Abstract
Purpose
We aimed to determine whether EEG background characteristics remain stable across discrete time periods during the acute period after resuscitation from pediatric cardiac arrest.
Methods
Children resuscitated from cardiac arrest underwent continuous conventional EEG monitoring. The EEG was scored in 12-hour epochs for up to 72-hours after return of circulation by an electroencephalographer using a Background Category with four-levels (normal, slow-disorganized, discontinuous/burst-suppression, or attenuated-featureless) or two-levels (normal/slow-disorganized or discontinuous/burst-suppression/attenuated-featureless). Survival analyses and mixed-effects ordinal logistic regression models evaluated whether the EEG remained stable across epochs.
Results
EEG monitoring was performed in 89 consecutive children. When EEG was assessed as the four-level Background Category, 30% of subjects changed category over time. Based on initial Background Category, one quarter of subjects changed EEG category by 24 hours if the initial EEG was attenuated-featureless, by 36 hours if the initial EEG was discontinuous or burst-suppression, by 48 hours if the initial EEG was slow-disorganized, and never if the initial EEG was normal. However, regression modeling for the four-level Background Category indicated that the EEG did not change over time (OR=1.06, 95%CI=0.96– 1.17, p=0.26). Similarly, when EEG was assessed as the two-level Background Category, 8% of subjects changed EEG category over time. However, regression modeling for the two-level category indicated that the EEG did not change over time (OR=1.02, 95%CI=0.91–1.13, p=0.75).
Conclusions
The EEG Background Category changes over time whether analyzed as four-levels (30% of subjects) or two-levels (8% of subjects) although regression analyses indicated no significant changes occurred over time for the full cohort. These data indicate that the Background Category is often stable during the acute 72 hours after pediatric cardiac arrest and thus may be a useful EEG assessment metric in future studies, but that some subjects do have EEG changes over time and therefore serial EEG assessments may be informative.
Keywords: EEG, cardiac arrest, pediatric
Introduction
Cardiac arrest occurs in over 16,000 children per year in the United States, and survivors often experience substantial neurobehavioral morbidity.1–9 Clinical and resuscitation characteristics are only moderately predictive of long-term neurobehavioral outcomes,10–14 likely because they do not directly assess brain function. Electroencephalographic (EEG) monitoring provides a robust method of assessing brain activity, and it is often acquired early after cardiac arrest to identify non-convulsive seizures.15,16 Further, single-center data indicate that specific early post cardiac arrest EEG features are associated with discharge neurologic outcomes,15,17–22 and the addition of EEG data to clinical information has been shown to significantly improve prognostication accuracy by clinicians.23 Thus, EEG features could serve as early, direct, reliable, standardized, and clinically available brain injury severity biomarkers. Recognizing these potential uses for EEG, the International Liaison Committee on Resuscitation (ILCOR) recommended that investigators should “examine a standardized approach to EEG analysis.”24
To establish a standardized EEG analysis approach, we previously assessed the inter-rater agreement of standardized EEG variables.25,26 However, a standardized EEG analysis approach also needs to determine when to assess the EEG and whether the EEG changes over time during the acute period. If the EEG is relatively stable during the acute period, then assessing the EEG during a single wide time span could be appropriate for subsequent prospective studies involving EEG assessment. In contrast, if the EEG changes substantially during the acute period in many patients, then EEG biomarker studies would need to assess the EEG in narrower and likely multiple time periods. Thus, we aimed to assess how the EEG background changes during the acute period in children resuscitated after cardiac arrest.
Methods
We enrolled consecutive children resuscitated after cardiac arrest who received care at the Children’s Hospital of Philadelphia (CHOP) in an Institutional Review Board approved prospective observational study of pediatric cardiac arrest. We collected demographic and clinical data related to prior medical history, cardiac arrest characteristics, resuscitation characteristics, and discharge outcome assessed from medical record notes using the Pediatric Cerebral Performance Category Score.27 As part of clinical care guided by an institutional pathway28 derived from recent guidelines and consensus statements regarding EEG monitoring in critically ill patients,16,29,30 all patients resuscitated after cardiac arrest underwent continuous conventional full-array EEG monitoring. We initiated EEG monitoring as soon as possible after resuscitation. We performed EEG monitoring with Grass video EEG equipment and the international 10–20 montage with modification for neonates as needed.
