Abstract
Wolbachia are widespread heritable endosymbionts of arthropods notorious for their profound effects on host fitness as well as for providing protection against viruses and eukaryotic parasites, indicating that they can interact with other microorganisms sharing the same host environment. Using the terrestrial isopod crustacean Armadillidium vulgare, its highly diverse microbiota (>200 bacterial genera) and its three feminizing Wolbachia strains (wVulC, wVulM, wVulP) as a model system, the present study demonstrates that Wolbachia can even influence the composition of a diverse bacterial community under both laboratory and natural conditions. While host origin is the major determinant of the taxonomic composition of the microbiota in A. vulgare, Wolbachia infection affected both the presence and, more importantly, the abundance of many bacterial taxa within each host population, possibly due to competitive interactions. Moreover, different Wolbachia strains had different impacts on microbiota composition. As such, infection with wVulC affected a higher number of taxa than infection with wVulM, possibly due to intrinsic differences in virulence and titer between these two strains. In conclusion, this study shows that heritable endosymbionts such as Wolbachia can act as biotic factors shaping the microbiota of arthropods, with as yet unknown consequences on host fitness.
Introduction
Heritable symbiotic bacteria are essential drivers of arthropod ecology and evolution. This is exemplified by the many species harbouring obligate or facultative vertically transmitted endosymbionts and the diversity of symbiont effects on host fitness1,2. These effects can be beneficial, e.g. providing essential nutrients lacking from the host’s diet or defence against natural enemies3–11 or parasitic, including reproductive parasitism12–14.
Bacteria of the genus Wolbachia are probably the most widespread heritable bacterial endosymbionts, infecting a wide range of arthropods (up to 65% of insect species are estimated to be infected15,16) and filarial nematodes17. Despite several cases of mutualism or dependence18–23, most arthropod-infecting Wolbachia are reproductive parasites: Being maternally transmitted, they manipulate their hosts’ reproduction in various ways (i.e. cytoplasmic incompatibility (CI), parthenogenesis, male-killing or the feminization of genetic males) to promote their own vertical transmission24–28. Although far from being the only bacteria inducing these phenotypes, Wolbachia are the most frequently encountered reproductive manipulators and cause the largest spectrum of reproductive phenotypes12,15,29.
In addition to our growing understanding of the diversity of symbiont-mediated effects on hosts, the focus of symbiosis research has recently broadened from the study of binary host-symbiont interactions to a more holistic view of a host and its associated microbial community30–34. From this perspective, symbioses are shaped by highly dynamic multipartite interactions, not only between the host and its symbionts but also between the different members of the symbiotic community35–39. The latter may be direct interactions, e.g. through competition for resources or space within the shared host35,39–41 or by promoting the evolution of cooperation or dependence between different bacteria36,42–44. Alternatively, particular taxa could provoke a host immune response, which in turn might affect the microbiota as a whole, with potential consequences for organismal function. Indeed, studies of the relatively simple gut microbiota of Drosophila melanogaster have revealed (i) the importance of certain commensal bacteria for larval growth and optimal nutrient metabolism as well as (ii) a fine-tuned equilibrium between the host’s innate immune response and the gut microbiota to maintain homeostasis and prevent the proliferation of bacterial pathogens45–49. This leads us to ask whether heritable intracellular bacterial symbionts such as Wolbachia, which can reach high titers in host tissues and have profound impacts on host fitness, may also interact with the rest of the bacterial community present in the same host. This is all the more relevant since, due to its wide arthropod host range, Wolbachia is a dominant member of the microbiota in many insects, including species of agricultural or medical importance such as whiteflies or mosquitoes50–55.
In the context of Wolbachia symbioses in arthropods, numerous studies have demonstrated a Wolbachia-mediated protection against various viruses and Plasmodium parasites in naturally infected Drosophila as well as in transfected mosquito species56–62. This protective phenomenon indicates that Wolbachia can indeed interact with other microorganisms in the same host environment, either indirectly via the induction of a general immune response, or via competition for resources and space. In contrast, relatively little is known regarding interactions between Wolbachia and other bacteria, except for several binary interactions with other highly abundant symbionts. Hence, male-killing Spiroplasma have been shown to negatively affect Wolbachia titers in D. melanogaster40 and recent studies have demonstrated a mutual competitive exclusion between Wolbachia and Asaia in the reproductive organs of Anopheles and Aedes mosquitoes, effectively inhibiting vertical transmission of the respective other symbiont63,64. However, Wolbachia’s influence on a larger commensal bacterial community has been investigated only recently in laboratory lines of D. melanogaster65,66, yielding conflicting results: While Wolbachia infection resulted in an overall decrease in taxonomic richness of the Drosophila gut microbiota, along with an increased abundance of the two bacterial families Leuconostocaceae (Firmicutes, Bacilli) and Acetobacteraceae (Alphaproteobacteria) in Ye et al.66, Simhadri et al.65 instead observed significantly reduced titers of Acetobacteraceae, notably Acetobacter pasteurianus. These results indicate that Wolbachia-microbiota interactions may be complex and dependent on both host genotype and Wolbachia strain.
The objective of the present study was to investigate the impact of Wolbachia on a diverse symbiotic bacterial community under both laboratory and natural conditions. To achieve this, we used the terrestrial isopod Armadillidium vulgare and its association with feminizing Wolbachia as a model system for several reasons: First, three different feminizing Wolbachia strains (wVulC, wVulM, wVulP) establish stable single-infections in this host67–70, allowing us to compare the impact of different Wolbachia strains without having to account for additional interactions between the different Wolbachia strains themselves. Second, our recent quantitative study revealed Wolbachia strain-specific tissue distribution patterns in this species71, possibly reflecting different co-evolutionary histories between the Wolbachia strains and A. vulgare69,72,73. Third, the strain wVulC has recently been shown to protect its host against two bacterial pathogens74, akin to the protective phenotypes against viruses and parasites in Drosophila and mosquitoes, suggesting that the presence of this strain indeed affects co-infecting bacteria. Finally, a recent in-depth characterization of the microbiota in various tissues of A. vulgare unveiled a highly diverse bacterial community compared to many insect species, comprising more than 200 bacterial genera even in the presence of highly abundant Wolbachia34,75. Moreover, microbiota composition differed between host populations due to an important share of environmental bacteria, resulting in a complex community of intracellular and intestinal symbionts as well as environmental passengers34,75. Herein, we investigate the specific impact of Wolbachia infection on microbiota composition in A. vulgare using 16S rRNA gene metabarcoding and genetic fingerprinting via Temperature Gradient Gel Electrophoresis (TGGE). Our results show that, although host origin is the major determinant, Wolbachia infection has a noticeable and strain-specific impact on microbiota composition within each host population, resulting in reduced abundances of many bacterial taxa, possibly due to competitive interactions.
