Abstract
Urethral stricture disease is increasingly common occurring in about 1% of males over the age of 55. The stricture tissue is rich in myofibroblasts and multi-nucleated giant cells which are thought to be related to stricture formation and collagen synthesis. An increase in collagen is associated with the loss of the normal vasculature of the normal urethra. The actual incidence differs based on worldwide populations, geography, and income. The stricture aetiology, location, length and patient's age and comorbidity are important in deciding the course of treatment. In this review we aim to summarise the existing knowledge of the aetiology of urethral strictures, review current treatment regimens, and present the challenges of using tissue-engineered buccal mucosa (TEBM) to repair scarring of the urethra. In asking this question we are also mindful that recurrent fibrosis occurs in other tissues—how can we learn from these other pathologies?
Keywords: Urethral strictures, Fibrosis, Tissue-engineered buccal mucosa, Augmentation urethroplasty
1. Introduction
Urethral strictures are an abnormal narrowing of the urethra. The origins of this fibrosis may be due to intrinsic conditions but commonly occur in response to damage or infection [1]. They represent a scarring of the vascular corpus spongiosum leading to fibrosis [2]. There are varying degrees of spongiofibrosis but obstruction of the urethra can cause infection, bladder calculi, fistulas, sepsis, and renal failure.
Overall the incidence of urethral strictures is about 1% in the males over the age of 55. The actual incidence differs based on worldwide populations, geography, and income [1], [3].
Management of strictures varies from less invasive techniques such as urethral dilatation and urethrotomy, to more invasive procedures such as anastomotic and substitution urethroplasty [4]. In patients who have extensive disease, obtaining sufficient graft can be challenging. For this reason, tissue-engineered buccal mucosa (TEBM) for the treatment of complex strictures was developed [5], [6].
2. Urethral stricture and histopathology
The normal urethra is lined by pseudo stratified columnar epithelium anchored to a basement membrane beneath which there is connective tissue composed of fibroblasts in an extracellular matrix composed of collagen, proteoglycans, elastic fibres and glycoproteins. Under this is the spongiosum composed of vascular sinusoids and smooth muscle. The pathological changes associated with strictures show that the normal epithelium becomes replaced with squamous metaplasia [7]. All strictures involve some injury to the epithelium of the urethra or corpus spongiosum and fibrosis occurs during the subsequent healing process.
This stricture tissue is rich in myofibroblasts and multi-nucleated giant cells which are thought to be related to stricture formation and collagen synthesis, respectively. An increase in collagen is associated with the loss of the normal vasculature of the normal urethra. Singh and Blandy [8] reported an experimental study in the rat to determine the role of extravasation of urine in the pathogenesis of urethral stricture. They observed that the ultrastructure of urethral stricture tissue suggested that some strictures were fibrous while others were more resilient, and the total amount of collagen increased in urethral strictures, resulting in dense fibrotic tissue with decreased smooth muscle tissue and decreased elasticity. In contrast, Baskin et al. [9] could not demonstrate an increase in the total amount of collagen in strictures compared with the normal urethra, but rather found that an alteration in the ratio of collagen type may explain the fibrotic, non-compliant nature of urethral stricture scar tissue. They found that the normal urethral spongiosum was composed of 75% type I collagen and 25% type III collagen. In contrast, the type III collagen in urethral stricture tissue was increased to 84% with a corresponding decrease in type I collagen (to 16%). These changes were accompanied by a decrease in the ratio of smooth muscle to collagen, as well as changes in the synthesis of nitric oxide in the urethral stricture tissue [10]. Glycosaminoglycans (GAGs) and collagens are major components of the extracellular matrix and they have key roles in fibrotic diseases. Da-Silva et al. [11] measured the GAG composition in the strictured urethral segment. They concluded that composition changes in GAGs could contribute to the non-compliant nature of urethral scar tissue and cause functional changes. Anterior urethral strictures normally occur after trauma or inflammation, and result in spongiofibrosis. Posterior urethral strictures generally result from iatrogenic injury or occur after pelvic fractures. These injures are contractures or stenosis of the urethra rather than true strictures.