This study evaluated 10-minute long EEG segments scored by a pediatric electroencephalographer blind to all clinical information. We removed all clinical annotations in the EEG tracing prior to blinded review. The video was not available, but the reviewer could adjust the montage, filters, and voltage settings. The reviewer scored the EEG using an electronic case report form in Research Electronic Data Capture (REDCap) which is a web-based electronic data application hosted at CHOP Research Institute.31 Use of the electronic case report form ensured there were no missing data. The reviewer assessed the EEG at the earliest time available after resuscitation and at up to six subsequent times relative to the time of return of circulation (12, 24, 36, 48, 60, and 72 hours). Thus, a maximum of 70 minutes of EEG were scored per subject. The reviewer scored the Background Category which consisted of: (1) normal (including sedated sleep), (2) slow-disorganized, (3) discontinuous (which had to be excessive for gestational age in neonates) or burst-suppression, and (4) attenuated-featureless. Prior studies of EEG monitoring in critically ill children have used this categorical system,20–22,32 and a prior study involving four pediatric electroencephalographers demonstrated almost perfect interrater agreement (kappa 0.89).26 The EEG background was also combined into two background categories including: (1) favorable (normal or slow-disorganized categories), and (2) unfavorable (discontinuous/burst-suppression or attenuated-featureless categories).
We performed statistical analyses using Stata 12 (College Station, Texas). We used standard descriptive statistics to summarize the data as medians (with interquartile ranges (IQR) or counts (with percentages) as appropriate. We aimed to assess whether the background remained stable for individual subjects. Because of the variability in EEG monitoring duration across subjects, we used survival analysis methods to evaluate the time from EEG initiation until a change in EEG to a better or worse category occurred for each individual subject. An event was defined as a change in EEG at the first of each subsequent evaluation times (12, 24, 36, 48, 60, and 72 hours), compared to the Background Category at the earliest EEG time. Subjects without a change in Background Category to a better or worse category were censored at the time closest to the time the EEG recording was discontinued. Since the entire study occurred in the acute period following resuscitation from cardiac arrest, no subjects were censored due to loss to follow-up. We generated Kaplan-Meier curves to show the estimated number of subjects without a change in EEG at various time points after EEG onset (12, 24, 36, 48, 60, and 72 hours after return of circulation). We performed separate survival analyses for the EEG data categorized into four-level and two-level categories. We created Heat Maps to visualize the EEG category over time for each individual subject. To assess whether the EEG background changed over time for the full cohort, we performed a mixed-effects ordinal logistic regression for the four-level EEG category data and a mixed-effects logistic regression for the two-level EEG category data. This method takes into account within-subject correlation due to repeated measures by including subject-specific random effects.
Results
The study included 89 subjects who experienced cardiac arrests between August 2012 and April 2016. Table 1 provides the demographic and clinical data from this cohort. The median patient age was 2.1 years (IQR 0.27, 9.1 years). The median duration of cardiopulmonary resuscitation was 10 minutes (IQR 4, 20 minutes), and 58 (65%) subjects had in-hospital cardiac arrests. The initial EEG was recorded 7.0 hours (IQR 4.4, 11.4 hours) after return of circulation.
Table 1.
