Abstract
Purpose
To examine differences in opioid prescribing by patient characteristics and variation in hydrocodone combination product (HCP) prescribing attributed to states, before and after the 2014 Drug Enforcement Administration’s reclassification of HCP from schedule III to the more restrictive schedule II.
Methods
We used 2013–2015 data for 9,202,958 patients aged 18 to 64 from a large nationally representative commercial health insurance program to assess the temporal trends in the monthly rate of opioid prescribing.
Results
HCP prescribing decreased by 26% from June 2013 to June 2015; the rate of prescriptions for any opioid decreased by 11%. Prescribing of non-hydrocodone schedule III opioids increased slightly while prescribing of non-hydrocodone schedule II opioids and tramadol was stable. Absolute decreases in HCP prescribing rates were larger in patients being treated for cancer (−2.26% vs −0.7% for non-cancer patients, P<0.0001) and in those with high comorbidities (−2.13% vs −0.55% for those with no comorbidity, P<0.0001). Differences in the absolute and relative changes in HCP prescribing rates among states were large; for example a relative decrease of 46.7% in Texas and a 12.7% increase in South Dakota. The variation in HCP prescribing attributable to the state of residence increased from 6.6% in 2013 to 8.7% in 2015.
Conclusions
The 2014 federal policy was associated with a decrease in rates of HCP and total opioid prescribing. The large decrease in the rates of HCP prescribing for patients with actively treated cancer may represent an unintended consequence.
Keywords: Opioids, Laws, Public Policy
INTRODUCTION
The rise in the prescribing of opioid analgesics for chronic non-cancer pain over the last decade has contributed to the epidemic of opioid-related addiction, overdose, and mortality in the US.1–4 State laws and federal policies implemented to curb this epidemic have had varying effects.5–14 The prescribing of schedule II opioids (classified as high abuse potential) is more tightly regulated by federal rules. Individual states have more input in regulating the prescribing of schedule III opioids (classified as moderate abuse potential). For example, states can make additional laws to make schedule III opioids prescribing almost as restrictive as schedule II.5, 7, 9–14 Previous studies showed that inter-state variation was smaller for schedule II than for schedule III opioids, suggesting a larger impact of federal policies on the former.7, 10
In 2014, the Drug Enforcement Administration (DEA) reclassified all hydrocodone combination products (HCP), the most frequently prescribed opioids in the US, from schedule III to schedule II.15–17 This limited all new HCP prescriptions to a maximum 30-day supply with no refills. A study based on data from US pharmacies showed a 22% decline in hydrocodone combination prescriptions and a 5% increase in non-hydrocodone opioid prescriptions within 12 months of the 2014 federal DEA policy.18 Studies from single center health systems or poison centers reported similar changes.19–24.The current study used data for patients aged 18 to 64 years from a large national commercial health insurance program to examine the impact of the 2014 federal policy on opioid prescribing by patient characteristics and across states.
Studying the effect of the 2014 federal policy in this population is important because persons aged 18 to 64 represent a high-risk population for opioid-related toxicity.25, 26 This population demonstrated a significant and progressive increase—from 2003 to 2013—in the prevalence of prescription opioid use disorders, frequency of use, and overdose deaths.26 In addition, the commercial insurance cohort under study represents a population with considerable access to prescription opioids. Any policy that restricts prescribing or affects refilling of specific prescription opioids, as in the 2014 federal policy, has potential to affect prescribing of opioid analgesics in this population.
We hypothesized that the 2014 federal policy would be associated with declines in HCP prescribing. We also hypothesized that there would be a larger decrease in HCP prescribing for non-cancer pain patients than for cancer patients.2, 3, 15 Lastly, we hypothesized that there would be a decrease in the state-to-state variation in HCP prescribing with the increase in federal regulation.
METHODS
Study Design and Data Sources
We conducted a retrospective cohort study using de-identified administrative health data from Clinformatics Data Mart™ (CDM, Optum Insight, Eden Prairie, MN).27 Data analyzed were from: the Member file, which includes information on demographic factors, region of residence, and insurance enrollment date; the Medical file, which includes all inpatient and outpatient encounter information, including diagnosis codes, procedure codes, and encounter dates; and the Pharmacy file, which includes medication name, date of fill, formulation, dose, quantity, and days of supply.
