Abstract
We examined the mechanism of synthesis in vitro of (1→3),(1→4)β-d-glucan (β-glucan), a growth-specific cell wall polysaccharide found in grasses and cereals. β-Glucan is composed primarily of cellotriosyl and cellotetraosyl units linked by single (1→3)β-linkages. The ratio of cellotriosyl and cellotetraosyl units in the native polymer is strictly controlled at between 2 and 3 in all grasses, whereas the ratios of these units in β-glucan formed in vitro vary from 1.5 with 5 μm UDP-glucose (Glc) to over 11 with 30 mm substrate. These results support a model in which three sites of glycosyl transfer occur within the synthase complex to produce the cellobiosyl-(1→3)-d-glucosyl units. We propose that failure to fill one of the sites results in the iterative addition of one or more cellobiosyl units to produce the longer cellodextrin units in the polymer. Variations in the UDP-Glc concentration in excised maize (Zea mays) coleoptiles did not result in wide variations in the ratios of cellotriosyl and cellotetraosyl units in β-glucan synthesized in vivo, indicating that other factors control delivery of UDP-Glc to the synthase. In maize sucrose synthase is enriched in Golgi membranes and plasma membranes and may be involved in the control of substrate delivery to β-glucan synthase and cellulose synthase.
The mixed-linked (1→3),(1→4)β-d-glucan (hereafter referred to as simply β-glucan) is a cell wall polysaccharide found only in grasses and cereals (Carpita, 1996). The β-glucan is not synthesized in dividing cells but accumulates specifically during cell enlargement (Carpita and Gibeaut, 1993). The β-glucan also accumulates in the walls of the endosperm of the developing grains and their surrounding maternal tissues (Fincher and Stone, 1986; Brown et al., 1997).
The β-glucan structure was established by use of a sequence-dependent Bacillus subtilis endoglucanase (lichenase) that cleaves (1→4)β-d-glucosyl units only if preceded by (1→3)β-units and yields primarily a diagnostic trisaccharide, cellobiosyl-(1→3)-d-Glc, and a tetrasaccharide, cellotriosyl-(1→3)-d-Glc (Anderson and Stone, 1975). It is an unbranched glucan and over 90% of the polymer consists of these cellotriosyl and cellotetraosyl units in ratios ranging from 2 to 3 in grasses, each connected by a single (1→3)β-linkage (Wood et al., 1991, 1994). The remainder of the polymer consists of longer runs of the cellodextrin interspersed within the polymer and connected by single (1→3)β-linkages (Staudte et al., 1985; Wood et al., 1994). The ratio of the odd cellodextrin oligomers is generally about 2-fold greater in abundance than the next-higher even-numbered oligomer in the series (Wood et al., 1991, 1994). Although not found in any other angiosperms, a similar mixed-linkage β-glucan is found in the lichen Cetraria islandica (Wood et al., 1994). In the lichen β-glucan, the cellotriosyl units comprise 86% of the polysaccharide.
Synthesis of β-glucan with cellular membranes was shown by digestion of the radioactive products with several enzymes, including the B. subtilis enzyme, and subsequent gel-permeation chromatography, HPAE-HPLC, or TLC of the hydrolysis products (Henry and Stone, 1982; Gibeaut and Carpita, 1993; Becker et al., 1995). Synthesis occurs strictly at the Golgi apparatus, uses UDP-Glc as substrate, and requires Mg2+ or Mn2+ as a cofactor (Gibeaut and Carpita, 1993). The proportions of (1→3)β- and (1→4)β-d-glucosyl linkages in total glucan products are altered substantially by reaction conditions in microsomal fractions from Lolium multiflorum (Meikle et al., 1991), and significant amounts of β-glucan are made only at UDP-Glc concentrations above 100 μm. A combination of gel permeation chromatography, linkage analysis, and enzymic digestion confirmed that entire tri- and tetrasaccharide units were synthesized and that the macromolecular β-glucan synthesized in vitro in the Golgi apparatus was similar to the cell wall polysaccharide (Gibeaut and Carpita, 1993).
Unlike the Golgi apparatus from nongramineous plants, the maize (Zea mays) Golgi apparatus also synthesizes a considerable proportion of callose in vitro (Gibeaut and Carpita, 1994). The Golgi membrane-associated callose synthase was not attributed to contamination with plasma membrane; this activity is inhibited slightly by Mg2+ and Mn2+ (Gibeaut and Carpita, 1993) and stimulated only 2-fold by CaCl2 compared with 7-fold for the plasma membrane-associated synthase (Gibeaut and Carpita, 1994). Compounds that disrupt membrane integrity, such as detergents and ionophores, abolish β-glucan synthase activity and increase the amount of callose synthesized. We have proposed that, like cellulose synthase, β-glucan synthase may revert to callose synthase when disrupted (Gibeaut and Carpita, 1994).
The structure and ratio of the cellotriosyl and cellotetraosyl units of β-glucan and the iteration of this ratio in higher order odd- and even-numbered oligomers are relatively constant within a species (Wood et al., 1994). How this consistency is maintained during synthesis is not known. The catalysis of polymerization of cellulose, β-glucans, and polymers containing (1→4)β-linked mannosyl and xylosyl units must overcome a steric problem, because the (1→4)β-linkage requires that each of these sugar units be rotated nearly 180° with respect to its neighbors. With a single site of glycosyl addition, the nonreducing acceptor sugar of the growing chain must be rotated with each addition, the active site of the substrate relative to the chain terminus must be rotated, or the sugar must be added in an activated configuration strictly at the O-4 position but then rotate into its proper 180° orientation as the chain extends.
As an alternative, we proposed that two sites of glucosyl transfer generate disaccharide units (Carpita et al., 1996; Carpita and Vergara, 1998). We proposed further that the trisaccharide unit of β-glucan can be formed if an ancestral cellulose synthase complex were modified to contain a third glucosyl transferase activity in addition to the cellobiose generating activity (Carpita et al., 1996). With an odd number of glucosyl units added, the nonreducing terminal sugar of the growing chain must always be oriented to expose the O-3 hydroxyl for attachment instead of the O-4 and, therefore, cellotriosyl units would always be linked by single (1→3)β-linkages. The experiments reported here were designed to resolve some of these possibilities. Our results indicated that cellotriosyl units are selectively enriched in abundance with respect to the higher cellodextrin series at more saturating substrate concentrations, and therefore support a three-site model of glucosyl addition.
Treatments designed to reduce UDP-Glc concentration in excised coleoptile sections did not result in marked changes in cellodextrin oligomeric ratios or in molecular size of the newly synthesized β-glucan. These data indicate that UDP-Glc supply to the β-glucan synthase is regulated by factors independent of cytosolic nucleotide sugar concentration. We immunodetected SuSy associated with Golgi membranes of maize but not those of soybean (Glycine max), whereas this UDP-Glc generating enzyme was found associated with the plasma membrane of both species. Amor et al. (1995) proposed such a role for SuSy in cellulose synthesis, and our data suggest that SuSy regulates delivery of UDP-Glc to the maize β-glucan synthase at the Golgi apparatus by a similar mechanism.
MATERIALS AND METHODS
Plant Material
Maize (Zea mays L.) caryopses were soaked overnight in deionized water bubbled with air at 30°C, sown in trays of moist, medium-grade vermiculite, and incubated in darkness at 30°C for 2 d. Soybean (Glycine max L. cv Williams 82) seeds were sown directly in trays of moist vermiculite and incubated in darkness at 25°C for 5 d. For preparation of Golgi and other membranes and for in vitro synthase reactions the upper two-thirds of the coleoptiles and 1-cm sections of soybean hypocotyls comprising the hook and elongation zone were collected in a chilled beaker. For pulse-labeling studies in vivo, the sections were floated at ambient temperature on an incubation buffer consisting of 5 mm potassium phosphate, pH 5.5, containing 5 mm KCl, 2× 10−5 m IAA, and 0.01% (w/v) tetracycline.
Synthesis of β-Glucan in Vivo under Conditions of Depleted UDP-Glc
For determinations of the incorporation of Glc into the cellotriosyl and cellotetraosyl units of β-glucan in vivo, the upper 11-mm sections of freshly isolated coleoptiles (approximately 30/sample), excluding 1 to 2 mm of the tip, were incubated immediately after harvest in 3 mL of incubation buffer with 66 μm d-Glc containing 50 μCi of d-[U-14C]Glc (252 mCi/mmol; Amersham) for 1.5 h at 30°C, and then frozen in liquid nitrogen. Additional coleoptile sections were floated on incubation buffer alone or on incubation buffer supplemented with 10 mm d-Gal or 100 mm d-Glc for 3 and 8 h at 30°C, rinsed in incubation buffer alone, and then incubated in incubation buffer with 66 μm d-Glc containing 50 μCi of the labeled Glc as before. For determination of Suc and nucleotide sugar content, coleoptile sections were incubated similarly but without the labeled Glc.