Subject characteristics (N=89)
| Clinical Variable | N (%) or Median [IQR] |
|---|---|
|
| |
| Age at Arrest (years) | 2.1 [0.27, 9.1] |
|
| |
| Sex: Male | 56 (62%) |
|
| |
| Race | |
| White | 46 (52%) |
| Black | 19 (21%) |
| Other | 24 (27%) |
|
| |
| Hispanic | 14 (16%) |
|
| |
| Pre-arrest PCPC Score | |
| 1 = Normal | 58 (65%) |
| 2 = Mild Disability | 12 (13%) |
| 3 = Moderate Disability | 8 (9%) |
| 4 = Severe Disability | 9 (10%) |
| 5 = Coma or Vegetative State | 2 (2%) |
|
| |
| Pre-Existing Medical Condition: no | 21 (24%) |
|
| |
| Cardiac Arrest location | |
| In-Hospital | 58 (65%) |
| Out-of-Hospital | 31 (35%) |
|
| |
| Bystander Cardiopulmonary Resuscitation for Out-of-Hospital Cardiac Arrest (n=31) | 25 (81%) |
|
| |
| Cardiopulmonary Resuscitation Duration (minutes) (n=70) | 10 [4, 20] |
|
| |
| Initial Rhythm | |
| Asystole/pea | 26 (29%) |
| Bradycardia | 33 (37%) |
| Ventricular fibrillation or tachycardia | 11 (13%) |
| Other/unknown | 19 (21%) |
|
| |
| Epinephrine Doses | |
| 0 | 14 (16%) |
| 1 | 15 (17%) |
| 2 | 10 (11%) |
| 3 | 18 (20%) |
| 4 | 7 (8%) |
| ≥5 | 20 (22%) |
| Unknown | 5 (6%) |
|
| |
| Induced Hypothermia | 10 (11%) |
|
| |
| Benzodiazepine Infusion (during EEG monitoring) | 69 (78%) |
|
| |
| Time from Cardiac Arrest to Electroencephalogram Initiation (hours) | 7 [4.4, 11.4] |
|
| |
| Mortality | 30 (34%) |
|
| |
| Post-arrest PCPC Score (Discharge) | |
| 1 = Normal | 16 (18%) |
| 2 = Mild Disability | 16 (18%) |
| 3 = Moderate Disability | 11 (12%) |
| 4 = Severe Disability | 16 (18%) |
| 5 = Coma or Vegetative State | 30 (34%) |
|
| |
| Unfavorable Outcome (Death or PCPC Score Change >1 Level) | 45 (51%) |
PCPC, pediatric cerebral performance category.
When the EEG Background Category was assessed with four-levels, 30% of subjects changed categories over time (Table 2). Figure 1a is the Kaplan-Meier curve. For all initial EEG background categories, fewer than 50% of subjects experienced changes in Background Category over time. Thus, the median number of subjects without a change in Background Category cannot be reported, and we instead summarized when 25% of subjects changed Background Category. One quarter of subjects changed Background Category by 24 hours if the initial EEG was attenuated-featureless, 36 hours if the initial EEG was discontinuous or burst-suppression, 48 hours if the initial EEG was slow-disorganized, and never if the initial EEG was normal. Differences in rates of subjects who did not have a change in their Background Category over time by initial Background Category were not statistically significant (log rank test, p=0.28). Although the survival analysis indicated 30% of subjects changed Background Category over time, the regression modeling indicated that there were no changes in Background Category over time for the full cohort (OR = 1.06, 95%CI = 0.96 – 1.17, p=0.26). Twenty-six subjects transitioned between the four-level categories including ten who worsened, twelve who improved, and four who briefly improved and then returned to initial worse category. Figure 2a is a Heat Map showing Background Category over time for each subject.
Table 2.
Summary of Kaplan-Meier Estimates for Stability (no change) of EEG Background Category at Each Timepoint Categorized as Four-Levels and Two-Levels.
| Duration Since Return of Circulation (hours) |
EEG Background Categorized as Four Levels | EEG Background Categorized as Two Levels | ||
|---|---|---|---|---|
| EEG Stablea (%) |
95% CI (%) |
EEG Stablea (%) |
95% CI (%) |
|
| 12 | 93 | 86–97 | 98 | 91–99 |
| 24 | 83 | 74–89 | 98 | 91–99 |
| 36 | 75 | 65–83 | 94 | 87–98 |
| 48 | 70 | 60–79 | 93 | 86–97 |
| 60 | 70 | 60–79 | 92 | 84–96 |
| 72 | 70 | 60–79 | 92 | 84–96 |
EEG stability is the equivalent of “survival” without a change in Background Category.