Study Population and Variables of Interest
During the study period (January 1, 2013 through December 31, 2015), 9,202,958 persons aged 18–64 years met the study’s inclusion criterion of having at least 13 months of continuous coverage. This study was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston.
Measures
Opioid Prescribing
We used National Drug Code (NDC) therapeutic class description and DEA class code from the 2015 RedBook Select Extracts database, to classify opioid treatment into hydrocodone combination products (HCP), non-hydrocodone schedule II opioids, non-hydrocodone schedule III opioids, and tramadol.28 Tramadol was in its own comparator category because prior studies showed a shift in prescribers’ behavior with a substitution of the less-restricted tramadol for the newly up-scheduled HCP.18–22, 24
The unit of measurement is the prevalence of opioid prescription defined as any opioid prescribed to enrollees, with at least one opioid prescription in the study year. Our unit of measurement is the monthly rate of opioid prescribing. To estimate the monthly prevalence of opioid prescribing for each of the four categories, we generated denominators of all insured adults aged 18–64 years for each month of observation from January 2013 through December 2015. To be included in the denominator for a given month, the beneficiary was required to have had complete enrollment for the month and for the 12 preceding months. For each patient at each month of study, we used a 90-day look back period to examine the most recent prescription date and the total duration (days) of that prescription period. Patients who had at least one day of opioid prescription available in the month under study contributed to the numerator of the prevalence estimate for that month. Patients who received prescriptions for more than one opioid category during a given month contributed to the numerator of each opioid category for that month.
Patient Characteristics
Patient age was categorized at each month of observation. Race or ethnicity are not reported in the CDM. We examined all conditions included in the Elixhauser comorbidity index, with the exception of cancer, using a 12-month lookback period.29 The Elixhauser comorbidity index comprises thirty conditions including drug and alcohol abuse, depression, and psychoses (eTable 2).29 Patients were classified as having cancer if they had an International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM) diagnosis code for any solid cancer or leukemia/lymphoma with the exception of non-melanoma skin cancers (eTable 1).30 Among patients with cancer, those who received radiation or chemotherapy treatment and those who were hospitalized with a primary diagnosis of cancer in the prior year were classified as ‘actively treated.30
Statistical Analysis
The proportion of patients with opioid prescribing in each of the four categories was calculated by month, from January 2013 to December 2015, and stratified by patient characteristics. Categorical variables were analyzed using chi-square tests. Absolute differences in rates of HCP prescribing by patient characteristics were assessed by testing the interaction of each covariate with year of prescription using General Estimating Equation (GEE) models. Multilevel multivariable analyses — using a hierarchical generalized linear mixed model (HGLMM) with a binomial distribution and logit link with patients nested within states and adjustment for patient clinical and demographic characteristics — were conducted to estimate variation in HCP prescribing attributed to states at two time points: 2013 versus 2015.
The time trend in HCP prescribing was examined by piecewise regression including five joinpoints. The first four joinpoints were pre-specified and represented the key dates associated with HCP rescheduling. The fifth joinpoint was selected based on our inspection of the data when the decline in the rate of HCP prescribing reached a plateau. Trends in the prescribing of other prescription opioids over time were analyzed using jointpoint regression analysis with a maximum of five possible jointpoints. A sequential application of the permutation test using 4,500 possible randomly permuted datasets and Bayesian information criterion methods were used to determine the optimal number of jointpoints.31 All tests of statistical significance were 2-sided. Analyses were performed by DA and YFK with SAS version 9.4 (SAS Inc., Cary, NC) and Jointpoint Regression Program, Version 4.4.0.0 (NCI).31 Maps were constructed using ArcGIS 9.3.
RESULTS
Figure 1 illustrates the monthly rate (%) of HCP prescribing for all enrollees from January 2013 through December 2015. Also shown are the rates for non-hydrocodone schedule II opioids, non-hydrocodone schedule III opioids, and tramadol. There was an overall decline in HCP prescribing during the three-year period, from 2.84% in the first quarter of 2013 to 2.04% in the first quarter of 2015, a 28.17% reduction (P<0.0001). Prescribing of opioid (regardless of schedule classification) decreased from 4.73% in June 2013 to 4.19% in June 2015, an 11.42% reduction, (P<0.0001).