Results
In the present study, we analysed the microbiota from a total of 77 A. vulgare collected from four laboratory lineages and two field sites in France (Availles and Plaine Mothaise, Table 1). Individuals from the laboratory lineages consisted of Wolbachia-uninfected males and females as well as females infected with either of the three feminizing Wolbachia strains wVulC, wVulM and wVulP. Specimens from the two natural populations consisted of uninfected males and wVulC-infected females (both sites), as well as wVulM-infected females (Availles only) and a single intersex male (the result of incomplete feminization) infected with wVulC from the Plaine Mothaise (Table 1). 16S rRNA gene amplicons were obtained from five different tissues (haemolymph, nerve cord, gonads, midgut caeca and hindgut) and biological replicates from the same tissue and sample type (origin x gender x Wolbachia strain) were pooled for sequencing. This resulted in 55 amplicon pools yielding 313 457 high-quality reads clustered into 1380 OTUs represented by ≥3 reads at the 97% similarity cut-off (see Supplementary Table S1 for details). These OTUs represented 19 bacterial phyla, 34 classes and 229 genera other than Wolbachia (see Supplementary Table S2 for a detailed taxonomy, Fig. 1). All reads identified as Wolbachia were excluded from the dataset for subsequent analyses and the tissue-specific data from the same sample type were merged in order to obtain a representative “whole animal” profile (Fig. 1), yielding an average of 14 800 reads clustered into 298.5 OTUs per sample type.
Table 1.
Origin, infection status and number of specimens used for 16S rRNA gene amplicon sequencing and/or TGGE fingerprinting. Individuals were pooled for amplicon sequencing.
| Lineage/Population | Gender (Wolbachia strain) | Number of Specimens | ||
|---|---|---|---|---|
| Sequencing | TGGE | |||
| Laboratory | Wolbachia-free | M | 10 | 9 |
| F | 10 | 7 | ||
| C lineage | F (wVulC) | 10 | 8 | |
| M lineage | F (wVulM) | 10 | 6 | |
| P lineage | F (wVulP) | 10 | 6 | |
| Field | Availles | M | 6 | 0 |
| F (wVulC) | 6 | 0 | ||
| F (wVulM) | 6 | 0 | ||
| Plaine Mothaise | M | 3 | 0 | |
| M intersex (wVulC) | 1 | 0 | ||
| F (wVulC) | 5 | 0 | ||
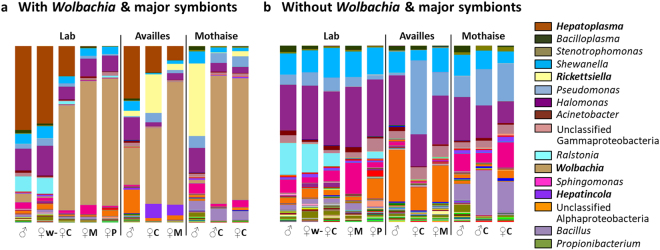
Figure 1.
Microbiota composition (%) at the genus level depending on host origin, gender and Wolbachia infection. The sequencing data from five different tissues were merged in order to obtain a representative profile for each sample type. The most abundant bacterial genera are specified in the legend, their order corresponding to the order of taxa in the barplots from top to bottom. (a) represents the complete bacterial community including Wolbachia and three other highly abundant terrestrial isopod symbionts (i.e. Hepatoplasma, Hepatincola, Rickettsiella, highlighted in bold in the legend). These highly abundant taxa were removed in (b) in order to obtain a less skewed representation of the rarer taxa.
Impact of Wolbachia infection on taxonomic richness and diversity
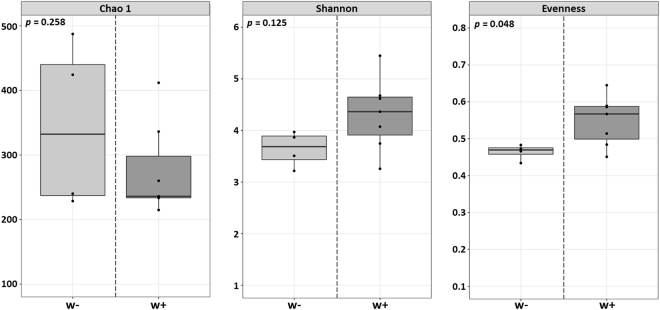
We first assessed whether Wolbachia infection affected bacterial taxonomic richness, diversity and community evenness, as estimated by the species richness estimator Chao1 and the Shannon Indices of diversity and evenness, respectively. Taxonomic richness and diversity were not significantly different between Wolbachia-free and Wolbachia-infected isopods (Fig. 2). However, there was a tendency towards a higher evenness in the bacterial communities from Wolbachia-infected animals (two sample t-test p = 0.048) (Fig. 2). These results suggest that Wolbachia infection did not affect species richness but may have induced changes in the abundance of certain bacterial taxa, resulting in more even bacterial communities in the presence of Wolbachia.
Figure 2.
Impact of Wolbachia infection on bacterial taxonomic richness and diversity. Boxplots representing taxonomic richness (Chao 1), diversity (Shannon Index) and evenness. Alphadiversity indices were compared depending on Wolbachia infection (i.e. between Wolbachia-infected and uninfected isopods) using two-sample t-tests (p-values in the top left corner of each panel). All indices were calculated at a depth of 5000 reads after merging the sequencing data from five different tissues.
Impact of Wolbachia infection on microbiota composition
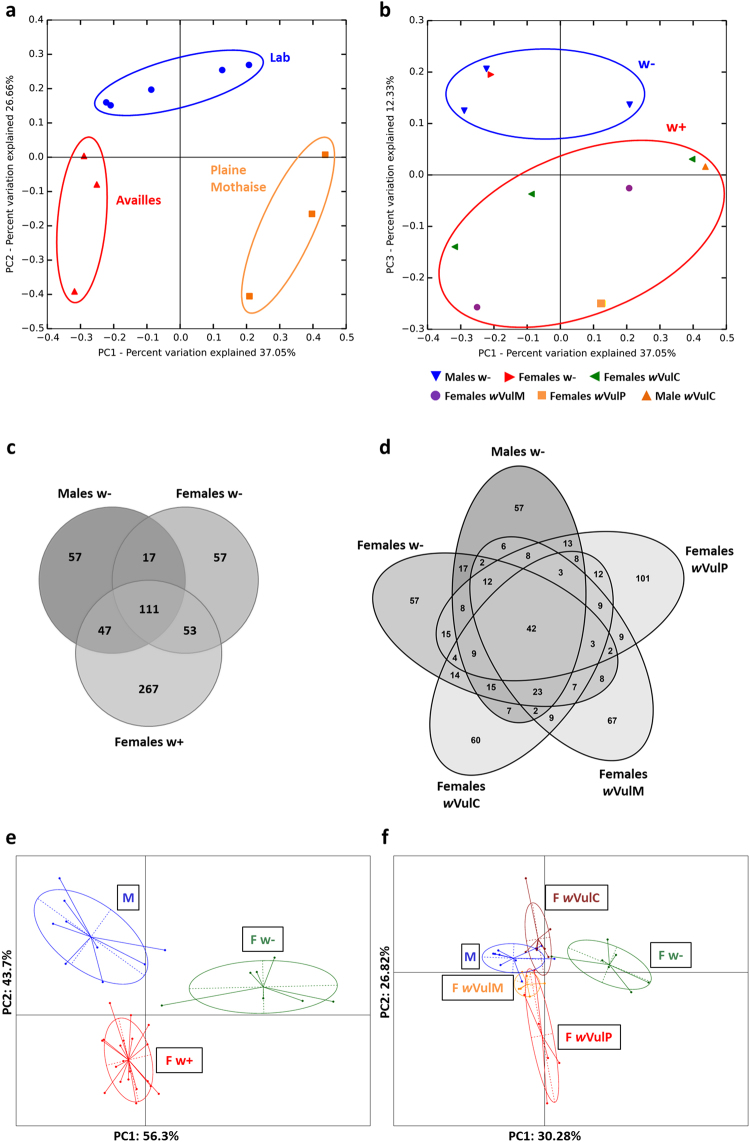
Taking into account that host origin is known to be a major factor shaping microbiota composition in terms of presence/absence of bacterial phylotypes in A. vulgare75, we first tested whether Wolbachia infection also had an impact on microbiota composition, independent of the host population (i.e. Laboratory, Availles, Plaine Mothaise). Principal Coordinates Analysis (PCoA) based on Bray-Curtis distances confirmed that host origin was indeed the major factor determining microbiota composition, with the first 2 principal components together explaining 63.71% of the variation (Fig. 3a). Nonetheless, Wolbachia infection was an additional factor influencing bacterial community composition, as the third principal component (explaining 12.33% of the variation) discriminated between Wolbachia-infected and uninfected isopods (Fig. 3b). In order to further investigate the impact of Wolbachia infection on microbiota composition independent of host origin, we next focused specifically on isopods from the laboratory lineages, since these (i) had been reared under controlled environmental conditions, (ii) had received the same food sources, and (iii) harboured all three feminizing Wolbachia strains. Interestingly, 43.84% (267/609) of the OTUs observed in these specimens occurred exclusively in Wolbachia-infected females, while only 9.36% (57/609) of the OTUs were specific for uninfected males and females, respectively, and 18.23% (111/609) were shared between all samples, independent of gender and infection status (Fig. 3c). Considering also the different Wolbachia strains, a higher number of OTUs (N = 101) were observed specifically in females infected with wVulP, compared with females harbouring wVulC (N = 60) or wVulM (N = 67) (Fig. 3d). These results indicate that not only Wolbachia infection itself, but also the different Wolbachia strains impact microbiota composition in A. vulgare under the same environmental conditions.
