Abstract
The total dinoflagellate cyst community and the cysts of Cochlodinium polykrikoides in the surface sediments of South Sea (Tongyeong coast), South Korea, were analysed using next-generation sequencing (NGS) and morphological approaches. Dinoflagellate cysts can be highly abundant (111–4,087 cysts g−1 dry weight) and have diverse species composition. A total of 35 taxa of dinoflagellate cysts representing 16 genera, 21 species (including four unconfirmed species), and 14 complex species were identified by NGS analysis. Cysts of Scrippsiella spp (mostly Scrippsiella trochoidea) were the most dominant and Polykrikos schwartzii, Pentapharsodinium dalei, Ensiculifera carinata, and Alexandrium catenella/tamarense were common. Thus, a combination of NGS and morphological analysis is effective for studying the cyst communities present in a given environment. Although C. polykrikoides developed massive blooms during 2013–2014, microscopy revealed low density of their cysts, whereas no cysts were detected by NGS. However, the vegetative C. polykrikoides not appeared during 2015–2017 in spite of the observation of C. polykrikoides cysts. This suggests that the C. polykrikoides blooms were not due to development of their cysts but to other factors such as currents transporting them to a marine environment suitable for their growth.
Introduction
Dinoflagellates are one of the most diverse and abundant groups of marine plankton. Some dinoflagellates are known to cause outbreaks of harmful algal blooms in coastal waters around the world, and can be extremely toxic, leading to significant damage of local aquaculture and wildlife. The life stages of dinoflagellates form an important component of their ecology and biogeography. Around 200 of the approximately 2000 existing species of marine dinoflagellates are known to form resting cysts as part of their life history1. Cysts are typically preserved in sediments, thereby providing an integrated record over time of the presence of cyst-producing dinoflagellates. Functions attributed to cysts include species dispersal, survival under unfavourable conditions, and bloom initialization2–4. Cyst surveys give information on history of harmful species in a given area, thereby providing an indication of the potential for future blooms, and it may reveal species rarely observed in the plankton, owing to rare, short-lived, or vegetative stages that are difficult to identify5.
In South Korea, Cochlodinium polykrikoides has been one of the most frequently occurring harmful red tide species responsible for fish kills since 19956. In particular, the Tongyeong coastal area, located in the South Sea of South Korea, is one of the most severely bloomed areas of C. polykrikoides7. Although, C. polykrikoides blooms frequently developed in many coastal countries during the last decade, cysts of C. polykrikoides are still understudied8–10. Our previous study reported the cyst morphology of C. polykrikoides in the natural surface sediment samples9, but the mechanisms of C. polykrikoides bloom formation by germination of cysts are mostly unknown.
Advances in DNA sequencing technology and bioinformatics have significant potential to strengthen biological monitoring in the ocean. The challenge of modelling and predicting ecological changes requires the linking of taxonomic data to the functional roles of individual microbial groups in biogeochemical cycles11. In other words, the challenge is not simply a matter of discovering ‘What is there’, but also ‘Why it is there?’ In this context, a major issue relates to how dinoflagellate cysts are temporally distributed and whether they follow any trends in their distribution.
To this end, we investigated the diversity and distribution of cysts in dinoflagellate communities and C. polykrikoides by focusing on 18S rDNA using next-generation sequencing (NGS) tool and morphological observation in samples harvested from the South Sea (Tongyeong coasts) during the red-tide and the non-red-tide season in South Korea.
Results
Dinoflagellate cyst composition based on next-generation sequencing analysis
The NGS results obtained for the eukaryotic organisms in surface sediments of Tongyeong coasts are summarized in Table 1. The number of OTUs varied among samples. The rarefaction curves (Supplementary Fig. S1), which plot the OTUs generated by the NGS runs against the number of reads, appeared to be nearing the asymptotic values. The amplicons generated 31,575–81,818 read counts from the six sequenced samples: The average read count in 2014 (59,894) were higher than in 2013 (42,383). In addition, the number of OTUs and alpha diversity (Chao1 richness, Shannon diversity, and Simpson evenness) showed a similar trend with changes in read counts (Table 1). Read counts, number of OTUs, and alpha diversity in the MG group in 2013 were higher than those in the IG and OG. However, the data of 2014 showed that the number of OTUs and alpha diversity of the OG were higher than in the other groups. Considering that eukaryotic organisms are present in sediments, the main group identified in the NGS data (97% cut off of read counts) was the Stramenopiles-Alveolata-Rhizaria (SAR) super group, with average proportion of 90.04% and 81.08% in 2013 and 2014, respectively (Fig. 1). Other groups, such as Opisthokonta, Archaeplastida, Amoebozoa, and incertae sedis were minor groups. In the SAR super group, Alveolata was the most abundant super kingdom with a average proportion of 60.78% in 2013 and 47.29% in 2014, followed by Rhizaria and Stramenopiles. Three diverse groups of unicellular eukaryotes were consisted of the Alveolata. Among these phyla, Dinoflagellata occupied an extremely high average proportion of 88.11% in 2013 and 76.82% in 2014 (Fig. 1).
Table 1.
