Abstract
While the mutational activation of oncogenes drives tumor initiation and growth by promoting cellular transformation and proliferation, increasing evidence suggests that the subsequent re-engagement of largely dormant developmental pathways contributes to cellular phenotypes associated with the malignancy of solid tumors. Genetic studies from a variety of organisms have defined many of the components that maintain epithelial planar cell polarity (PCP), or cellular polarity in the axis orthogonal to the apical-basal axis. These same components comprise an arm of non-canonical Wnt signaling that mediates cell motility events such as convergent extension movements that are essential to proper development. In this review, we summarize the increasing evidence that the Wnt/PCP signaling pathway plays active roles in promoting the proliferative and migratory properties of tumor cells, emphasizing the importance of PCP component subcellular localization and protein-protein interactions in regulating cell properties associated with malignancy. Specifically, we discuss the upregulation of Wnt/PCP pathway components in cancer, and the functional consequences of aberrant expression, focusing on Wnt ligands, Frizzled (Fzd) receptors, the tetraspanin-like proteins Vangl1 and Vangl2, and the Prickle1 (Pk1) scaffold protein. In addition, we discuss Wnt/PCP pathway negative regulation, with particular emphasis on the Nrdp1 E3 ubiquitin ligase. We hypothesize that the engagement of Wnt/PCP pathway after tumor initiation drives malignancy by promoting cellular proliferation and invasiveness, and that the ability of Wnt/PCP signaling to supplant oncogene addiction may contribute to tumor resistance to oncogenic pathway-directed therapeutic agents.
Keywords: planar cell polarity, Vangl, Prickle, Nrdp1, cancer, metastasis
1. Introduction
Tremendous effort over the last four decades has been put into unraveling the molecular mechanisms that contribute to cancer initiation and tumor development, which has led to the identification of a plethora of mutationally activated oncogenes that drive cellular transformation and proliferation. However, most attempts to therapeutically inhibit oncogenic drivers and their associated growth signaling pathways have yielded only incremental improvements in patient outcomes. While these observations suggest that secondary mutations in oncogenic driver pathways subvert therapeutic efficacy, another possibility is that tumors engage alternative pathways that promote malignancy and contribute to drug resistance.
Aggressive, invasive tumors arise not only from dysregulated proliferation and differentiation pathways, but also from the breakdown of cellular polarity programs. Cellular architecture within the simple epithelial sheets lining many tissues is organized along two axes: (1) the commonly studied apical-basal axis responsible for the separation of apical components associated with epithelial function from basal components involved in adhesion and signaling, and (2) the planar axis orthogonal to the apical-basal axis that organizes cell polarity across the surface of the sheet. Cell polarity is intimately connected to homeostatic cell function, and disruptions to apical-basal polarity genes can sensitize cells to oncogenic transformation [1, 2]. Recent studies have revealed critical contributions of under-appreciated planar cell polarity (PCP) signaling to tumor progression, and suggest that a deeper understanding of this pathway may yield valuable insights into tumor biology and the development of novel therapeutic strategies.
Given the critical importance of PCP signaling in not only maintaining polarized epithelial tissue organization but also in promoting migration and convergent extension processes during development, the emerging role for aberrant pathway activity in tumor malignancy should not be unexpected. Tumor cells often engage dormant developmental processes to facilitate their aggressiveness. Here we will describe the association between the dysregulation of core Wnt/PCP signaling complexes and tumor progression, including a previously overlooked role for Wnt/PCP signaling in the regulation of cancer cell proliferation. Additionally, we will highlight the importance of negative regulators of Wnt/PCP signaling in promoting localized signaling and in restraining aberrant pathway activation, and highlight Nrdp1, a novel negative regulator of Wnt/PCP signaling in glioblastoma multiforme (GBM). Finally, we will discuss potential crosstalk between the Wnt/PCP pathways and other developmentally important signaling pathways in the context of tumor progression. Our focus on recent contributions to the field will emphasize cancer biology studies that describe in mechanistic detail the complexes that affect Wnt/PCP signaling, and that may yield important insights into analogous developmental processes.
2. PCP is a Developmentally Conserved Polarity Pathway
Classic PCP generates tissue-wide epithelial organization along the planar axis by propagating cellular asymmetry across multiple cell distances. This is accomplished by establishing asymmetric protein localization within a single cell and transmitting that asymmetry to its neighbors through intercellular protein-protein interactions. Mechanistically, intracellular asymmetry in PCP signaling is maintained by a branch of non-canonical Wnt signaling (Figure 1A). Unlike canonical Wnt signaling, which follows a well-characterized signaling cascade culminating in the stabilization and nuclear translocation of β-catenin, non-canonical Wnt signaling encompasses a collection of β-catenin-independent pathways that are often categorized into Wnt/Ca2+, Wnt-Ror, and PCP branches [3]. Based largely on Drosophila genetic studies, PCP signaling is perhaps the best understood non-canonical Wnt pathway, and has prominent roles in the organized development of multiple tissue types.
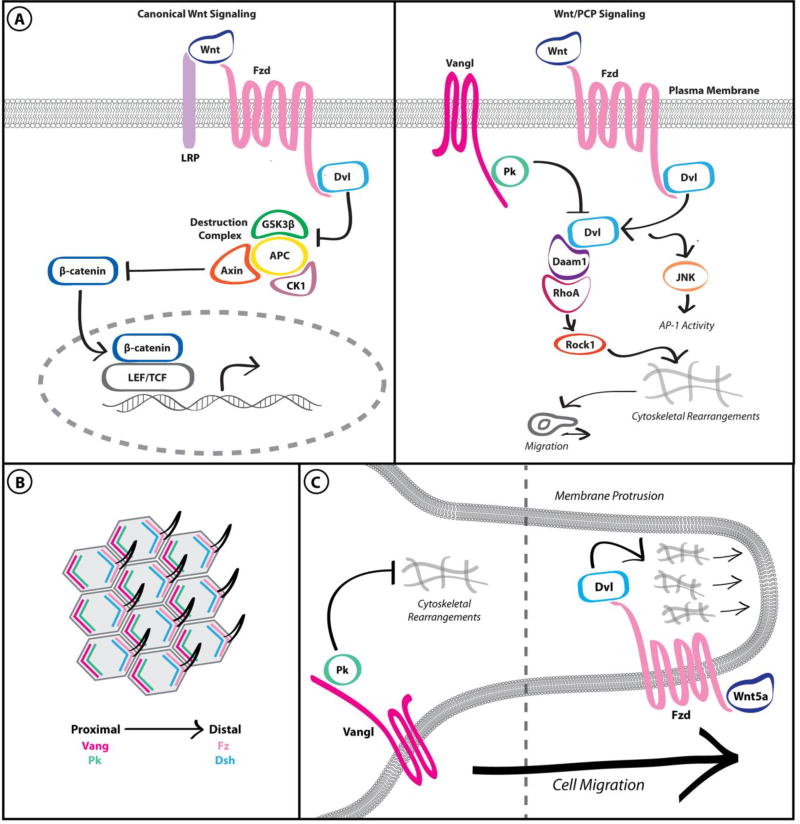
Figure 1. Wnt/PCP signaling is distinct from canonical Wnt signaling and is adapted to promote cancer cell migration.