2.1. Aetiology of urethral strictures
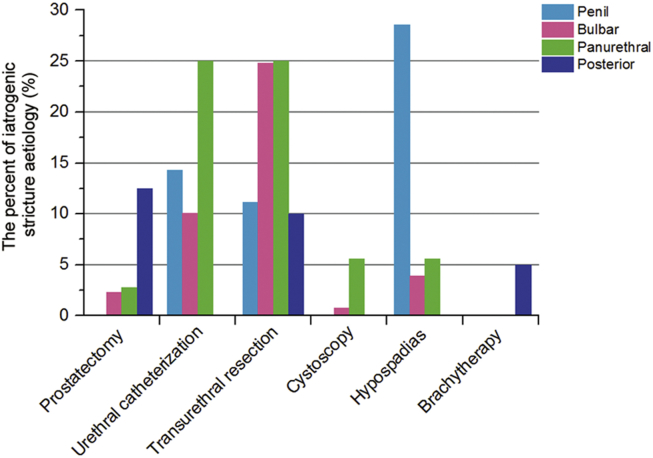
Urethral stricture disease can occur due to several different aetiologies (Table 1) [12]. Strictures can be due to iatrogenic, idiopathic, inflammatory or traumatic causes. The largest category is actually iatrogenic resulting from urethral manipulations, related to placing of indwelling catheters, transurethral manipulation, surgery for hypospadias, prostatectomy, and brachytherapy [13], [14] (Fig. 1). Strictures can also occur due to trauma associated with pelvic fractures and in approximately 60% of patients the function of the distal sphincter mechanism and hence continence depends on the integrity of the bladder neck. Moving on to infection, untreated gonorrhoea and chlamydia causing urethritis can lead to strictures. Another inflammatory disease associated with urethral stricture is balanitis xerotica obliterans. This is a chronic inflammatory disease whose aetiology is still unknown [15].
Table 1.
Stricture aetiology by location [12].
| Penile, % | Bulbar, % | |
|---|---|---|
| Iatrogenic | 40 | 35 |
| Idiopathic | 15 | 40 |
| Inflammatory | 40 | 10 |
| Traumatic | 5 | 15 |
Figure 1.
Iatrogenic stricture aetiology (%) by location [13].
3. Clinical evaluation
The first important step in the evaluation of a patient and the decision about treatment is to obtain a thorough history to get as much information as possible about the aetiology behind the urethral stricture. This requires documenting the onset and severity of obstructive and storage-related voiding symptoms. In addition to this history, uroflowmetry is widely used in the assessment of the urethral stricture. Retrograde urethrography is used to provide information on stricture location and length. Moreover, retrograde and antegrade cystourethrographies are recommended to assess posterior urethral strictures and bladder neck function [16]. Cystoscopy can show the location and degree of the stricture, but if the stricture cannot be passed, no information can be obtained. Another diagnostic procedure is ultrasonography which can be helpful in the assessment of the stricture length and the degree of spongiofibrosis [17].
4. Management
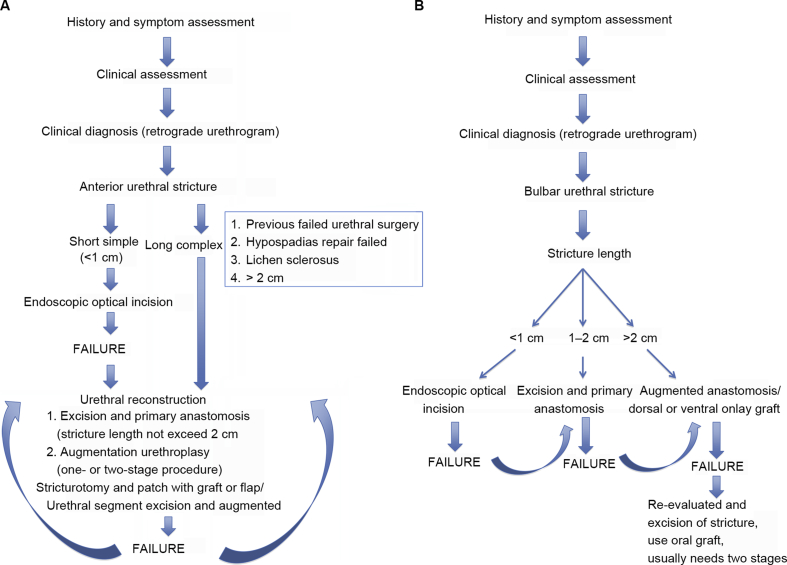
Treatment of urethral strictures depends on stricture aetiology, localisation (anterior or posterior), the length of stricture, the degree of spongiofibrosis, the previous history of treatment, and the patient's age. Theoretically, short simple strictures are treated endoscopically, however long complex strictures often require one- or two-stage urethroplasty (Fig. 2).