Number of read counts and operational taxonomic units (OTUs) obtained from next-generation sequencing analysis and the alpha-diversity indices in this study.
| Sampling date | group | Total bases | Read Count (Cut off: 97%) |
OTUs | Chao1 richness | Shannon diversity | Simpson evenness | Remark |
|---|---|---|---|---|---|---|---|---|
| December 2013 | IG | 13,141,208 | 31,575(5,504) | 175 | 190 | 4.82 | 0.87 | Non red-tide |
| MG | 25,586,779 | 61,246(11,159) | 227 | 242 | 5.28 | 0.91 | ||
| OG | 14,315,268 | 34,327(6,502) | 208 | 217 | 5.24 | 0.90 | ||
| September 2014 | IG | 18,935,579 | 45,435(8,336) | 256 | 271 | 5.61 | 0.94 | Red-tide |
| MG | 34,212,216 | 81,818(14,646) | 264 | 287 | 4.91 | 0.92 | ||
| OG | 21,940,670 | 52,428(10,173) | 273 | 292 | 5.42 | 0.92 |
IG, MG, and OG indicate inner group, middle group, and outer groups, respectively, of sample sites in Tongyeong coast (South Sea) of South Korea during December 2013 and September 2014.
Figure 1.

Taxonomic distribution of super group, super kingdom, and phylum levels revealed by next-generation sequencing analysis in surface sediments of Tongyeong coast (South Sea) of South Korea during December 2013 and September 2014. IG, inner group; MG, middle group; OG, outer group; SAR, Stramenopiles-Alveolata-Rhizaria super kingdom.
The composition of the dinoflagellate cyst assemblages is shown in Fig. 2a (97% cut-off of read counts) and Supplementary Tables S1 and S2. A total of 35 taxa (or complex species) of cysts representing 16 genera, 21 species (including four unconfirmed species), and 14 groups (complex species) were identified from all the samples. The Pentapharsodinium-Scrippsiella complex, which included Pentapharsodinium tyrrhenicum, P. dalei, Scrippsiella trochoidea, S. sweeneyae, and S. erinaceus, constituted the most predominant taxa with average proportions of 63.68% in 2013 and 62.20% in 2014. Gonyaulax spinifera (3.94% in 2013 and 11.99% in 2014), Alexandrium complex species I, including Alexandrium pacificum, A. catenella/tamarense, and A. fundyense (5.49% in 2013 and 6.50% in 2014), Woloszynskia complex species, including Woloszynskia cincta and W. halophila (6.09% in 2013 and 4.17% in 2014), Alexandrium complex species II, including A. minutum, A. insuetum, A. tamutum, and A. lusitanicum (3.23% in 2013 and 3.74% in 2014), Gymnodinium aureolum (3.47% in 2013 and 2.65% in 2014), Gyrodinium impudicum (2.29% in 2013 and 0.99% in 2014), and S. hangoei (2.31% in 2013 and 0.62% in 2014) were the next most abundant species and represent more than 1% of the total read counts of OTUs. These major taxa account for more than 90% of the total read counts of cysts of dinoflagellates. In particular, G. spinifera was present in higher proportion in 2014 compared to 2013.
Figure 2.
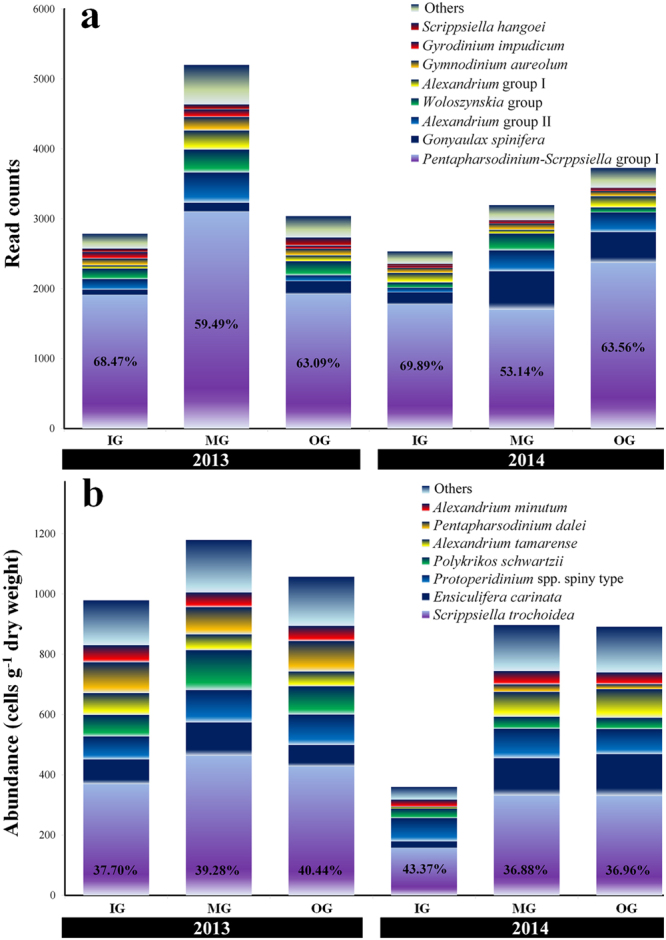
Common species (or genus) composition-inferred assemblages of dinoflagellate cysts based on next-generation sequencing (a) and morphological data (b) in surface sediments of Tongyeong coast (South Sea) of South Korea during December 2013 and September 2014. IG, inner group; MG, middle group; OG, outer group.