Figure 1.
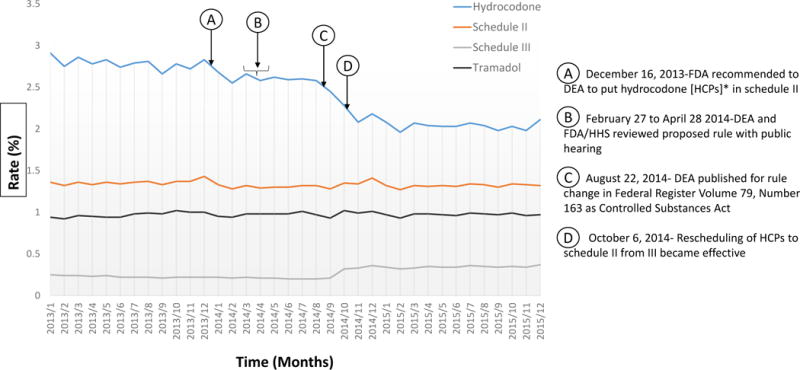
Change in opioid prescriptions from January 2013 to December 2015, stratified by drug categories. [See enlarged version in appendix for better visualization].
*HCPs – Hydrocodone combination products
Table 1 summarizes the joinpoints where the slopes in rate of opioid prescribing changed during 2013–2015. HCP prescribing rates slightly decreased during 2013, with a significantly larger decrease in early 2014 (Arrows A and B in Figure 1), followed by stable prescribing from March to August 2014 (Arrows B and C in Figure 1). This stable period was followed by the largest decrease between August and October of 2014 (Arrows C and D in Figure 1). After October 2014, the rate of HCP prescribing continued to decrease but the slope of the decrease was less after March of 2015 (the fifth joinpoint).
Table 1.
Joint points in trend of the monthly percentage of opioid prescribing between January 2013 and December 2015.
| Opioids | Joint Point | Fitted Line | ||
|---|---|---|---|---|
| Time | 95% CI | Time Period | Slopeˆ (p-value) | |
| Hydrocodone-HCP* | 12/2013 | ~12/2013 | −0.0095 (0.0491) | |
| 03/2014 | 12/2013~03/2014 | −0.0462 (0.0100) | ||
| 08/2014 | 03/2014~08/2014 | 0.0012 (0.9203) | ||
| 10/2014 | 08/2014~10/2014 | −0.1903 (<0.0001) | ||
| 03/2015 | 10/2014~03/2015 | −0.0413 (0.0006) | ||
| ~03/2015 | 0.0034 (0.5615) | |||
| Schedule II** | No Joint Point | No Joint Point | No Joint Point | No Joint Point |
| Schedule III** | 08/2014 | 07/2014, 09/2014 | ~08/2014 | −0.0101(0.0000) |
| 11/2014 | 10/2014, 12/2014 | 08/2014~11/2014 | 0.1776(0.0015) | |
| 11/2014~ | 0.0028(0.2259) | |||
| Tramadol** | 10/2013 | 06/2013, 01/2014 | ~10/2013 | 0.0067(0.0200) |
| 10/2013~ | −0.0007(0.2378) | |||
Based on the concurrency dates associated with hydrocodone combination product (HCP) schedule change, joinpoints for HCP were pre-specified in the piecewise regression model.
Joinpoint analyses were conducted to locate the optimal joint points.
Slope is expressed as percentage change in opioid prescribing rate, for example −0.0095 means 0.95% decrease in monthly opioid prescribing rate.
Schedule II (non-hydrocodone) opioids had no identified joinpoints—the joinpoint analysis found no significant changes in the slope of schedule II opioid prescribing over the 2013–2015 period (Table 1). Non-hydrocodone schedule III opioid prescribing showed a significant increase in slope during August to November 2014. The joinpoint analysis found a significant deflection in tramadol prescribing around October of 2013. This is not obvious on inspection of Figure 1, which is basically flat over the three-year period.