For determinations of nucleotide sugars, frozen coleoptile sections were homogenized in ice-cold 10% (w/v) TCA, centrifuged at 10,000g for 10 min, and the supernatant was collected. The TCA was removed by two partitionings against trioctylamine in 1,1,2-trichlorotrifluoroethane, as described previously (Kanabus et al., 1986). Nucleotides and nucleotide sugars in the neutralized aqueous fraction were separated by HPAE-HPLC and detected by UV A260 essentially as described by Liljebjelke et al. (1995). UDP-Glc was also assayed enzymically with UDP-Glc dehydrogenase (Keppler and Decker, 1981). The UDP-sugar fraction from HPLC was collected, and the sugars were hydrolyzed by 2 m trifluoroacetic acid. The trifluoroacetic acid was then evaporated in a stream of air and the sugars were separated by HPAE-HPLC and detected by pulsed amperometry (Gorshkova et al., 1997), or alditol acetate derivatives were prepared and separated by GLC (Gibeaut and Carpita, 1991). In parallel experiments, Suc and other small molecules were extracted from the frozen coleoptile sections with 80% (v/v) ethanol at 45°C for 30 min with gentle agitation, and the extract was collected by pipette and dried under a stream of nitrogen at 35°C. Suc and Glc were quantified in samples with and without invertase with a coupled hexokinase/Glc-6-P dehydrogenase assay (Carpita and Kanabus, 1987).
The cell walls pelleted in ice-cold TCA were resuspended in 50 mm sodium acetate, 50 mm NaCl, and 30 mm ascorbate, pH 5.5, filtered on nylon mesh, and washed sequentially with 100 mm NaCl, water, acetone, 100 mm NaCl, and water. A portion of the wall preparation was saved for further analysis, and the remainder (11–15 mg) was extracted sequentially with 20 mL each of 0.5% (w/v) ammonium oxalate, pH 7.0 (90°C with occasional stirring), and 0.08 n NaOH, 0.8 n NaOH, and 4 n NaOH (ambient temperature with continuous stirring). Each alkali solution was supplemented with 3 mg mL−1 NaBH4 to prevent reducing-end elimination, and extractions were made at atmospheric N2. All extractions were for 1 h except the 4 n NaOH fractionation, which was for 15 h. After each extraction the unextracted wall was pelleted by centrifugation and the supernatant liquid was filtered through glass-fiber mats to remove small amounts of suspended wall material. Alkali-soluble fractions were chilled to ice temperature and acidified with glacial acetic acid to about pH 5.0. All fractions were dialyzed extensively against running deionized water and freeze-dried. The α-cellulose remaining was acidified to pH 5.0 with glacial acetic acid and then washed several times with deionized water. Radioactivity in these fractions was determined by liquid scintillation spectroscopy. The freeze-dried materials were suspended in 2.5 mL of water, and 0.5 mL was assayed for radioactivity by liquid scintillation spectroscopy.
A Bacillus subtilis endoglucanase preparation was added to portions of wall and fractionation material in water to digest material into cellodextraoyl-(1→3)-d-Glc oligosaccharides. These oligosaccharides were separated by HPAE-HPLC in a NaOH/sodium acetate gradient designed to optimize separation of oligosaccharides to DP 10 (Gibeaut and Carpita, 1993). One-half-milliliter fractions were collected in 1.0 mL of 2 n acetic acid, and radiolabeled oligosaccharides were determined by liquid scintillation spectroscopy. The remainder of the neutralized alkali extracts were dialyzed and freeze-dried for gel permeation chromatography.
Preparation of Membranes
Freshly isolated 2-d-old maize coleoptiles and 5-d-old soybean hypocotyl sections (20 g fresh mass) were collected in a chilled beaker and overlaid with an equal volume of ice-cold homogenization buffer consisting of 84% (w/v) Suc in 20 mm HEPES[KOH], pH 7.2, containing 20 mm KCl, 5 mm EDTA, 5 mm EGTA, 10 mm DTT, and 0.2 mm PMSF. In addition, 2 g of activated charcoal was sprinkled on coleoptiles before addition of homogenization buffer to absorb inhibitory phenolics released by the maize coleoptiles during mashing (Gibeaut and Carpita, 1993). The material from maize and soybean was gently stirred in homogenization buffer to coat each coleoptile with the Suc for about 5 min before gentle mashing in a chilled mortar and pestle (Gibeaut and Carpita, 1994). The homogenates, which were about 45% (w/v) Suc, were squeezed through a nylon mesh (47-μm2 pores).
About 15 mL of the homogenate was pipetted into a 38.5-mL centrifuge tube (Ultraclear, Beckman) onto a cushion of 50% (w/v) Suc in a gradient buffer containing 20 mm HEPES[Bis-Tris-propane], pH 7.6, and overlaid with 8 mL each of 34% and 25% (w/v) Suc, 7 mL of 18% (w/v) Suc in gradient buffer, and the remaining volume was made up with 9.5% (w/v) Suc in the same buffer. After Suc gradient centrifugation at 140,000g in a rotor (model SW28, Beckman) for 45 min, the interfaces containing the enriched Golgi apparatus were collected with a Pasteur pipette or by a displacement fractionator. These membranes were used directly for β-glucan synthase reactions at low substrate concentration or were diluted with 1.08 m Suc in gradient buffer and pelleted by centrifugation at 140,000g for 30 min in the rotor. The membrane pellet was gently resuspended with a camel-hair paintbrush into 2 mL of 1.08 m Suc in gradient buffer for β-glucan synthase reactions at high substrate concentration.
Samples of the other layers were also taken for protein determination and enzyme marker assays. For immunolocalization of SuSy, portions of the mitochondrial- (47%/34% interface), Golgi- (34%/25% interface), and ER/tonoplast-enriched membranes (25%/9.5% interface) were diluted with 1.08 m Suc in gradient buffer, pelleted at 140,000g, and the pellets were dissolved by heating in a PAGE sample buffer containing 10% (w/v) SDS and 100 mm DTT. For preparation of enriched plasma membrane, coleoptile and soybean tissues were gently mashed with a mortar and pestle in homogenization buffer containing 9.5% (w/v) Suc, the homogenate was cleared of cell debris by centrifugation at 3,000g for 10 min, and the total membranes were then pelleted at 140,000g in the rotor.
A portion of the supernatant was saved for immunolocalization of soluble SuSy, and the membrane pellets were gently resuspended in 2 mL of a two-phase buffer consisting of 6.5% (w/v) PEG 3350 and 6.5% (w/v) dextran 500T in 5 mm potassium phosphate, pH 7.2, 3 mm KCl, 10 mm DTT, and 0.2 mm PMSF. The suspension was frozen, thawed, and gently sheared by six strokes in a smooth glass tube with a Teflon piston. Additional two-phase buffer was added, and the plasma membrane was prepared by two rounds of partitioning (100 inversions followed by centrifugation at 400g for 5 min at 4°C). The membranes were washed twice in 20 mm HEPES[KOH], pH 7.2, containing 1.08 m Suc and 10 mm KCl, and pelleted by centrifugation at 140,000g. Marker assays were performed after resuspension in the dilution buffer. Soluble and membrane proteins were dissolved by heating in a PAGE sample buffer containing 10% (w/v) SDS and 100 mm DTT.
Protein was determined by bicinchoninic acid assay (Pierce), and assays for IDPase (Golgi), NADH oxidase (mitochondria), vanadate-sensitive ATPase (plasma membrane), NADP reductase (ER), and NO3−-sensitive ATPase (vacuole) were performed as optimized for maize from published methods described in Gibeaut and Carpita (1994).