Dinoflagellate cyst composition based on microscopic analysis
In microscopic observations, the total abundance of dinoflagellate cysts at the sampling sites varied between 253–4,087 cysts g−1 dry weight (average = 1,072 cysts g−1 dry weight) in 2013 and 111–1,532 cysts g−1 dry weight (average = 716 cysts g−1 dry weight) in 2014 (Fig. 3). Thus, the dinoflagellate cysts in 2013 showed higher abundance than in 2014 (Fig. 3). In particular, the abundance of cysts of the MG in both 2013 and 2014 was higher than in the other groups. Thus, our samples of the dinoflagellate cysts in the surface sediments of the Tongyeong coast revealed high abundance and diverse species composition, compared to the abundance and composition in many coastal countries worldwide (Supplementary Table S3). In addition, abundances recorded in this study were higher than those reported previously for other coastal areas of South Korea (Supplementary Table 3). The composition of the dinoflagellate cysts is shown in Fig. 2b and Supplementary Table S4. A total of 25 taxa of cysts representing 13 genera and 25 species (including five unconfirmed species) were identified in 2013, and 27 taxa representing 13 genera, 27 species (including five unidentified cysts), and one unidentified taxon in 2014. The cysts of Scrippsiella spp (mostly Scrippsiella trochoidea) were the most predominant at average proportions of 40.72% (27.93% for S. trochoidea) in 2013 and 43.55% (21.05% for S. trochoidea) in 2014. Moreover, the predominant cysts (>5% in total cyst abundances) in 2013 were of Polykrikos schwartzii (9.65%), Protoperidinium sp. (spiny form, 9.01%), Pentapharsodinium dalei (8.82%), Ensiculifera carinata (8.24%), Phaeopolykrikos hartmanii (5.23%), and Alexandrium catenella/tamarense (5.07%) and the predominant cysts in 2014 were of Ensiculifera carinata (13.77%), Protoperidinium sp (spiny form, 11.37%), and Alexandrium catenella/tamarense (8.94%). Thus, the proportion of total common cysts (average cyst abundances > 5% in 2013 and 2014) were 77.92 and 77.63%, respectively (Fig. 3 and Supplementary Fig. S2).
Figure 3.
Distribution of total and common dinoflagellate species revealed by morphological analysis in surface sediments of Tongyeong coast (South Sea) of South Korea during December 2013 (50 sampling sites) and September 2014 (44 sampling site). Scrippsiella spp., including S. trochoidea, S. tridida, S. precaria, S. crystalline, S. rotunda, and S. operosa; Alexandrium spp., including A. catenella/tamarense and A. minutum; Protoperidinium spp., including P. latissimum, P. leonis, P. minutun, P. americanum, and Protoperidinium spp. spiny type; Gymnodinium spp., including G. catenatum and Gymnodinium spp.; Polykrikos spp., including P. schwartzii and P. kofoidii. All maps were generated with Surfer v12.2.705 (http://www.goldensoftware.com/products/surfer).
Similarity in dinoflagellate cyst distribution based on the NGS and microscopic analysis
The results of the MDS analysis of distributions of the OTUs based on the NGS and morphology in the group samples are shown in Fig. 4. The dinoflagellate cysts identified using NGS were clearly divided into two groups (similarity = 76%) associated with years (2013 and 2014) and merged into a single group (70%). Based on microscopical analysis, they could be divided into two groups (IG-, MG-, OG-2013 and MG-, OG-2014; IG-2014) (76%) and merged into a single group (65%).
Figure 4.
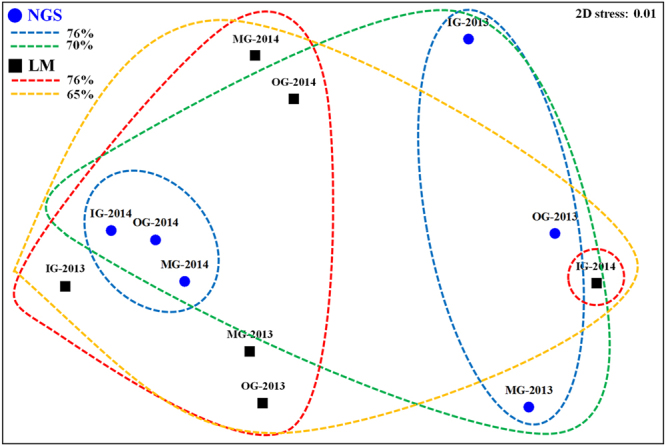
Non-metric multidimensional scaling plot based on next-generation sequencing (NGS as blue circles) and microscopy (LM as black square) results of the Bray–Curtis dissimilarity at the stress of 0.01 level. IG, inner group; MG, middle group; OG, outer group.