Figure 3.
Impact of Wolbachia infection on microbiota composition. (a,b) PCoA based on Bray-Curtis distances showing the impact of host origin (a) and Wolbachia infection (b) on microbiota composition. Each data point represents the microbiota of a given sample type after merging the sequencing data from five different tissues. (c,d) Distribution of OTUs in isopods from laboratory lineages depending on Wolbachia infection (c) and infection with different Wolbachia strains (d). (e,f) Between-Class-Analysis of TGGE profiles showing differences in microbiota composition depending on Wolbachia infection (e) and infection with different Wolbachia strains (f) for specimens from laboratory lineages. Each data point represents the merged profile from five different tissues of an individual isopod.
Considering that the sequencing data represented pooled amplicons from several individuals and therefore did not allow us to investigate inter-individual variations, we complemented the metabarcoding data with individual bacterial community profiles for the same specimens from the laboratory lineages using Temperature Gradient Gel Electrophoresis (TGGE) of the V3 region of the 16S rRNA gene. A total of 46 distinct bands (excluding the band corresponding to Wolbachia) were observed across all TGGE profiles, but none of these bands was found in all individuals. Indeed, the majority of bands occurred only in a relatively low number of individuals (mean ± SE = 3.52 ± 0.33 individuals) and the band corresponding to Wolbachia was detected at the highest frequency, being present in the 20 infected females. This suggests a relatively high level of inter-individual variation in microbiota composition in A. vulgare. Between-Class-Analysis of these profiles based on gender and Wolbachia infection resulted in three distinct clusters corresponding to the microbiotas of uninfected males, uninfected females and Wolbachia-infected females (Fig. 3e). When considering also the different Wolbachia strains, the TGGE profiles of females infected with either of the three Wolbachia strains formed separate clusters (Fig. 3f and Supplementary Figure S1). Interestingly, the microbiota of wVulM-infected females formed a tight cluster at the intersection between the two more variable clusters representing the microbiotas of females harbouring wVulC or wVulP (Fig. 3f and Supplementary Figure S1). Overall, the microbiota of Wolbachia-infected females appeared to be more similar to the microbiota of uninfected males than to those of uninfected females. However, Supplementary Figure S1 (an interactive 3D version of Fig. 3f) shows that uninfected males and uninfected females were discriminated by the first principal component, while Wolbachia-infected females and uninfected males were discriminated by the third principal component. The percentage of variation explained by the first three principal components was very similar (PC1: 30.28%, PC2: 26.82%, PC3: 23.26%), suggesting that both Wolbachia infection and host gender may influence microbiota composition. Unfortunately, since to date there is no reliable method to determine the genetic sex of A. vulgare, we could not determine which of the Wolbachia-infected individuals used in this study were indeed feminized genetic males.
Identification of differentially abundant bacterial taxa
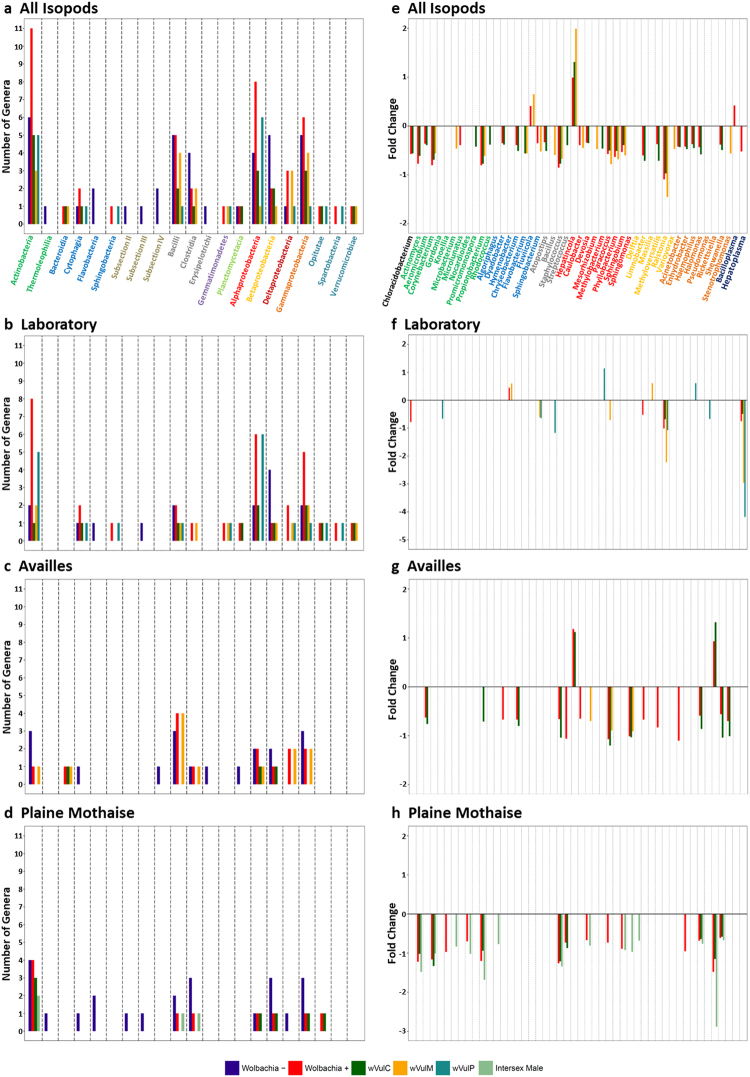
To investigate which bacterial phylotypes within the highly diverse isopod microbiota were specifically affected by Wolbachia (both positively and negatively), we identified bacterial genera which were either (i) present or absent depending on Wolbachia infection or (ii) present in both Wolbachia-infected and uninfected isopods but differentially abundant based on DESeq. 2 analysis76. This was done for each population separately, but also after combining the data from all host populations in order to identify more general patterns (Fig. 4). For the latter, we only considered the strains wVulC and wVulM, which occurred in isopods from at least two different origins.