Figure 1.
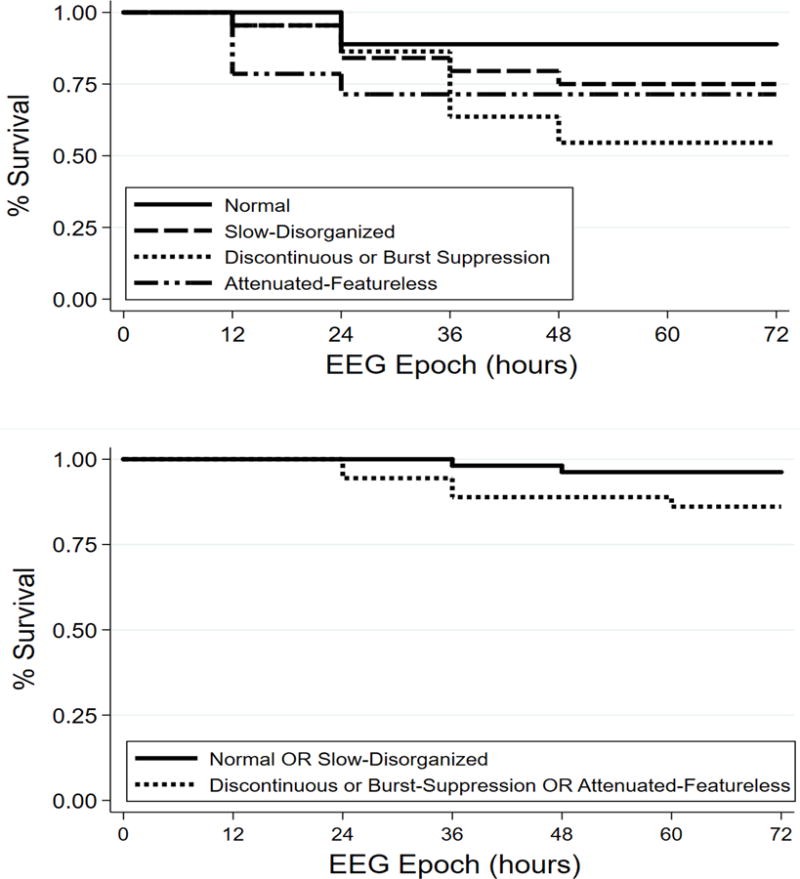
Kaplan-Meier survival curves by initial Background Category indicating the percentages of subjects with unchanged EEGs at various time-points after return of circulation with the (a) Background Category scored as four-levels or (b) two-levels. Subjects are censored the first time they change categories or when EEG monitoring ends.
Figure 2.
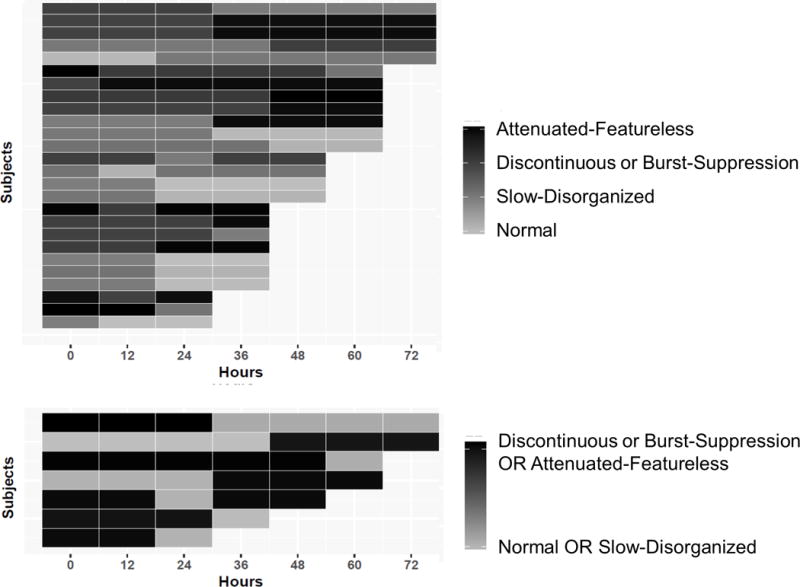
Heat maps illustrating the EEG Background Category by initial Background Category at various time-points after return of spontaneous circulation for subjects who changed category with the EEG categorized into (a) four-levels or (b) two-levels.