Immunolocalization of SuSy
The polypeptides extracted in a high SDS-DTT sample buffer were concentrated by precipitation in 80% (v/v) ethanol chilled to −80°C. The pelleted proteins were dried in vacuo and redissolved in 6× SDS-gel sample buffer at a concentration of 10 μg μL−1. The polypeptides (10 μg/lane) were separated by SDS-PAGE (10% [w/v] gel) and electroblotted onto cellulose nitrate membranes by semidry blotting. The blots were probed with polyclonal antisera directed against maize SuSy, which was provided by Drs. P. Chourey and Karen Koch (University of Florida, Gainesville). Owing to differences in the apparent avidity of the antibody to soybean and maize SuSy, the blotted maize proteins were probed at a 1:500 dilution, whereas the soybean proteins were probed at a 1:200 dilution. Alkaline phosphatase-conjugated goat anti-rabbit IgG (1:3,000; Bio-Rad) was used as secondary antibody, and color was developed with 100 mm Tris-HCl, pH 9.5, 100 mm NaCl, 5 mm MgCl2, 1% of 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt in dimethylformamide, and 1% of p-nitroblue tetrazolium chloride in 100 mm Tris [HCl], pH 9.5, containing 75% (v/v) dimethylformamide. After color development the cellulose nitrate membranes were washed with water several times before scanning.
The developed blots were scanned (QuickScan, Epson, Torrance, CA) at 254 grayscale units and 600 dpi, and the band intensities were quantified with an image analysis program (Scanalytics, Vienna, VA). SuSy was also detected in a standard series of 0.1 to 10 μg of total soluble protein to determine the linearity of signal with protein content.
Glucan Synthase Reactions
Reactions were performed with freshly isolated membranes at 30°C for up to 2 h. Reactions at low substrate concentrations were performed in 4-mL borosilicate glass vials and contained, at the final substrate concentrations indicated, 0.5 to 2.0 μCi of UDP-d-[U-14C]Glc (250 mCi/mmol; ICN Radiochemicals), 20 mm KCl, 1.08 m Suc, 10 mm MgCl2, 10 mm HEPES[Bis-Tris-propane], pH 7.6. One-half milliliter each of reaction buffer and the membrane fraction (50–100 μg of protein) were mixed in vials and placed at 30°C. Reactions without radioactivity at high substrate concentrations were performed with concentrated membranes (200 μg of protein), and MgCl2 concentrations were 1.5 times that of the UDP-Glc concentration. Reactions were stopped with 3 mL of ethanol, capped, and placed in an oven at 105°C for 5 min. The reaction mixtures were cooled to room temperature, and then centrifuged for 5 min at 10,000g. The pellet was washed extensively with hot 80% (w/v) ethanol after heating and recentrifuged.
Digestion with B. subtilis Endoglucanase as a Specific Assay for (1→3),(1→4)β-d-Glucan
The washed and dried products of the glucan synthase reactions, and cell walls from excised coleoptiles labeled in vivo, were suspended in 200 μL of water and 200 μg of barley β-glucan (Sigma) was added as carrier. Ten to 50 μL of a preparation of B. subtilis endo-β-d-glucanase (Gibeaut and Carpita, 1993) in 20 mm sodium acetate, 20 mm NaCl, pH 5.5, was added, and the samples were incubated for 3 h at 37°C. The products released from digestion of purified β-glucan were mostly cellobiosyl- and cellotriosyl-(1→3)β-d-Glc, with decreasingly smaller amounts of cellotetraosyl- and cellopentaosyl-(1→3)β-d-Glc. The reactions were terminated by heating for 2 min in a boiling water bath, cooled to ambient temperature, and centrifuged at 10,000g for 5 min. Radioactivity in β-glucan was estimated as the difference in disintegrations per minute in the supernatants between digested and undigested samples. The remaining insoluble radioactivity from in vitro synthase reactions, which is greater than 90% (1→3)-linked glucosyl units was attributed to callose (Gibeaut and Carpita, 1993).
HPAE-HPLC
The oligomers from digestion of the in vitro reaction products were separated on an anion-exchange column (Carbo-Pac PA1, Dionex) equilibrated in 0.5 n NaOH and eluted in a linear gradient of sodium acetate in 0.5 n NaOH as described previously (Gibeaut and Carpita, 1993). Using the reaction products from low concentrations of UDP-Glc, the radioactivity in 0.5-mL fractions collected in 1.0 mL of 2 n acetic acid was determined by liquid scintillation spectroscopy, whereas the β-glucan products of high UDP-Glc concentrations and native β-glucans from alkali extracts were determined by pulsed amperometric detection.
Gel Permeation Chromatography
The alkali extracts, the β-glucan products from high-UDP-Glc concentration reactions, and radioactive polysaccharides from the low-UDP-Glc reactions (spiked with 200 μg of barley β-glucan) were applied to a 2.5- × 40-cm column of Sepharose 4B (Sigma) equilibrated in McIlvaine's buffer (50 mm citric acid and 100 mm Na2HPO4), pH 5.5. Fractions (4 mL) were collected, 1.0 mL was assayed for total radioactivity by liquid scintillation spectroscopy, and 200 μL was assayed for sugar by the phenol-sulfuric acid method (Dubois et al., 1956). The remaining β-glucans were combined in four fractions, dialyzed against running deionized water, and lyophilized. The dry materials were dissolved in 200 μL of water and digested with the B. subtilis endoglucanase as described earlier. In separate column runs, either dextran standards ranging from 17.5 to 500 kD (Sigma) or 5 mL of a 0.5% (w/v) solution of barley β-glucan were run under conditions identical to those for the labeled products.
RESULTS
β-Glucan Synthesis in Vitro
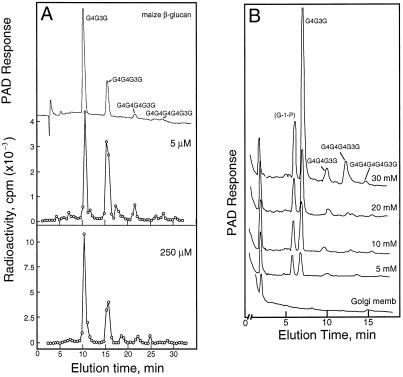
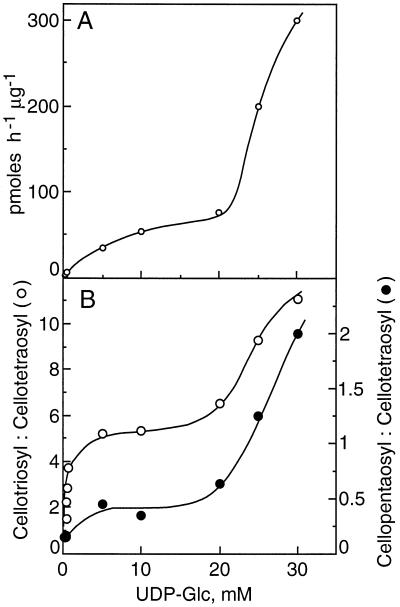
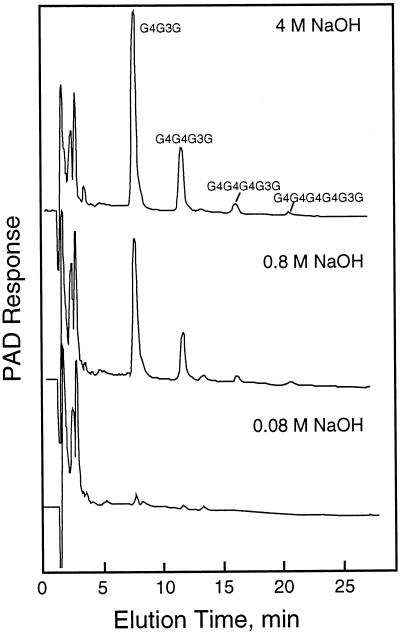
A gentle mashing of the maize coleoptiles in an equal portion of buffer containing 84% (w/v) Suc yielded a homogenate with a final concentration equivalent to about 45% (w/v) Suc. Separation of the membranes and organelles by flotation centrifugation gave an enriched Golgi membrane fraction in about 1 h after harvest of the tissue. In preliminary experiments, the β-glucan formed at suboptimal UDP-Glc concentrations of 5 to 255 μm was determined. The incorporation of radiolabel into the cellodextrin-(1→3)β-d-glucan oligomers digested by the B. subtilis endoglucanase and their separation by HPAE-HPLC provided the specificity needed for these determinations (Fig. 1). Based on the specific activity of the UDP-Glc provided, the incorporation of Glc into polymer increased from 0.021 pmol μg−1 protein h−1 when provided with 5 μm UDP-Glc to 0.82 pmol μg−1 protein h−1 with 255 μm substrate (Fig. 2A). In subsequent experiments the concentrations of UDP-Glc ranged from 5 to 30 mm, and the Glc incorporated could be determined by pulsed amperometry rather than by incorporation of radioactivity (Fig. 1B). While the incorporation of Glc into β-glucan appeared to saturate between 5 and 10 mm UDP-Glc, the amount of product formed increased markedly above 20 mm UDP-Glc. Total incorporation of Glc into β-glucan increased proportionally with substrate concentrations, from 55 pmol μg−1 protein h−1 at 5 mm UDP-Glc to 300 pmol μg−1 protein h−1 with 30 mm substrate (Fig. 2A).