Figure 4.
Differentially abundant bacterial taxa. (a–d) Histograms showing the distribution of genera per bacterial class which were specifically present or absent depending on Wolbachia infection, for all isopods independent of host origin (a) as well as for each population separately (b–d). (e–h) show the fold changes of the 48 genera identified as differentially abundant depending on Wolbachia infection using DESeq. 2, for all isopods independent of host origin (e) as well as for each population separately (f–h). The taxonomic identifications shown in (a) and (e) apply to all other panels and are colour-coded depending on bacterial phylum (or class for Proteobacteria): Acidobacteria = black, Actinobacteria = dark green, Bacteroidetes = light blue, Cyanobacteria = brown, Firmicutes = grey, Gemmatimonadetes = purple, Planctomycetes = light green, Alphaproteobacteria = red, Betaproteobacteria = light orange, Deltaproteobacteria = dark red, Gammaproteobacteria = dark orange, Tenericutes = dark blue, Verrucomicrobia = Cyan.
86 out of the 229 identified genera (37.55%) were indeed systematically present or absent depending on Wolbachia infection across all isopod populations (Supplementary Table S3). However, these genera were all of low abundance and together accounted for only 0.47% of all reads. Moreover, only four of these genera were observed in all host populations (Amycolatopsis (Actinobacteria), Spirosoma (Bacteroidetes), Granulicatella (Firmicutes) and Cupriavidus (Betaproteobacteria)) and all four were only present in Wolbachia-free isopods. Despite the fact that the other genera did not occur in all host populations, several patterns emerged when looking at higher taxonomic levels: Hence, genera belonging to the phylum Cyanobacteria and to the classes Thermoleophilia (phylum Actinobacteria), Flavobacteria (Bacteroidetes) and Erysepilotrichi (Firmicutes) were only observed in Wolbachia-free isopods, while genera belonging to the phyla Gemmatimonadetes and Verrucomicrobia as well as to the classes Bacteroidia and Sphingobacteria (Bacteroidetes) were only observed in Wolbachia-infected specimens (Fig. 4a). Moreover, a higher number of genera belonging to the Actinobacteria and Alphaproteobacteria were specifically associated with Wolbachia-infected isopods, while more genera of the Betaproteobacteria were only present in Wolbachia-free isopods (Fig. 4a). Interestingly, most of the specifically Wolbachia-associated genera of the Actinobacteria were observed in isopods from the laboratory lineages and the Plaine Mothaise population, i.e. primarily in specimens infected with the wVulC or wVulP strains (Fig. 4a–d). Similarly, 75% of the Alphaproteobacteria genera specifically present in Wolbachia-infected animals only occurred in isopods harbouring wVulC or wVulP (Fig. 4a). In contrast, more genera of the Bacilli (Firmicutes) and Deltaproteobacteria specifically present in Wolbachia-infected animals occurred in isopods infected with wVulM from the Availles population (Fig. 4a,c).
In addition, 48 genera (20.96%) were found to be differentially abundant depending on Wolbachia infection across all populations, mostly belonging to the Actinobacteria (11 genera), Alphaproteobacteria (9 genera) and Gammaproteobacteria (8 genera) (Fig. 4a, Supplementary Table S4). In contrast to the genera found to be specifically present or absent, the differentially abundant genera accounted for 87.54% of all reads, due to several highly abundant genera (i.e. Propionibacterium (Actinobacteria), Bacillus (Firmicutes), Ca. Hepatincola and Sphingomonas (Alphaproteobacteria), Ralstonia (Betaproteobacteria), Halomonas, Pseudomonas, Rickettsiella and Shewanella (Gammaproteobacteria) and Ca. Hepatoplasma (Tenericutes)). Both Wolbachia infection as well as different Wolbachia strains shaped bacterial abundance patterns, since more differentially abundant genera were detected in wVulC-infected isopods than in those harbouring wVulM (wVulC: 27, wVulM: 17). This was due to a higher number of differentially abundant genera belonging to the Actinobacteria and Gammaproteobacteria in wVulC-infected animals from the Plaine Mothaise and Availles, respectively (Fig. 4c,d). However, differences due to host origin were also apparent. Thus, more differentially abundant genera were detected in isopods from the two field populations than in specimens from laboratory lineages (Laboratory: 13, Availles: 18, Plaine Mothaise: 20) (Fig. 4b–d).
Several taxa need to be highlighted since they are well-known isopod symbionts: Ca. Hepatoplasma crinochetorum (Mollicutes) and Ca. Hepatincola porcellionum (Alphaproteobacteria), both facultative extracellular symbionts of the midgut caeca34,77–79, and the bacterial pathogen Rickettsiella (Gammaproteobacteria)34,80–82. Each of these genera was identified as differentially abundant depending on Wolbachia infection in certain host populations: Ca. Hepatoplasma was found to decrease in abundance in Wolbachia-infected laboratory lineages (particularly in the lineages harbouring wVulM and wVulP), Ca. Hepatincola and Rickettsiella increased in abundance in wVulC-infected isopods from Availles and Rickettsiella decreased in abundance in Wolbachia-infected specimens from the Plaine Mothaise (Fig. 4b–d). However, at least in the case of Ca. Hepatoplasma and Rickettsiella, it is more likely that this result is due to a sampling artefact rather than an actual interaction with Wolbachia. Indeed, Ca. Hepatoplasma is known to occur in the laboratory lineages derived from the A. vulgare population initially sampled in Helsingör (Denmark), while being almost absent from the two lineages derived from French populations71,75, which explains the observed statistically significant decrease in abundance. Moreover, a previous study found no differences in Hepatoplasma titer based on quantitative PCR between the Wolbachia-free and wVulC-infected laboratory lineages (i.e. all lineages established from the same Danish population)75. Similarly, only a single male from the Plaine Mothaise population was infected with Rickettsiella while all females were found uninfected based on diagnostic PCR, which again explains the statistically significant decrease in abundance in Wolbachia-infected isopods from this population but not the increase in abundance in the Availles population, where Rickettsiella infection is more widespread in both males and females75. Hence, it remains to be investigated whether Hepatincola and Rickettsiella do indeed interact with Wolbachia.
