ABSTRACT
Several adverse events have been associated with the infusion of hemoglobin-based oxygen carriers (HBOCs), including transient hypertension, gastrointestinal, pancreatic/liver enzyme elevation, and cardiac/renal injury in humans. Although several mechanisms have been suggested, the basis of HBOC toxicity is still poorly understood. Scavenging of vascular endothelial nitric oxide (NO) and heme-mediated oxidative side reactions are thought to be the major causes of toxicity. However, based on more recent preclinical studies, oxidative pathways (driven by the heme prosthetic group) seem to play a more prominent role in the overall toxicity of free Hb or HBOCs. HBOCs display a diversity of physicochemical properties, including molecular size/cross-linking characteristics leading to differences in oxygen affinity, allosteric, redox properties, and even oxidative inactivation by protein/heme clearing mechanisms. These diverse characteristics can therefore be manipulated independently, leaving open the possibility of engineering a safe and effective HBOC. To date, several antioxidative strategies have been proposed to counteract the redox side reactions of current generation HBOCs.
Keywords: Hemoglobin, hemoglobin-based oxygen carriers, oxidative toxicity
CURRENT GENERATION HBOCs
Several chemically or genetically engineered hemoglobin-based oxygen carriers (HBOCs) have been developed with diverse oxygen-binding properties, circulatory half-lives, and oncotic properties. The purpose of these chemical/genetic alterations was to primarily serve two functions: first, to stabilize the hemoglobin (Hb) molecule (which dimerizes readily in dilute solutions) in tetrameric or polymeric form, and second, to improve Hb oxygen carrying capabilities. However, no allowance in the design of these products has been made to compensate for the red blood cells (RBCs) own protective mechanisms that prevent Hb dimerization and heme iron oxidation. RBCs contain highly concentrated Hb (stabilized in the tetrameric form) of and highly efficient enzymatic machinery that maintains Hb in the functionally active ferrous form (1). Oxygen affinity, as reflected by P50 values (when Hb is half-saturated with oxygen), varies among engineered HBOCs, ranging from as low as P50 of 4.0 mmHg to as high as 40 mmHg. This article discusses current generation HBOCs (created by different modification strategies) that have been tested clinically in humans (Table 1).
Table 1.
Biochemical characterization of HBOCs
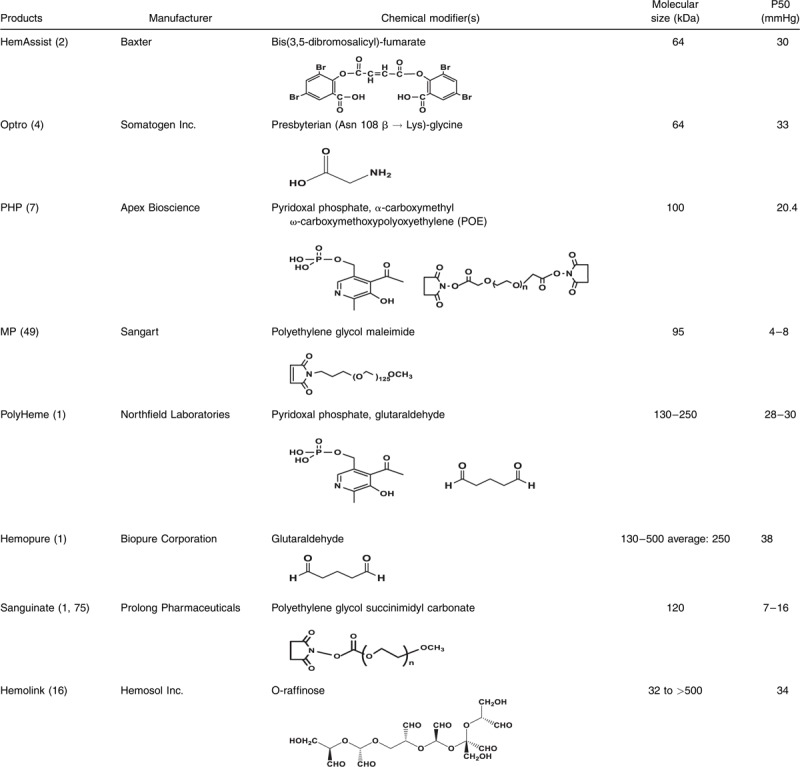
Chemically and genetically cross-linked tetrameric hemoglobins
Diaspirin crossed-linked Hb (DCLHb) (HemAssist), also commonly known as DBBF-Hb, was developed independently by Baxter International Inc. and the United States Army. The reagent (3,5-diobromo-salicyl)-fumarate was used to specifically cross-link stroma free Hb (SFH) in the deoxy conformation, between two alpha subunits (Lys99 α1 and Lys99 α2) (2). This HBOC had a substantially longer half-live than unmodified SFH and a P50 value of approximately 30 mmHg, close to that of normal red blood cell. DCLHb had been extensively tested in various preclinical and clinical studies; however, in Phase III studies, patients treated with DCLHb had significantly higher mortality rates than control groups (3). Somatogen Inc. opted to engineer another cross-linked tetramer using recombinant technology. This HBOC was expressed in Escherichia Coli in the deoxy form of a low oxygen affinity mutant, Hb Presbyterian (βN109K) using glycine to bridge the two α-subunits (di-α-gly-α] (Optro) (4). Like DCLHb, Optro had a P50 value close to that of normal RBCs. Some phase I/II clinical trials in elective surgeries with Optro (5 g Hb/dL) were conducted but discontinued due to the hypertensive effects and other related adverse events (5). Unlike Optro, extensive biochemical analysis and animal studies were carried out on chemically modified tetramers by several independent laboratories due to availability of the Army's version of DCLHb, i.e., DBBF-Hb (for review, see Ref. (6))
Conjugated “decorated” tetrameric hemoglobins
Both human and bovine Hbs have been modified by nonprotein entities such as polyethylene glycol (PEG) or polyoxyethylene (POE) to primarily increase their retention time in circulation and to maintain low oxygen affinity capabilities. One such HBOC product, pyridoxylated Hb conjugated with POE (PHP), was tested in humans initially as an oxygen carrier (7). Apex Inc. later developed this HBOC as a nitric oxide (NO) scavenger to correct hypotensive effects in response to septic shock (8). Sangart Inc. innovated this PEG modification approach by including site-specific modification of the thiol surface with imniothiolane (followed by PEGylation with PEG-50) to produce their commercial product, MP4 or Hemospan (9). This particular Hb unlike other HBOCs had very high oxygen affinity (P50 = 4.0 mmHg) and was introduced specifically to counter the autoregulatory responses associated with first generation HBOCs. Furthermore, MP4 was indicated for use in elective surgery and had gone through early clinical trials in orthopedic patients in Sweden (10). Sangart failed to secure new funding and had to terminate development operations (11).
