |
| Additionally adjusted for ln hs-CRP | 1.60 (0.88, 2.91) | 0.86 (0.27, 2.75) | 1.55 (0.91, 2.63) | -- |
| Additionally adjusted for e-selectin | 1.43 (0.76, 2.68) | 0.84 (0.26, 2.70) | 1.52 (0.89, 2.61) | -- |
| Additionally adjusted for ln PAI-1 | 1.51 (0.79, 2.88) | 0.85 (0.24, 2.95) | 1.55 (0.91, 2.64) | -- |
| Overweight or obese (N=643) | ||||
| Base model† | 2.40 (1.61, 3.58) | 4.50 (2.14, 9.48) | 1.75 (1.34, 2.29) | 3.12 (2.39, 4.05) |
| Additionally adjusted for adiponectin | 2.06 (1.33, 3.17) | 3.92 (1.78, 8.62) | 1.59 (1.19, 2.12) | 3.01 (2.31, 3.92) |
| Additionally adjusted for ln hs-CRP | 1.88 (1.24, 2.84) | 3.08 (1.51, 6.29) | 1.50 (1.12, 2.00) | 2.83 (2.18, 3.68) |
| Additionally adjusted for e-selectin | 1.53 (1.01, 2.33) | 2.13 (1.11, 4.08) | 1.42 (1.05, 1.91) | 2.77 (2.13, 3.61) |
| Additionally adjusted for ln PAI-1 | 1.20 (0.74, 1.96) | 2.02 (1.09, 3.77) | 1.26 (0.91, 1.73) | 2.62 (1.93, 3.56) |
The model for PNFI does not include adjustment for age, waist circumference, or triglyceride levels.
The base model includes adjustment for age, sex, household income, parental education, Mexican background, field center, physical activity, SBP percentile, LDL-c, HDL-c, ln of triglycerides, and pubertal status
Discussion
Among 8- to 16-year-old children of Hispanic/Latino background, insulin resistance was associated with elevated liver enzymes and PNFI. These associations were only seen in children who were overweight or obese and only remained statistically significant for AST and PNFI after mutual adjustment for several biomarkers of glucose homeostasis, inflammation, and endothelial dysfunction. Our findings suggest that liver disease may begin to develop early in the life course among obese children, and may be related to the development of insulin resistance and diabetes risk. This suggests an important focus for prevention and awareness in young Hispanics/Latinos, a population that has a high prevalence of overweight/obesity.
ALT and AST are intracellular enzymes located in the hepatocytes, although AST is less specific to hepatocytes and is abundant in muscle tissue, red blood cells, and other tissues. When detected in circulation, they indicate enzyme leakage due to liver injury. Elevated levels of GGT are associated with biliary disease and, in one pediatric study, directly correlate with the degree of hepatic fibrosis.40,41 The PNFI is a noninvasive index of fibrosis that has been assessed in children.4 However, it should be noted that children with elevated PNFI may not actually have NAFLD, and that associations of obesity-related insulin resistance with NAFLD could potentially be attributed to associations with central adiposity, which is part of the definition of PNFI. ALT levels have been shown to be highest in Hispanic/Latino adolescents compared to those of other races/ethnicities,24,25 and higher in males than females,24 suggesting more liver damage among Hispanics/Latinos and among males. We observed here that boys were more likely to have elevated liver enzymes than girls.
The mechanism by which insulin resistance may be associated with elevated liver enzymes has not been fully delineated. The liver plays a major role in glucose metabolism. Conversely, it has been hypothesized that insulin resistance may increase both lipogenesis and the amount of free fatty acids in the liver, resulting in increased hepatic fat accumulation. This, in turn, may lead to liver injury.1,23,40,42 Hyperglycemia, inflammation, and decreased endothelial function may contribute to hepatocyte injury independently. Alternatively, hyperglycemia may induce inflammation and endothelial dysfunction,43 which may in turn lead to decreased liver function.23,44 We demonstrated that sequential adjustment for biomarkers of inflammation and endothelial dysfunction attenuated the association of HOMA-IR with elevated liver enzymes, which suggests that they may be in the pathway between insulin resistance and liver injury. Furthermore, the relationship between insulin resistance and elevated liver enzymes may not be linear, and may propagate a cycle of worsening liver disease and insulin resistance.20
We did not observe an association of glycemia with liver enzyme elevation in our analysis. Our study population consisted of children and adolescents, and the levels of glycemia may not have been great enough to see an association even if it were to exist. Furthermore, HOMA-IR is a better early detector of glucose homeostasis abnormalities than either fasting glucose or HbA1c, since it precedes hyperglycemia,45,46 which may explain the consistency of associations we saw for HOMA-IR with liver enzymes and PNFI. Indeed, insulin resistance tends to be exacerbated during adolescence. During puberty, levels of growth hormone, IGF-1, and other related hormones are high, and a limitation of this study is the lack of measurements that may have captured the influence of these variables on the relationship between insulin resistance and NAFLD. Further studies may be able to help identify the specific period of adolescence that may be associated with the greatest risk for developing NAFLD and its metabolic sequelae.