We next examined the decrease in HCP prescribing as a function of specific patient characteristics (Table 2). For this, we compared HCP prescribing in June 2013 to June 2015. The unadjusted rates are given for each characteristic, along with the absolute and relative differences in use between the two time points. The absolute declines in HCP prescribing were greater in the older groups, (e.g., 0.87% drop in 56–64 year olds vs 0.52% in 18–35 year olds). Women had higher rates of receiving HCP prescriptions than men did initially, and a larger absolute decrease in receiving HCP after the 2014 policy. Patients with higher comorbidity scores had the highest initial rate of being prescribed HCP, and greatest absolute decreases.
Table 2.
Patient characteristics and hydrocodone combination product (HCP) prescribing before (June 2013) and after (June 2015) policy implementation in October 2014.
| Characteristics | Hydrocodone Prescribing 2013 | Hydrocodone Prescribing 2015 | Absolute difference | % Reduction | p-value** | ||
|---|---|---|---|---|---|---|---|
| N | Rate (%) | N | Rate (%) | ||||
| Overall | 4846500 | 2.74 | 4182504 | 2.03 | −0.71 | 25.91 | |
| Age Group | <0.0001 | ||||||
| 18–35 | 1630799 | 1.69 | 1450431 | 1.17 | −0.52 | 30.77 | |
| 36–45 | 1096662 | 2.53 | 936011 | 1.88 | −0.65 | 25.69 | |
| 46–55 | 1208413 | 3.41 | 1015371 | 2.55 | −0.86 | 25.22 | |
| 56–64 | 910626 | 3.99 | 780691 | 3.12 | −0.87 | 21.80 | |
| Sex* | <0.0001 | ||||||
| Male | 2397800 | 2.48 | 2125942 | 1.83 | −0.65 | 26.18 | |
| Female | 2447931 | 3.00 | 2056057 | 2.23 | −0.77 | 25.67 | |
| Cancer | <0.0001 | ||||||
| Yes (with Trt.) | 21278 | 8.80 | 17253 | 6.54 | −2.26 | 25.68 | |
| Yes (no Trt.) | 45323 | 5.21 | 38131 | 3.89 | −1.32 | 25.34 | |
| No Cancer | 4779899 | 2.69 | 4127120 | 1.99 | −0.70 | 26.02 | |
| Number of Comorbidity*** | <0.0001 | ||||||
| 0 | 3794704 | 1.93 | 3293678 | 1.38 | −0.55 | 28.50 | |
| 1 | 671760 | 4.64 | 563387 | 3.59 | −1.05 | 22.63 | |
| 2 | 250196 | 6.40 | 211618 | 5.00 | −1.40 | 21.88 | |
| 3 or more | 129840 | 9.46 | 113821 | 7.33 | −2.13 | 22.52 | |
Note: All the characteristics were significant at 0.01 level.
Trt. =Treatment
Does not add up to total N because of missing values.
p-value was determined by multinomial model for long vs. long-term prescribing characteristic, and by GEE for all other characteristics.
All conditions in the Elixhauser comorbidity index, with the exception of cancer, were included. The index comprised 30 conditions including drug and alcohol abuse, depression, and psychoses.29
Among patients with a cancer diagnosis, we examined rates of HCP prescribing by whether or not they were being actively treated for cancer. Both groups had higher initial HCP prescribing rates (e.g., 8.8% for actively treated cancer patients, and 5.2% for not actively treated cancer patients, vs 2.7% for those who did not have a cancer diagnosis). Active cancer patients had a much larger absolute reduction in HCP prescribing than did non-cancer patients (-2.26 vs -0.70), resulting in very similar relative reductions in prescribing (25.7% vs 26.0%).
Figure 2 shows the rates of HCP prescribing by state before (A) and after (B) the change of HCP from schedule III to II. The colors of the maps divide states by quintile of 2013 HCP prescribing rates. States varied considerably in 2013, with the lowest rate in New Jersey (0.91%) and the highest rate in Alabama (5.66%). By 2015, only 9 of the states were in the top two quintiles and 33 were in the bottom two quintiles. There were also large differences in the relative changes in use among states, from a 46.7% decrease in Texas to a 12.7% increase in South Dakota (eTable 2).
Figure 2.