Similar results occurred when the EEG was categorized as two-levels. When the EEG Background Category was assessed as two-levels, 8% of subjects changed categories over time (Table 2). Figure 1b is the Kaplan-Meier curve. Although the survival analysis indicated 8% of subjects changed Background Category over time, the regression modeling indicated there were no changes in Background Category over time for the full cohort (OR = 1.02, 95%CI = 0.91 – 1.13, p=0.75). Seven subjects transitioned between the two-level categories including two who worsened, four who improved, and one who briefly improved and then worsened again. Figure 2b is a Heat Map showing Background Category over time for each subject.
Discussion
The Background Category EEG variable has been used in pediatric prognostication systems,15,17–22 and we have previously shown that that assessments of Background Category by four pediatric electroencephalographers had almost-perfect interrater agreement.26 Thus, it may be a useful EEG exposure variable for future large multi-center studies assessing EEG as a biomarker of early brain injury severity after pediatric cardiac arrest. The current study adds two key findings which impact use of the Background Category EEG variable as a standardized EEG system in future studies. First, a meaningful proportion of individual subjects have changes in their Background Category assessments during the initial 72 hours after cardiac arrest. The Background Category changes for 30% of subjects when analyzed as four-levels and 8% of subjects when analyzed as two-levels. Thus, future studies assessing EEG as a brain injury severity biomarker or using EEG for neuroprognostication purposes may benefit from EEG assessment at multiple timepoints and analysis of changes in EEG over time. Several studies in adults after cardiac arrest have demonstrated that changes in EEG over time may be informative in terms of outcome prediction with improvements in EEG assessments associated with more favorable outcomes44–47 Second, and somewhat contradictory, is the finding that when analyzed as a cohort, no statistically significant changes in EEG were identified using Background Category as either four-levels or two-levels. Establishing that EEG background categories do not significantly change over time when analyzed among the full cohort may indicate that this is a reasonably stable assessment system. This approach could serve as a standardized and reliable system to EEG scoring for future multicenter prospective pediatric cardiac arrest trials aiming to stratify subjects using EEG as an early and clinically available biomarker of initial brain injury severity.
In considering survival analysis requirements,48 clinical management leading to study data did not change during the study, the risk of consequences did not change during the study, and events occurred uniformly over the follow-up intervals. However, subjects represented by censored data may not have experienced the same consequences as those remaining under study. Patients with very normal or very abnormal EEGs may have been censored since EEG monitoring might have been discontinued earlier in patients who had a return to normal mental status or progressed to death, respectively. Thus, the uncensored subjects with mid-level EEG abnormalities tended to undergo longer durations of EEG monitoring. As a result, the survival analyses might underestimate the stability of EEG findings since more stable patients were more likely to be censored.