A. Canonical Wnt signaling results in inhibition of the “destruction complex” and β-catenin-dependent transcription (left panel). In contrast, Wnt/PCP signaling results in mutual antagonism between Fzd/Dvl and Vangl/Pk complexes, leading to cytoskeletal rearrangements (right panel). B. The asymmetric distribution of core PCP complexes in the developing Drosophila wing results in consistent distal localization of the wing hair throughout the tissue. C. Wnt/PCP pathway activation within individual breast cancer cells results in asymmetric localization of core PCP components in a manner analogous to PCP in polarized epithelial cells. These complexes respectively promote and restrain actin cytoskeletal dynamics, resulting in directed migration.
Observations of hair and sensory bristle defects on the wings of Drosophila mutants led to the first descriptions of PCP [4]. Subsequent genetic studies identified evolutionarily conserved core PCP protein complexes, which are asymmetrically distributed on the apical surface of a single polarized cell and are mutually antagonistic (Figure 1B). In classic PCP, the distal plasma membrane is marked by a complex of the seven-pass transmembrane receptor Frizzled (Drosophila Fz/mammalian Fzd), the atypical cadherin Flamingo (Fmi/Celsr), and the intracellular scaffolds Dishevelled (Dsh/Dvl) and Diego, while the proximal plasma membrane is marked by Fmi, the tetraspanin Van Gogh (Drosophila Vang or Stb/mammalian Vangl) and the intracellular scaffold Prickle (Pk). Correct localization of these complexes is reinforced by intracellular antagonism between the opposing complexes and by intercellular interactions with the opposing complex on adjacent cells [5]. Importantly, the mechanisms affecting intracellular antagonism are incompletely understood but may involve selective endocytosis of excess or improperly localized complexes governed by post-translational modifications [6–8]. Thus, local negative regulation of PCP complexes is essential to generating global positive polarity signals.
Core PCP components are also critical to tissue organization in vertebrate development and are highly conserved from Drosophila. For example, asymmetric localization of PCP complexes is crucial to correct orientation of limb bud chondrocytes [9], hair follicles [10] and cochlear stereocilia [11] in the developing mouse. However, a more thorough understanding of the contributions of PCP to adult tissue homeostasis in vertebrates has been hindered by both the high degree of redundancy in mammalian PCP components (for example, there are 3 Dvl, 10 Fzd, and 19 Wnt paralogs in mice and humans) and the perinatal lethality of loss-of-function mutations to non-redundant PCP components. Interestingly, vertebrate PCP signaling regulates cell migration in embryonic development in addition to its evolutionarily conserved roles in tissue organization and polarity. This PCP-mediated migration is driven by non-canonical Wnt ligands and antagonism between core PCP complexes, resulting in context- and tissue-specific cytoskeletal rearrangements [9, 12]. Notably, defects in vertebrate PCP signaling result in impaired convergent extension and delays in neural tube closure [13–17]; human birth defects associated with mutations in PCP genes appear to phenocopy these effects [18, 19]. Aberrant PCP signaling, promoted either by transcriptional elevation of core PCP components or loss of key negative regulators, may therefore result in both loss of tissue organization and dysregulated cell migration, both of which are associated with tumor progression.
3. Wnt/PCP Components are Dysregulated in Cancer
Expression of many core PCP components is elevated in aggressive tumors and is often correlated with poor patient prognosis, consistent with roles for developmental PCP signaling promoting tumor progression. Table 1 provides an abbreviated overview of PCP component dysregulation in human tumors. While canonical Wnt signaling has well-documented roles in regulating the proliferation and differentiation of stem/progenitor cells in both normal and malignant tissues [20, 21], non-canonical Wnt signaling is typically associated with cell migration and invasion. However, recent studies have also suggested that signaling through PCP components in tumors may expand beyond regulation of cell motility to also promote proliferation. Because the classic tissue-wide phenotypic depiction of PCP is lacking in the context of a single motile tumor cell, we here denote PCP signaling outside of the polarized tissue as Wnt/PCP signaling. In perhaps the most striking dissection of the localization of PCP components in a migrating cancer cell, Luga et al. demonstrated that Wnt/PCP pathway activation in breast cancer cells results in the asymmetric distribution of core PCP components in a manner analogous to planar-polarized epithelial cells, with Fzd and Dvl co-localized at the leading edge and Vangl and Pk co-localized at the neck of actin-rich protrusions (Figure 1C) [22]. This study provides an intriguing framework to understand the effects of tumor-associated alterations in individual Wnt/PCP pathway components on cell motility. Such perturbations can disrupt the balance between mutually antagonistic PCP complexes, regulating directed migration within the context of a single motile cell.
Table 1.