Presence of Cochlodinium polykrikoides cyst
C. polykrikoides bloomed in late July, peaked in early August, sustained in mid-August, and gradually decreased in late August of 2013 (Supplementary Fig. S3). In particular, the greatest density of this species was detected at density of 5,780 cells mL−1 on August 6, 2013. The blooming patterns of C. polykrikoides in 2014 were similar to that in 2013, but their strength was weaker than in 2013. Cysts of C. polykrikoides were not detected by NGS analysis (Supplementary Tables S1 and S2) and all PCR results were negative except for sample from the cultured C. polykrikoides (Supplementary Fig. S4). However, in microscopic analysis, the cysts of C. polykrikoides were observed at seven sampling sites (7–48 cysts g−1 dry weight in 2013; average cyst density of 4 cysts g−1 dry weight across total sampling sites), and five sampling sites had an abundance of 8–14 cyst g−1 dry weight in 2014 (average = 2 cysts g−1 dry weight) (Fig. 5).
Figure 5.
Cyst distribution of Cochlodinium polykrikoides (revealed by morphological analysis) in surface sediments of Tongyeong coast (South Sea) of South Korea during December 2013 (50 sampling sites) and September 2014 (44 sampling site). All maps were generated with Surfer v12.2.705 (http://www.goldensoftware.com/products/surfer).
Discussion
Recently environmental DNA(eDNA) has begun to be employed in aquatic environmental research12, and it is also being used to monitor marine biodiversity13,14. However, we are unaware of any reports that have attempted to use eDNA to monitor dinoflagellate cysts in surface sediments. Approximately 200 dinoflagellate species produce resting cysts during their life cycle15 and these are deposited in the sediments. The cyst population can serve as inocula for the recurrence of blooms of harmful plankton species2. The dinoflagellate cysts in samples from surface sediments of the Tongyeong coast revealed a high abundance and diverse species composition (Supplementary Table S3). It is well known that dinoflagellate cysts occur at higher concentrations in muddy sediments10. Muddy particles are present in the surface sediments of Tongyeong coast10,16. Geological characteristics, such as currents along coastlines, and also determine the abundance of cysts17. As showing the characteristics of currents along Tongyeong coasts18 the cysts in Tongyeong coastal area may be introduced by currents. Moreover, Tongyeong area is one of the richest ecosystems and highest primary productivities in the Korean coast19,20. Thus, the abundance of dinoflagellate cysts in Tongyeong coastline is favoured by marine characteristics, such as the effects of sediment type, currents, and nutrient richness.
Small and orthoperidinoid dinoflagellate species were the most common. Cysts of S. trochoidea (the most predominant cyst) and Pentapharsodinium dalei in morphological results occurred at average proportions of 25.4 and 5.9%, respectively (Supplementary Table S4). These cysts are the most frequently encountered of the potentially harmful species. In particular, S. trochoidea is a cosmopolitan dinoflagellate species in coastal waters and distributed worldwide. Late summer–autumn peaks of calcareous cyst production were recorded in the Gulf of Naples21, and early autumn peaks of S. trochoidea were recorded in Onagawa Bay in Japan4. Cysts belonging to potentially toxic species of the genus Alexandrium, which cause paralytic shellfish poisoning, were recorded in 11.9% of the sediment samples. These taxa are commonly found in coastal zones across the world22. In the study area during 2013–2014, vegetative S. trochoidea and A. catenella/tamarense were frequently observed by two samplings per month at a high average abundance of 232 and 477 cells mL−1, respectively (unpublished data). In particular, they occurred frequently in spring and summer. In the NGS analysis, the assemblage composition is dominated by the Pentapharsodinium-Scrppsiella group, Gonyaulax spinifera, Woloszynskia group, Alexandrium group, and Gymnodinium aureolum. These dominant species detected by NGS were consistent with the morphological results. However, Pentapharsodinium spp. and some Scrppsiella spp. showed very high genetic similarity. Many researchers23–26 reported that Pentapharsodinium is a sister group or primitive lineage of Scrippsiella species based on molecular phylogeny of Calciodinellaceae. In this study, NGS analysis detected 35 taxa compared to only 27 cysts identified using microscopic observation. Thus, NGS analysis can allow researchers to detect higher number of species than microscopic analysis. This is because cysts have high morphological similarity and therefore are difficult to discriminate.