Figure 1.
HPAE-HPLC separation of labeled cellodextrin-(1→3)-d-Glc oligosaccharides from β-glucan synthesized in vitro. A, Separation of the β-glucan cellodextrin products synthesized at low substrate concentrations. Radioactivity from 0.5-mL fractions was determined by liquid scintillation spectroscopy after acidification of the NaOH with acetic acid. Upper trace, Pulsed amperometric detection of cellodextrin-(1→3)-d-Glc oligosaccharides from β-glucan. B, Separation of the β-glucan cellodextrin products synthesized at high substrate concentrations. The cellodextrin-(1→3)-d-Glc oligosaccharides from β-glucan made in vitro were quantified by pulsed amperometric detection (PAD).
Figure 2.
A, Rates of β-glucan synthase activity dependent on UDP-Glc concentration. Product made was determined from total radioactivity and specific activity of substrate or from calibration of the pulsed amperometric signal. Values in the range of 5 to 255 μm represent the averages of three experiments, whereas those in the range of 5 to 30 mm are from two experiments. B, Molar ratios of cellotriose to cellotetraose and cellopentaose to cellotetraose are dependent on the UDP-Glc concentration. The molar ratios based on incorporation of radioactivity were corrected based on the number of glucosyl units per oligosaccharide, whereas the mass detected by pulsed amperometry was calibrated by direct assay of eluted sugars.
The values for total incorporation per microgram of membrane protein were proportional to the ratios of both cellotriose to cellotetraose and cellopentaose to cellotetraose. The relative amounts of cellotriose formed increased with increasing concentrations of UDP-Glc provided, but exhibited an apparent saturation at concentrations of UDP-Glc below 5 mm (Fig. 2B). At 5 μm substrate, the ratio of the cellotriose and cellotetraose units was only 1.5 but increased to 3.7 at 255 μm UDP-Glc. The ratio of cellotriose to the less-abundant cellopentaose also rose with increasing substrate concentration, from about 8.0 to 26, and the cellopentaose to cellotetraose ratio was nearly constant at 0.15 (Fig. 2B). The cellopentaose to cellohexaose rose only slightly, from 2.5 to 4.0, over the same concentration range. However, at substrate concentrations between 5 and 30 mm, the ratio of cellotriose to cellotetraose units increased markedly again, from about 5.0 with 15 to 20 mm UDP-Glc to over 11 with 30 mm UDP-Glc (Fig. 2B). The ratio of cellotriose to cellopentaose units was about 15 with 10 mm UDP-Glc, but then fell to only about 5.0 with 30 mm substrate. Concomitant with the marked increase in cellotriose units between 15 and 30 mm UDP-Glc, the ratio of cellopentaose to cellotetraose units increased even more strongly, from about 0.5 at 5 mm UDP-Glc to 2.0 at 30 mm. The cellopentaose units are a small fraction of the total at suboptimal UDP-Glc but become twice as abundant as cellotetraose units at high substrate concentrations.
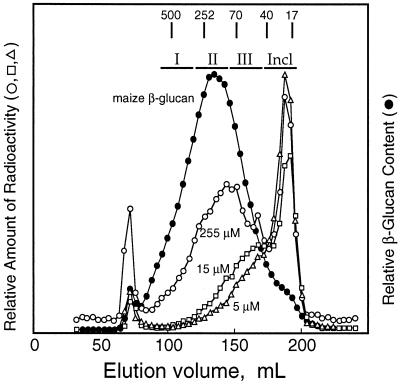
Separation of the radiolabeled β-glucan synthesized in vitro by gel permeation chromatography revealed that polymers less than 50 kD were formed at substrate concentrations of 15 μm and less. The product made at 80 μm was about 100 kD, and that formed at 255 μm was about 250 kD, which is about the size of native maize β-glucan (Fig. 3).
Figure 3.
Gel-permeation chromatography showing the molecular mass distribution of native maize β-glucan compared with β-glucan synthesized in vitro at various low substrate concentrations. The native β-glucan and the products of the in vitro reactions were dissolved in McIlvaine's buffer, pH 5.5, and loaded onto a 2.5- × 40-cm column of Sepharose 4B equilibrated in the same buffer. Four-milliliter fractions were collected, and a portion was digested with the B. subtilis endoglucanase enzyme for HPAE-HPLC. The β-glucan was detected either by total sugar (Dubois et al., 1956) or by radioactivity as assayed by liquid scintillation spectroscopy. The distribution of native β-glucan observed here was essentially identical for β-glucans extracted from excised coleoptile sections incubated with labeled Glc as described in Tables II and III. Two experiments were run with essentially identical results. Fractions from in vivo labeling experiments were pooled (designated I, II, III, and Incl. [included]), dialyzed against deionized water, and assayed for cellodextrin distribution in β-glucan as described in Table III. Peak fractions of dextran standards for molecular mass (in kD) are marked.
Synthesis of β-Glucans in Vivo
To determine if the ratios of the cellodextrin units could be altered in vivo, coleoptile sections were incubated under conditions designed to lower endogenous UDP-Glc concentrations. The length of the coleoptile sections increased almost 50% in 9.5 h when incubated in a buffer containing IAA in the presence or absence of d-Glc (Table I). However, incubation in 10 mm d-Gal inhibited the auxin-induced growth by about one-half. The cytosolic sugar and UDP-sugar concentrations cannot be estimated accurately because of the inherent uncertainty of relative compartmentation between cytosol and vacuole. The amounts of Glc and Suc were 2 to 3 orders of magnitude greater than the UDP-Glc per gram fresh mass (Table I).
Table I.
Changes in Glc, Suc, and UDP-Glc content and relative concentration during growth of excised coleoptile sections
| Sample | Final Length | Glc | Suc | UDP-Glc |
|---|---|---|---|---|
| mm | nmol mg−1 fresh mass | pmol mg−1 fresh mass | ||
| Fresh sections | 11.0 | 32.9 | 5.7 | 36.7 |
| 4.5 h, no sugar | 14.7 ± 0.4 | 23.2 | 1.9 | 11.9 |
| 9.5 h, no sugar | 15.9 ± 0.3 | 19.9 | 1.7 | 7.9 |
| 9.5 h, 100 mm Glc | 16.4 ± 0.3 | 40.6 | 3.9 | 13.8 |
| 9.5 h, 10 mm Gal | 14.5 ± 0.3 | 23.3 | 1.8 | 4.7 |
Sections exactly 11 mm long were sliced with a razor blade 1 to 2 mm below the tip of 3-d-old coleoptiles about 2 to 2.5 cm long. Sections were floated on a medium containing 5 mm potassium phosphate, pH 5.5, 5 mm KCl, 0.01% tetracycline, and 2 × 10−5 m IAA, and, in some instances, supplemented with 100 mm Glc or 10 mm Gal. The sections were transferred either immediately or after 4.5 or 9.5 h of incubation at 30°C. Matching sets of samples were transferred either immediately or after 3 or 8 h of incubation at 30°C to the same respective media containing radiolabeled Glc and incubated an additional 1.5 h at 30°C. After incubations the sections were rinsed with water and frozen in liquid nitrogen. Length measurements are from 20 coleoptile sections. Values are typical of three independent experiments.
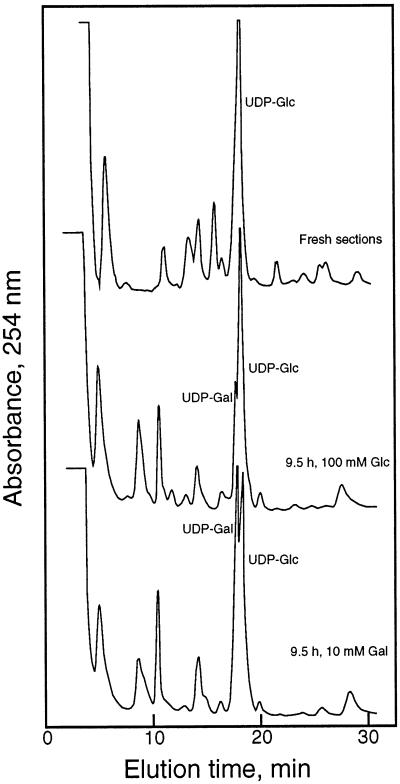
After incubation for 9.5 h without sugar, the levels of all metabolites were diluted, but the UDP-Glc concentrations were substantially lowered in sections incubated without sugar or in the presence of growth-inhibiting Gal. UDP-Glc was the major UDP-sugar in the presence or absence of Glc, representing 85% of the fraction, whereas incubation with Gal resulted in UDP-Gal concentrations representing 60% of the total UDP-sugar (Fig. 4). Relative Glc and Suc concentrations also decreased during incubations without sugar or in the presence of Gal but to a lesser extent than the marked decreases in nucleotide sugar. The relative concentrations of Glc and Suc could be maintained to a certain extent by incubation of the coleoptile sections with 100 mm Glc, but UDP-Glc levels fell to about one-third that of the freshly isolated coleoptiles.