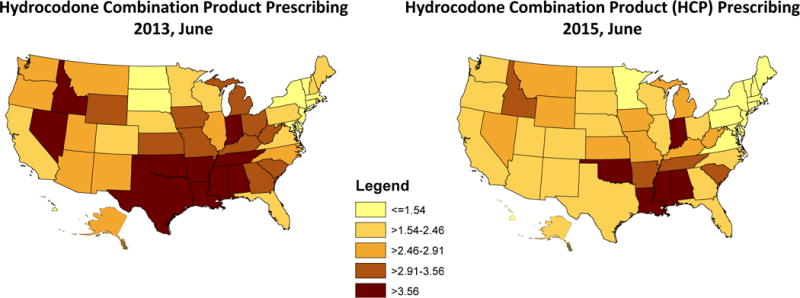
Hydrocodone combination product (HCP) prescriptions before and after federal reschedule in US states.
We further examined the variation among states in HCP prescribing before and after the change in regulations using a multilevel model to estimate Intra-class Correlation Coefficients (ICCs) indicating the proportion of variance in HCP prescribing attributed to the state of residence. Variation attributable to state was actually higher after the change in HCP scheduling than before (ICC of 6.6% in June 2013 vs 8.7% in June 2015).
DISCUSSION
The implementation of the 2014 federal policy restricting hydrocodone combination products (HCP) resulted in substantial decreases in the rates of opioid prescribing in the US. Between June 2013 and June 2015, the rate of HCP prescribing decreased by 26%; overall opioid prescribing (regardless of schedule) decreased by 11%—mostly driven by the decline in HCP prescribing. The decrease in refills, resulting from the up-scheduling of HCPs to schedule II and concomitant elimination of refills, is one possible explanation for the decrease in HCP prescribing. The publication and implementation of the final federal policy rule coincided with a sharp drop in the rate of HCP prescribing from August to October 2014. Coinciding with this drop was a small but significant increase in the non-hydrocodone schedule III opioid prescribing, mostly codeine-combination products. The rate of prescribing of non-hydrocodone schedule II opioids and tramadol was stable over the three-year study period.
Unlike the previously reported increase in tramadol prescribing after the 2014 HCP up-scheduling,14–20 we did not find this increase in our study population. Our national cohort included men and women aged < 65 with access to commercial insurance that covers prescription medications. Most prior studies were based on predominantly indigent, pediatric or veteran populations or from single health systems, hospitals, or poison centers.16, 32, 33 It is possible that commercial health insurance medication carriers have specific programs to blunt the substituting of one opioid for another, as reported in prior studies.18–24, 34 In particular, the health insurance carrier for the population who contributed data for our study implemented several programs (e.g. limiting number of refills of any opioid and requiring prior authorization) to reduce inappropriate opioid prescribing among its enrollees.34 It is thus possible that the upticks in tramadol prescribing previously reported in other studies were mitigated in our sample by the impact of these programs.
Consistent with prior studies, increasing age, female gender, and high comorbidity scores were associated with higher rates of HCP prescribing.7, 35–37 Patients with higher comorbidity scores had the highest initial rate of receiving prescribed opioids, and their absolute decreases were greatest. Actively treated cancer patients had substantially larger absolute reductions and similar relative reductions in the rate of HCP prescribing compared to non-cancer patients.24, 38, 39 Several studies have raised concerns about the potential for unintended consequences of the federal policy on the adequacy of pain treatment in cancer patients undergoing chemotherapy and other cancer-specific interventions.24, 39–42
There were large inter-state variations in both the absolute and relative changes in the rates of HCP prescribing before and after the October 2014 federal policy, ranging from a 46.7% decrease in Texas to a 12.7% increase in South Dakota. The variation in HCP prescribing attributable to the state of residence may, in part, reflect quantitative and qualitative differences in laws and policies regulating prescription opioids at the state level, as well as the degree of enforcement of these laws.5–10, 12, 13, 43 For example, the degrees of enforcement of Prescription Drug-Monitoring Programs in all US states vary from states to states with respect to the degree of inter-state data sharing, enrollment, access mandates, law enforcement access, and data collection interval.13 Also, as of 2010, Florida had six categories of laws while Georgia has only one law regulating prescribing of opioid analgesics.7 A careful examination of different state laws and opioid safe-use programs is key to understanding key components of effective state programs for possible adaptation and adoption by other states.