Figure 2.
Algorithm of anterior urethral stricture treatment (A) and bulbar urethral stricture treatment (B) [4].
4.1. Urethral dilation and internal urethrotomy
There are several methods for urethral dilatation. These encompass sequential dilatation with metal sounds though filiform dilations, followers, dilation with a balloon, and self-dilation with catheters. The success of these all depends on the regeneration of epithelium without it leading to further restenosis. For patients with an epithelial stricture without spongiofibrosis, dilatation can be curative and overall there is no difference in recurrence rates following internal urethrotomy versus urethral dilation [18], [19].
Internal urethrotomy [19] is performed by a cold-knife transurethral incision to release stricture tissue. The goal is to provide a minimally invasive treatment that achieves a patent urethra to allow unobstructed voiding with minimal side effects. For the urethra to remain patent, reepithelialization must occur at a faster rate than wound contracture [20]. For patients who are carefully selected with optimal stricture characteristics, primary bulbar strictures of <1 cm which are soft, then a stricture-free rate of up to 50%–70% can be achieved. Thus, urethrotomy remains the first-line therapy for these selected patients [21], [22]. Steenkamp et al. [19] reported long-term success rates of only 20%. Patients with longer (>2 cm in length), multiple, penile or distal strictures and extensive periurethral spongiofibrosis typically do not respond well to repeat incisions. Thus repeat internal urethrotomy offers no real chance of a cure after a third incision or if the stricture recurs within 3 months of the first incision. Such patients should be offered urethroplasty [23], [24].
Some studies have evaluated the efficacy of agents injected into the scar tissue at the site of stricture area as an internal urethrotomy procedure to decrease recurrence rates. For example mitomycin C was used for anterior urethral stricture and bladder neck contractions. Authors found that after 15 months mean follow-up urethral stricture recurred in 10% urethral stricture patients in the mitomycin C treated group and in 50% of patients in the untreated group [25], [26]. Another study evaluated the use of triamcinolone injection and showed a significant decrease in recurrence rate [27], [28]. Incision/dilation followed by long-term self- or office dilation is an alternative option for men with severe comorbidities or limited life expectancy, or for those who have failed prior reconstruction with no further available surgical options.
Internal urethrotomy can have complications such as urethral hemorrhage, perineal hemorrhage and extravasation of irrigation into perispongiosal tissues, scrotal oedema, creation of a false passage, rectal perforation, epididymo-orchitis, meatal stenosis, incontinence, fever, bacteremia, urinary sepsis, and scrotal abscess. With deep incisions at the 10 o'clock and 2 o'clock positions, there is also a risk of entering the corpus cavernous and creating fistulas between corpus spongiosum and cavernous, leading to erectile dysfunction [28], [29], [30], [31]. Overall, however, the incidence of these complications is around 2%–10% but in addition there can be recurrence of the urethral strictures.
While the procedure can be done with cold-knife there have been studies [32], [33], [34] looking at the use of lasers for the treatment of urethral strictures. Many different types of lasers have been used including argon, carbon dioxide, excimer, diode, KTP and Nd:YAG lasers. Unfortunately the bottom line is that the addition of lasers has not improved success rates compared to cold-knife urethrotomy.
5. Urethroplasty
5.1. Excision and primary anastomosis
The lowest re-stricture rate with least complications is achieved by excising the stricture, particularly where this is a short bulbar urethral stricture of <2 cm in length and achieving an anastomosis of the two healthy ends on either side [2], [35]. Success rates are reported to be between 90% and 95% [36], [37], [38], [39].
The length that can be gained depends on the anatomy of the individual patient, as well as the length and elasticity of the distal urethral segment, and more particularly, the size of the penis and urethra. By separating the corpora or freeing the urethra from the corpus cavernous up to the peno-scrotal junction as much as 2–4 cm in length can be gained. Additionally, younger men may have better tissue compliance, increasing the chances of successful primary re-anastomosis for long strictures [38].