Figure 4.
Separation of nucleotide sugars by HPLC. Nucleotide sugars were extracted with ice-cold 10% (w/v) TCA, and the TCA was partitioned into a mixture of trioctylamine in 1,1,2-trichlorofluoroethane. The neutralized aqueous extract was passed through a 0.22-μm membrane filter and injected onto a column equilibrated in 20 mm ammonium formate, pH 6.0. The nucleotides and nucleotide sugars eluted in a nonlinear gradient to 1 m ammonium formate, pH 6.0, and were detected and quantified from UV absorbance based on standards. Profiles are typical of three experiments.
After incubation in radiolabeled sugars, the coleoptile walls were separated into pectic and several alkali-soluble fractions. Most of the label was incorporated into polysaccharides of the alkali-soluble fractions, and the β-glucans were found primarily in the 0.8 and 4 n NaOH fractions (Fig. 5). The ratios of the cellotriosyl to cellotetraosyl units in these two fractions were similar in the individual samples depending on the incubation conditions (Table II). When the 4 n NaOH fractions were separated by gel permeation chromatography, the molecular mass of β-glucan and the distribution of radioactivity in the newly synthesized glucan were each centered at about 250 kD regardless of the incubation conditions (as in Fig. 3). The ratio of newly synthesized cellotriosyl to cellotetraosyl units in each of three fractions, representing the larger third to the smaller third of the included polymers and the total included volume, were similar to that expected in the total β-glucan digested from the fraction, although slightly lower ratios were obtained with the smallest of polysaccharides (Table III). Based on pulsed amperometric detection, the molar ratios of cellotriosyl to cellotetraosyl units in β-glucan mass digested from the coleoptile was about 2.9 in most fractions from gel permeation chromatography.
Figure 5.
Relative amounts of β-glucan in the alkali extracts of depectinated maize coleoptile walls determined by HPAE-HPLC of the cellodextrin-(1→3)-d-Glc oligosaccharides. The β-glucan oligomers were separated and quantified as described in Figure 1B. PAD, Pulsed amperometric detection.
Table II.
Ratio of cellodextrin oligomeric products synthesized in maize coleoptile cell wall β-glucans synthesized in vivo from [14C]Glc
| Treatment | 3-mer:4-mer | 3-mer:5-mer | 5-mer:4-mer |
|---|---|---|---|
| Freshly excised | |||
| 0.8 n NaOH | 3.0 | 19.2 | 0.16 |
| 4 n NaOH | 2.9 | 22.7 | 0.13 |
| 9.5 h, no sugar | |||
| 0.8 n NaOH | 3.0 | 18.5 | 0.16 |
| 4 n NaOH | 2.9 | 19.8 | 0.15 |
| 9.5 h, 100 mm Glc | |||
| 0.8 n NaOH | 2.8 | 18.2 | 0.15 |
| 4 n NaOH | 2.8 | 19.0 | 0.15 |
| 9.5 h, 10 mm Gal | |||
| 0.8 n NaOH | 2.6 | 17.2 | 0.15 |
| 4 n NaOH | 2.7 | 21.0 | 0.13 |
| Maize β-glucan | 2.9 ± 0.1 | 19.0 ± 1.1 | 0.14 ± 0.003 |
β-Glucan was digested with the B. subtilis enzyme, and the oligomeric products were separated by HPAE-HPLC and quantified by liquid scintillation spectroscopy. Treatment of coleoptile sections was as described in Table I. Ratios of treated samples are based on radioactivity incorporated after adjustment for molar ratio; ratio for maize β-glucan is made by pulsed amperometry, with empirical calibration of the signal from sugar determinations (Dubois et al., 1956).
Table III.
Molar ratio of the cellotriosyl and cellotetraosyl units based on incorporation of radioactivity from [14C]Glc into several size fractions of β-glucans synthesized by excised coleoptile sections
| Peak Fraction | Treatment
|
|||
|---|---|---|---|---|
| 0 Time | 9.5 h, Control | 9.5 h, Glc | 9.5 h, Gal | |
| 3-mer:4-mer | ||||
| I | 3.3 | 2.9 | 3.2 | 3.0 |
| II | 3.1 | 2.9 | 2.9 | 2.7 |
| III | 3.1 | 2.6 | 2.8 | 2.8 |
| Included | 2.9 | 2.4 | 2.5 | 2.5 |
Immunolocalization of SuSy in Maize Coleoptiles versus Soybean Hypocotyls
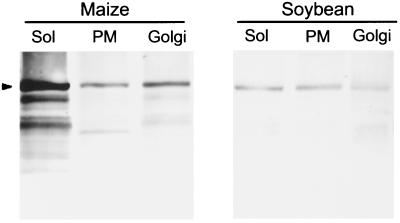
The upper phases from two-phase aqueous partitioning of both soybean and maize membranes were enriched in plasma membrane, as judged by vanadate-sensitive ATPase (Table IV). The Golgi-enriched fractions from both soybean and maize had IDPase activities nearly an order of magnitude greater than the plasma membrane fraction. Although the Golgi fraction contained considerable vanadate-sensitive ATPase activity, the activity represented about 70% of the total phosphatase activity of the plasma membrane-enriched fractions but only 20% to 40% of the Golgi-enriched fractions (Table IV). SuSy was immunodetected in soluble and membrane fractions, and when quantified by densitometry, the maize Golgi contained more than the plasma membrane fraction, whereas that quantified in soybean membranes was proportional to the amount of contaminating vanadate-sensitive ATPase (Fig. 6; Table IV).
Table IV.
Comparison of marker assays of membrane purity with quantitation of intensity of the immunogel blot signal with SuSy antisera
| Membrane Source | Vanadate-Sensitive ATPase | IDPase | SuSy Intensity |
|---|---|---|---|
| nmol Pi min−1 μg−1 | arbitrary units | ||
| Soybean | |||
| Plasma membrane | 126.7 | 181.5 | 17.2 |
| Golgi membranes | 37.7 | 1763.1 | 4.6 |
| Soluble protein | NDa | ND | 15.5 |
| Maize | |||
| Plasma membrane | 96.9 | 114.4 | 29.7 |
| Golgi membranes | 37.8 | 1091.9 | 38.4 |
| Soluble protein | ND | ND | 67.1 |
The soluble protein of the homogenate and the Golgi membranes were prepared from flotation centrifugation as adapted from Gibeaut and Carpita (1994), whereas the plasma membrane was obtained by two-phase aqueous partitioning of total membranes. The vanadate-sensitive ATPase for soybean membranes represented 70% and 23% of the total phosphatase activity of the plasma membranes and Golgi membranes, respectively. This activity for maize membranes represented 66% and 41% of the total phosphatase activity of the plasma membranes and Golgi membranes, respectively. The blot intensities of the SuSy bands revealed by Western analysis (Fig. 6) were digitally scanned and quantified after subtraction of background. Values are typical of four independent experiments.
ND, Not determined.
Figure 6.
Immunodetection of SuSy in soluble (Sol), and plasma membrane (PM)- and Golgi membrane-enriched fractions of elongating maize coleoptiles and soybean hypocotyls. The Golgi membranes and soluble fraction were obtained after flotation centrifugation as described by Gibeaut and Carpita (1994), whereas the plasma membrane was obtained by two-phase aqueous partitioning. Ten micrograms of protein per lane was separated by SDS-PAGE in 10% (w/v) gels, and electroblotted onto cellulose nitrate by semidry blotting. SuSy was detected with polyclonal antisera at a 1:500 dilution for maize proteins and a 1:200 dilution for soybean proteins. Values are typical of four determinations.
DISCUSSION
The Synthesis of β-Glucans in Vitro
Two distinct rates of β-glucan synthesis are observed in vitro with Golgi membranes: one that appears to saturate at about 10 mm UDP-Glc, and a second that increases markedly above 20 mm substrate (Fig. 2A). The increases in the ratios of the cellotriosyl and cellopentaosyl units to cellotetraosyl units parallel these two activities (Fig. 2B). Suboptimal UDP-Glc concentrations favor the synthesis of the longer cellodextrin units in β-glucan, particularly the cellotetraosyl unit, whereas the proportion of cellotriose increases specificially as the UDP-Glc concentration is raised.