C. polykrikoides causes red tides in Tongyeong coast almost every year7. In particular, they developed massive blooms with density greater than 5,000 cells mL−1 in summer of 2013 (Supplementary Fig. S3). In our previous study, one of the several cysts isolated from surface sediments in the same study area germinated into C. polykrikoides cells, which was confirmed via 28S rDNA sequences9. In the present study, the cysts of C. polykrikoides were detected at ranges between 7 and 48 cysts g−1 dry weight based on morphological analysis. However, the cysts could not be detected in the NGS and PCR analyses. We could not perform replicate NGS analyses because the amount of gDNA of dinoflagellate cysts in the surface sediment was small. This is a weak point in the present study. This is very important to obtain accurate result and to evaluate the reproducibility of NGS for the identification of species, in particular rare species such as C. polykrikoides. Park et al.27 detected C. polykrikoides cysts by quantitative PCR using surface sediments of the same study area, but they found only five cysts in 93 samples. This result is similar to our microscopic findings. Thus, the non-detection of C. polykrikoides in our NGS and conventional PCR analyses could likely be due to the presence of very low amounts of C. polykrikoides cysts in the surface sediments. Tang and Gobler8 found resting cysts of C. polykrikoides in culture. If C. polykrikoides can cause massive blooms in summer, high abundance of cysts can be expected after termination of the blooms. However, relatively low densities of C. polykrikoides cysts were detected as compared with the massive blooms of the vegetative cells. In our preliminary study, NGS analysis of the sediments below the surface layer revealed a high proportion of C. polykrikoides cysts (>70%; Supplementary Fig. S5). However, in the preliminary study extraction of genomic DNA was carried out immediately after sampling, without long-term storage for six months. The high proportions of C. polykrikoides might be attributed to the presence of submerged dead C. polykrikoides and DNA debris by decomposition of C. polykrikoides vegetative cells, although cysts might have constituted a small portion (Fig. 5). Hattenrath-Lehmann et al.28 reported that low cyst densities after high-density blooms may be affected by several physical, chemical, and biological factors, which suggest that a small portion of the vegetative population is involved in sexual reproduction22. Moreover, the other factors affecting density of cysts include grazing of cysts in sediments29, bacterial degradation of cysts8, and/or the dispersal of cysts/vegetative cells out of the bloom region via currents30, followed by deposition of these cysts elsewhere31.
Evidently, the patterns of abundance of C. polykrikoides cysts in the present study cannot represent the prior blooming and might not predict future blooms. Thus, how blooms develop in spite of lower density of C. polykrikoides cysts remains unclear. Alexandrium fundyense, a cyst-forming dinoflagellate, can produce massive blooms from low cyst densities22,32. Hattenrath-Lehmann et al.28 suggested that the modest densities of cysts are important as ‘seed banks for initiating blooms’. However, the present results indicate some distinct patterns of C. polykrikoides cysts, as reported by Hattenrath-Lehmann et al.24. According to the hypothesis of Hattenrath-Lehmann et al.28, vegetative cells of C. polykrikoides were present during 2015, as evident from observation of the sediments in Tongyeong area in 2014. However, vegetative cells of C. polykrikoides were not detected during the summers of 2015 and 2017 (Supplementary Fig. S3). Matsuoka et al.30 and Jeong et al.33 emphasised that C. polykrikoides blooms developed after accumulation of cysts transported from other places. In particular, Lee et al.7 and Park et al.34 mentioned that the mechanism of bloom initiation of C. polykrikoides might be related to the transport of cells into the study area via lateral mixing of water of this area with that of offshore currents containing a high density of C. polykrikoides cells. Thus, the presence of C. polykrikoides might be determined by currents transporting them to a marine environment suitable for their growth.
Methods
Study area and sample collection
The study area is located in Tongyeong coastal waters in the southern coast of South Korea (34°45′N and 128°23′E). This costal water is a eutrophic system that is subjected to strong mixing between the surface and bottom layers, in addition to supplementation of nutrients by food supply from fisheries. Currents in the studied area are not strong, because water depths are generally shallow (<20 m), and many islands are present. In order to analyse the distribution and composition of the dinoflagellate cysts morphologically and using NGS technology, we sampled the surface sediments from 50 sites during the winter, in December 2013 (i.e. after termination of C. polykrikoides bloom) and 44 sites during summer in September 2014 (i.e. during the blooming period of C. polykrikoides) using a gravity core sampler (diameter: 5 cm). The top 2 cm of the core samples were sliced and preserved immediately in the dark at 4 °C (Fig. 6).
Figure 6.
Map showing the sampling sites of surface sediments in Tongyeong coast (South Sea) of South Korea during December 2013 and September 2014. IG, inner group; MG, middle group; OG, outer group. All maps were generated with Surfer v12.2.705 (http://www.goldensoftware.com/products/surfer).
Morphological analysis
The core samples for morphological analysis were collected as described by Li et al.9. One gram of sample was put into a beaker with pure seawater and processed with an ultrasonic probe for 30 s, followed by sieving with 125- and 10-μm stainless steel mesh. The residues on the 10-μm mesh stainless steel mesh were distributed equally in 10-mL aliquots with pure seawater. Dinoflagellate cysts were observed on a 1-mL sub-sample of the 10-mL aliquots in Sedgwick-Rafter counting chamber under a light microscope (Zeiss, Axio Imager A2, Hallbergmoos, Germany), and then photographed using an AxioCam MRm digital camera (Zeiss). Ten grams of sample was weighed in wet condition and dried in an oven to 110 °C for 1 day to measure the water content, and cyst concentrations in this study were estimated as the sum of living and empty cysts, which were presented as the number of cysts per gram of dry sediment.
Genomic DNA extraction and amplification of 18S rDNA
The core samples were stored in dark and cool conditions (at 4 °C) for at least six months for decomposition of organic matter and organisms. Ten grams (wet weight) of the stored samples from each site were dried at 60 °C for 1 d in a drying oven. Thereafter, the dried sub-samples (2 g dry weight) from approximately 10–20 sites were merged and divided into the inner group (IG), middle group (MG), and outer group (OG) from the inner to outer sea, for the 44–50 sampling sites in the survey area (Fig. 6). To remove large-sized particles, the samples were subjected to a prefiltering step using a stainless steel mesh 80-μm pores. The samples after the removal of large-sized particles were harvested onto a collection filter with 10-μm pores (TCTP04700, Millipore, Billerica, MA, USA) and to remove DNA debris from the samples, filtered sediments were washed with hot sterilised seawater at approximately 70 °C and approximately 60-kPa pressure. Then, the harvested samples after removal of large particles and DNA debris were frozen at −80 °C before DNA extraction.