This excision and primary anastomosis appears to have a negligible effect on penile shortening or chordae if more than 2 cm of urethra is excised. Another important complication is sexual dysfunction. Erickson et al. [40] found that when patients did report postoperative erectile dysfunction after urethral reconstruction, it tended to be transient, with the vast majority of patients recovering preoperative erectile function within 6 months of surgery. Erectile function may be influenced by patient age, stricture length and location, and the method of reconstruction.
5.2. Augmentation urethroplasty
Augmentation urethroplasty is traditionally used for strictures longer than 2 cm for which an anastomotic urethroplasty is not suitable for surgery. These techniques are recommended to achieve a tension free anastomosis and to avoid chordae. Augmentation urethral reconstruction can be a one-stage or a two-stage procedure.
There are three potential options with a one-stage procedure:
-
1)
An augmented anastomotic procedure; Stricture excision and then restore a roof or floor strip of native urethra augmented with a patch.
-
2)
An onlay augmentation procedure. This is incision of stricture with an onlay patch to the urethral roof or floor strip.
-
3)
A tube augmentation: Excise the stricture and put in a circumferential patch. This procedure is associated with high recurrence rates [41], [42].
A two-stage procedure involves excision of the stricture and the abnormal urethra and reconstruction of a roof strip, which is allowed to heal prior to second-stage tubularisation.
Another approach to urethroplasty is the use of a graft or flap. This was viewed as controversial but it is now clear from a review of the literature that the stricture recurrence rate is 14.5%–15.7% using either a flap or graft [43]. Thus there is no advantage in the use of a flap over a straightforward graft in terms of stricture recurrence. In carrying out an augmentation procedure, one must also consider whether full-thickness tissue or partial-thickness tissue should be used; Partial-thickness tissue has a greater propensity to contract than does full-thickness tissue. This is exactly the same as what is found with graft contraction in split-thickness skin where thinner skin grafts contract to a much greater extent than do thicker skin grafts [44].
A range of materials have been used for grafting including penile skin, scrotal skin, oral mucosa, bladder mucosa, and colonic mucosa. From these oral mucosa grafts have become the most clinically accepted due to their graft short harvest time, a lack of hair, low morbidity, and their high clinical success rates [45], [46], [47], [48]. Oral mucosa is taken as full-thickness and most patients can provide an adequate donor area. Oral mucosa can be harvested from the cheek (buccal mucosa), from the lip (labial mucosa), or from the undersurface of the tongue (lingual mucosa). Labial mucosa can be managed in a similar fashion, but is much thinner and more difficult to handle, and has become associated with greater morbidity. Reported complications of oral mucosal grafts include intraoperative hemorrhage, postoperative infection, pain, swelling, and damage to salivary ducts. In some cases, patients note initial limitation of oral opening, although this is usually transient. Occasionally there can be loss or alteration of sensation within the cheek. Barbagli et al. [49] reported that 98.4% would undergo the surgery again and concluded that harvesting from a single cheek with closure of the donor site was a safe procedure with high patient satisfaction.
There are several different approaches to the augmentation procedure. For onlay augmentation, the option is a ventral, lateral, or dorsal approach. Dorsal and ventral onlay grafts have comparable success rates of 88% at 3 years [50]. There is likely to be less bleeding from an incision in this plane and potentially less interference with blood supply as one extends into the proximal and distal normal urethra. Barbagli et al. [51] described lateral onlay augmentation and success rate remained at 83% over the follow-up period.
Two-stage reconstruction should be considered after hypospadias repair or in the presence of lichen sclerosus (LS) or when there are other concerns about the success of any reconstructive procedure in the penile urethra. Several months elapse while the first-stage reconstruction heals and only when it is adequate for closure is the urethra retubularised. After first-stage urethral reconstruction 10%–39% of patients show contraction because of scarring initial graft unfortunately [52]. For example, nearly 15% of men treated for urethral strictures have a history of failed hypospadias repair [53]. Treatment of adults with recurrent strictures is difficult because of the poor blood supply, urethral shortening from prior surgery, the degree of inflammation and the scarring itself.
In complex strictures due to previously failed hypospadias or LS, these may require second-stage urethroplasty with buccal mucosa. Commonly these patients require large areas of buccal mucosa grafting.