Discussion
The terrestrial isopod Armadillidium vulgare harbours a highly diverse bacterial community consisting of ≥200 bacterial genera34,75 and is frequently infected by feminizing Wolbachia26,69,83. Apart from the reproductive manipulation, these Wolbachia have profound effects on host life history traits, e.g. reducing fecundity and immunocompetence72,73. In addition, the feminizing strain wVulC has recently been shown to protect its host against two bacterial pathogens74, indicating that Wolbachia can affect other co-infecting bacteria in this species. Using A. vulgare and its three feminizing Wolbachia strains (wVulC, wVulM, wVulP) as a model system, the present study shows for the first time that Wolbachia not only interacts with certain bacterial pathogens, viruses or eukaryotic parasites, but can even influence a diverse bacterial community as a whole under both laboratory and natural conditions. While Wolbachia infection had no impact on bacterial taxonomic richness and diversity, it influenced the taxonomic composition of the microbiota and, more importantly, the abundance of many bacterial taxa. Thus, 48 genera (20.96% of all identified genera), mainly belonging to the phyla Actinobacteria and Proteobacteria, were found to be differentially abundant depending on Wolbachia infection. Moreover, different Wolbachia strains also had different impacts on microbiota composition. In particular, a higher number of differentially abundant genera were detected in wVulC-infected isopods than in those harbouring wVulM, especially in specimens from a natural population in which both strains were present. This is of interest in light of previous studies suggesting that these two Wolbachia strains represent different co-evolutionary histories with A. vulgare and that wVulC has higher virulence, transmission rate and feminizing capacity than wVulM69,84. wVulM also reaches lower titers than wVulC in most host tissues71, which may explain both its lower virulence and the weaker impact on other bacterial taxa observed here. The wVulP strain is presumably the result of a recombination event between wVulC and wVulM, with wVulC as its major parent67. While wVulP has indeed similar tissue-specific titers as wVulC71, the microbiota of the laboratory-reared isopods harbouring this strain was clearly different from lineages infected with wVulC or wVulM, due to several genera belonging to the Actinobacteria and Alphaproteobacteria which were specifically associated with the wVulP lineage. Unfortunately, we did not find any isopods harbouring this strain in natural populations to corroborate this effect in a different host genetic background.
Nonetheless, it has to be pointed out that all the genera which were specifically present or absent and most genera identified as differentially abundant depending on Wolbachia infection were low-abundance genera, making it less likely that the observed Wolbachia-mediated changes could be sufficiently strong to influence the performance of terrestrial isopods as keystone species in soil ecosystems. Interestingly, several better-studied and highly abundant terrestrial isopod facultative symbionts (Ca. Hepatoplasma crinochetorum (Mollicutes), Ca. Hepatincola porcellionum (Alphaproteobacteria) and Rickettsiella (Gammaproteobacteria)) were also identified as differentially abundant depending on Wolbachia infection. While this is likely a sampling artefact in the case of Ca. Hepatoplasma, potentially a nutritional symbiont enhancing host survival on low-quality diets85, it remains to be investigated using specific quantitative methods whether Ca. Hepatincola and Rickettsiella (a deadly isopod pathogen) are indeed more abundant in Wolbachia-infected individuals. If this were the case, it could suggest some kind of facilitation due to the presence of Wolbachia (e.g. reduction in host immunocompetence72,73) and the absence of Wolbachia-mediated resistance against the natural isopod pathogen Rickettsiella. Alternatively, Rickettsiella might reach higher titers in Wolbachia-infected individuals due to a Wolbachia-mediated tolerance as previously observed in D. simulans60, allowing the host to survive with higher pathogen loads compared to Wolbachia-free specimens.
Since the first discoveries of Wolbachia-mediated protection against pathogens in Drosophila and mosquitoes, it has been hypothesized that these protective phenotypes might be due to (i) an enhanced immune response, especially in transfected non-native hosts57,86,87, or (ii) competition between Wolbachia and other microorganisms for resources and space in the shared host environment, resulting in titer-dependent protection60,88–90. Several factors make it more likely that the observed differences in the microbiotas of Wolbachia-infected A. vulgare are predominantly due to competitive interactions: (i) Previous studies demonstrated a reduction in several immune effectors in Wolbachia-infected isopods, arguing against immune priming72,73, (ii) total bacterial loads increase in some, but not all tissues of Wolbachia-infected individuals, suggesting a competition for resources or space between Wolbachia and other bacteria71, and (iii) in line with the latter, the present study shows that most differentially abundant bacterial phylotypes indeed decreased in abundance.
Based on the data presented here and in our previous study75, Wolbachia infection is not the only factor shaping microbiota composition in A. vulgare. In particular, host origin had been previously identified as a major factor determining the taxonomic composition of the microbiota in this species75. While this could be due to both host genotype and/or different environments (e.g. in terms of soil or food-associated bacteria), we argue that the latter is the more important driver in A. vulgare. First, although the laboratory lineages used in this study were established from three different natural populations with very different genotypes71, their microbiotas are more homogenous in taxonomic composition after many years of controlled laboratory rearing on the same food sources than their conspecifics from natural populations75. This pattern was also obvious in the present study, since different bacterial presence/absence and differential abundance patterns were observed for each population. Second, environmental bacteria have an important share in the taxonomic richness of the microbiota in A. vulgare, potentially acquired from the prevailing food sources or the surrounding soil environment75. This has been corroborated by a recent study in the terrestrial isopod Porcellio scaber, whose microbiota composition was found to vary depending on different plant food sources in a controlled feeding experiment91. Host gender may be an additional confounding factor, especially in the present case where (i) only females harbour Wolbachia, (ii) it is impossible to distinguish Wolbachia-infected genetic females from feminized genetic males since no sex-specific markers are currently available for terrestrial isopods, and (iii) uninfected genetic females are rare in infected populations. Hence, it is impossible to determine which of the Wolbachia-infected specimens used in this study were genetically male or female, thus precluding any firm conclusions regarding the genetic sex as a driver of microbiota composition. Nonetheless, our data indicate that Wolbachia infection has a distinct impact on microbiota composition, independent of the genetic sex of the host: While the microbiota of uninfected females from the laboratory could not always be distinguished from that of uninfected males of the same genotype (i.e. the two microbiotas had distinct TGGE profiles but clustered together in PCoA of 16S rRNA gene amplicon data, which is a much more powerful technique to capture bacterial diversity), the microbiota associated with wVulC-infected females from the same initial population was different from both uninfected males and females in both analyses. Moreover, the microbiotas of uninfected males from several populations were different from those of infected females (which may be feminized genetic males), indicating a more general trend independent of host genotype, host genetic sex or environmental factors. Lastly, the microbiota of the Wolbachia-infected intersex male was most similar to the microbiota of infected females from the same natural population instead of clustering with the uninfected males.
Taken together, the composition of the bacterial microbiota associated with A. vulgare appears to be shaped by several factors: Host origin (via environmental bacteria and nutrition), Wolbachia infection status and host gender. While the respective impacts of these factors are not easily disentangled, similarly complex multifactorial patterns likely underlie many animal-bacteria symbioses under ecologically realistic conditions.