Polymerized human and bovine hemoglobins
Intravascular retention times of HBOCs can be further increased by polymerization of the protein (1). For example, glutaraldehyde (a five-carbondialdehyde nonsite-specific reagent) forms a Schiff-base with Hb's lysine amino acid side chains and was thus used to routinely polymerize human Hb (Polyheme, produced by Northfield) and bovine Hb (Hemopure, produced by Biopure). Both HBOCs advanced to phase 3 clinical trials in humans. Polyheme was initially evaluated in phase II clinical studies in acute hemorrhage and later in a pivotal phase III prehospital trauma study (12, 13). Hemopure is approved in South Africa for the treatment of adult surgical patients who are acutely anemic and for the purpose of eliminating, reducing, or delaying the need for allogeneic red cell transfusion in these patients. Hemopure was tested in the United States for a similar indication in orthopedic surgical patients (14, 15).
Hemolink (produced by the Canadian company Hemosol) was another polymerized Hb with similar oxygen carrying characteristics (P50 is around 38–40 mmHg) but additionally possessed noncooperative behavior; this HBOC possessed a Hill coefficient (n) = 1, compared with 2.5–2.9 which is characteristic of the oxygen-binding cooperativity associated with normal human Hb. Hemolink is produce by an intra- and intermolecular cross-linking Hb with the activated sugar O-raffinose; studies have shown that this HBOC is unstable, likely due to the nonsite-specific nature of this form of modification (16). Hemolink has been tested in a series of clinical trials including in patients undergoing elective coronary artery bypass graft surgery (CABG) (17, 18).
SAFETY PROFILES OF HBOCs
Safety concerns with the development of HBOCs as viable oxygen therapeutics have challenged industry as well as research and regulatory communities. A safety summary on a number of HBOCs evaluated in several clinical settings including surgery and trauma was presented at a public workshop in 2008, and was subsequently published in the clinical literature (19). Adverse events, including cardiac, gastrointestinal, hepatic, pancreatic, central nervous systems, and renal effects, have been noted in a number of these trails and have been variously ascribed to HBOC-mediated nitric oxide scavenging, hyperoxygenation, and heme-mediated toxicity. Potential heme-mediated mechanisms (including the binding of heme to NO) were suggested by some researchers to play a role in some organ toxicities, such as gastrointestinal (pancreatitis, esophageal spasm), cardiovascular (increased incidence of myocardial infraction), and renal systems (nephrotoxicity due to generation of reactive oxygen species [ROS] and redox reactions related to the availability of iron) (for full review of the workshop summary, see Ref. (19)). In addition, there was also a large body of published preclinical data on the use of some of these HBOCs or their analogues in the literature (20).
Biochemical mechanisms of HBOC toxicity
The levels of HBOCs in human circulation were reported to be approximately 250 to 1000 μM (heme) after infusion, with half-life estimations (based on animal studies) for some HBOCs to range from 8 to 17 h. The extended persistence of HBOCs in circulation exposes Hb to the body's degradative processes and Hb oxidative pathways that current chemical engineering designs failed to take into account. Three major biochemical mechanisms have been proposed by researchers to explain the basis of free Hb-mediated toxicity that would otherwise have been suppressed inside RBCs. These are (1) scavenging of endothelial-derived nitric oxide (NO), a signaling molecule and a vasodilator; (2) oversupply of oxygen; and (3) heme-mediated oxidative reactions (for review see Refs. (21,22)).
Hemodynamic imbalances (as manifested in blood pressure elevation) in response to HBOC infusion are primarily due to NO scavenging by Hb and subsequent vasoconstriction and hypertension. An alternative mechanistic explanation to NO scavenging is the hypothesis of premature offloading of oxygen to tissues. This potentially results in autoregulatory vasoconstriction and/or through the formation of ROS and local destruction of NO (21). This hypothesis introduces complex physiological responses to free Hb in circulation and (if true) the need for a new HBOC design that can withstand these autoregulatory mechanisms; however, currently there is very little data from independent researchers to support this hypothesis. To date, less-studied pseudoenzymatic activities initiated by endogenous oxidants (as they react with the heme moiety of Hb) may have shown to have more lasting tissue-damaging effects than the other two mechanisms (22, 23, 24).
Interferences of HBOCs with the NO pathways
As the discovery of NO (a diatomic gas and important signaling and vasodilator of the vascular system), there has been a substantial increase in research focused on the interference of free Hb with normal hemodynamic and NO-mediated signaling mechanisms. Research on HBOCs provided an ideal platform for investigators to apply some of these newly discovered NO signaling pathways to a number of model systems together with potential therapies that counteract the rapid dioxygenase reaction of NO with Hb.