To analyse dinoflagellate cysts, the 18S rDNA V4-V5 gene was targeted for the NGS analysis. The genomic DNA from cysts collected was extracted using beads in a Power Soil DNA Isolation kit (MoBio, Laboratories, Solana Beach, CA, USA) and diluted to a final concentration of 20 ng μL−1. Amplification of the 18S rDNA gene was performed using 25-μL reaction mixtures containing 200 μmol L−1 each dNTP, 1.5 mmol L−1 MgCl2, 0.3 μmol L−1 each primer, 2.5 U Taq DNA polymerase (TaKaRa, EX Taq, Kyoto, Japan), and 1 μL DNA template. The V4–V5 region of the 18S rDNA gene was targeted using the universal primers TAReuk454FWD1 (5′-CCA GCA SCY GCG GTA ATT GG-3′) and TAReukREV3 (5′-ACT TTC GTT CTT GAT YRA-3′)35. Three PCR reactions were performed in distinct tubes, and then mixed to obtain more accurate NGS results. The target region (V4–V5) was chosen because of its high coverage of almost all phyla in conventional and metagenomic studies36. Each primer was tagged using Illumina pre-adapter and locus-specific primers following the manufacturer’s instructions (Mi-Seq, Illumina, San Diego CA, USA). The reaction profile consisted of an initial denaturation step at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 52 °C for 45 s, and extension at 72 °C for 1 min, and a final extension step of 72 °C for 5 min. Under these conditions, 800–1,000 ng PCR product was obtained using a BioRad T100 thermal cycler (BioRad, Hercules, CA, USA). Products from triplicate reactions were pooled and electrophoresed in 1.0% agarose gels, stained with ethidium bromide, and visualized under ultraviolet transillumination. Before NGS analysis, the amplified PCR products were individually purified using an UltraClean PCR Clean-up Kit (MoBio), and their DNA concentration was measured in a Bio-analyzer 2100 (Agilent Technologies, Palo Alto, CA, USA).
Bioinformatics and data analysis
Similar amounts of PCR product from each sample were analysed using a Mi-Seq platform. The data were pre-processed using MiSeq Control Software (MCS) v2.4.1. Raw sequences were first analysed using FastQC37 to check basic statistics, such as GC%, per base quality score distribution, and poor-quality sequences were flagged. Also, ambiguous and chimeric reads also were removed and noised sequences (denoising), which involved OTUs with read 1, 2, and 3, were removed at cut-off of 97% (Supplementary Table S5). The processed pair-end reads were then merged using the fast length adjustment of short reads (FLASH) software tool38. After each sequencing procedure, a quality check was performed to remove short sequence reads (<150 bp), low-quality sequences (score <33), singletons, non-target sequences. Using Basic Local Alignment Search Tool (BLAST)39, all of the sequence reads were compared with those at the National Center for Biotechnology Information (NCBI) database. Sequence reads with an E-value <0.01 were considered for further analysis. A pairwise global alignment was performed on selected candidate hits to find the most similar sequences. The taxonomy of the sequence with the highest similarity was assigned to the sequence read (species or genus levels with >97% or > 94% similarity, respectively). To analyse operational taxonomic units (OTUs), the CD-HIT-OTU software40 was used for clustering, and metagenomic functional information to calculate alpha-diversity, including Shannon-Weaver diversity, Chao richness, and Simpson evenness based on the OTU table was generated using the closed-reference protocol in Mothur41 and QIIME42. All the six datasets were deposited in the Sequence Read Archive database at the NCBI under accessions SRS2412382 (PRJNA397358).
To test the significance of similarity between the six sequenced samples, the multidimensional scaling (MDS) analysis was performed using PRIMER 643 based on Bray-Curtis distance matrices obtained from the read count of OTUs.
Electronic supplementary material
Acknowledgements
The stored genomic DNA samples and fixed phytoplankton samples were obtained from the Library of Marine Samples, Korea Institute of Ocean Science & Technology, South Korea. This work was supported by the Public Welfare & Safety Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Republic of Korea (Development of autonomous microalgal identification system and feasibility analyses of developed HAB control technologies: NRF-2013M3A2A1067529), supported by the Research Program of Korea Institute of Marine Science & Technology (KIMST) funded by the Ministry of Oceans and Fisheries of Republic of Korea (Establishment and demonstration of red tide detection and prediction system for minimizing red tide damage: PM 60650), and supported by the Research Program of Korea Institute of Ocean Science and Technology (Development of marine cold water layer-based multi-culture technology for marine biological resources: PE99628).