There were several strengths and limitations of this analysis to consider. This was a cross-sectional study, so we were unable to address temporality of these relationships and could not distinguish between mediators and confounders. We were unable to include 2-hour glucose results as one of the measures of glycemia. Nor were we able to use a euglycemic hyperinsulinemic clamp technique to directly estimate insulin resistance. However, this technique is challenging to use in large epidemiologic studies and is less relevant in clinical practice. The use of HOMA-IR as a surrogate measure is justified by its linear relationship with estimates of insulin resistance as measured by the glucose clamp.47 Lastly, liver enzymes and PNFI are not diagnostic of NAFLD. Nonetheless, they are commonly used noninvasive surrogate markers of NAFLD. An important strength of this study was that the sample was population-based and drawn from a heterogeneous mix of children and adolescents from various Hispanic/Latino backgrounds. SOL Youth is unique in that it is one of the largest studies of cardiometabolic health in Hispanic/Latino children. In the pediatric population, it is relatively uncommon for external insults such as alcohol consumption or viral liver infection to affect liver enzyme levels, and we had available an array of clinical and behavioral variables to control for confounding. Furthermore, we showed similar results among those children and adolescents who had not recently consumed alcohol.
In conclusion, we found that Hispanic/Latino children and adolescents had a high prevalence of elevated liver enzymes in general, and that the prevalence was particularly high in those who were male, overweight/obese, or exhibited central adiposity. Insulin resistance was associated with surrogate markers of NAFLD in overweight or obese Hispanic/Latino children and adolescents, and this association was partially mediated by inflammation and endothelial dysfunction. Furthermore, associations were consistent across Hispanic/Latino background. This suggests a potential target for prevention of liver disease in Hispanic/Latino youth, which is particularly important given the high prevalence of liver damage in Hispanic/Latino adults. Identifying overweight or obese Hispanic/Latino children and adolescents with insulin resistance could help detect those who are at highest risk for developing NAFLD. Further longitudinal study of these associations could better elucidate the temporal relationship between obesity, insulin resistance, and liver enzyme elevation, including the complex interplay of inflammation on glucose homeostasis.
Supplementary Material
Acknowledgments
Funding and acknowledgments: SOL Youth was supported by Grant Number R01HL102130 from the National Heart, Lung, and Blood Institute. The children in SOL Youth are drawn from the study of adults: The Hispanic Community Health Study/Study of Latinos, which was supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. Dr. Qi is supported by a Scientist Development Award (K01HL129892) from the NHLBI.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- GGT
gamma-glutamyl transpeptidase
- HbA1c
hemoglobin A1c
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- HPLC
high performance liquid chromatography
- hs-CRP
high-sensitivity C-reactive protein
- IGF-1
insulin-like growth factor-1
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PAI-1
plasminogen activator inhibitor-1
- PNFI
pediatric NAFLD fibrosis index
- PR
prevalence ratio
- SOL Youth
Study of Latino Youth
Footnotes
Disclosures: There are no conflicts of interest to disclose.
Author contributions: CMP: designed the study, conducted the analysis, interpreted the data, and drafted the manuscript; BJR, ML, LCG, BT, SJC, QQ, TS, DCV, HDS, and RCK: interpreted the data and critically revised the manuscript; and CRI: designed the study, acquired the data, interpreted the data, and critically revised the manuscript. All authors gave final approval of the version to be submitted for publication and agreed to be accountable for all aspects of the work.