Contrary to our hypothesis, the variation in HCP prescribing attributable to states was actually higher after the 2014 federal policy than before. Others have noted similar unexpected and paradoxical effects after implementation of opioid-related policies.14 For example, an analysis of the impact of prescription monitoring programs aimed at reducing prescription opioid dispensing from retail pharmacies in Canada showed significant increases in prescription opioids dispensing over the study period (2005 to 2010) in most Canadian provinces and a widening of the inter-provincial variations in prescription opioids dispensing.14 These unexpected results underscore the need for long-term monitoring and evaluation of the impact of opioid-related policy at provider, patient and health-system levels.
Of note, the decrease in HCP prescribing started months before the final implementation of the law on October 2014. This decrease likely reflects the effects of media activities and myriad public hearings on prescribing behaviors of physicians before the official implementation date. Prior studies have described similar effects.14, 43,44 For example, the reported decrease in prescription opioid use and misuse among the Canadian residents of Ontario reflected not just the policy interventions but also the effects of media reporting and public hearings on prescription use disorders surrounding the policy implementations.14, 44 The media reporting not only affect MD prescribing behaviors but also patient expectations regarding pain control.44
Our study has limitations. First, information on severity of pain was not available. Second, the study population examined — aged 18 to 64 and members of commercial insurance plans — is not representative of the entire US population. Third, we were restricted to measuring opioids obtained by prescriptions. Other sources of opioids may be particularly prevalent among working-age populations.4, 45 Fourth, prescription claims reflect what was dispensed, not whether it was consumed. Finally, information on race, ethnicity, and socioeconomic status was not available for the study population. Past studies showed an association of socio-demographic factors with rates of use, misuse, and toxicity of prescription and non-prescription opioids.25, 45–47
Our study also has important strengths, including a large sample size in all US states. The present study added to the existing body of literature in by improving our understanding of: a) the differential effects of the 2014 federal policy in subpopulations at high risk of pain under-treatment—the actively treated cancer patients and patients with multiple comorbidities or/and multiple sources of pain-causing conditions and; b) the extent to which these effects vary by state. The changes in HCP prescribing before and after the federal policy allows for natural experiments of examining how characteristics such as different state opioid-related laws interact with the 2014 federal policy. The large decrease in opioid prescribing for patients undergoing active cancer treatment is surprising; the CDC federal guidelines specifically addressed over-prescribing of opioid analgesics for “chronic pain outside of active cancer treatment, palliative care, and end-of-life care.”2, 38, 42
Conclusions
The 2014 federal policy was associated with a decrease in rates of hydrocodone prescribing. The large decrease in rates of hydrocodone prescriptions in patients with actively treated cancer was unexpected. This may represent an unintended consequence of the federal policy on the adequacy of pain treatment in cancer patients. It will be important to assess whether the restriction of hydrocodone prescribing increase the rates of illicit opioid use.
Supplementary Material
Key Points.
Question: Do opioid prescribing rates before and after the 2014 federal hydrocodone combination product (HCP) rescheduling policy vary by patient characteristics or state of residence?
Findings: In the retrospective cohort study of 9,202,958 privately-insured patients, HCP prescribing decreased by 26% from 2013 to 2015; any opioid prescribing decreased by 11%; and variation by state of residence increased substantially. Patients with multi-morbidities and actively-treated cancer patients had largest decreases in HCP prescribing.
Meaning: HCP prescribing decreased after the 2014 policy. The large decrease in HCP prescribing in actively-treated cancer patients was unexpected, and may represent an unintended consequence.
Acknowledgments
This work was supported by Grants R01-DA039192, R01-AG033134, K05-CA134923, P30-AG024832, and UL1TR001439 from the National Institutes of Health, and R24-HS022134 from the Agency for Healthcare Research and Quality.
Appendix Figure 1a*.