This study has several notable strengths. The EEG studies were obtained from a cohort of consecutive children resuscitated after cardiac arrest, so the EEG patterns likely represented the full spectrum of findings and the true prevalence of the various EEG patterns. The EEG reviewer could modify any settings, which mimics real-world EEG reading. However, there are also limitations. First, the EEG epochs were only assessed by one pediatric electroencephalographer, and interpretation may have varied by reviewer. However, our prior interrater agreement data in this cohort indicated almost-perfect agreement for the EEG categorization system.26 Second, we assessed one overarching categorization system and did not assess each EEG component such as voltage, frequency, continuity, reactivity, or variability. Future work assessing individual EEG components is important since they might provide additional and more nuanced information than the categorical approach. Third, we assessed ten-minute segments of EEG data within each 12-hour epoch. This approach has been used in similar studies of adult cardiac arrest cohorts.45 However, more frequent EEG assessments might have shown changes or fluctuations not identified by this analysis. Several similar adult cardiac arrest cohorts performed analysis on 5-minute epochs per hour46,47 and several studies showed EEG changes over time periods shorter than 12-hour epochs were informative in terms of prognosis.45–47
Overall, these data indicate that the EEG Background Category changes over time in some subjects. Thus, repeated assessments may be needed for the highest accuracy in future studies assessing whether EEG predicts neurobehavioral outcomes in individual patients. However, for the full cohort, statistically significant changes in the Background Category were not identified indicating that the initial EEG assessment may provide a relatively stable assessment of early brain function which could be used to stratify patients by early brain injury severity in future neuroprotection trials.
References
- 1.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41:1057–66. doi: 10.1007/s00134-015-3789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Zellem L, Utens EM, Legerstee JS, et al. Cardiac Arrest in Children: Long-Term Health Status and Health-Related Quality of Life. Pediatr Crit Care Med. 2015;16:693–702. doi: 10.1097/PCC.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 3.Michiels EA, Dumas F, Quan L, Selby L, Copass M, Rea T. Long-term outcomes following pediatric out-of-hospital cardiac arrest*. Pediatr Crit Care Med. 2013;14:755–60. doi: 10.1097/PCC.0b013e31829763e2. [DOI] [PubMed] [Google Scholar]
- 4.Gelberg J, Stromsoe A, Hollenberg J, et al. Improving Survival and Neurologic Function for Younger Age Groups After Out-of-Hospital Cardiac Arrest in Sweden: A 20-Year Comparison. Pediatr Crit Care Med. 2015;16:750–7. doi: 10.1097/PCC.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 5.Meert KL, Slomine BS, Christensen JR, et al. Family Burden After Out-of-Hospital Cardiac Arrest in Children. Pediatr Crit Care Med. 2016;17:498–507. doi: 10.1097/PCC.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slomine BS, Silverstein FS, Christensen JR, et al. Neurobehavioral Outcomes in Children After Out-of-Hospital Cardiac Arrest. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017;376:318–29. doi: 10.1056/NEJMoa1610493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med. 2016;44:798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–9. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 11.Topjian AA, Clark AE, Casper TC, et al. Early lactate elevations following resuscitation from pediatric cardiac arrest are associated with increased mortality*. Pediatr Crit Care Med. 2013;14:e380–7. doi: 10.1097/PCC.0b013e3182976402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starling RM, Shekdar K, Licht D, Nadkarni VM, Berg RA, Topjian AA. Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest. Pediatr Crit Care Med. 2015;16:542–8. doi: 10.1097/PCC.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42:1518–23. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med. 2015;16:146–54. doi: 10.1097/PCC.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–40. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler SK, Topjian AA, Gutierrez-Colina AM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2011;14:37–43. doi: 10.1007/s12028-010-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav. 2015;49:238–44. doi: 10.1016/j.yebeh.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topjian AA, Sanchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest. Pediatr Crit Care Med. 2016;17:547–57. doi: 10.1097/PCC.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostendorf AP, Hartman ME, Friess SH. Early Electroencephalographic Findings Correlate With Neurologic Outcome in Children Following Cardiac Arrest. Pediatr Crit Care Med. 2016;17:667–76. doi: 10.1097/PCC.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschen MP, Topjian AA, Hammond R, Illes J, Abend NS. Neuroprognostication after pediatric cardiac arrest. Pediatr Neurol. 2014;51:663–8 e2. doi: 10.1016/j.pediatrneurol.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Caen AR, Maconochie IK, Aickin R, et al. Part 6: Pediatric Basic Life Support and Pediatric Advanced Life Support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations (Reprint) Pediatrics. 2015;136(Suppl 2):S88–119. doi: 10.1542/peds.2015-3373C. [DOI] [PubMed] [Google Scholar]
- 25.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15–9. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abend NS, Massey SL, Fitzgerald M, et al. Interrater Agreement of EEG Interpretation after Pediatric Cardiac Arrest Utilizing Standardized Critical Care EEG Terminology. Journal of Clinical Neurophysiology. doi: 10.1097/WNP.0000000000000424. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 28.The Children’s Hospital of Philadelphia, Critical Care Pathway for EEG Monitoring. 2016 Accessed February 1, 2016, 2016, at http://www.chop.edu/clinical-pathway/critical-care-pathway-eeg-monitoring-clinical-pathways.