Contribution of PCP components to human cancer.
| PCP Component |
Function in Wnt/PCP Signaling |
Cancer Type | Contribution to Tumorigenesis | Reference |
|---|---|---|---|---|
| WNT5A | Secreted ligand, activates Wnt/PCP signaling | Promote or suppress in context dependent manner | ||
| Breast | Promote cell migration and invasiveness | [25],[26] | ||
| Gastric | Promote cell migration and invasiveness | [27] | ||
| Melanoma | Promote cell motility and invasiveness | [32] | ||
| Colon | Suppress cell proliferation and EMT | [36] | ||
| Hematopoeitic Malignancies | Suppress cell proliferation (inhibit canonical Wnt signaling) | [38] | ||
| Thyroid Carcinoma | Suppress cell proliferation, migration, and invasiveness (inhibits canonical Wnt signaling | [39] | ||
| WNT11 | Secreted ligand, activates Wnt/PCP signaling | Promote or suppress in context dependent manner | ||
| Breast | Promote cell motility and tumor metastasis | [22] | ||
| Colon | Promote cell proliferation, migration and invasiveness | [30] | ||
| Prostate | Promote cell differentiation, survival and migration | [31] | ||
| Hepatocellular Carcinoma | Supress cell proliferation and migration (inhibits canonical Wnt signaling | [37] | ||
| FZD7 | Transmembrane Wnt receptor | |||
| Ovarian | Promote cell migration and invasiveness | [42] | ||
| HepatocellularCarcinoma | Promote cell migration | [44] | ||
| Colorectal | Promote cell migration | [45] | ||
| VANGL1 | Transmembrane scaffold for Wnt/PCP signaling | |||
| Glioblastoma | Promotes cell migration, scaffold for PCP negative regulator Nrdp1 | [43] | ||
| Colon | Promote cell migration | [46] | ||
| Head and Neck Squamous Cell Carcinoma | Promote cell migration and metastasis | [47] | ||
| Breast | Promote cell migration | [48] | ||
| VANGL2 | Transmembrane scaffold for Wnt/PCP signaling | |||
| Breast | Promotes cell migration and proliferation | [12] | ||
| PRICKLE1 | Cytoplasmic scaffold for Wnt/PCP signaling | |||
| Breast | Promote cell migration, proliferation and metastasis | [22], [50], [51] | ||
3.1. Dysregulation of Wnt Ligands and Fzd Receptors
Wnt/PCP signaling is activated primarily by the binding of non-canonical Wnt ligands such as Wnt5a and Wnt11 to Fzd receptors. However, both tumorigenic and tumor suppressive activities have been attributed to non-canonical Wnt ligands. These apparently divergent sets of observations may in part be due to the well-established ability of non-canonical Wnt signaling to suppress canonical Wnt signaling [23, 24]. Thus, non-canonical Wnt ligands may impair progression in tumors dependent on canonical Wnt signaling while promoting Wnt/PCP-mediated migration and invasion in other contexts.
Consistent with the developmental roles of PCP signaling in cell motility, the tumor-promoting effects of non-canonical Wnt ligands primarily impact cell migration and invasion. Autocrine Wnt11 secretion from breast cancer cells cooperates with fibroblast-derived exosomes to promote segregation of Fzd and Vangl complexes and promote cell motility and metastasis [22]. Wnt5a can also exert a tumor promoting effect in breast cancer, augmenting breast cancer cell migration and invasiveness [25, 26]. Further, high expression of Wnt5a in gastric cancers results in activation of the downstream Wnt/PCP effectors Rac and JNK to promote cell migration and invasiveness; treatment with a Wnt5a-targeted antibody impairs the appearance of liver metastases from gastric tumors in mice [27–29]. Elevated Wnt11 expression in colon cancer is associated with increased cell proliferation, migration, and invasiveness, accompanied by activation of JNK and c-Jun, suggesting that Wnt11 promotes tumor progression through a non-canonical Wnt pathway [30]. Aberrant non-canonical Wnt expression has also been reported in prostate cancer [31], and is elevated in melanoma and cervical cancers, where high expression predicts poor patient outcome [32–35].
While non-canonical Wnt ligands primarily impact invasion and metastasis, tumor suppressive functions attributed to these ligands largely affect cell proliferation. This observation may suggest that in these contexts, non-canonical Wnt ligands are suppressing canonical Wnt signaling. For example, in colorectal cancer patients with non-metastatic disease, higher expression of Wnt5a correlates with better clinical outcomes, and colorectal cancer cell lines overexpressing Wnt5a displayed less tumorigenicity and proliferative capacity in a xenograft model [36]. Impaired tumor progression associated with non-canonical Wnt ligands in hepatocellular carcinoma, lymphoma, and thyroid cancers has also been attributed to inhibition of canonical Wnt signaling [37–39].
Of the 10 members of the Fzd family, Fzd7 has received much attention for its ability to potentiate developmental PCP signaling via RhoA and JNK [40, 41]; Fzd7 expression is also linked with cancer cell migration and invasion. Fzd7 expression and PCP/Wnt signaling are associated with an aggressive subtype of ovarian cancer, and Fzd7 knockdown impairs ovarian cancer cell migration through modulation of downstream PCP effectors RhoA and Rac1 [42]. Fzd7 is also transcriptionally upregulated and correlates with poor patient outcome in GBM [43] and promotes the migration of hepatocellular carcinoma and colon cancer cells [44, 45]. However, because individual Fzd family members are likely not specific for only canonical or non-canonical Wnt signaling, further investigation is required to determine whether the effects of Fzd7 modulation in tumors is specific for effects on Wnt/PCP signaling. Similarly, assessment of transcriptional alterations to the three Dvl family members contributing to dysregulated Wnt/PCP signaling during tumor progression has been hindered by the critical role of these scaffolds in canonical Wnt signaling. However, multiple post-translational regulators of Dvl have been identified. At least one of these, the E3 ubiquitin ligase Nrdp1, specifically modulates Wnt/PCP signaling and is dysregulated in multiple tumors, and is discussed in further detail below.
3.2 Dysregulation of the Vangl Transmembrane Scaffolds
In vertebrates, the Vangl family of tetraspanin-like scaffolding proteins consists of Vangl1 and Vangl2. Studies involving the Vangl proteins are of particular interest when assessing the contributions of Wnt/PCP signaling to cancer biology, as they are thought to be specific for this branch of non-canonical Wnt signaling. Vangl1 expression is elevated in the gastric tumor mucosa and in lymph node metastases of head and neck squamous cell carcinomas [46, 47]. Vangl1 also promotes the migratory properties of breast, colon, and neck squamous cell carcinoma and GBM cell lines [43, 46–48]. Data from our laboratory indicate that both Vangl1 and Vangl2 are amplified and transcriptionally upregulated in ovarian and uterine cancers [49], and that high expression of either Vangl transcript predicts poor patient outcome in GBM [43]. In addition, we and others have reported that elevated Vangl1 is associated with poor patient outcomes in both estrogen receptor (ER)-positive and all breast cancers, and that elevated Vangl2 is associated with poor patient outcomes in breast cancer [12, 22, 48, 49]. Together, these studies suggest that Vangl dysregulation may be a feature common to the malignancy of many solid tumors.