Other enzymatic and nonenzymatic reactions of Hb with NO such as nitrite reductase and nonenzymatic Hb S-nitrosylation have also been suggested to play a role in overall NO physiology. The reaction of NO with Hb primarily occurs at the heme prosthetic group, which can be completed within a few seconds with profound consequences (i.e., blood vessel constriction) and elevation in systematic and pulmonary blood pressures (approximate mean arterial blood pressure changes ranged between 15 and 30 mmHg) (25). However, blood pressure elevations seen in animals and in humans seem to follow a predictable path that can return to normal within 2 h. In fact, animal studies have shown that observed short-term side effects can be explained by NO scavenging, including transient heart lesions (26), histoimmunological changes in kidneys (27), and gastrointestinal effects in humans (28).
Several strategies either focused on controlling hemodynamic imbalances (after infusion of HBOCs using NO donors) or inhibiting/enhancing NO synthetic pathways seemed to have blunted these responses, but with little or no long-term tangible improvements on organ toxicities (29). Some short-term strategies to control blood pressure elevation included the transformation of Hb into an NO carrier (S-nitrosylation of βCys93 residue) or enzymatically transforming Hb in the presence of nitrite into a source for NO (nitrite reductase) (30, 31). Although imaginative, these approaches have also failed to resolve long-term toxicities associated with HBOCs. In fact, infusion of nitrite with an HBOC produced a profound cytotoxicity in the lungs of a swine animal model (32); nitrite is known to accelerate Hb oxidation and to induce tissue toxicity (33). Similar approaches designed to control pulmonary blood pressure (triggered by free Hb) have resulted in a disappointing clinical trial that involved investigating similar NO-modulating strategies in sickle cell disease (SCD) (34).
Oxidative pathways and toxicity of HBOCs
Hb oxidative toxicity and the consequences of these redox side reactions are difficult to study in living systems but recent animal studies have confirmed the involvement of oxidation reactions in the initiation of inflammatory responses (35, 36). Hb undergoes oxidation, in which the oxygen-bound ferrous heme iron (FeII) is transformed nonenzymatically to the ferric/metHb (FeIII) state (a process known as autooxidation), generating a mixture of protonated and anionic superoxide radicals (O2−). Hydrogen peroxide (H2O2) is ultimately produced during Hb autooxidation by spontaneous and enzymatic dismutation of superoxide. Hb autooxidation is to be associated with subsequent globin dysfunction and instability due to H2O2 generation resulting from dismutation of the initial superoxide products (37). When H2O2 is in excess (i.e., under oxidative stress conditions), a pseudoperoxidase catalytic cycle of Hb begins with three distinct steps in which H2O2 is ultimately and completely consumed: initial ferrous (HbFeII) oxidation to a higher oxidation ferryl Hb (HbFeIV); autoreduction of the ferryl intermediate to ferric (HbFeIII); and reaction of HbFeIII(metHb) with an additional H2O2 molecule to regenerate the ferryl intermediate/ferryl protein radical (.HbFeIV = O). This radical may migrate and further damage the protein, including the irreversible oxidation of βCys93 and other “hotspots” amino acids (38, 39). These internal reactions if not controlled also result in the modification of heme and its attachment to nearby amino acids. Irreversible oxidation of βCys93 to cysteic acid results in a destabilizing effect which contributes to Hb unfolding, dissociation of Hb into dimers, higher autooxidation rates, and rapid heme loss. Although the Hb ferryl state and its associated radicals are more damaging than the ferric form, we have recently shown that heme loss from the ferric was much higher than from the ferryl protein (40).
Due to the nature of chemical and/or genetic modifications used in the first-generation HBOCs, heme iron autooxidation and subsequent oxidative changes have been observed to occur at higher rates than those of unmodified Hb (1). Lowered oxygen affinities (due to these modifications) have also been shown to enhance autooxidation rates (41), redox potential (42), and heme loss (43). These issues have led many researchers to design countermeasures that can retard and/or control heme/iron oxidation in HBOCs. This ranged from either directly adding antioxidants (or reductants) to the HBOC solutions or even chemically cross-linking some of these antioxidants to the protein (44).
Measuring autooxidation and oxidative changes of HBOCs in circulation is difficult to monitor and is dependent on animal species used in these investigations. For example, the in vitro oxidation measurements of some HBOCs have been reported to be less of a predictor of Hb in vivo oxidation in rats (commonly used animal model) (45), whereas in sheep almost 30% to 40% metHb was accumulated after infusion of HBOCs (46). Guinea pigs, on the contrary, have been successfully used as a model for examining the Hb oxidative processes because they (similar to humans and unlike rats) lack the enzymatic ability to produce ascorbate, a powerful reductant capable of controlling intravascular Hb oxidation (24).
It has also been demonstrated in the guinea pig model that autooxidation after infusion of Oxyglobin (a bovine Hb polymerized with gltutaraldehyde approved by the FDA for veterinary use) can compromise the ability of Hb to carry oxygen, as reflected by the suppression of hypoxia inducible factor (HIF-1α) (an oxygen sensor molecule) in kidney tissues (4–6 after infusion) (47). Furthermore, renal HO-1 induction and l-ferritin expression were accompanied by significant iron deposition after Oxyglobin infusion. In a follow-up experiment, evidence was presented to show that Oxyglobin transfusion suppressed renal antioxidant enzyme expression at the gene and protein level, possibly through epigenetic alterations involving DNA methylation (27). In massive transfusion of stored blood (∼10 units) it was also reported that the Hb-driven pathologies (as consequence of the RBC storage lesions) were observed in guinea pigs that were attenuated by coinfusion of haptoglobin (Hp) (48).