Methods
Terrestrial isopod samples and Wolbachia infection status
The 77 Armadillidium vulgare used in this study were sampled from four laboratory lineages and two field sites in France (Table 1) and were partly the same as those used in previous studies71,75. In the laboratory, animals were reared at 20 °C and natural photoperiod in plastic breeding boxes on moistened potting mix and fed ad libitum with lime tree leaves and carrot slices. One laboratory lineage was Wolbachia-free, i.e. both males and females from this lineage do not carry Wolbachia. In the other three lineages, natural infections with either of the Wolbachia strains wVulC, wVulM or wVulP have been stably maintained for at least 7 years (30 years for the oldest lineage). The Wolbachia-free lineage and the wVulC lineage were established from the same original population sampled in Helsingör, Denmark in 1982. The other two lineages carrying wVulM or wVulP derive from specimens sampled in Mery-sur-Cher (France) in 1999 and in Poitiers (France) in 2007, respectively. 10 males and 10 females (pairs of brothers and sisters) were randomly chosen from the Wolbachia-free lineage, as well as 10 females each from the wVulC, wVulM and wVulP lineages. Additional isopods from two natural populations in France (Availles-Thouarsais (46° 51′ 37′′N, 0° 8′ 28′′E) and Plaine Mothaise (46° 21′ 21′′N, 0° 06′ 32′′E)) were sampled in autumn 2011 and 2012. The collected specimens were kept in plastic boxes with soil and leaves from their respective sampling site until dissection.
Prior to dissection, all isopods were surface-sterilized using sodium hypochlorite and haemolymph was collected after piercing the dorsal cuticle with a sterile needle. For each specimen, DNA was extracted from the collected haemolymph and four different tissues (gonads, nerve cord, midgut caeca and hindgut) using phenol–chloroform92. Wolbachia infection status as well as Wolbachia titers in all host tissues of the specimens used in this study have been determined previously using diagnostic as well as quantitative PCR of the wsp gene71,75. Females collected from Availles harboured either wVulC or wVulM, while all females collected from the Plaine Mothaise were infected with wVulC. In addition, one individual from the Plaine Mothaise was identified as an intersex male infected with wVulC: While the external morphological characters were male, the androgenic glands were hypertrophied, which is an indication of Wolbachia infection with incomplete feminization93,94.
16S rRNA gene metabarcoding
The 16S rRNA gene amplicon sequences analysed in this study derive from the same pyrosequencing dataset used for the initial characterization of the microbiota of A. vulgare in different host tissues and populations75 (Accession Number PRJEB8160). Briefly, a 526 bp-fragment spanning the variable regions V1-V3 of the 16S rRNA gene was amplified using the universal primers 27 F and 520 R. Primers were adapted for 454 pyrosequencing by adding the 454 Adapter A and a 10-bp Multiplex Identifier sequence (MID) to the reverse primer 520 R as well as the 454 Adapter B to the forward primer 27 F. Amplicons were obtained from five different tissues (haemolymph, nerve cord, gonads, midgut caeca and hindgut) and up to 10 biological replicates from the same tissue and sample type (origin x gender x Wolbachia strain) were pooled for sequencing, resulting in 55 amplicon pools (see Supplementary Table S1 for details). The amplicon pools were purified using AMPure Beads (Agencourt Bioscience Corporation), quantified using PicoGreen (Invitrogen) and sequenced on a 454 GS FLX sequencer (Roche, 454 Life Sciences) by GenoScreen (Lille, France) as well as on a GS Junior sequencer (Roche, 454 Life Sciences) at the University of Poitiers (France).
Metabarcoding Data Analysis
The 16S rRNA gene pyrotags were analysed using QIIME version 1.9.195 and R (R Project 3.3.2). Briefly, the flowgrams were denoised with AmpliconNoise96,97 and chimeras were removed using Perseus97. All reads shorter than 250 bp were discarded and the remaining reads were clustered into Operational Taxonomic Units (OTUs) at 97% similarity using uclust98. Representative sequences were aligned against the Silva reference alignment (release 108,99) using PyNAST100 and identified using the RDP Classifier101. Rare OTUs (i.e. singletons and doubletons) were discarded, resulting in 1380 OTUs represented by ≥3 reads (see Supplementary Table S1 for details). All reads identified as Wolbachia were excluded from the dataset for subsequent analyses and the data from the five different tissues of the same sample type were merged in order to obtain a representative “whole animal” profile. Taxonomic richness, diversity and evenness were determined using the nonparametric species richness estimator Chao 1 and the Shannon Index of diversity and evenness, after subsampling of 5000 sequences per sample. Alpha diversity indices were compared between Wolbachia-infected and uninfected isopods using two-sample t-tests with 1000 Monte Carlo permutations. Betadiversity was analysed using Principal Coordinates Analysis (PCoA) based on Bray-Curtis distances. Venn diagrams were produced in R using the VennDiagram package. Differentially abundant taxa were determined after data normalization using the DESeq. 2 Wald Test76 as implemented in QIIME.
Temperature Gradient Gel Electrophoresis (TGGE)
Considering that the sequencing data from pooled amplicons did not allow us to investigate inter-individual variations, the metabarcoding data was complemented with individual bacterial community profiles from the same specimens from the laboratory lineages using Temperature Gradient Gel Electrophoresis (TGGE). A 196 bp-fragment of the variable region V3, also included in the fragment used for amplicon sequencing, was amplified from the tissue samples using a nested PCR approach: First, a 795-bp fragment was amplified using primers 27 F and 786 R102, followed by a second amplification targeting the V3 region using primers 338F-GC and 520 R103. Primer 338 F contained a 42-nucleotide GC-clamp preventing the complete denaturation of the DNA strands during TGGE103. All PCR amplifications were confirmed by electrophoresis in 1.5% agarose gels. TGGE was performed using the DCode Universal Mutation Detection System™ (Bio-Rad) following a protocol modified from104. Briefly, 20 μl of the final PCR products were run across a temperature gradient from 38 °C to 70 °C (ramping of 1.3 °C/h) at 60 V on 10% polyacrylamide gels. A standard containing V3 16S rRNA gene fragments from several reference bacteria (Bacillus megaterium, Escherichia coli, Listeria ivanovii, Micrococcus luteus, Salmonella typhimurium and Wolbachia spp.) was loaded on each gel to allow the standardization of bands between gels. This also allowed a visual screening of Wolbachia infection across all samples via the presence of a band at the position corresponding to Wolbachia spp. in the standard. After electrophoresis, gels were stained with ethidium bromide and photographed under UV. Banding patterns on each gel were standardized based on the position of the reference bands using the GelAnalyzer 2010 software (www.gelanalyzer.com). Banding patterns across all gels were then analysed using an in-house Perl script: Bands with highly similar positions across all gels were grouped into a single normalized band, resulting in a presence/absence matrix of each normalized band per tissue sample. Finally, bacterial community profiles of each individual were established by merging the tissue-specific profiles into a single presence/absence profile per individual. The band corresponding to Wolbachia was removed from the dataset and bacterial community composition was analysed via principal component analysis (PCA) followed by between-class analysis (BCA)105 in R using the ade4 package. The 3D image of the BCA was made using the function scatter3d of the car package.
Data availability
The 16S rRNA gene dataset used in this study is accessible in the European Nucleotide Archive under the Accession Number PRJEB8160.
Electronic supplementary material
Acknowledgements
The authors would like to thank Sébastien Leclercq for the Perl script performing the grouping of TGGE bands between gels, Jérôme Lesobre for performing emPCR and sequencing on the GS Junior, Bouziane Moumen for assistance with computing resources as well as Caroline Bedeau, Nicolas Bech, Sophie Beltran-Bech, Damien Deschamps, Gaël Freyssinel and Catherine Souty-Grosset for help with field sampling. This work was funded by the 2015–2020 State-Region Planning Contracts (CPER), the European Regional Development Fund (FEDER) and intramural funds from the Centre National de la Recherche Scientifique and the University of Poitiers. JD was supported by PhD grants from the CNRS and the Région Poitou-Charentes.