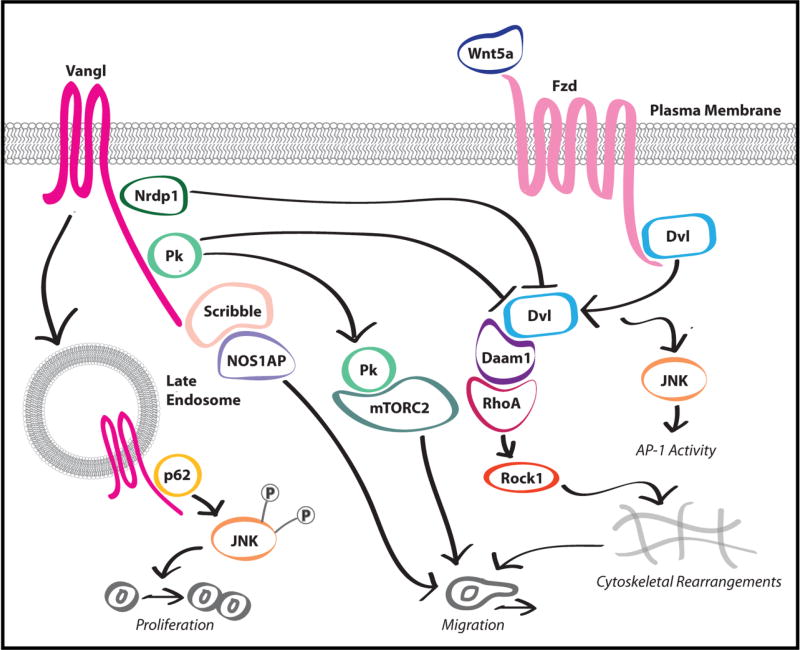
Several recent studies have identified Vangl protein complexes that regulate cellular properties associated with malignancy, providing additional mechanistic insight into how dysregulation of Wnt/PCP signaling promotes tumor progression. We recently identified Nrdp1 as a negative regulator of Wnt/PCP signaling that interacts with Vangl proteins, and found that loss of Nrdp1 promotes GBM progression (see below) (Figure 2) [43]. Further, Anastas et al. [48] identified a complex containing Vangl1, NOS1AP, and Scribble that localizes to the leading edge of migrating breast cancer cells (Figure 3). Disruption of this complex both reduced tumor cell migration and impaired Vangl localization to the leading migratory edge. Interestingly, NOS1AP is absent from the membrane of confluent mammary epithelial cells [48], suggesting that the Vangl1-NOS1AP-Scribble ternary complex does not promote PCP in homeostatic tissues but instead represents an adaptation of the tumorigenic state to promote malignancy.
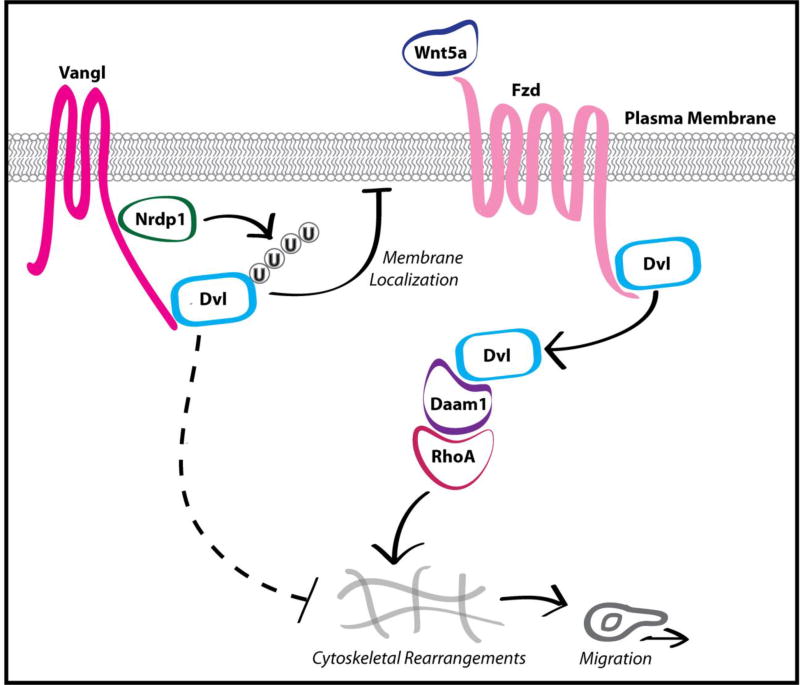
Figure 2. Nrdp1 promotes the Vangl-dependent poly-ubiquitination of Dvl.
Nrdp1 negatively regulates Wnt/PCP signaling by promoting the Vangl-dependent K63-linked polyubiquitination of Dvl. Polyubiquitination inhibits Wnt/Fzd-mediated Dvl recruitment to the plasma membrane and concomitant activation, resulting in the suppression of downstream Wnt/PCP signaling.
Figure 3. Vangl and Pk are critical molecular scaffolds for Wnt/PCP signaling.
Protein complexes assembled around the Wnt/PCP-specific scaffolds Vangl and Pk are implicated in tumor progression. The Vangl-Scribble-NOS1AP complex promotes cancer cell migration, and the Vangl-p62/SQSTM1 complex promotes cancer cell proliferation and migration. Conversely, the Vangl-Nrdp1 complex negatively regulates Wnt/PCP signaling and impairs cancer cell migration. Pk1 modulates cancer cell migration through complexes with mTORC2 or RhoA GAPs.