OXIDATIVE COUNTERMEASURES AND INTERVENTION STRATEGIES
Nature has provided a multitude of protective mechanisms that can effectively detoxify decompartmentalized Hb under normal physiological conditions. Both erythroid and mature RBCs have very efficient reductive machinery that maintains the integrity of Hb. During early genesis of RBCs, the chaperone protein α-hemoglobin stabilizing protein (AHSP) protects vulnerable α-subunits from oxidative side reactions before assembling with β-subunits to form Hb tetramers (49). RBC antioxidative enzymes activities (primarily SOD and catalase) maintain Hb in the ferrous functional form and their activities increase in response to ROS-induced oxidative stress. Glutathione (GSH) is an important scavenger of free radicals and a potent endogenous antioxidant, which helps to protect RBCs from oxidative injury. Other important proteins also involved in antioxidant protection and H2O2 elimination include glutathione peroxidase1 (GPX1) and peroxiredoxin (50).
Co-administering the detoxifiers of hemoglobin and heme with HBOCs
During hemolysis, haptoglobin (Hp) chaperones Hb subunits (αβ dimers) in the circulation to macrophages for safe degradation through the CD163 receptors on the macrophage membrane. Complete degradation of heme into carbon monoxide (CO), bilirubin, and biliverdin byproducts occurs in these cells (51). The Hp protein is a tetramer consisting of two α-chains (each approximately 9 kDa) and two β-chains (each approximately 33 kDa). In mammals these α- and β-chains are covalently linked by disulfide bond, and in most species, a disulfide bond cross-links the α-chain of the two αβ-subunits. The binding between Hp and Hb is among the strongest noncovalent interactions known in biological systems. When released in plasma, Hb is instantly captured with high avidity by Hp to form the Hb–Hp complex. This strong binding detoxifies Hb and restricts Hb's peroxidative side reactions known to be toxic to tissues. Moreover, the Hb–Hp complex impairs filtration and clearance of Hb dimers by the kidney, and instead directs Hb to CD163 on macrophages for a process of endocytosis and final degradation (for review, see Ref. (51)).
Our recent work on Hp interactions with Hb has advanced the field in understanding the molecular details behind Hp action in protecting Hb against its own oxidative toxicity in circulation. Apart from site-specific oxidative protection of Hb's hotspots amino acids (i.e., βCys93), Hp has been shown to allow the heme active site to operate unhindered leading to the elimination of oxidants (52, 53). As the inner-sphere oxidative attack by H2O2 proceeds, Hp also diffuses the radical chemistry emanating from the heme as a consequence of this reaction. Recent mutagenesis studies have confirmed that a tyrosine residue at the Hb α-subunit (αTyr42) acts as a conduit for electron transfer to and from the heme which facilitates the autoreduction of the ferrylHb (54). EPR studies have also shown that this electron transfer is diverted to another Tyr residue, namely, βTyr145 when Hb is complexed with Hp in the presence of H2O2 in a process that stabilizes the ferryl-induced free radicals on βTyr145. This radical reactivity may ultimately be directed to the Hp molecule resulting in a safer redox inactive Hb molecule (54). Another advantage of complexing Hb with Hp is that this newly formed complex retards heme release for a considerable amount of time (55).
Although Hb binds to Hp with a high affinity via Hb αβ dimerization, nondissociable Hb tetramers may also form protein complex with Hp. Hb tetramers cross-linked between two β-subunits retain an R-state like conformation and display much higher Hp affinity than that of α-subunit cross-linked Hb tetramers. Because the binding of ββ XLHb to Hp like unmodified Hb retains its ability to decrease the propagation of damaging ferryl radicals, site-specific cross-linking of the ββ-subunits may provide a basis for an improved design of Hb-based oxygen therapeutics (56).
Hemopexin (HPX) is an acute phase plasma protein, and varies in concentrations from 8 to 21 mM in circulation (57). HPX serves as a specific carrier of plasma heme and participates in clearance by transporting it to the liver, and like Hp, HPX functions as a key plasma protector against Hb oxidation by-products. Importantly, the Heme–HPX complex is completely inactive as an oxidant; unlike the Hp–Hb complex, however, the heme–HPX complex is unable to bind or consume ligands such as NO, O2, and H2O2(57). This is because the heme in HPX is in a hexacoordinated configuration with the low spin iron (FeIII), thus unable to bind ligands.
When compared with other plasma heme scavengers (such as albumin, high- and low-density lipoproteins, and α1-microglobin), HPX has the tightest linkage (Ka ∼1.9 × 1013 M−1) with heme via a bis–His complex that is stabilized by hydrophobic and electrostatic interactions within the heme pocket. In addition, HPX also competes with LDL for heme; by reducing the amount of available heme for LDL binding HPX effectively plays a regulatory role by reducing Hb oxidative activity (57). After HPX transports the heme through a receptor on parenchymal cells, intracellular HPX is recycled to its intact free form and released into the bloodstream (57). The crystal structure of the equimolar heme–HPX complex (58) revealed a unique coordination of the heme with the protein which contributes to one of the highest affinities known (57). Thus, HPX is the key defense against the deleterious effects of heme on cells, particularly in the liver, immune system, and vascular endothelium (57). Recent animal studies have confirmed that Hb compartmentalization (rather than short-lived NO-based therapies) may be useful in countering vasoactive and oxidative toxicities associated with free Hb in hemolytic anemias (35). In dog, guinea pig, and sickle cell mice, Hp and HPX have been shown to limit the toxic effects of infused cell-free Hb (59). In addition, data obtained from these models have also revealed that Hb–Hp complex formation attenuates the hypertensive response during Hb exposure, and prevents Hb peroxidative toxicity in extravascular compartments, such as the kidney (48). However, chemical or genetic manipulations of Hb and/or Hp molecules were required to allow effective binding of the two proteins (Hp binds avidly to Hb dimers and only weakly to β-cross-linked HBOCs) (56).