References
- 1.Giorgio V, Prono F, Graziano F, et al. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013;13(1):40. doi: 10.1186/1471-2431-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohli R, Sunduram S, Mouzaki M, et al. Pediatric Nonalcoholic Fatty Liver Disease: A Report from the Expert Committee on Nonalcoholic Fatty Liver Disease (ECON) J Pediatr. 2016 May;172:9–13. doi: 10.1016/j.jpeds.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: A global perspective. Vol. 28, Seminars in Liver Disease. 2008:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 4.Nobili V, Alisi A, Vania A, et al. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. doi: 10.1186/1741-7015-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousefzadeh G, Shokoohi M, Yeganeh M, et al. Role of gamma-glutamyl transferase (GGT) in diagnosis of impaired glucose tolerance and metabolic syndrome: A prospective cohort research from the Kerman Coronary Artery Disease Risk Study (KERCADRS) Diabetes Metab Syndr Clin Res Rev. 2012;6(4):190–4. doi: 10.1016/j.dsx.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet F, Ducluzeau PH, Gastaldelli A, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes. 2011;60(6):1660–7. doi: 10.2337/db10-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulum T, Kolarić B, Duvnjak L, et al. Nonalcoholic fatty liver disease markers are associated with insulin resistance in type 1 diabetes. Dig Dis Sci. 2011;56(12):3655–63. doi: 10.1007/s10620-011-1807-7. [DOI] [PubMed] [Google Scholar]
- 8.Schneider ALC, Lazo M, Ndumele CE, et al. Liver enzymes, race, gender and diabetes risk: The atherosclerosis risk in communities (ARIC) study. Diabet Med. 2013;30(8):926–33. doi: 10.1111/dme.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko S-H, Baeg MK, Han K-D, et al. Increased liver markers are associated with higher risk of type 2 diabetes. World J Gastroenterol. 2015;21(24):7478–87. doi: 10.3748/wjg.v21.i24.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Jiang CQ, Schooling CM, et al. Liver enzymes and incident diabetes in China: a prospective analysis of 10 764 participants in the Guangzhou Biobank Cohort Study. J Epidemiol Community Health. 2015 doi: 10.1136/jech-2015-205518. jech – 2015–205518. [DOI] [PubMed] [Google Scholar]
- 11.Katoh S, Peltonen M, Wada T, et al. Fatty liver and serum cholinesterase are independently correlated with HbA1c levels: Cross-sectional analysis of 5384 people. J Int Med Res. 2014;42(2):542–53. doi: 10.1177/0300060513517485. [DOI] [PubMed] [Google Scholar]
- 12.Calanna S, Scicali R, Di Pino A, et al. Alpha- and beta-cell abnormalities in haemoglobin A1c-defined prediabetes and type 2 diabetes. Acta Diabetol. 2014;51(4):567–75. doi: 10.1007/s00592-014-0555-5. [DOI] [PubMed] [Google Scholar]
- 13.Rocha R, Cotrim HP, Bitencourt AGV, et al. Nonalcoholic fatty liver disease in asymptomatic Brazilian adolescents. World J Gastroenterol. 2009;15(4):473–7. doi: 10.3748/wjg.15.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell M, Flores YN, Zhang ZF, et al. Prevalence and predictors of alanine aminotransferase elevation among normal weight, overweight and obese youth in Mexico. J Dig Dis. 2013;14(9):491–9. doi: 10.1111/1751-2980.12072. [DOI] [PubMed] [Google Scholar]
- 15.Lee K, Yang JH. Which Liver Enzymes Are Better Indicators of Metabolic Syndrome in Adolescents: The Fifth Korea National Health and Nutrition Examination Survey, 2010. Metab Syndr Relat Disord. 2013;11(4):229–35. doi: 10.1089/met.2012.0153. [DOI] [PubMed] [Google Scholar]
- 16.Kelishadi R, Cook SR, Adibi A, et al. Association of the components of the metabolic syndrome with non-alcoholic fatty liver disease among normal-weight, overweight and obese children and adolescents. Diabetol Metab Syndr. 2009;1:29. doi: 10.1186/1758-5996-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang RC, Mori TA, Burke V, et al. Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care. 2009;32(4):695–701. doi: 10.2337/dc08-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SY, Sung E, Chang Y. Elevated serum gamma-glutamyltransferase is a strong marker of insulin resistance in obese children. Int J Endocrinol. 2013;2013 doi: 10.1155/2013/578693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhang J, Wang B, et al. New evidence for an association between liver enzymes and pancreatic islet β-cell dysfunction in young obese patients. Endocrine. 2013:688–95. doi: 10.1007/s12020-013-9937-7. [DOI] [PubMed] [Google Scholar]