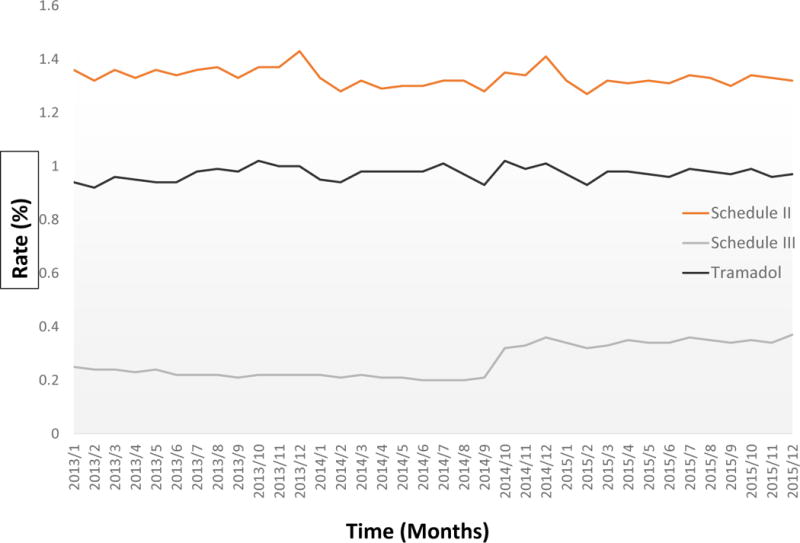
Change in opioid prescriptions from January 2013 to December 2015, stratified by opioid categories *The Figure 1a was enlarged version of figure 1 for easier visualization of the curves for schedule II, III, and tramadol. The Y-axis is from 0% to 1.6%.
Footnotes
Conflicts of Interest: None.
References
- 1.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 5.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014 Dec;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff (Millwood) 2016;35(6):1045–1051. doi: 10.1377/hlthaff.2015.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129(2):221.e21–e30. doi: 10.1016/j.amjmed.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood) 2016;35(7):1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meara M, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375(1):44–53. doi: 10.1056/NEJMsa1514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines – United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–568. [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–331. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 12.Gomes T, Juurlink D, Yao Z, Camacho X, Paterson JM, Singh S, Dhalla I, Sproule B, Mamdani M. Impact of legislation and a prescription monitoring program on the prevalence of potentially inappropriate prescriptions for monitored drugs in Ontario: a time series analysis. CMAJ Open. 2014 Oct 1;2(4):E256. doi: 10.9778/cmajo.20140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster LR, Grabois M. Current Regulations Related to Opioid Prescribing. Vol. 7. PM&R; 2015. pp. 11–S236. [DOI] [PubMed] [Google Scholar]
- 14.Fischer B, Jones W, Krahn M, Rehm J. Differences and over-time changes in levels of prescription opioid analgesic dispensing from retail pharmacies in Canada, 2005–2010. Pharmacoepidemiol Drug Saf. 2011;20(12):1269–77. doi: 10.1002/pds.2190. [DOI] [PubMed] [Google Scholar]
- 15.Drug Enforcement Administration. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. 21 CFR part 1308 [docket no. DEA 389] final rule. Fed Regist. 2014;79(163):49661–49682. Available at https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0822.htm. Accessed March 13, 2017. [PubMed] [Google Scholar]
- 16.Office of Diversion Control, Drug & Chemical Evaluation Section. Hydrocodone. Drug Enforcement Administration. 2014 Oct; Available at https://www.deadiversion.usdoj.gov/drug_chem_info/hydrocodone.pdf. Accessed March 13, 2017.
- 17.IMS Health. Top 25 Medicines by Dispensed Prescriptions (US) 2013 Available at https://www.imshealth.com/files/web/Corporate/News/Top-Line%20Market%20Data/US_Top_25_Medicines_Dispensed_Prescriptions.pdf. Accessed March 13, 2017.