At the highest UDP-Glc concentrations tested, the cellotriose comprised nearly 80% of the total polymer synthesized. The increase in the proportion of cellotriose occurs relative to all other cellodextrins at low substrate concentrations, but the proportion of cellopentaosyl units also increases concomitantly at high substrate concentrations. While the cellotetraosyl units exceed the cellopentosyl units by about 7:1 at concentrations of UDP-Glc below 1 mm, the cellopentaosyl units exceed the cellotetraose units by 2:1 when the substrate concentration is 30 mm (Fig. 2B). The distribution of cellodextrin units in β-glucan synthesized in vitro at 30 mm UDP-Glc becomes similar to that of the β-glucan from the lichen Cetraria islandica, in which the cellotriose unit is 86% of the polysaccharide, and the cellopentaose is second in abundance—twice that of cellotetraose (Wood et al., 1994).
These data indicate that the mechanism of synthesis occurs in such a way as to favor the formation of cellotriosyl and odd-numbered cellodextrin units at saturating substrate concentrations. Thus, the synthesis of β-glucan resembles that of cellulose synthesis, but cellotriosyl units are linked by single (1→3)β-linkages instead of two cellobiosyl units linked by a (1→4)β-linkage. Regardless of the UDP-Glc-dependent variation in the ratio of cellodextrins, the single (1→3)β-linkages between them is a constant, and a biochemical mechanism of synthesis to explain our data must also account for these two features.
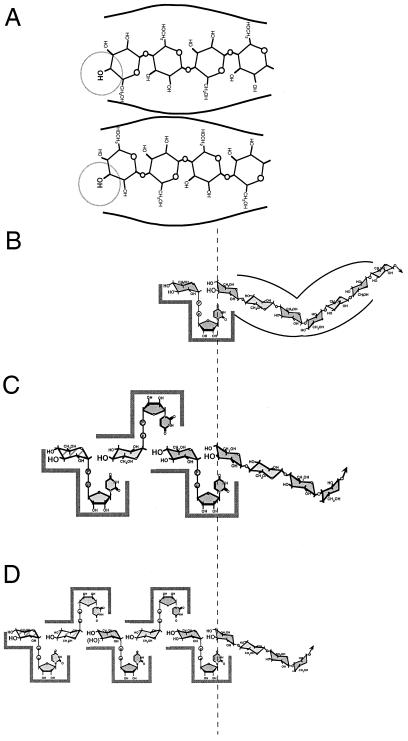
The orientation of the two Glc units in a (1→3)β- or (1→4)β-linkage are so different that it may seem difficult to imagine how both can be made interchangeably in a single synthase. However, Glc units in a linear (1→4)β-linked chain are slightly angled such that the O-4 or O-3 hydroxyl of the nonreducing terminal sugar will fill an acceptor position merely by inverting the chain (Fig. 7A). If the positioning of the terminal sugar is controlled at the chain-holding portion of the synthase, then the introduction of (1→4)β- or (1→3)β-linkages can be strictly regulated. For cellulose synthase, we and others have proposed that cellobiosyl units are added to the nonreducing end of a growing chain in which the O-4 hydroxyl is in the acceptor position (Carpita et al., 1996; Koyama et al., 1997; Carpita and Vergara, 1998). When cellobiose units are added, the chain is never reoriented but instead glides through the chain-holding portion of the synthase until the new nonreducing terminal O-4 hydroxyl is positioned for the next round of synthesis. This model of synthesis also provides a simple explanation for how a callose synthesis can “default” by damage to the cellobiose generating synthase. If the second glucosyl transferase fails to function, then only a single glucosyl unit will glide into the nonreducing end and the O-3 hydroxyl will be positioned in the acceptor position of the synthase (Carpita et al., 1996). Because the (1→3)β-linkage does not invert one sugar with respect to its neighboring sugars, every nonreducing sugar afterward will have its O-3 hydroxyl in the acceptor position and callose will be the only product.
Figure 7.
A, Two possible positions of the nonreducing terminal acceptor glucosyl unit of a growing chain can be made by rotating the chain over or by processively adding a single glucosyl unit from a fixed UDP-Glc site. Both (1→4)β- and (1→3)β-linkages are possible, so positioning of the nonreducing terminal sugar to expose either the O-3 or O-4 must be tightly controlled in glucans with a defined linkage structure. B, A single fixed site of glucosyl transfer requires the growing chain to be “read” by the enzyme to determine when the chain is rotated to direct linkage structure at the O-3 or O-4 position, as defined in A. C, Three glucosyl transferases working processively would always link a cellotriosyl unit to the O-3 of the nonreducing acceptor sugar. The third site is of lower affinity than the cellobiosyl generating glucosyl transferases, and failure to fill it produces the longer cellodextrins. Formation of cellotriosyl units should form almost exclusively at saturating UDP-Glc concentrations. D, Five glucosyl transferases work processively to form mostly cellotriosyl units, but occasionally cellotetraosyl and cellopentaosyl units, because the fourth and fifth sites are of lower affinity for substrate binding. Longer cellodextrins would be favored at saturating UDP-Glc concentrations. In B through D, the arrows indicate the reducing end and continuation of the synthesized polymer.
How does the mechanism of synthesis of β-glucan parallel that of cellulose synthesis? We formulated three possible mechanisms of β-glucan synthesis. In all models, the synthase is considered to have two domains: one or more active sites for substrate binding and a single chain-holding domain that positions the nonreducing terminal sugar to form the proper glycosidic linkage. To synthesize (1→4)β-d-glucosyl linkages with a single UDP-Glc site, either the substrate-binding domain or the chain-holding domain must rotate or toggle with respect to each other with each round of glycosyl transfer. Alternatively, the glucosyl unit is guided into the proper orientation by a steric mechanism within the chain-holding domain (Fig. 7B). However, to synthesize a mixed-linkage β-glucan, the chain-holding domain must also direct the proper ratio of cellotriosyl and cellotetraosyl units and the placement of the (1→3)-β-d-glucosyl units. Thus, the chain-holding domain functions as a “chain-reading” domain.
Such a model of synthesis must be independent of UDP-Glc concentration because the information needed to direct the synthesis of the proper ratios of cellodextrin units and their proper spacing by (1→3)β-linkages must come from polymer already synthesized (Fig. 7B). Although our data seem to rule out this model, variations of the single-site model excluding the chain-reading function are possible. Because there is considerable freedom of rotation of the nonreducing terminal glucosyl unit around the glycosidic bond, one could argue that there is a statistical probability of forming a (1→3)β-linkage that is dependent on substrate concentration. However, the strict unit structure, the lack of cellobiosyl units, and the lack of contiguously linked (1→3)β-units strongly argue against any model of random introduction of (1→4)β- and (1→3)β-linkages.
In our second model of synthesis, several glucosyl transferase activities are associated with the synthase; the chain-holding domain positions the nonreducing end of the polymer but exhibits no chain-reading activity. Three UDP-Glc transferase activities would result in cellotriose units and positioning of the nonreducing end of the chain in a conformation that always places the O-3 hydroxyl in the acceptor position (Fig. 7C). In such a model, the higher cellodextrins would be made by occasional failure to fill the third site of substrate binding, resulting in the formation of a cellobiose unit instead of cellotriose. In some instances, the cellobiosyl unit slides through the chain-holding domain and a subsequent complete cellotriosyl unit formation in the next round would result instead in the formation of a cellopentaosyl unit. However, the formation of one cellobiosyl unit must greatly favor the formation of a second cellobiosyl unit to form the more abundant cellotetraosyl unit (Fig. 7C). As the third site is occasionally unfilled, its affinity for UDP-Glc must be lower than that of the cellobiosyl generating sites. In contrast to the single-site model, the ratio of cellodextrin units is dependent on the substrate concentration and not on chain reading. Suboptimal UDP-Glc should result in decreased proportions of cellotriose because of more frequent failure to fill the third site. Our data support this three-site model of glycosyl transfer.
A third model for β-glucan synthesis places additional substrate binding sites and transferases to account for the longer cellodextraoyl units (Fig. 7D). These sites would possess affinities for UDP-Glc much lower than the first three sites to account for the smaller proportions of the higher cellodextrin units. In contrast to the three-site model, suboptimal UDP-Glc should result in increased proportions of cellotriose because of more frequent failure to fill the fourth and fifth sites. Our results show that the opposite occurs.