Interestingly, Vangl2 and Wnt/PCP signaling have recently been implicated in cancer cell proliferation. A study by Puvirajesinghe et al. [12] reports that knockdown of Vangl2 in breast cancer cells results in impaired proliferation and xenograft growth. Investigation into the mechanism through which Vangl2 contributes to cell proliferation identified a Vangl2-p62/SQSTM1 complex as an evolutionarily conserved Wnt/PCP signaling effector (Figure 3). Maintenance of this complex is required for proper convergent extension in developing Xenopus and promotes JNK-dependent cancer cell proliferation. Elevated expression of the Vangl2-p62/SQSTM1-JNK signaling axis is upregulated in breast cancer patients and is associated with with shorter survival. Intriguingly, Vangl2 and p62/SQSTM1 co-localize at the late endosome of poorly polarized cells, suggesting that endocytosis of Vangl2 is necessary for this complex to promote PCP/Wnt signaling, but interact only weakly in polarized cells. This suggests that, like the Vangl1-Scribble interaction previously described, the Vangl2-p62/SQSTM1 interaction may represent the repurposing of a developmental state not observed in normal tissue homeostasis, which has been hijacked to promote tumor malignancy.
Collectively, these studies implicate Vangl as an important scaffold upon which complexes containing crucial interacting proteins, such as Scribble-NOS1AP and p62/SQSTM1, are assembled to exploit Wnt/PCP signaling and promote a malignant state. Interestingly, these observations suggest that Vangl localizes to the late endosome [12] or to the leading migratory cell membrane [48] to elicit its effects, rather than at the neck of cell protrusions as has also been documented [22]. Additional study is required to determine the mechanisms governing selection of each complex, and whether complex selection impacts subcellular localization of Vangl.
3.3 Dysregulation of the Pk Cytoplasmic Scaffold
Like Vangl, the cytoplasmic scaffold Pk is thought to be a specific Wnt/PCP signaling component not involved in other branches of non-canonical Wnt signaling. Upregulation of Pk1 has been reported in basal breast cancers and is associated with poor metastasis-free survival [50]. The correlation between Pk1 expression and tumor progression is also supported by the observation that Pk1 promotes breast cancer cell proliferation, motility, and metastasis in a non-canonical Wnt-dependent manner [22, 50]. Recently, several studies have demonstrated that Pk1 is a critical scaffolding protein that supports the localization of complexes supporting tumor progression.
Zhang et al. have reported with additional detail the mechanism through which Pk1 regulates cancer cell migration [51]. Pk1 is enriched at the plasma membrane adjacent to lamellipodia, which in response to Wnt/PCP signaling undergo rapid protrusion/retraction cycles that correlate with cell motility. Interestingly, depletion of Pk1 resulted in protrusive membrane ruffling around the entire cell periphery, suggesting that Pk1 acts to locally impair actin cytoskeletal dynamics and result in directed migration. In support of this hypothesis, subsequent experiments demonstrated that Pk1-mediated cell motility requires a Pk1-Arhgap21/23 complex which limits the activity of the critical cytoskeletal regulator RhoA to actin-enriched lamellipodia (Figure 3). Further, Pk1 is excluded from these protrusions by the E3 ubiquitin ligase Smurf2, which has previously been demonstrated to promote Dvl-dependent proteasomal degradation of Pk1 [52]. Zhang et al. [51] provide strong evidence in support of a model in which RhoA-dependent cytoskeletal dynamics are limited to specific subcellular regions by Pk1, and which suggests that careful control of Pk1 protein levels is required to control directed migration. Disruption of the PK1 balance results in two distinct cellular phenotypes, both of which impair directed migration. Reductions in Pk1 protein levels lead to unrestrained RhoA activity throughout the cell periphery, resulting in excessive and futile actin dynamics which do not generate net directed cellular motility. Conversely, accumulation of Pk1 inhibits RhoA throughout the cell periphery resulting in impaired actin cytoskeletal dynamics and inhibited motility. In the context of previous reports [22, 52], this study illustrates how the mutual antagonism between Fzd/Dvl and Vangl/Pk1 complexes could control directed migration by defining subcellular regions permissive to pro-migratory cytoskeletal rearrangements.
Crosstalk between mammalian target of rapamycin complex 2 (mTORC2) and Wnt/PCP signaling mediated by the serine/threonine kinase MINK1 in cancer cells has recently been investigated by Daulat et al. (Figure 3) [50]. Asymmetric localization of Pk1 to the leading edge of migrating cancer cells is dependent on Pk1 phosphorylation by MINK1, and depletion of either Pk or MINK1 results in decreased cell motility and a distinctive flattened cellular morphology. The requirement for MINK1-mediated phosphorylation for asymmetric Pk1 distribution has also been observed in developmental PCP [53]. In breast cancer cells, the Pk1-MINK1 complex associates specifically with components of mTORC2 to promote phosphorylation of Akt. Disruption of this Pk1-MINK1-mTORC2 signaling axis results in impaired cell migration and in vivo tumor growth. This study suggests a model whereby Pk1 controls the localized activation of Akt through an interaction with mTORC2 at the leading edge of a migratory cell to promote directed motility.
Although both studies identify Pk1 as a scaffold for assembly of tumor-promoting complexes, they support models requiring slightly divergent roles for Pk1 in regulating cell migration. The findings of Zhang et al. [51] suggest that Pk1 promotes the local negative regulation of Wnt/PCP signaling to enable global directed migration, while the Pk1-mTORC2 interaction reported by Daulat et al. [50] suggest that Pk1 has a more direct role at the leading edge of a motile cell. Further investigation is required to reconcile these models, although both localizations could operate simultaneously. For example, Pk1 may both restrict RhoA activity to the leading migratory edge and promote endocytosis of Fzd/Dvl complexes, an action which has been attributed to Vangl and may be necessary for Wnt/PCP signaling [54]. It is also not clear whether membrane localization of these Pk1 complexes is supported by an interaction with Vangl, despite localization to the same plasma membrane regions.
4. Negative Regulation of PCP Complexes
Drosophila genetic experiments first recognized that mutual antagonism is fundamental to the partitioning of Fzd/Dvl and Vangl/Pk complexes that drives PCP. In a polarized epithelial sheet, intercellular interactions between PCP complexes may be sufficient to maintain polarity, however, additional intracellular circuits that actively exclude excess or mislocalized core PCP complexes have recently been identified. These negative regulatory pathways may be even more important in maintaining Wnt/PCP signaling in the tumor context, where planar polarity cues reinforced by neighboring cells are absent and mutual antagonism between complexes occurs along the leading migratory edges of the cell rather than on opposing proximal and distal boundaries. Because none of the core PCP components have documented enzymatic activity, accessory proteins promote the endocytosis or degradation of excess or mislocalized core PCP components. For example, endocytosis or degradation of PCP proteins at cell-cell junctions is regulated by interactions between core PCP scaffolds and multiple E3 ubiquitin ligases, which are critical effectors of developmental PCP [6–8, 52, 55].