Author Contributions
J.D. and D.B. designed the study, J.D. analysed the data and wrote the manuscript, all authors reviewed and finalised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25450-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–90. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 2.Moya A, Pereto J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–29. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 3.Thao ML, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol. 2004;70:3401–6. doi: 10.1128/AEM.70.6.3401-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran NA, Dunbar HE, Wilcox JL. Regulation of transcription in a reduced bacterial genome: nutrient-provisioning genes of the obligate symbiont Buchnera aphidicola. J Bacteriol. 2005;187:4229–37. doi: 10.1128/JB.187.12.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pais R, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74:5965–74. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci USA. 2009;106:15394–9. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukasik P, van Asch M, Guo H, Ferrari J, Godfray HC. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett. 2013;16:214–8. doi: 10.1111/ele.12031. [DOI] [PubMed] [Google Scholar]
- 8.Scarborough CL, Ferrari J, Godfray HC. Aphid protected from pathogen by endosymbiont. Science. 2005;310:1781. doi: 10.1126/science.1120180. [DOI] [PubMed] [Google Scholar]
- 9.Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA. 2003;100:1803–7. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–5. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida T, et al. Symbiotic bacterium modifies aphid body color. Science. 2010;330:1102–4. doi: 10.1126/science.1195463. [DOI] [PubMed] [Google Scholar]
- 12.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–51. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 13.Duron O, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurst, G. D. & Frost, C. L. Reproductive Parasitism: Maternally Inherited Symbionts in a Biparental World. Cold Spring Harb Perspect Biol7 (2015). [DOI] [PMC free article] [PubMed]
- 15.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? - A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–20. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandi C, Anderson TJ, Genchi C, Blaxter ML. Phylogeny of Wolbachia in filarial nematodes. Proc Biol Sci. 1998;265:2407–13. doi: 10.1098/rspb.1998.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedeine F, et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA. 2001;98:6247–52. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pannebakker BA, Loppin B, Elemans CP, Humblot L, Vavre F. Parasitic inhibition of cell death facilitates symbiosis. Proc Natl Acad Sci USA. 2007;104:213–5. doi: 10.1073/pnas.0607845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA. 2010;107:769–74. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brownlie JC, et al. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009;5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikoh N, et al. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci USA. 2014;111:10257–62. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stouthamer R, Breeuwert JA, Luck RF, Werren JH. Molecular identification of microorganisms associated with parthenogenesis. Nature. 1993;361:66–8. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 25.Hurst, G.D., Jiggins, F.M. & Majerus, M.E. Inherited microorganisms that selectively kill male hosts: The hidden players of insect evolution? in Insect Symbiosis (eds Bourtzis, K. & Miller, T.A.) 177–197 (CRC Press, 2003).
- 26.Bouchon, D., Cordaux, R. & Grève, P. Feminizing Wolbachia and the evolution of sex determination in isopods. In Insect Symbiosis, Vol. 3 (eds Bourtzis, K. & Miller, T. A.) 273–296 (CRC Press, Boca Raton, USA, 2008).
- 27.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 28.Narita S, Kageyama D, Nomura M, Fukatsu T. Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl Environ Microbiol. 2007;73:4332–41. doi: 10.1128/AEM.00145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sicard M, Dittmer J, Greve P, Bouchon D, Braquart-Varnier C. A host as an ecosystem: Wolbachia coping with environmental constraints. Environ Microbiol. 2014;16:3583–3607. doi: 10.1111/1462-2920.12573. [DOI] [PubMed] [Google Scholar]
- 30.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–35. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 31.Dittmer J, et al. Disentangling a Holobiont - Recent Advances and Perspectives in Nasonia Wasps. Front Microbiol. 2016;7:1478. doi: 10.3389/fmicb.2016.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFall-Ngai M, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110:3229–36. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–88. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchon D, Zimmer M, Dittmer J. The Terrestrial Isopod Microbiome: An All-in-One Toolbox for Animal-Microbe Interactions of Ecological Relevance. Front Microbiol. 2016;7:1472. doi: 10.3389/fmicb.2016.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo N, Shimada M, Fukatsu T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett. 2005;1:488–91. doi: 10.1098/rsbl.2005.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouton L, et al. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics. 2004;168:181–9. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc Biol Sci. 2003;270:2543–50. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver KM, Moran NA, Hunter MS. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc Biol Sci. 2006;273:1273–80. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol. 2005;71:4069–75. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goto S, Anbutsu H, Fukatsu T. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol. 2006;72:4805–10. doi: 10.1128/AEM.00416-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen SB, Boye M, Nash DR, Boomsma JJ. Dynamic Wolbachia prevalence in Acromyrmex leaf-cutting ants: potential for a nutritional symbiosis. J Evol Biol. 2012;25:1340–50. doi: 10.1111/j.1420-9101.2012.02521.x. [DOI] [PubMed] [Google Scholar]
- 42.Vautrin E, Vavre F. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 2009;17:95–9. doi: 10.1016/j.tim.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Vautrin E, Genieys S, Charles S, Vavre F. Do vertically transmitted symbionts co-existing in a single host compete or cooperate? A modelling approach. J Evol Biol. 2008;21:145–61. doi: 10.1111/j.1420-9101.2007.01460.x. [DOI] [PubMed] [Google Scholar]
- 44.Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol. 2010;19:414–25. doi: 10.1111/j.1365-294X.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee KA, et al. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Ryu JH, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–82. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 47.Shin SC, et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–4. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 48.Storelli G, et al. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–14. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Newell PD, Douglas AE. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl Environ Microbiol. 2014;80:788–96. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zouache K, et al. Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus. Plos One. 2009;4:e6388. doi: 10.1371/journal.pone.0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zouache K, et al. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol Ecol. 2011;75:377–89. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 52.Gueguen G, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol. 2010;19:4365–4378. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- 53.Gottlieb Y, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. Faseb J. 2008;22:2591–9. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- 54.Baldini F, et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun. 2014;5:3985. doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minard G, et al. French invasive Asian tiger mosquito populations harbor reduced bacterial microbiota and genetic diversity compared to Vietnamese autochthonous relatives. Front Microbiol. 2015;6:970. doi: 10.3389/fmicb.2015.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–78. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 58.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum In Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bian G, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–51. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 60.Osborne SE, Leong YS, O’Neill SL, Johnson KN. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. Plos Pathog. 2009;5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci USA. 2012;109:255–60. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 63.Hughes, G.L. et al. Native microbiome impedes vertical transmission of Wolbachia In Anopheles mosquitoes. Proc Natl Acad Sci U S A (2014). [DOI] [PMC free article] [PubMed]