Author Contributions
Jung, S.W., Kang, D., Lee, T.-K. designed the research plan; Jung, S.W., Kim, H.J., Shin, H.H., Park, J.S., Park, S.Y. performed the experiments; Jung, S.W., Kang, D., Kim, H.J., Park, J.S., Lee, T.-K., Park, S.Y. discussed the results; Jung, S.W., Kang, D., Kim, H.J., Lee, T.-K. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Seung Won Jung and Donhyug Kang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25345-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seung Won Jung, Email: diatoms@kiost.ac.kr.
Taek-Kyun Lee, Email: tklee@kiost.ac.kr.
References
- 1.Head, M. J. Modern dinoflagellate cysts and their biological affinities in Palynology: Principles and Applications (eds Janosnius, J. & McGregor, D. C.) 1197–1248 (AASP Foundation, 1996).
- 2.Anderson DM. Red tides. Sci. Am. 1994;271:62–68. doi: 10.1038/scientificamerican0894-62. [DOI] [PubMed] [Google Scholar]
- 3.Cembella A, Turgeon J, Theriault JC, Béland P. Spatial distribution of Protogonyaulax tamarensis resting cysts in nearshore sediments along the north coast of the lower St. Lawrence estuary. J. Shellfish Res. 1988;7:597–609. [Google Scholar]
- 4.Ishikawa A, Taniguchi A. Contribution of benthic cysts to the population dynamics of Scrippsiella spp. (Dinophyceae) in Onagawa Bay, northeast Japan. Mar. Ecol. Prog. Ser. 1996;140:169–178. doi: 10.3354/meps140169. [DOI] [Google Scholar]
- 5.Hesse, K. J., Tillmann, U., Nehring, S. & Brockmann, U. Factors controlling phytoplankton distribution in coastal waters of the German Bight (North Sea) in Oceanographic Literature Review (eds Eleftheriou, A., Ansell, A. D. & Smith, C. J.) 11–22 (Olsen & Olsen, 1996).
- 6.Kim C, Kim HG, Kim CH, Oh HM. Life cycle of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korean Coastal waters. Harmful Algae. 2007;6:104–111. doi: 10.1016/j.hal.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Park TG, Park YT, Lim WA. Monitoring and trends in harmful algal blooms and red tides in Korean coastal waters, with emphasis on Cochlodinium polykrikoides. Harmful Algae. 2013;30:S3–S14. doi: 10.1016/j.hal.2013.10.002. [DOI] [Google Scholar]
- 8.Tang YZ, Gobler CJ. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae. 2012;20:71–80. doi: 10.1016/j.hal.2012.08.001. [DOI] [Google Scholar]
- 9.Li Z, Han MS, Matsuoka K, Kim SY, Shin HH. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J. Phycol. 2015;51:204–210. doi: 10.1111/jpy.12252. [DOI] [PubMed] [Google Scholar]
- 10.Pospelova V, Kim SJ. Dinoflagellate cysts in recent estuarine sediments from aquaculture sites of southern South Korea. Mar. Micropaleontol. 2010;76:37–51. doi: 10.1016/j.marmicro.2010.04.003. [DOI] [Google Scholar]
- 11.Gifford SM, Sharma S, Moran MA. Linking activity and function to ecosystem dynamics in a coastal bacterioplankton community. Front. Microbiol. 2014;5:185. doi: 10.3389/fmicb.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rees HC, Maddison BC, Middleditch DJ, Patmore JRM, Gough KC. The detection of aquatic animal species using environmental DNA - a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014;51:1450–1459. doi: 10.1111/1365-2664.12306. [DOI] [Google Scholar]
- 13.Miya M, et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: detection of more than 230 subtropical marine species. R.Soc.Open Sci. 2015;2:150088. doi: 10.1098/rsos.150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, et al. Environmental DNA metabarcoding reveals local fish communities in a species-rich coastal sea. Sci. Rep. 2017;7:40368. doi: 10.1038/srep40368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka, F. & Head, M. Clarifying cyst-motile stage relationships in dinoflagellates. In Biological and Geological Perspectives of Dinoflagellates (eds. Lewis, J. M., Marret, F. & Bradley, L.) 325–350 (The Geological Society, 2013).
- 16.Cho HJ, Kim CH, Moon CH, Matsuoka K. Dinoflagellate cysts in recent sediments from the southern coastal waters of Korea. Bot. Mar. 2003;46:332–337. [Google Scholar]
- 17.Goodman, D.K. Dinoflagellate cysts in ancient and modern sediments in The Biology of Dinoflagellates, Botanical Monographs (ed. Taylor, F. J. R.) 649–722 (Blackwell, 1987).
- 18.Park KS, et al. Development of the operational oceanographic system of Korea. Ocean Sci. J. 2015;50:353–369. doi: 10.1007/s12601-015-0033-1. [DOI] [Google Scholar]
- 19.Jung SW, Park JG, Jeong DH, Lim DI. Seasonal changes in water masses and phytoplankton communities in the Western part of South coastal waters, Korea. Korean J. Environ. Biol. 2012;30:328–338. doi: 10.11626/KJEB.2012.30.4.328. [DOI] [Google Scholar]
- 20.Jung, S. W., Kwon, O. Y., Joo, H. M. & Lee, J. H. Variations of phytoplankton standing crops affecting by environmental factors in the marine ranching ground of Tongyeong coastal waters from 2000 to 2007. Korean J. Environ. Biol. 25, 303–312 (2007).