Reduction of oxidized HBOCs by ascorbate
Vitamin C (ascorbic acid) and vitamin E supplementation have been used in SCD patients who are known to have considerable amount of free Hb in circulation (60). A pilot clinical trial has demonstrated that a cocktail consisting of daily doses of ascorbic acid may be beneficial to SCD patients. Ascorbic acid supplementation efficiently decreases ROS production, increases GSH concentration, and prevents H2O2-induced hemolysis. These supplements have also been shown to inhibit dense RBC formation and decrease lipid peroxidation in SCD patients (61–63).
Ascorbate is among the most important antioxidants (inside and outside the RBC) that protect Hb directly against autooxidation by reducing both ferric and ferryl heme iron (64). Photometric and EPR studies have characterized ascorbate as an active reductant capable of controlling Hb oxidative toxicity. It was indicated that ascorbate removes key precursors to Hb oxidative damage in vitro and in vivo(65). Specifically, ascorbate reduces the ferryl iron through interactions with the redox active α Tyr-42 (66). However, dehydroascorbic acid (an oxidizing species formed as a result of this one electron reduction step of Hb) is efficiently re-reduced by the erythrocyte membrane-bound reductase donor (50). Therefore, extra precaution must be taken into account when these oxidative intermediates are formed as a result of ascorbate infusion to ensure that sufficient healthy RBCs of the receipt subjects are present to rejuvenate the added ascorbate.
A recent case of compassionate use of HBOC-201 was reported in a severely injured Jehovah's Witness patient, for whom survival was considered unlikely. Severe anemia and cardiac hypoxia were reversed after slow coinfusion of this Hb with ascorbic acid (1 g twice daily). No vasoactive side effects were associated with the treatment, possibly due to slow infusion, and the patient survived (67). Ascorbate was given over 8 h to minimize any adverse events related to volume overload, vasoactivity, and accumulation of methemoglobin, by-product of Hb oxidation (67).
Engineering of oxidative stability in HBOCs
Mutant Hbs, which have been subjected over time to a variety of oxidative stresses, may develop resistance to oxidative stress or in some cases succumb to evolutionary pressure. A case in point is sickle cell Hb which is known to be oxidatively less stable than HbA (38). Our laboratory has searched for naturally occurring Hb variants which may have enhanced resistance to oxidative damage (by H2O2) and used rational protein engineering strategies to design oxidatively stable recombinant Hbs that may serve as a candidate HBOC. In particular, we have focused on the ability of a given Hb to cyclically consume H2O2 as an efficient pseudoperoxidase to prevent formation of long-lived ferryl species and protein radicals (39). Toward this goal, we have previously shown that Hb Providence (HbProv) (βK82D) from the patient's blood is much more resistant to H2O2 damage than native HbA; this is likely due to its ability to better internalize radicals through an efficient ferric/ferryl pseudoperoxidase cycle (68). In an earlier study, Weickert et al. (69) reported that the recombinant Hb Prov variant significantly improved soluble globin accumulation during expression in E. coli compared with wild-type recombinant Hb (called rHb0.0). The Hb Providence βK82D mutation likely confers markedly more resistance to H2O2 degradation by markedly inhibiting oxidation of the βCys93 side chain, particularly in cross-linked tetramers.
Redox-active tyrosine residues can facilitate electron transfer between endogenous antioxidants and oxidative ferryl heme species. A suitable residue is present in the α-subunit (Y42) of Hb, but absent from the homologous position in the β-subunit (F41). Recently, this residue was replaced with a tyrosine (βF41Y, found in the mutant Hb Mequon). These results showed that cross-linking and introducing a βF41Y into human facilitated electron transfer between endogenous antioxidants and oxidative ferryl heme species (70). In the presence of ascorbate, this mutation significantly reduced lipid peroxidation as measured by conjugated diene and singlet oxygen formation following the addition of ferric Hb to liposomes.
SECOND-GENERATION PRODUCTS AND INTERVENTION STRATEGIES THAT TARGET OXIDATIVE TOXICITY
Ligation of carbon monoxide to HBOCs
Carbon monoxide (CO) has always been recognized as a toxic gas, due to its high affinity binding to Hb and mitochondrial cytochromes. However, recent studies have revealed an intriguing role for CO as an endogenous signaling molecule which may exert many cellular activities similar to NO, including anti-inflammatory, antiapoptotic, and antiproliferative activities (71). As a result, there are several ongoing investigations of the biological applications of CO and CO-releasing molecules (CORMs) in inflammatory and vascular diseases (72).
Blood substitute manufactures have also tapped into this expanding field and began to explore the benefits of CO ligation to the Hb protein. Hb CO ligation undergoes little or no autooxidation of its heme group, as long as CO remains ligated to the heme iron. MP4 or Hemospan was initially developed as an oxygen carrier but has recently been reevaluated as a CO carrier (CO-MP4) (73). In a rat model of myocardial infract (in contrast to oxy-MP4) CO-MP4 reduced infract size when administrated before the induction of ischemia (51). In another experiment, CO-MP4 was found to modulate heme oxygenase-1 (HO-1), inflammation, and vasoocclusion in transgenic sickle cell mice. These effects were mediated by Nrf2, an important transcriptional regulator of HO-1 (74). Recently, however, a planned phase 2 study was withdrawn and the sponsor has ceased operations (11). Another example of a study involving CO-ligated Hb is Sanguinate, a purified bovine Hb manufactured by Prolong Pharmaceuticals, NJ; this protein is conjugated with 5,000 Da molecular weight PEG residues on the surface lysines and is ligated to CO (75). The unligated form of this HBOC has a high oxygen affinity (a P50 of approximately 11 mmHg). In a topload transfusion rat model, PEG-CO (<1 g/dL) produced noticeable reduction in infarct volume (76). In a phase 1 trial, three cohorts of eight healthy volunteers received single ascending doses of Sanguinate (80, 120, or 160 mg/kg) that were well tolerated. Phase 1b studies have been completed in stable patients with SCD, but no published data are available (77).