Thus far, only p62/SQSTM1-dependent endocytosis of Vangl2 and Smurf2-dependent regulation of Pk1 have been demonstrated to clearly impact Wnt/PCP signaling in both the tumor and developmental contexts [12, 51, 52]. Further investigation into the role of negative regulation in promoting PCP-mediated cancer migration is critical to future efforts to therapeutically manipulate this pathway, given that core pathway components lack enzymatic activity and may be difficult to specifically target. In addition, a detailed understanding of the developmental and homeostatic impacts of negative regulators will be necessary to minimize side effects arising from pathway manipulation.
We recently demonstrated that aberrant Wnt/PCP signaling promotes invasiveness of GBM cells, and identified the TRIM family E3 ubiquitin ligase Nrdp1 as a novel negative regulator of this signaling pathway [43]. NRDP1 expression is strikingly diminished in GBM patient samples compared to normal brain tissue, and those patients with tumors expressing the lowest levels of NRDP1 transcript have a worsened prognosis. Restoration of Nrdp1 to GBM cell lines results in flattened cells reminiscent of the effects of Pk1 or MINK1 depletion in breast cancer cells [51] and decreases cell migration in a Vangl-dependent manner. We hypothesize that that loss of the Wnt/PCP pathway-restraining effects of Nrdp1 combined with aberrant expression of pathway-promoting Wnt/PCP signaling components observed in these tumors contributes to the aggressive invasion characteristic of GBM.
Mechanistically, Nrdp1 interacts with Vangl proteins and inhibits Wnt/PCP signaling by promoting the Vangl-dependent ubiquitination of Dvl (Figure 2). Nrdp1-mediated Dvl ubiquitination does not result in Dvl degradation or internalization of Fzd/Dvl complexes, but rather impairs the ability of Dvl to couple signaling events at the plasma membrane with downstream effectors such as Rho and Jnk. We demonstrated that Nrdp1-mediated ubiquitination of Dvl impairs the ability of Dvl to interact with the phospholipid phosphatidic acid in the plasma membrane, which is necessary for downstream non-canonical Wnt signaling. We propose that Vangl-dependent, Nrdp1-mediated ubiquitination sterically hinders the ability of Dvl to localize to the plasma membrane and engage with Fzd receptors, thereby inhibiting pro-migratory Wnt/PCP signaling. Interestingly, our findings represent at least the fourth mechanism by which post-translational ubiquitin modifications to Dvl regulate Dvl function, distinct from previously characterized effects such as degradation [56], oligomerization [57], and endocytosis [55]; this underscores both the critical importance of Dvl as a signaling hub for Wnt signaling and the versatility of ubiquitin modifications. Unlike previously discussed negative regulatory mechanisms of Wnt/PCP signaling, Nrdp1-mediated Dvl inhibition does not potentiate pathway activity but rather appears to dampen global Wnt/PCP signaling. This distinct role of Nrdp1 is underscored by its transcriptional loss in GBM tumors, whereas expression of most Wnt/PCP pathway activators is elevated in malignancies. Alternatively, it is possible that levels of Nrdp1 must be tightly controlled in a manner similar to Pk1 [51]; that is, the expression level in normal cells restrains directed migration while complete loss of Nrdp1 would result in futile undirected motility. Future studies with Nrdp1 conditional knockout animals could provide insight into such questions.
5. Crosstalk between PCP/Wnt Signaling and Other Dysregulated Signaling Pathways in Cancer
Dysregulation of essential signaling pathways in tumors results in extensive overlap and crosstalk between developmentally distinct circuits. Wnt/PCP signaling is no exception to this paradigm. In addition to interplay between Wnt pathways observed in development [9, 23, 24], the identification of novel protein complexes involving core Wnt/PCP components has expanded the possibilities for crosstalk. For example, the Pk1-mTORC2 complex adds a layer of complexity to the well-characterized regulation of Akt in cancer cells by suggesting that the Wnt/PCP pathway limits the subcellular localization of Akt phosphorylation [50].
The epithelial-mesenchymal transition (EMT) is a process fundamental to both embryonic development and tumor progression in which polarized epithelial cells acquire mesenchymal phenotypes characterized by reduced cell-cell adhesion, loss of polarity, and increased migratory and invasive ability [58]. The EMT transcriptional program is maintained by pleiotropic transcription factors such as Slug, Twist, and Snail which synergistically or redundantly contribute to tumor progression and metastasis. Given that both EMT and Wnt/PCP signaling promote cancer cell migration, possible interplay between these pathways should be investigated. In support of Wnt/PCP signaling triggering EMT, elevation of Fzd2 and the non-canonical Wnt ligands Wnt5a and Wnt5b correlates with the expression of EMT markers in metastatic liver, breast, lung, and colon cancer cell lines. Interestingly, Fzd2-dependent EMT in these cells was dependent upon two novel non-canonical Wnt pathway components, Fyn and Stat3. Further, treatment with a Fzd2-targeted antibody impaired cell invasion and in vivo tumor growth and metastasis [59], demonstrating that activation of non-canonical Wnt signaling in this context is sufficient to induce EMT.
Conversely, increased Wnt5a expression has been reported in multiple systems undergoing EMT [60, 61], although it is unclear whether Wnt5a is a direct transcriptional target of EMT-driving transcription factors such as Slug, Snail and Twist. Both Ras-mediated transformation and TGF-β treatment of MDCK epithelial cells is sufficient to stimulate Wnt5a expression, and Wnt5a knockdown decreases migration in Ras-transformed cells [61]. However, this study did not investigate the role of Wnt5a in TGF-β-stimulated migration, and the possibility that Wnt/PCP signaling is a critical effector of EMT-dependent tumor progression remains a topic worthy of further investigation.
Wnt/PCP signaling and apical basal polarity may be subject to shared regulatory circuits, as several proteins interact with components of both pathways. Scribble, which forms a cell density-dependent interaction with Vangl1 to promote breast cancer cell migration [48], is a well-characterized marker of the basolateral domain in an apical-basal polarized cell [62]. Conversely, Vangl2 interacts with aPKC, a marker of the apical domain [62], and regulates apical-basal polarity during early Xenopus development [63]. Finally, the E3 ubiquitin ligase Nrdp1 both inhibits Wnt/PCP signaling though the ubiquitination of Dvl in GBM [43] and promotes apical-basal polarity in response to phosphorylation by the basolateral marker Par1b in 3D cultures of breast epithelial cells [64]. These examples indicate that crosstalk between the two polarity pathways may be critical to normal tissue homeostasis and dysregulated in malignancy.