- 21.Montresor M, Zingone A, Sarno D. Dinoflagellate cyst production at a coastal Mediterranean site. J. Plankton Res. 1998;20:2291–2312. doi: 10.1093/plankt/20.12.2291. [DOI] [Google Scholar]
- 22.Anglès S, Garcés E, Hattenrath-Lehmann TK, Gobler CJ. In situ life-cycle stages of Alexandrium fundyense during bloom development in Northport Harbor (New York, USA) Harmful Algae. 2012;16:20–26. doi: 10.1016/j.hal.2011.12.008. [DOI] [Google Scholar]
- 23.Montresor M, Sgrosso S, Procaccini G, Kooistra W. Intraspecific diversity in Scrippsiella trochoidea (Dinophyceae): evidence for cryptic species. Phycologia. 2003;42:56–70. doi: 10.2216/i0031-8884-42-1-56.1. [DOI] [Google Scholar]
- 24.Gottschling M, et al. A molecular phylogeny of Scrippsiella sensu lato (Calciodinellaceae, Dinophyta) with interpretations on morphology and distribution. Eur. J. Phycol. 2005;40:207–220. doi: 10.1080/09670260500109046. [DOI] [Google Scholar]
- 25.Craveiro SC, Calado AJ, Daugbjerg N, Hansen G, Moestrup O. Ultrastructure and LSU rDNA-based phylogeny of Peridinium lomnickii and description of Chimonodinium gen. nov. (Dinophyceae) Protist. 2011;162:590–615. doi: 10.1016/j.protis.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Zinssmeister C, Soehner S, Facher E, Kirsch M. Catch me if you can: the taxonomic identity of Scrippsiella trochoidea (F.STEIN) A.R.LOEBL. (Thoracosphaeraceae, Dinophyceae) Syst. Biodivers. 2011;9:145–157. doi: 10.1080/14772000.2011.586071. [DOI] [Google Scholar]
- 27.Park TG, Kim JJ, Kim WJ, Won KM. Development of real-time RT-PCR for detecting viable Cochlodinium polykrikoides (Dinopyceae) cysts in sediment. Harmful Algae. 2016;60:36–44. doi: 10.1016/j.hal.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Hattenrath-Lehmann TK, Zhen Y, Wallace RB, Tang YZ, Gobler CJ. Mapping the distribution of cysts from the toxic dinoflagellate Cochlodinium polykrikoides in bloom-prone estuaries by a novel fluorescence In Situ hybridization assay. Appl Environ Microb. 2016;82:1114–1125. doi: 10.1128/AEM.03457-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson A, Rosenberg R. Impact of grazing and bioturbation of marine benthic deposit feeders on dinoflagellate cysts. Harmful Algae. 2003;2:43–50. doi: 10.1016/S1568-9883(03)00003-9. [DOI] [Google Scholar]
- 30.Matsuoka K, et al. Seed populations of a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef in the East China Sea. Harmful Algae. 2010;9:548–556. doi: 10.1016/j.hal.2010.04.003. [DOI] [Google Scholar]
- 31.Genovesi B, et al. Influences of sedimentation and hydrodynamics on the spatial distribution of Alexandrium catenella/tamarense resting cysts in a shellfish farming lagoon impacted by toxic blooms. Harmful Algae. 2013;25:15–25. doi: 10.1016/j.hal.2013.02.002. [DOI] [Google Scholar]
- 32.Hattenrath TK, Anderson DM, Gobler CJ. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae. 2010;9:402–412. doi: 10.1016/j.hal.2010.02.003. [DOI] [Google Scholar]
- 33.Jeong HJ, et al. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: I. Temporal variations in three-dimensional distributions of red-tide organisms and environmental factors. Algae. 2017;32:101–130. doi: 10.4490/algae.2017.32.5.30. [DOI] [Google Scholar]
- 34.Park GH, et al. A sulfur hexafluoride-based Lagrangian study on initiation and accumulation of the red tide Cochlodinium polykrikoides in southern coastal waters of Korea. Limnol. Oceanogr. 2005;50:578–586. doi: 10.4319/lo.2005.50.2.0578. [DOI] [Google Scholar]
- 35.Stoeck T, et al. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010;19:21–31. doi: 10.1111/j.1365-294X.2009.04480.x. [DOI] [PubMed] [Google Scholar]
- 36.Rzeznik-Orignac J, et al. Comparison of meiofaunal diversity by combined morphological and molecular approaches in a shallow Mediterranean sediment. Mar. Biol. 2017;164:40. doi: 10.1007/s00227-017-3074-4. [DOI] [Google Scholar]
- 37.Andrews, S. FQC A quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 38.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 40.Li RW, Connor EE, Li C, Baldwin VIRL, Sparks ME. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ. Microbiol. 2012;14:129–39. doi: 10.1111/j.1462-2920.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 41.Schloss PD, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke, K. R., & Warwick, R. M. Change in marine communities: An approach to statistical analysis and interpretation. (2nd edition) Primer-e Ltd, Plymouth (2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.