Polynitroxylated-pegylated hemoglobin
Polynitroxylated-pegylated hemoglobin (PNPH), developed by SynZyme Technologies, is an HBOC currently being developed for use as a small-volume resuscitation solution designed to mitigate oxidative toxicity. PNPH is a pegylated bovine carboxyhemoglobin (CO-Hb) covalently labeled with nitroxide moieties. The developers claim that this HBOC possess mimetic activities of superoxide dismutase (SOD) (scavenger of superoxide) and catalase (scavenger of peroxide) as well as the ability to prevent NO scavenging, whereas the polyethylene glycol (PEG) side chains create a “hydrating shell” with a marked oncotic effect that is useful in resuscitation (78). The published preclinical data demonstrated that PNPH acts as a neurovascular protective multifunctional therapeutic in an animal model simulating prehospital resuscitation of traumatic brain injury (TBI) with hemorrhagic shock (HS) (79).
ErythroMer blood substitute
ErythroMer is a novel blood substitute composed of patented nanobialys nanoparticles developed by a team at Washington University, St Louis. ErythroMer has multiple unique advantages by design: a toroidal morphology resembling RBCs; physiologic O2 binding and release; a simple system to inhibit Hb autooxidation; limited NO sequestration; and its amenable to lyophilization and reconstitution. As a validation of these advantages, ErythroMer has been shown to demonstrate superior performance than other blood substitutes in a rodent model (https://otm.wustl.edu/technologies/erythromer-blood-substitute/).
HEMOXY carrier
Natural giant extracellular Hbs from polychaete annelids are currently being investigated by Hemarina (HEMARINA SA, Morlaix, France) as promising oxygen carriers. One of these proteins is a high molecular weight acellular Hb found in the marine invertebrate Arenicola marina. This extracellular high molecular weight protein (∼3600 kDa) is composed of 156 globins and 44 nonglobin linker chains. It has a large oxygen-binding capacity, carrying up to 156 oxygen molecules when saturated (80). This new HBOC caused no allergic reaction or kidney damage after injection in mice. Infusion of this HBOC in animals showed no significant vasoconstriction, hypertension, or modification of heart rate (81). An interesting additional feature is the absence of a free cysteine residue in this Hb (80) which may also explain the reported oxidative stability of this Hb. As discussed in detail in this review, the free cysteine 93 of human β-chains has been shown to be a critical target of Hb's own radical chemistry (38).
Concluding remarks
In spite of years of research and development, no HBOC product has been licensed in the United States due in large part to safety concerns that plagued these products. Attempts have been made by industry and the research community (including short term fixes) to control hemodynamic imbalances, specifically vasoconstriction and hypertension associated with the infusion of these products. However, very little attention has been directed toward long-term heme-mediated oxidative toxicity typically seen with modified and unmodified Hbs in free environment. Moreover, the diverse chemistry of HBOC modifications and its impact on Hb redox side reactions is not fully understood. A better understanding of the complex chemistry of HBOCs is emerging, together with specific countermeasures designed to slow down and/or prevent HBOCs oxidative pathways that may ultimately provide a platform for delivering a safe and effective product.
Footnotes
This work was supported by National Institutes of Health NHLBI Grant P01-HL110900 and grants from the United States Food and Drug Administration (MODSCI).
The author reports no conflicts of interest.
REFERENCES
- 1.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med 30:381–389, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Nelson D, Azari M, Brown R, Burhop K, Bush S, Catarello J, Chuang H, Downing C, Estep T, Loewen A, et al. Preparation and characterization of diaspirin cross-linked hemoglobin solutions for preclinical studies. Biomater Artif Cells Immobil Biotechnol 20:423–427, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Sloan EP, Koenigsberg M, Weir WB, Clark JM, O’Connor R, Olinger M, Cydulka R. Emergency resuscitation of patients enrolled in the US Diaspirin Cross-linked Hemoglobin (DCLHb). Clinical Efficacy Trial Prehosp Disaster Med 1:54–61, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman SJ, Looker DL, Roehrich JM, Cozart PE, Durfee SL, Tedesco JL, Stetler GL. Expression of fully functional tetrameric human hemoglobin in coli. Proc Natl Acad Sci USA 87:8521–8525, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloan EP, Koenigsberg M, Clark JM, Weir WB, Philbin N. Shock index and prediction of traumatic hemorrhagic shock 28-day mortality: data from the DCLHb resuscitation clinical trials. West J Emerg Med 7:795–802, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agnillo F, Alayash AI. Site-specific modifications and toxicity of blood substitutes: the case of diaspirin cross-linked hemoglobin. Adv Drug Deliv Rev 40:199–212, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Washita Y. Relationship between chemical properties and biological properties of pyridoxalated hemoglobin polyoxyethylene. Biomat Artif Cell Immbol 20:299–307, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Kinasewitz GT, Privalle CT, Imm A, Steingrub JS, Malcynski JT, Balk RA, DeAngelo J. Multicenter, randomized, placebo-controlled study of the nitric oxide scavenger pyridoxalated hemoglobin polyoxyethylene in distributive shock. Crit Care Med 36:1999–2007, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs 9:800–806, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Olofsson CI, Górecki AZ, Dirksen R, Kofranek I, Majewski JA, Mazurkiewicz T, Jahoda D, Fagrell B, Keipert PE, Hardiman YJ, et al. Study 6084 clinical investigators. Evaluation of MP4OX for prevention of perioperative hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia: a randomized, double-blind, multicenter study. Anesthesiology 114:1048–1063, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Weiskopf RB. Hemoglobin-based oxygen carriers: disclosed history and the way ahead: the relativity of safety. Anesth Analg 119:758–760, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, DeWoskin R, Moss GS. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg 187:113–122, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Gould SA, Moore EE, Hoyt DB, Burch JM, Ness PM, Norris EJ, Carson JL, Hides GA, Freeman IH, DeWoskin R, et al. The life sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg 195:445–452, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Levy JH, Goodnough LT, Grelich PE, Parr GV, Stewart RW, Gratz I, Wahr J, Williams J, Comunale ME, Doblar D, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg 124:35–42, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Sprung J, Kindscher JD, Wahr JA, Levy JH, Monk TG, Moritz MW, O’ Hara PJ: The use of bovine hemoglobin glutamer-250 (Hemopure) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg 94:799–808, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Boykins RA, Buehler PW, Jia Y, Venable R, Alayash AI. O-raffinose crosslinked hemoglobin lacks site-specific chemistry in the central cavity: structural and functional consequences of beta93Cys modification. Proteins 59:840–855, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael FJ, Ali AC, Campbell JA, Langlois SF, Biro GP, Willan AR, Pierce CH, Greenburg AG. A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit Care Med 28:2283–2292, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hill S, Gottschalk LI, Grichnik K. Safety and preliminary efficacy of hemoglobin raffimer for patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth 16:695–702, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion 49:2495–2515, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Buehler PW, Alayash AI. Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion 44:1516–1530, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol 17:545–549, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Alayash AI, Cashon RE. Hemoglobin and free radicals: implications for the development of a safe blood substitute. Mol Med Today 1:122–127, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal 13:1087–1123, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther 323:49–60, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Hess JR, MacDonald VW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol 74:1769–1778, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Burhop K, Gordon D, Estep T. Review of hemoglobin-induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol 32:353–374, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Rentsendorj O, Zhang X, Williams MC, Buehler PW, D’Agnillo F. Transcriptional suppression of renal antioxidant enzyme systems in guinea pigs exposed to polymerized cell-free hemoglobin. Toxics 2016; 4 1: pii: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskopf RB, Beliaev AM, Shandler A, Guinn NR, Cap AP, Ness PM, Silverman TA. Addressing the unmet need of life-threatening anemia with hemoglobin-based oxygen carriers. Transfusion 57:207–214, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med 36:685–697, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Hess DT, Qian Z, Hausladen AF, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA 112:6425–6430, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide 38:58–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon-Massat P, Scultetus A, Arnaud F, Brown A, Haque A, Saha B, Kim B, Sagini E, McGwin G, Jr, Auker C, et al. Effect of HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury 43:638–647, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Baek JH, Zhang X, Williams MC, Hicks W, Buehler PW, D’Agnillo F. Sodium nitrite potentiates renal oxidative stress and injury in hemoglobin exposed guinea pigs. Toxicology 333:89–99, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Machado RJ, Barst NA, Yovetich KL, Hassell GJ, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 118:855–864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121:1276–1284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123:377–390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollan TL, Alayash AI. Redox reactions of hemoglobin: mechanisms of toxicity and control. Antioxid Redox Signal 18:2251–2253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassa T, Jana S, Strader MB, Meng F, Jia Y, Wilson MT. Alayash, A. I. Sickle cell hemoglobin in the ferryl state promotes βCys-93 oxidation and mitochondrial dysfunction in epithelial lung cells (E10). J Biol Chem 289:22342–22357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia Y, Buehler PW, Boykins RA, Venable RM, Alayash AI. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J Biol Chem 282:4894–4907, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Kassa T, Jana S, Meng F, Alayash AI. Differential heme release from various hemoglobin redox states and the upregulation of cellular heme oxygenase-1. FEBS Open Bio 6:876–884, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonaventura C, Henkens R, Alayash AI, Crumbliss AL. Allosteric effects on oxidative and nitrosative reactions of cell-free hemoglobins. IUBMB Life 59:498–505, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Bonaventura C, Henkens R, Alayash AI, Banerjee S, Crumbliss AL. Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxid Redox Signal 18:2298–2313, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagababu E, Ramasamy S, Rifkind JM, Jia Y, Alayash AI. Site-specific cross-linking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry 41:7407–7415, 2002. [DOI] [PubMed] [Google Scholar]
- 44.D’Agnillo F, Chang TM. Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol 16:667–671, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, Young MA, Samaja M, Winslow RM. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J 3:463–471, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol 79:236–242, 1985. [DOI] [PubMed] [Google Scholar]