6. Concluding Remarks
Accumulating evidence implicates Wnt/PCP signaling as a fundamental developmental pathway dysregulated in malignancy. Given the critical importance of developmental Wnt/PCP signaling to both tissue polarity and cell migration, the hijacking of this pathway to promote tumor progression comes as no surprise. Recent studies have contributed additional mechanistic insight in support of a model in which the mutual antagonism between core Wnt/PCP protein complexes drives Wnt/PCP pathway activity. Interestingly, many of these findings were observed in the context of a motile cancer cell rather than in an epithelial sheet, and it remains to be seen the extent to which these mechanisms are also engaged during development or are specialized adaptations to the malignant state.
Vangl and Pk1 increasingly appear to function as scaffolds for the assembly of several complexes that regulate tumor progression (Figure 3). The subtle context-specific circumstances, perhaps dictated by post-translational modifications or relative levels of Wnt/PCP pathway activity, that either permit or restrict the assembly of these complexes remain to be elucidated. Similar complexes are likely essential to regulation of the Wnt/Fzd/Dvl complex, however, careful dissection of these interactions is necessary due to the overlap between Wnt pathways. Given the lack of enzyme activity in core PCP components, these protein complexes are essential to both our understanding of the contributions of Wnt/PCP signaling to tumor biology and attempts to therapeutically modulate this pathway.
While multiple studies have demonstrated that expression of Wnt/PCP components is elevated in tumors and often is associated with worsened patient prognosis, many fundamental aspects of Wnt/PCP signaling in cancer remain unknown. For example, we have little understanding of how Wnt/PCP components are transcriptionally regulated in malignancies, both with respect to the events resulting in initial aberrant expression and the dynamics of expression throughout tumor progression. Given the role of Wnt/PCP signaling in cell migration, it is possible that a process analogous to the mesenchymal-to-epithelial transition is necessary to dampen Wnt/PCP pathway activity during metastatic colonization.
Translationally, the connection between Wnt/PCP signaling and invasive or metastatic lesions suggests that core pathway components have potential as biomarkers for predicting likelihood of metastasis or patient response to therapy. The development of a specific marker of Wnt/PCP signaling, akin to phospho-specific antibodies commonly employed to monitor growth factor signaling cascades, would be immensely useful in determining the extent of pathway activation in tumor types such as carcinomas that might constitutively express core PCP proteins. Moreover, the observation that aberrant Wnt/PCP signaling actively contributes to tumor cell proliferation and invasiveness raises the possibility that this pathway acts in parallel to canonical oncogenic driver pathways to facilitate malignancy. If so, Wnt/PCP engagement could supplant tumor dependency on initiating oncogenes, resulting in resistance to therapeutic agents that target conventional oncogenic pathway components. The development of strategies and agents that interfere with aberrantly engaged Wnt/PCP signaling could offer avenues for better outcomes to patients with particularly aggressive tumors. For example, antibody-based Wnt/PCP inhibition strategies have been described that target Wnt5a [28] and Fzd2 [59] and inhibit metastasis in mouse models; humanized versions of these or similar agents could ultimately be incorporated into therapeutic regimens for patients whose tumors aberrantly engage these components. However, such agents are limited to specific ligand-receptor signaling systems and may not be universally applicable to all Wnt/PCP-driven tumors. Alternatively, further exploration of downstream signaling mechanisms could uncover kinases, phosphatases, E3 ubiquitin ligases, or other enzymes essential for Wnt/PCP signaling in response to an array of ligand-receptor drivers, and that may be targeted using small molecule approaches. As suggested above, pathway activation biomarkers could identify patients whose tumors might be susceptible to such an approach.
Acknowledgments
Funding
Salary support for the authors was provided by NIH fellowships CA210467 (KV) and CA165758 (JH), training grant GM0099608 (AB), and by NIH grants CA123541 and CA166412 (KC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
Conflicts of Interest: None
References
- 1.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22(21):5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392(6674):402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 3.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 5.Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20(8):964–71. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Cho B, Pierre-Louis G, Sagner A, Eaton S, Axelrod JD. Clustering and negative feedback by endocytosis in planar cell polarity signaling is modulated by ubiquitinylation of prickle. PLoS Genet. 2015;11(5):e1005259. doi: 10.1371/journal.pgen.1005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013;140(8):1693–702. doi: 10.1242/dev.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strutt H, Thomas-MacArthur V, Strutt D. Strabismus promotes recruitment and degradation of farnesylated prickle in Drosophila melanogaster planar polarity specification. PLoS Genet. 2013;9(7):e1003654. doi: 10.1371/journal.pgen.1003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20(2):163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10(11):1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306(1):121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puvirajesinghe TM, Bertucci F, Jain A, Scerbo P, Belotti E, Audebert S, Sebbagh M, Lopez M, Brech A, Finetti P, Charafe-Jauffret E, Chaffanet M, Castellano R, Restouin A, Marchetto S, Collette Y, Goncalves A, Macara I, Birnbaum D, Kodjabachian L, Johansen T, Borg JP. Identification of p62/SQSTM1 as a component of non-canonical Wnt VANGL2-JNK signalling in breast cancer. Nat Commun. 2016;7:10318. doi: 10.1038/ncomms10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127(10):2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 14.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405(6782):76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 15.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405(6782):81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 16.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4(8):610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26(8):2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei YP, Zhang T, Li H, Wu BL, Jin L, Wang HY. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;362(23):2232–5. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- 19.Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356(14):1432–7. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 20.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 21.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134(3):479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 24.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–30. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103(14):5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMillan CD, Leong HS, Dales DW, Robertson AE, Lewis JD, Chambers AF, Tuck AB. Stage of breast cancer progression influences cellular response to activation of the WNT/planar cell polarity pathway. Sci Rep. 2014;4:6315. doi: 10.1038/srep06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66(21):10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 28.Hanaki H, Yamamoto H, Sakane H, Matsumoto S, Ohdan H, Sato A, Kikuchi A. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer Ther. 2012;11(2):298–307. doi: 10.1158/1535-7163.MCT-11-0682. [DOI] [PubMed] [Google Scholar]