A three-site model of substrate attachment is attractive because a β-glucan can be made in vitro almost exclusively with cellotriose units, which are the most abundant units in cereal β-glucans (Fig. 1B and 2B). The Poales are the only taxonomic order among angiosperms to make these mixed-linkage β-glucans (Smith and Harris, 1999), but the lichen Cetraria islandica also synthesizes a β-glucan that is almost exclusively cellotriose units (Wood et al., 1994). Like the maize β-glucan made in vitro at very high substrate concentrations, the lichen β-glucan contains cellopentaosyl units in greater abundance than the cellotetraosyl units.
The mechanism also includes an apparently absolute requirement for the spacing of the units with a single (1→3)β-linkage. β-Glucans with alternating (1→3)β- and (1→4)β-linked glucosyl units are not observed under any conditions. Both of these features demand a cellodextrin unit spacing mechanism, which is provided by the three-site model. This model also predicts that the cellopentaosyl units will also be favored over cellotetraosyl units at near-saturating UDP-Glc concentrations, and this is exactly what we observed (Figs. 1B and 2B).
The three-site model also requires that the formation of the (1→3)β-linkage be restricted to the attachment of the cellodextrin unit to the nonreducing end of the growing chain. If the (1→3)β-linkage were specified at the outer site, then failure to fill the sites would result in a minimum of a cellopentaosyl unit and no possibility to form a cellotetraosyl unit. If the (1→3)β-linkage were specified at the middle site, then failure to fill the outer site would result in a cellobiosyl unit, and these units are never observed under any conditions. In all multiple-site models of synthesis for cellulose and β-glucan, loss of all but one of the glycosyl transferase activities would result in the nonreducing end of the chain always being oriented with the O-3 in the acceptor position, which would form callose (Carpita et al., 1996).
Although a “three-site” mechanism of glucosyl transfer is indicated by our data, we certainly have not defined how the active site and the transferase activities are organized. The discovery of a higher plant cellulose synthase gene (CelA) by Pear et al. (1996) has made it possible to compare the deduced amino acid structure of the active site with rational models of catalysis. First, the CelA gene family is extensive and consists of several subclasses (Cutler and Somerville, 1997). A “true” CelA gene encodes a large polypeptide with the presumed active site comprising a large cytosolic domain with four highly conserved domains. Each of the first three contain an essential aspartyl residue and the fourth possesses a QxxRW motif. In addition, there are two plant-specific sequences, one of which exhibits extensive amino acid variability (Pear et al., 1996).
This entirely cytosolic domain is sandwiched between two membrane-spanning domains on the N-terminal side, and four to six membrane-spanning domains on the C-terminal side, which may fold together to form a channel to facilitate extrusion of the glycan chain. The four U motifs of the CelA gene, i.e. the three aspartyl residues and the QxxRW motif, are iterated in the genes encoding several processive glycosyl transferases in which repeating β-glycosyl unit structures are synthesized (Saxena et al., 1995). For example, the U motifs are found in hyaluronan synthases (DeAngelis et al., 1993), chitin synthases (Bulawa et al., 1986), and the NodC synthase (Geremia et al., 1994), all of which make consecutive β-glycosidic linkages in which one sugar is oriented nearly 180° with respect to each neighboring sugar. Hyaluronan is a repeating disaccharide unit of β-d-GlcUA and GlcNAc, whereas chitin and the Nod factor are composed of (1→4)β-d-GlcNAc units. Hyaluronate synthase is a bifunctional transferase, with both glycosyl transferase activities contained within the single polypeptide (Spicer et al., 1997); it remains to be demonstrated if the active site consists of two distinct glycosyl transferases or if a single transferase toggles between two conformation states to produce the two distinct kinds of glycosyl units. By analogy, the β-glucan synthase could have a single glucosyl transferase that toggles between three conformational modes to produce the cellotriosyl units at saturating substrate levels.
Other nonprocessive glycosyl transferases contain the first two U motifs but lack the QxxRW motif. One example of this kind of enzyme is the Escherichia coli K5 glycosyl transferase, which makes the polysaccharide N-acetylheparosan with the repeating disaccharide unit (1→4)α-d-GlcA-(1→4)β-d-GlcNAc. Using site-directed mutagenesis, Griffiths et al. (1998) showed that the aspartyl residues of the first two U motifs were essential for the β-glycosyl transfer but independent from the UDP-GlcNAc α-transferase activity. Experiments with truncated polypeptides showed that the α-transferase activity is within the first 300 residues of the N terminus and upstream from the U-1 motif.
This raises the possibility that processive β-glycosyl transferases with the D, D, D, and QxxRW motifs may accommodate as many as three glycosyl transferase activities, as was predicted by our kinetic data for the β-glucan synthase. However, one important exception is the Agrobacterium tumefaciens (1→3)β-glucan synthase gene. This synthase contains the four U motifs, even though the Glc units are not inverted during synthesis (Stasinopoulos et al., 1999). Such a gene may have derived from an ancestral cellulose synthase, but the encoded synthase may have lost the function of one of the two glycosyl transfer activities. The hypothesis that cellulose synthase and callose synthase are one and the same enzyme (Delmer, 1977) and our hypothesis explaining mechanistically how callose can be formed when one of the two glycosyl transferase activities is lost (Carpita et al., 1996) are both consistent with the finding that cellulose and callose synthases are from related genes.
The diversity of polysaccharide structures encoded by the four U motif structures and the size of the CelA gene family suggest that in plants some members of the CelA gene family synthesize polysaccharides other than cellulose. Virtually all cross-linking glycans of plants possess linear chain structures of β-glycosyl units with one sugar inverted almost 180° with respect to each neighbor. These include xyloglucans, glucomannans, arabinoxylans, and the mixed-linked β-glucan. The enormity of the CelA gene family suggests that some of the members are involved in synthesis of these noncellulose β-glycans, but identification of mutants lacking these genes or experiments to define the biochemical functions of individual CelA genes are just now being undertaken.
The Synthesis of β-Glucans in Vivo
While β-glucan synthase can produce a wide range of distributions of cellodextrin units in vitro, the distribution of the units in vivo is tightly regulated within a specific grass species. Treatments that induce a large decrease in UDP-Glc concentration result in small alterations in the ratio of cellotriose units to cellotetraose units. This finding indicates that the pool of UDP-Glc directed to β-glucan synthase is distinct from that of the bulk cytosolic pool. In affinity labeling experiments with [32P]UDP-Glc, an 84-kD polypeptide was found to be associated with the plasma membrane containing the highest activity of callose synthase (Delmer et al., 1991). This polypeptide turned out to be SuSy.
Subsequent confirmation of membrane association was made immunocytochemically (Amor et al., 1995). Delmer and Amor (1995) proposed that the association of SuSy represented a UDP-Glc delivery mechanism to cellulose synthase. β-Glucan microfibrils were synthesized from Suc and UDP on immobilized tobacco plasma membrane sheets (Hirai et al., 1998). These results strengthen the idea of an association of SuSy directly with plasma membrane synthases. Furthermore, the products were callose, not cellulose, additional evidence that callose synthase is a default activity from cellulose synthesis.
Conditions that greatly lowered UDP-Glc concentration in maize coleoptiles lowered Suc and Glc concentrations to a much lesser extent (Table I). Regardless, concentrations of these sugars were 3 orders of magnitude higher than that of UDP-Glc. In maize, SuSy was detected immunocytochemically in both plasma membrane-enriched and Golgi membrane-enriched fractions, whereas in soybean, the detection at the Golgi membranes was more equivocal (Fig. 6; Table IV). Xyloglucan synthase, the major synthase in dicots such as soybean, does not require SuSy for substrate delivery because the active site of the synthase is fully contained within the lumen of the Golgi apparatus and is dependent on a UDP-Glc translocator for its nucleotide-sugar substrates (Muñoz et al., 1996).
Our results are consistent with the hypothesis that β-glucan synthase is derived from an ancestral cellulose synthase and therefore shares features of the mechanism of synthesis. Part of this mechanism may include the association of SuSy to control the supply of UDP-Glc to the active site(s) of glucan synthase. Although we could detect SuSy at the Golgi membranes, it appears not to function in vitro because β-glucan synthesis was absolutely dependent on UDP-Glc. In preliminary experiments, we were unable to observe incorporation of label from [U-14C]Suc into β-glucan in vitro in the presence or absence of UDP. We need to explore ways to preserve the SuSy:β-glucan synthase interaction in vitro before we can begin to understand how the substrate delivery controls precise ratio determination of the cellodextrin oligomer units in the native β-glucan structure.