- 47.Manalo DJ, Buehler PW, Baek JH, Butt O, D’Agnillo, Alayash AI. Acellular haemoglobin attenuates hypoxia-inducible factor-1alpha (HIF-1alpha) and its target genes in haemodiluted rats. Biochem J 414:461–469, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122:1444–1458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mollan TL, Banerjee S, Wu G, Parker Siburt CJ, Tsai AL, Olson JS, Weiss MJ, Crumbliss AL, Alayash AI. α-Hemoglobin stabilizing protein (AHSP) markedly decreases the redox potential and reactivity of α-subunits of human HbA with hydrogen peroxide. J Biol Chem 288:4288–4298, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety, and transfusion: capturing the “radical” menace. Antioxid Redox Signal 14:1713–1728, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Alayash AI. Haptoglobin: old protein with new functions. Clin Chim Acta 412:493–498, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Alayash AI, Andersen CB, Moestrup SK, Bülow L. Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol 31:2–3, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bülow L, Cooper CE, Wilson MT. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem 283:30780–33087, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper CE, Schaer DJ, Buehler PW, Wilson MT, Reeder BJ, Silkstone G, Svistunenko DA, Bulow L, Alayash AI: Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine β145. Antioxid Redox Signal 18:2264–2267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mollan TL, Jia Y, Banerjee S, Wu G, Kreulen RT, Tsai AL, Olson JS, Crumbliss AL, Alayash AI. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic Biol Med 69:265–277, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.H Jia Y, Wood F, Buehler PW, Alayash AI. Haptoglobin preferentially binds β but not α subunits cross-linked hemoglobin tetramers with minimal effects on ligand and redox reactions. PLoS One 8 3:e59841, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith A, McCulloh RJ. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol 6:187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hrkal Z, Vodrázka Z, Kalousek I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem 43:73–78, 1974. [DOI] [PubMed] [Google Scholar]
- 59.Vercellotti GM, Zhang P, Nguyen J, Abdulla F, Chen C, Nguyen P, Nowotny C, Steer CJ, Smith A, Belcher JD. Overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Mol Med 22:437–451, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva DG, Belini E, Jr, Alves de Almeida E, Regina C, Bonini-Domingos CR. Oxidative stress in sickle cell disease: an overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free Rad Biol Med 65:1101–1109, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Marangon K, Devaraj S, Tirosh O, Packer L, Jialal I. Comparison of the effect of α-lipoic acid and α-tocopherol supplementation on measures of oxidative stress. Free Radic Biol Med 27:1114–1121, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, Figueiredo MS. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol 160:688–700, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Muskiet FA, Muskiet FD, Meiborg G, Schermer JG. Supplementation of patients with homozygous sickle cell disease with zinc, alpha-tocopherol, vitamin C, soybean oil, and fish oil. Am J Nutr 54:736–744, 1991. [DOI] [PubMed] [Google Scholar]
- 64.Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, Cooper CE. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J 399:513–524, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper CE, Silaghi-Dumitrescu R, Rukengwa M, Alayash AI, Buehler PW. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC). Biochim Biophys Acta 1784:1415–1420, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Silkstone G, G. Silkstone RS, Wilson MT, Simons M, Bülow L, Kallberg K, Ratanasopa K, Ronda L, Mozzarelli A, Reeder BJ, et al. Engineering tyrosine electron transfer pathways decreases oxidative toxicity in hemoglobin: implications for blood substitute design. Biochem J 473:3371–3383, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzgerald MC, Chan JY, Ross AW, Liew SM, Butt WW, Baguley D, Salem HH, Russ MK, Deasy C, Martin KE, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma. Med J Aust 194:471–473, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Abraham B, Hicks W, Jia Y, Baek JH, Miller JL, Alayash AI. Isolated Hb Providence beta82Asn and beta82Asp fractions are more stable than native HbA0 under oxidative stress conditions. Biochemistry 50:9752–9766, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Weickert MJ, Pagratis M, Glascock CB, Blackmore R. A mutation that improves soluble recombinant hemoglobin accumulation in Escherichia coli in heme excess. Appl Environ Microbiol 65:640–647, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bulow L, Cooper CE, Wilson MT. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem 283:30780–30787, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Araujo JA. HO-1 and CO: fighters vs sickle cell disease? Blood 122:2535–2536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji X, Damera K, Zheng Y, Yu B, Otterbein LE, Wang B. Toward carbon monoxide-based therapeutics: critical drug delivery and developability issues. J Pharm Sci 105:406–416, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, Winslow RM. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol 154:1649–1661, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belcher JD, Young M, Chen C, Nguyen J, Burhop K, Tran P, Vercellotti GM. MP4CO, a pegylated hemoglobin saturated with carbon monoxide, is a modulator of HO-1, inflammation, and vaso-occlusion in transgenic sickle mice. Blood 122:2757–2764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nho K, Glower D, Bredehoeft S, Shankar H, Shorr R, Abuchowski A. PEG-bovine hemoglobin: safety in a canine dehydrated hypovolemic-hemorrhagic shock model. Biomater Artif Cells Immobil Biotechnol 20:511–524, 1992. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Cao S, Kwansa H, Crafa D, Kibler KK, Koehler RC. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol 113:1709–1717, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abuchowski A. PEGylated bovine carboxyhemoglobin (SANGUINATE™): results of clinical safety testing and use in patients. Adv Exp Med Biol 876:461–467, 2016. [DOI] [PubMed] [Google Scholar]
- 78.Hsia CJ, Ma L. A hemoglobin-based multifunctional therapeutic: polynitroxylated pegylated hemoglobin. Artif Organs 36:215–220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brockman EC, Bayır H, Blasiole B, Shein SL, Fink EL, Dixon C, Clark RS, Vagni VA, Ma L, Hsia CJ, et al. Polynitroxylated-pegylated hemoglobin attenuates fluid requirements and brain edema in combined traumatic brain injury plus hemorrhagic shock in mice. J Cereb Blood Flow Metab 33:1457–1464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marchand A, Crepin N, Roulland I, Semence F, Domergue V, Zal F, Polard V, Coquerel A. Application of HBOCs electrophoretic method to detect a new blood substitute derived from the giant extracellular haemoglobin of lugworm. Drug Test Anal 9 (11–12):1762–1767, 2017. [DOI] [PubMed] [Google Scholar]
- 81.Le Gall T, Polard V, Rousselot M, Lotte A, Raouane M, Lehn P, Opolon P, Leize E, Deutsch E, Zal F, et al. In vivo biodistribution and oxygenation potential of a new generation of oxygen carrier. J Biotechnol 187:1–9, 2014. [DOI] [PubMed] [Google Scholar]