- 29.Ara H, Takagishi M, Enomoto A, Asai M, Ushida K, Asai N, Shimoyama Y, Kaibuchi K, Kodera Y, Takahashi M. Role for Daple in non-canonical Wnt signaling during gastric cancer invasion and metastasis. Cancer Sci. 2016;107(2):133–9. doi: 10.1111/cas.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishioka M, Ueno K, Hazama S, Okada T, Sakai K, Suehiro Y, Okayama N, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Possible involvement of Wnt11 in colorectal cancer progression. Mol Carcinog. 2013;52(3):207–17. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- 31.Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, Waxman J, Kypta RM. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer. 2010;9:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 33.Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14(18):5825–32. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 34.Dissanayake SK, Olkhanud PB, O'Connell MP, Carter A, French AD, Camilli TC, Emeche CD, Hewitt KJ, Rosenthal DT, Leotlela PD, Wade MS, Yang SW, Brant L, Nickoloff BJ, Messina JL, Biragyn A, Hoek KS, Taub DD, Longo DL, Sondak VK, Hewitt SM, Weeraratna AT. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68(24):10205–14. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin L, Liu Y, Zhao W, Sun B, Chen Q. Wnt5A expression is associated with the tumor metastasis and clinical survival in cervical cancer. Int J Clin Exp Pathol. 2014;7(9):6072–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol. 2014;229(12):1908–17. doi: 10.1002/jcp.24566. [DOI] [PubMed] [Google Scholar]
- 37.Toyama T, Lee HC, Koga H, Wands JR, Kim M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2010;8(2):254–65. doi: 10.1158/1541-7786.MCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4(5):349–60. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 39.Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24(13):2144–54. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 40.Medina A, Reintsch W, Steinbeisser H. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech Dev. 2000;92(2):227–37. doi: 10.1016/s0925-4773(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 41.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27(4):606–17. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, Virshup D, Thiery JP, Huang RY. FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis. 2014;5:e1346. doi: 10.1038/cddis.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wald JH, Hatakeyama J, Printsev I, Cuevas A, Fry WHD, Saldana MJ, VanderVorst K, Rowson-Hodel A, Angelastro JM, Sweeney C, Carraway KL., 3rd Suppression of planar cell polarity signaling and migration in glioblastoma by Nrdp1-mediated Dvl polyubiquitination. Oncogene. 2017 doi: 10.1038/onc.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127(4):1110–22. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10(7):697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Park SR, Chay KO, Seo YW, Kook H, Ahn KY, Kim YJ, Kim KK. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res. 2004;64(12):4235–43. doi: 10.1158/0008-5472.CAN-04-0275. [DOI] [PubMed] [Google Scholar]
- 47.Lee JK, Bae JA, Sun EG, Kim HD, Yoon TM, Kim K, Lee JH, Lim SC, Kim KK. KITENIN increases invasion and migration of mouse squamous cancer cells and promotes pulmonary metastasis in a mouse squamous tumor model. FEBS Lett. 2009;583(4):711–7. doi: 10.1016/j.febslet.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, Angers S, Moon RT. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene. 2012;31(32):3696–708. doi: 10.1038/onc.2011.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatakeyama J, Wald JH, Printsev I, Ho HY, Carraway KL., 3rd Vangl1 and Vangl2: planar cell polarity components with a developing role in cancer. Endocr Relat Cancer. 2014;21(5):R345–56. doi: 10.1530/ERC-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daulat AM, Bertucci F, Audebert S, Serge A, Finetti P, Josselin E, Castellano R, Birnbaum D, Angers S, Borg JP. PRICKLE1 Contributes to Cancer Cell Dissemination through Its Interaction with mTORC2. Dev Cell. 2016;37(4):311–25. doi: 10.1016/j.devcel.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Luga V, Armitage SK, Musiol M, Won A, Yip CM, Plotnikov SV, Wrana JL. A lateral signalling pathway coordinates shape volatility during cell migration. Nat Commun. 2016;7:11714. doi: 10.1038/ncomms11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137(2):295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 53.Daulat AM, Luu O, Sing A, Zhang L, Wrana JL, McNeill H, Winklbauer R, Angers S. Mink1 regulates beta-catenin-independent Wnt signaling via Prickle phosphorylation. Mol Cell Biol. 2012;32(1):173–85. doi: 10.1128/MCB.06320-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20(2):177–91. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sewduth RN, Jaspard-Vinassa B, Peghaire C, Guillabert A, Franzl N, Larrieu-Lahargue F, Moreau C, Fruttiger M, Dufourcq P, Couffinhal T, Duplaa C. The ubiquitin ligase PDZRN3 is required for vascular morphogenesis through Wnt/planar cell polarity signalling. Nat Commun. 2014;5:4832. doi: 10.1038/ncomms5832. [DOI] [PubMed] [Google Scholar]
- 56.Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9(18):3700–9. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Groot RE, Ganji RS, Bernatik O, Lloyd-Lewis B, Seipel K, Sedova K, Zdrahal Z, Dhople VM, Dale TC, Korswagen HC, Bryja V. Huwe1-mediated ubiquitylation of dishevelled defines a negative feedback loop in the Wnt signaling pathway. Sci Signal. 2014;7(317):ra26. doi: 10.1126/scisignal.2004985. [DOI] [PubMed] [Google Scholar]
- 58.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159(4):844–56. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S, Nagayama M. Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci. 2003;94(7):593–7. doi: 10.1111/j.1349-7006.2003.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YS, Mathias RA, Mathivanan S, Kapp EA, Moritz RL, Zhu HJ, Simpson RJ. Proteomics profiling of Madin-Darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGF-beta-mediated epithelial-mesenchymal transition. Mol Cell Proteomics. 2011;10(2) doi: 10.1074/mcp.M110.001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2011;12(1):23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 63.Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138(18):3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewandowski KT, Piwnica-Worms H. Phosphorylation of the E3 ubiquitin ligase RNF41 by the kinase Par-1b is required for epithelial cell polarity. J Cell Sci. 2014;127(Pt 2):315–27. doi: 10.1242/jcs.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]