ACKNOWLEDGMENTS
We thank Anna Olek for technical assistance, and we are grateful to Larry Dunkle, Maureen McCann, and Charles Woloshuk for helpful discussions and critical review of the manuscript.
Abbreviations:
- HPAE-HPLC
high-pH anion-exchange HPLC
- SuSy
Suc synthase
Footnotes
This work was supported by contract DE–FG02–88ER13903 from the U.S. Department of Energy, Energy Biosciences (to N.C.C.) and a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (to M.S.B.). This is journal paper 16,002 of the Purdue Agricultural Experiment Station.
LITERATURE CITED
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Stone BA. A new substrate for investigating the specificity of β-glucan hydrolases. FEBS Lett. 1975;52:202–207. doi: 10.1016/0014-5793(75)80806-4. [DOI] [PubMed] [Google Scholar]
- Becker M, Vincent C, Reid JSG. Biosynthesis of (1,3)(1,4)-β-glucan and (1,3)-β-glucan in barley (Hordeum vulgare L.): properties of the membrane-bound glucan synthases. Planta. 1995;195:331–338. doi: 10.1007/BF00202589. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Stone BA, Olsen O-A. Cell wall (1→3)- and (1→3,1→4)-β-glucans during early grain development in rice (Oryza sativa L.) Planta. 1997;202:414–426. doi: 10.1007/s004250050145. [DOI] [PubMed] [Google Scholar]
- Bulawa CE, Slater M, Cabib E, Au-Young J, Sburlati A, Adair WL, Jr, Robbins PW. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell. 1986;46:213–225. doi: 10.1016/0092-8674(86)90738-5. [DOI] [PubMed] [Google Scholar]
- Carpita N, McCann M, Griffing LR. The plant extracellular matrix: news from the cell's frontier. Plant Cell. 1996;8:1451–1463. doi: 10.1105/tpc.8.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of the primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Kanabus J. Extraction of starch by dimethyl sulfoxide and quantitation by enzymatic assay. Anal Biochem. 1987;161:132–139. doi: 10.1016/0003-2697(87)90662-2. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Kanabus J. Chemical structure of the cell walls of dwarf maize and changes mediated by gibberellin. Plant Physiol. 1988;88:671–678. doi: 10.1104/pp.88.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Vergara CE. A recipe for cellulose. Science. 1998;279:672–673. doi: 10.1126/science.279.5351.672. [DOI] [PubMed] [Google Scholar]
- Cutler S, Somerville C. Cellulose synthesis: cloning in silico. Curr Biol. 1997;7:R108–R111. doi: 10.1016/s0960-9822(06)00050-9. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL, Papaconstantinou J, Weigel PH. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem. 1993;268:19181–19184. [PubMed] [Google Scholar]
- Delmer DP. Biosynthesis of cellulose and other plant cell wall polysaccharides. Recent Adv Phytochem. 1977;11:105–153. [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Solomon M, Read SM. Direct labeling with [32P]UDP-glucose for identification of a subunit of cotton fiber callose synthase. Plant Physiol. 1991;95:556–563. doi: 10.1104/pp.95.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Fincher GB, Stone BA (1986) Cell walls and their components in cereal grain technology. In Y Pomerantz, ed, Advances in Cereal Science and Technology, Vol 8. American Association of Cereal Chemists, St. Paul, MN, pp 207–295
- Geremia RA, Mergaert P, Geelen D, Van Montagu M, Holsters M. The NodC protein of Azorhizobium caulinodans is an N-acetylglucosaminyltransferase. Proc Natl Acad Sci USA. 1994;91:2669–2673. doi: 10.1073/pnas.91.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Carpita NC (l991) Tracing the biosynthesis of the cell wall in intact cells and plants: selective turnover and alteration of cytoplasmic and cell wall polysaccharides of proso millet cells in liquid culture and Zea mays seedlings. Plant Physiol 97: 551–561 [DOI] [PMC free article] [PubMed]
- Gibeaut DM, Carpita NC. Synthesis of (1→3),(1→4)-β-d-glucan in the Golgi apparatus of maize coleoptiles. Proc Natl Acad Sci USA. 1993;90:3850–3854. doi: 10.1073/pnas.90.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Carpita NC. Improved recovery of (1→3),(1→4)-β-d-glucan synthase activity from Golgi apparatus of Zea mays (L.) using differential flotation centrifugation. Protoplasma. 1994;180:92–97. [Google Scholar]
- Gorshkova TA, Chemikosova SB, Lozovaya VV, Carpita NC. Turnover of galactans and other cell wall polysaccharides in developing flax plants. Plant Physiol. 1997;114:723–729. doi: 10.1104/pp.114.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Cook NJ, Gottfridson E, Lind T, Lidholt K, Roberts IS. Characterization of the glycosyltransferase enzyme from the Escherichia coli K5 capsule gene cluster and identification and characterization of the glucuronyl active site. J Biol Chem. 1998;273:11752–11757. doi: 10.1074/jbc.273.19.11752. [DOI] [PubMed] [Google Scholar]
- Henry RJ, Stone BA. Factors influencing β-glucan synthesis by particulate enzymes from suspension-cultured Lolium multiflorum endosperm cells. Plant Physiol. 1982;69:632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai N, Sonobe S, Hayashi T. In situ synthesis of β-glucan microfibrils on tobacco plasma membrane sheets. Proc Natl Acad Sci USA. 1998;95:15102–15106. doi: 10.1073/pnas.95.25.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus J, Bressan RA, Carpita NC. Carbon assimilation in carrot cells in liquid culture. Plant Physiol. 1986;82:363–368. doi: 10.1104/pp.82.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D, Decker K. Uridine-5′-diphosphoglucose. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis, Vol 1. Berlin: Springer-Verlag; 1981. pp. 2225–2228. [Google Scholar]
- Koyama M, Helbert W, Imai T, Sugiyama J, Henrissat B. Parallel-up structure evidences the molecular directionality during biosynthesis of bacterial cellulose. Proc Natl Acad Sci USA. 1997;94:9091–9095. doi: 10.1073/pnas.94.17.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljebjelke K, Adolphson R, Baker K, Doong RL, Mohnen D. Enzymatic synthesis and purification of uridine diphosphate [14C]galacturonic acid: a substrate for pectin biosynthesis. Anal Biochem. 1995;225:296–304. doi: 10.1006/abio.1995.1158. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Ng KF, Johnson E, Hoogenraad NJ, Stone BA. The β-glucan synthase from Lolium multiflorum: detergent solubilization, purification using monoclonal antibodies, and photoaffinity labeling with a novel photoreactive pyrimidine analogue of uridine 5′-diphosphoglucose. J Biol Chem. 1991;266:22569–22581. [PubMed] [Google Scholar]
- Muñoz P, Norambuena L, Orellana A. Evidence for a UDP-glucose transporter in Golgi apparatus-derived vesicles from pea and its possible role in polysaccharide biosynthesis. Plant Physiol. 1996;112:1585–1594. doi: 10.1104/pp.112.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM. Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci USA. 1996;93:12637–12642. doi: 10.1073/pnas.93.22.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena IM, Brown RM, Jr, Fevre M, Geremia RA, Henrissat B. Multidomain architecture of β-glycosyl transferases: implication for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BG, Harris PJ. The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochem Syst Ecol. 1999;27:33–53. [Google Scholar]
- Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997;272:8957–8961. doi: 10.1074/jbc.272.14.8957. [DOI] [PubMed] [Google Scholar]
- Stasinopoulis SJ, Fisher PR, Stone BA, Stanisich VA. Detection of two loci involved in (1→3)-β-glucan (curdlan) biosynthesis by Agrobacterium sp. ATCC31749, and comparative sequence analysis of the putative curdlan synthase gene. Glycobiology. 1999;9:31–41. doi: 10.1093/glycob/9.1.31. [DOI] [PubMed] [Google Scholar]
- Staudte RG, Woodward JR, Fincher GB, Stone BA. Water-soluble (1→3),(1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. III. Distribution of cellotriosyl and cellotetraosyl residues. Carbohydr Polym. 1985;3:299–312. [Google Scholar]
- Wood PJ, Weisz J, Blackwell BA. Structural studies of (1→3),(1→4)-β-d-glucans by 13C-nuclear magnetic resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chem. 1994;71:301–307. [Google Scholar]
- Wood PJ, Weisz J, Mahn W. Molecular characterization of cereal β-glucans. II. Size-exclusion chromatography for comparison of molecular weight. Cereal Chem. 1991;68:530–536. [Google Scholar]