- 29.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Crit Care Med. 2013;41:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroink H, Schimsheimer RJ, de Weerd AW, et al. Interobserver reliability of visual interpretation of electroencephalograms in children with newly diagnosed seizures. Dev Med Child Neurol. 2006;48:374–7. doi: 10.1017/S0012162206000806. [DOI] [PubMed] [Google Scholar]
- 34.Piccinelli P, Viri M, Zucca C, et al. Inter-rater reliability of the EEG reading in patients with childhood idiopathic epilepsy. Epilepsy Res. 2005;66:195–8. doi: 10.1016/j.eplepsyres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Azuma H, Hori S, Nakanishi M, Fujimoto S, Ichikawa N, Furukawa TA. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin Neurosci. 2003;57:485–9. doi: 10.1046/j.1440-1819.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- 36.Little SC, Raffel SC. Intra-rater reliability of EEG interpretations. J Nerv Ment Dis. 1962;135:77–81. doi: 10.1097/00005053-196207000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Williams GW, Luders HO, Brickner A, Goormastic M, Klass DW. Interobserver variability in EEG interpretation. Neurology. 1985;35:1714–9. doi: 10.1212/wnl.35.12.1714. [DOI] [PubMed] [Google Scholar]
- 38.Gerber PA, Chapman KE, Chung SS, et al. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophysiol. 2008;25:241–9. doi: 10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 39.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol. 1988;5:161–74. doi: 10.1097/00004691-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Young GB, McLachlan RS, Kreeft JH, Demelo JD. An electroencephalographic classification for coma. Can J Neurol Sci. 1997;24:320–5. doi: 10.1017/s0317167100032996. [DOI] [PubMed] [Google Scholar]
- 41.Westhall E, Rosen I, Rossetti AO, et al. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol. 2015;126:2397–404. doi: 10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Ronner HE, Ponten SC, Stam CJ, Uitdehaag BM. Inter-observer variability of the EEG diagnosis of seizures in comatose patients. Seizure. 2009;18:257–63. doi: 10.1016/j.seizure.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Wusthoff CJ, Sullivan J, Glass HC, et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia. 2017;58:429–35. doi: 10.1111/epi.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fantaneanu TA, Sarkis R, Avery K, et al. Delayed Deterioration of EEG Background Rhythm Post-cardiac Arrest. Neurocrit Care. 2017;26:411–9. doi: 10.1007/s12028-016-0355-6. [DOI] [PubMed] [Google Scholar]
- 45.Sivaraju A, Gilmore EJ, Wira CR, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–72. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 46.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–75. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 47.Hofmeijer J, Beernink TM, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, van Putten MJ. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85:137–43. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang TA, Secic M. Assessing Time-to-Event as an Endpoint - Reporting Survival Analyses How to Report Statistics in Medicine. 2nd. Philadelphia: American College of Physicians; 2006. pp. 115–24. [Google Scholar]