- 20.Gruben N, Shiri-Sverdlov R, Koonen DPY, et al. Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta - Mol Basis Dis. 2014;1842(11):2329–43. doi: 10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Valenti L, Bugianesi E, Pajvani U, et al. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int. 2016 Jun 8; doi: 10.1111/liv.13185. [DOI] [PubMed] [Google Scholar]
- 22.Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218(3):R25–36. doi: 10.1530/JOE-13-0201. [DOI] [PubMed] [Google Scholar]
- 23.Pasarín M, La Mura V, Gracia-Sancho J, et al. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser A, Longnecker MP, Lawlor DA. Prevalence of Elevated Alanine Aminotransferase Among US Adolescents and Associated Factors: NHANES 1999–2004. Gastroenterology. 2007;133(6):1814–20. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deboer MD, Wiener RC, Barnes BH, et al. Ethnic Differences in the Link Between Insulin Resistance and Elevated ALT. Pediatrics. 2013;132(3):e718–26. doi: 10.1542/peds.2012-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallwitz ER, Daviglus ML, Allison MA, et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin Gastroenterol Hepatol. 2015;13(3):569–76. doi: 10.1016/j.cgh.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among hispanics/latinos from diverse backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2014;37(8):2233–9. doi: 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isasi CR, Parrinello CM, Ayala GX, et al. Sex differences in cardiometabolic risk factors among Hispanic/Latino Youth. Findings from the Study of Latino Youth (SOL Youth) J Pediatr. (in press) [Google Scholar]
- 29.Isasi CR, Carnethon MR, Ayala GX, et al. The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth): Design, objectives, and procedures. Ann Epidemiol. 2014;24(1):29–35. doi: 10.1016/j.annepidem.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010 Aug;20(8):629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988 Apr;17(2):117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 33.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States : Methods and Development. Vital Heal Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 34.NHLBI/NHBPEP. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 35.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Heal Stat. 2012;11(252):1–40. [PubMed] [Google Scholar]
- 36.Romanzini M, Petroski EL, Ohara D, et al. Calibration of ActiGraph GT3X, Actical and RT3 accelerometers in adolescents. Eur J Sport Sci. 2014;14(1):91–9. doi: 10.1080/17461391.2012.732614. [DOI] [PubMed] [Google Scholar]
- 37.Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology. 2010;138(4):1357–64. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrows R, Reyes M, Blanco E, et al. Healthy Chilean Adolescents with HOMA-IR ≥ 2. 6 Have Increased Cardiometabolic Risk : Association with Genetic, Biological, and Environmental Factors. J Diabetes Res. 2015;2015 doi: 10.1155/2015/783296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Association Diabetes. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Supplement 1):S13–22. [Google Scholar]
- 40.Harris EH. Elevated Liver Function Tests in Type 2 Diabetes. Clin Diabetes. 2005;23(3):115–9. [Google Scholar]
- 41.Nobili V, Siotto M, Bedogni G, et al. Levels of serum ceruloplasmin associate with pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2013;56(4):370–5. doi: 10.1097/MPG.0b013e31827aced4. [DOI] [PubMed] [Google Scholar]
- 42.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–61. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 43.Oguntibeju OO, editor. Pathophysiology and Complications of Diabetes Mellitus. 2012. [Google Scholar]
- 44.Nozaki Y, Fujita K, Wada K, et al. Deficiency of eNOS exacerbates early-stage NAFLD pathogenesis by changing the fat distribution. BMC Gastroenterol. 2015;15:177. doi: 10.1186/s12876-015-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warram JH, Martin BC, Krolewski AS, et al. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990 Dec 15;113(12):909–15. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 46.Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988 May 12;318(19):1217–25. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- 47.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1(2):36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