5.3. Graft contraction or failure
While contraction of grafted tissue post urethral surgery is a relatively common postoperative complication which usually requires further surgery, this is not a unique situation. Skin grafts, for example, commonly contract.
The mechanism of skin or oral mucosa contraction is a normal physiological phenomena which reduces the area of the graft. The graft contraction occurs in two-stages. When the graft is first harvested from the donor side, it undergoes an initial reduction in size called primary contraction. This can range from 9% to 22% dependent on the thickness of the graft [54]. The thicker the skin grafts are, the more elastin fibres will be present in the dermis and the greater these contract. Thus full-thickness grafts exhibit the greatest degree of primary contraction and split-thickness grafts with less elastin fibres contract less and pure epidermal grafts fail to contract [54]. When grafts are placed on their recipient wound beds then they undergo secondary contraction. This is the contraction that is of clinical concern. This contraction reduces both the size of the graft and the circumference of the graft at its periphery, with the edges of the graft contracted towards the centre [55]. For secondary graft contraction, split-thickness grafts contract more than full-thickness grafts [56]. It is thought that this is due to the difference in matrix composition between the dermal layers within the grafts [57]. Epidermal hyperplasia and dermal fibrosis are less prominent in full-thickness grafts than split-thickness grafts at 4 weeks after grafting [57].
Certainly it is known that the graft bed and the recipient exert a major influence on the degree of contraction. Grafting onto more mobile tissues results in more contraction. Also age plays a big role. Grafts in paediatric patients contract more frequently and to a greater extent than in adults. This may be related to the growth factor profile of children compared to adults [47], [58], [59]. Looking at the cellular mechanisms of contraction there is considerable evidence that contraction occurs secondary to the differentiation of fibroblasts to form myofibroblasts with expression of alpha-actin filament bundles. These myofibroblasts possess intrinsic contractile properties similar to smooth muscle cells. As the myofibroblasts are adherent both to one another and to the fibronectin-rich wound bed, the entire mass of granulation tissue contract [60], [61], [62]. Keratinocytes are also capable of contracting collagen gels in vitro and their role is increasingly recognised in vivo [63]. Keratinocytes possess strong intercellular adhesions, with cultured confluent sheets of keratinocytes rapidly contracting to 70% of their original area following detachment from tissue culture plastic in vitro [64]. Keratinocytes are also effective at contracting collagen gels when they are seeded on the top of the gel. This mimics the in vivo situation, in which the keratinocytes migrate across the wound surface during reepithelialisation. At relatively low densities of surface-seeded keratinocytes, the contraction is equivalent to that seen with much higher densities of gel-incorporated human dermal fibroblasts [65].
Our group has developed a 3D tissue engineered model of human skin, based on sterilised human dermis. This is seeded with laboratory-expanded human keratinocytes and fibroblasts and cultured at an air–liquid interface [66], [67]. This tissue-engineered skin is based on normal mature human cross-linked collagen. It retains a basement membrane [68], to which keratinocytes attach firmly and form a stratified epithelium, while fibroblasts penetrate and migrate through the dermis. This tissue engineered skin contracts by 25%–40% during 10 days of culture in vitro [69], [70] and by up to 60% over 30 days of culture [67]. It appears that keratinocytes contract the dermis as they differentiate. Increasing keratinocyte differentiation with Vitamin C provokes premature differentiation and hyperkeratosis with a marked increase in keratinocyte-driven contraction of the tissue-engineered skin [70]. This change appears to be mediated by covalent crosslinking of adjacent collagen fibrils. Culture of tissue-engineered skin with β-aminopropionitrile (β-APN), an inhibitor of the lysyl oxidase crosslinking enzyme, leads to a reduction in contraction in vitro [67]. These findings have also been seen in a fibroblast-impregnated collagen gel model where lysyl oxidase-catalysed collagen crosslinking is observed during the contraction process and again this contraction can be inhibited by β-APN [71]. Interestingly this keratinocyte-mediated contraction can be reduced in vitro by suturing the tissue engineered skins to a rigid frame for a number of days [72]. It is known that skin graft contracture is more severe in children than in adults, although the reason for this is not clear. There are changes in transforming growth factor-β (TGF-β) expression with age and a reduction in expression of TGF-β1 and TGF-β2 expression with ageing, accompanied by an increase in TGF-β3 [41]. Up-regulations of TGF-β1 and TGF-β2 have been proposed as a primary mechanism for hypertrophic and keloid scarring [73]. Insulin-like growth factor-1 (IGF-1) plays a major role in wound healing [74]. Synthesis of IGF-1 leads to increased collagen synthesis and deposition [75]. It shares many fibrogenic characteristics with TGF-β1 and is found in elevated levels in hypertrophic scar tissue when compared with patient matched normal skin [76]. Using a tissue engineered model of skin in vitro, IGF-1 was found to have no significant effect on contraction [67]. Similarly addition of exogenous basic fibroblast growth factor (bFGF), tumour necrosis factor-α (TNFα) and prostaglandin-E2 (PGE2) had no effect on contraction [67]. Interestingly, culture with estradiol resulted in a marked increase in contraction, although the reason for this is unclear.