- 64.Rossi P, et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit Vectors. 2015;8:278. doi: 10.1186/s13071-015-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simhadri, R.K. et al. The Gut Commensal Microbiome of Drosophila melanogaster Is Modified by the Endosymbiont Wolbachia. mSphere2(2017). [DOI] [PMC free article] [PubMed]
- 66.Ye YH, Seleznev A, Flores HA, Woolfit M, McGraw EA. Gut microbiota in Drosophila melanogaster interacts with Wolbachia but does not contribute to Wolbachia-mediated antiviral protection. J Invertebr Pathol. 2017;143:18–25. doi: 10.1016/j.jip.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Verne S, Johnson M, Bouchon D, Grandjean F. Evidence for recombination between feminizing Wolbachia in the isopod genus Armadillidium. Gene. 2007;397:58–66. doi: 10.1016/j.gene.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Verne S, Johnson M, Bouchon D, Grandjean F. Effects of parasitic sex-ratio distorters on host genetic structure in the Armadillidium vulgare-Wolbachia association. J Evol Biol. 2012;25:264–76. doi: 10.1111/j.1420-9101.2011.02413.x. [DOI] [PubMed] [Google Scholar]
- 69.Cordaux R, Michel-Salzat A, Frelon-Raimond M, Rigaud T, Bouchon D. Evidence for a new feminizing Wolbachia strain in the isopod Armadillidium vulgare: evolutionary implications. Heredity. 2004;93:78–84. doi: 10.1038/sj.hdy.6800482. [DOI] [PubMed] [Google Scholar]
- 70.Rigaud T, Souty-Grosset C, Raimond R, Mocquard JP, Juchault P. Feminizing endocytobiosis in the terrestrial crustacean Armadillidium vulgare Latr. (Isopoda): recent acquisitions. Endocyt Cell Res. 1991;7:259–273. [Google Scholar]
- 71.Dittmer J, et al. Host tissues as microhabitats for Wolbachia and quantitative insights into the bacterial community in terrestrial isopods. Mol Ecol. 2014;23:2619–35. doi: 10.1111/mec.12760. [DOI] [PubMed] [Google Scholar]
- 72.Braquart-Varnier C, et al. Wolbachia mediate variation of host immunocompetence. PLoS One. 2008;3:1–6. doi: 10.1371/journal.pone.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sicard M, et al. Variations of immune parameters in terrestrial isopods: a matter of gender, aging and Wolbachia. Naturwissenschaften. 2010;97:819–26. doi: 10.1007/s00114-010-0699-2. [DOI] [PubMed] [Google Scholar]
- 74.Braquart-Varnier C, et al. The Mutualistic Side of Wolbachia-Isopod Interactions: Wolbachia Mediated Protection Against Pathogenic Intracellular Bacteria. Front Microbiol. 2015;6:1388. doi: 10.3389/fmicb.2015.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dittmer, J., Lesobre, J., Moumen, B. & Bouchon, D. Host origin and tissue microhabitat shaping the microbiota of the terrestrial isopod Armadillidium vulgare. FEMS Microbiol Ecol92, In press (2016). [DOI] [PubMed]
- 76.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Brune A, Zimmer M. Bacterial symbionts in the hepatopancreas of isopods: diversity and environmental transmission. FEMS Microbiol Ecol. 2007;61:141–52. doi: 10.1111/j.1574-6941.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, et al. Candidatus Hepatoplasma crinochetorum, a new, stalk-forming lineage of Mollicutes colonizing the midgut glands of a terrestrial isopod. Appl Environ Microbiol. 2004;70:6166–72. doi: 10.1128/AEM.70.10.6166-6172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Stingl U, Anton-Erxleben F, Zimmer M, Brune A. Candidatus Hepatincola porcellionum gen. nov., sp. nov., a new, stalk-forming lineage of Rickettsiales colonizing the midgut glands of a terrestrial isopod. Arch Microbiol. 2004;181:299–304. doi: 10.1007/s00203-004-0655-7. [DOI] [PubMed] [Google Scholar]
- 80.Bouchon, D., Cordaux, R. & Grève, P. Rickettsiella, intracellular pathogens of arthropods in Manipulative Tenants: Bacteria Associated with Arthropods (eds Zchori-Fein, E. & Bourtzis, K.) 127–148 (CRC Press, Boca Raton, USA, 2011).
- 81.Kleespies RG, Federici BA, Leclerque A. Ultrastructural characterization and multilocus sequence analysis (MLSA) of ‘Candidatus Rickettsiella isopodorum’, a new lineage of intracellular bacteria infecting woodlice (Crustacea: Isopoda) Syst Appl Microbiol. 2014;37:351–9. doi: 10.1016/j.syapm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Chandler C. Candidate pathogenicity islands in the genome of Candidatus Rickettsiella isopodorum, an intracellular bacterium infecting terrestrial isopod crustaceans. PeerJ. 2016;4:e2806. doi: 10.7717/peerj.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchon D, Rigaud T, Juchault P. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc Biol Sci. 1998;265:1081–90. doi: 10.1098/rspb.1998.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Clec’h W, Raimond M, Bouchon D, Sicard M. Strength of the pathogenicity caused by feminizing Wolbachia after transfer in a new host: Strain or dose effect? J Invertebr Pathol. 2014;116:18–26. doi: 10.1016/j.jip.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Fraune S, Zimmer M. Host-specificity of environmentally transmitted Mycoplasma-like isopod symbionts. Environ Microbiol. 2008;10:2497–504. doi: 10.1111/j.1462-2920.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 86.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan X, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O’Neill SL, Johnson KN. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl Environ Microbiol. 2012;78:6922–9. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu P, Bian G, Pan X, Xi Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis. 2012;6:e1754. doi: 10.1371/journal.pntd.0001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chrostek E, et al. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet. 2013;9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horvathova, T., Babik, W. & Bauchinger, U. Biofilm feeding: Microbial colonization of food promotes the growth of a detritivorous arthropod. Zookeys, 25-41 (2016). [DOI] [PMC free article] [PubMed]
- 92.Kocher TD, et al. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 1989;86:6196–200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Legrand JJ, Juchault P. Le déterminisme de l’intersexualité chez les Crustacés Isopodes terrestres; corrélation entre intersexualité et monogénie. C R Acad Sci Hebd Seances Acad Sci D. 1969;268:1647–1649. [Google Scholar]
- 94.Legrand JJ, Juchault P, Mocquard JP. Analyse préliminaire du mécanisme de l’intersexualité féminisante chez le Crustacé Armadillidium vulgare Latr. (Isopode Oniscoïde) C R Acad Sci Hebd Seances Acad Sci D. 1974;278:2979–2982. [Google Scholar]
- 95.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quince C, et al. Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods. 2009;6:639–41. doi: 10.1038/nmeth.1361. [DOI] [PubMed] [Google Scholar]
- 97.Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing noise from pyrosequenced amplicons. BMC Bioinformatics. 2011;12:38. doi: 10.1186/1471-2105-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 99.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caporaso JG, et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–7. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dittmer J, Lesobre J, Raimond R, Zimmer M, Bouchon D. Influence of changing plant food sources on the gut microbiota of saltmarsh detritivores. Microb Ecol. 2012;64:814–25. doi: 10.1007/s00248-012-0056-4. [DOI] [PubMed] [Google Scholar]
- 105.Culhane AC, Perriere G, Considine EC, Cotter TG, Higgins DG. Between-group analysis of microarray data. Bioinformatics. 2002;18:1600–8. doi: 10.1093/bioinformatics/18.12.1600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA gene dataset used in this study is accessible in the European Nucleotide Archive under the Accession Number PRJEB8160.