- 18.Jones CM, Lurie PG, Throckmorton DC. Effect of US Drug Enforcement Administration’s rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med. 2016;176(3):399–402. doi: 10.1001/jamainternmed.2015.7799. [DOI] [PubMed] [Google Scholar]
- 19.Oehler EC, Day RL, Robinson DB, Brown LH. Has the rescheduling of hydrocodone changed ED prescribing practices? Am J Emerg Med. 2016;34(12):2388–2391. doi: 10.1016/j.ajem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Schultz S, Chamberlain C, Vulcan M, Rana H, Patel B, Alexander JC. Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J Opioid Manag. 2016;12(2):119–122. doi: 10.5055/jom.2016.0323. [DOI] [PubMed] [Google Scholar]
- 21.Seago S, Hayek A, Pruszynski J, Newman G. Change in prescribing habits after federal rescheduling of hydrocodone combination products. Proc Baylor Univ Med Cent. 2016;29(3):268–270. doi: 10.1080/08998280.2016.11929431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes A, Kleinschmidt K, Forrester MB, Young A. Trends in analgesic exposures reported to Texas poison centers following increased regulation of hydrocodone. Clin Toxicol (Phila) 2016;54(5):434–440. doi: 10.3109/15563650.2016.1148720. [DOI] [PubMed] [Google Scholar]
- 23.García MC, Dodek AB, Kowalski T, et al. Declines in opioid prescribing after a private insurer policy change — Massachusetts, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(41):1125–1131. doi: 10.15585/mmwr.mm6541a1. [DOI] [PubMed] [Google Scholar]
- 24.Pergolizzi JV, Breve F, Taylor R, Zampogna G, LeQuang JA. The aftermath of hydrocodone rescheduling: Intentional and unintended consequences. Int J Anesth Res. 2017;5(1):377–382. [Google Scholar]
- 25.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci USA. 2015;112(49):15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han B, Compton WM, Jones CM, Cai R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. JAMA. 2015;314(14):1468–1478. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 27.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RED BOOK Select Extracts. Ann Arbor, MI: Truven Health Analytics; 2011. [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris RD, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.SEER-Medicare: Identification of Diagnosis & Procedure Codes. National Cancer Institute; Division of Cancer Control and Population Sciences. HealthCare Delivery Research program: 2017. Available at https://healthcaredelivery.cancer.gov/seermedicare/considerations/identification.htm. Accessed March 13, 2017. [Google Scholar]
- 31.Joinpoint Regression Program, version 3.5. Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 32.Chumpitazi CE, Rees CA, Camp EA, Bernhardt BM. Decreased opioid prescribing in a pediatric emergency department after the rescheduling of hydrocodone. J Emerg Med. 2016 Oct 07; doi: 10.1016/j.jemermed.2016.08.026. In press. Accessed March 13, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Grasso MA, Dezman ZDW, Comer AC, Jerrard DA. The decline in hydrocodone/acetaminophen prescriptions in emergency departments in the Veterans Health Administration between 2009 to 2015. West J Emerg Med. 2016;17(4):396–403. doi: 10.5811/westjem.2016.5.29924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long-term use of opioid prescriptions and prospects for innovation in prevention, treatment and pain management. Available at http://www.unitedhealthgroup.com/~/media/UHG/PDF/2017/UNH-Clinical-Innovation-Report-Opioids.ashx?la=en. Accessed October 5, 2017.
- 35.Frenk SM, Porter KS, Paulozzi LJ. NCHS data brief. Hyattsville, MD: National Center for Health Statistics; 2015. Prescription opioid analgesic use among adults: United States, 1999–2012. (no 189). [PubMed] [Google Scholar]
- 36.Vital Signs: Overdoses of prescription opioid pain relievers and other drugs among women — United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(26):537–542. [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 39.Alford DP. Opioid prescribing for chronic pain – achieving a right balance through education. N Engl J Med. 2016;374(4):301–303. doi: 10.1056/NEJMp1512932. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21(1):3–19. doi: 10.1002/ejp.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: pain management attitudes and practice. Cancer. 2000;88(8):1929–1938. [PubMed] [Google Scholar]
- 42.Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165(14):1574–1580. doi: 10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 43.Spiller HA, Scaglione JM, Aleguas A, Foster H, Durback-Morris L, Scharman EJ, Baker SD. Effect of scheduling tramadol as a controlled substance on poison center exposures to tramadol. Ann Pharmacother. 2010;44:1016–1021. doi: 10.1345/aph.1P064. [DOI] [PubMed] [Google Scholar]
- 44.Nelson LS, Juurlink DN, Perrone J. Addressing the Opioid Epidemic. JAMA. 2015;314(14):1453–1454. doi: 10.1001/jama.2015.12397. [DOI] [PubMed] [Google Scholar]
- 45.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 47.Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11:e0159224. doi: 10.1371/journal.pone.0159224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