In 2008 there was a randomized comparative study of 30 patients who were treated either with native buccal mucosa or an acellular bladder matrix graft. It was found that the results were related to the number of previous interventions. In patients who had less than two prior operations, the success rate of the bladder matrix graft was eight out of nine cases but for those who had more than two operations, four out of the six patients were unsuccessful. This study showed that the best results were obtained in patients with a healthy urethral bed, no spongiofibrosis and good urethral mucosa [77].
In 2008 we reported the first use of tissue-engineered buccal mucosa for extensive substitution urethroplasty in five patients and here we reported on a 3-year follow-up. We found that initial results were good in all five patients with rapid vascularisation of the grafts and retubularisation of all five patients occurred as though native buccal mucosa had been used. However, after 8 and 9 months respectively, two of the five patients' grafts developed contraction and fibrosis. One graft was completely removed and one was partially removed and replaced by native buccal mucosa. Histology of the two excised grafts showed pronounced epithelial hyperproliferation and fibrosis [5].
A recent 9-year follow-up in 2014 showed no further fibrosis for the four patients who had tissue engineered buccal mucosa still in place. In this study all the patients had same aetiology with significant LS [6].
In 2011 there was a report of the use of tissue engineered autologous urethrografts for patients who needed reconstruction. Another cell-seeded scaffolds were used in a series of five pediatric patients with posterior urethral stricture, a tissue biopsy was taken from each patient, and the muscle and epithelial cells were seeded onto tubularised polyglycolic acid:poly (lactide-co-glycolide acid) scaffolds. Patients then underwent urethroplasy with tissue engineered scaffolds. Median follow-up was 71 months and successful in 4/5 patients, and 1/5 patients required transurethral incision 4 weeks after surgery. The patient was able to void well without further interventions [72]. In 2012 a report was published of six pediatric patients with severe hypospadias who were treated with urothelial cells seeded onto an acellular dermis graft. Follow-up was for a median of 7.25 years. Five out of the six patients had a good cosmetic appearance and outcome. In one patient an internal urethrotomy was performed 1 year after urethroplasy [78].
After these mixed clinical results we decided we needed to undertake further investigation of the mechanism of contraction of TEBM in vitro before going forward clinically.
Our first study of contraction of TEBM showed that they lost a mean of 45.4% of their original surface area over 28 days of culture. Treating TEBM with glutaraldehyde, β-APN, or mechanical restraint during culture all significantly inhibited graft contraction. Glutaraldehyde treatment was most effective during culture reduction graft contraction [79].
Several studies reported long-term success rates of urethroplasty with patient's buccal mucosa graft [41], [42], [43], [45], [46], [47], [48], [49], [50], [51]. A few studies published multivariate analysis of urethroplasy out-comes examining preoperative parameters predictors of recurrence (Table 2). Breyer et al. [80] reported their cohort of 445 patients undergoing urethroplasy with mean 5.8 years follow-up period. They determined an overall recurrence rate of 21%. A history of smoking and prior urethral surgery (internal urethrotomy or urethroplasty) were found to be significant predictors for recurrence. The authors also noted that most of the recurrence occurred within the first 2 years. Another study found the average time to recurrence was 11.7 months, with recurrence occurring between 2 weeks and 77 months and these authors also noted that recurrences generally occurred early within the first 6 months [81]. Multivariate analysis showed that long stricture length (>5 cm), LS, iatrogenic and infection were all associated with recurrence [81]. Warner et al. [82] maintained that second-stage urethroplasties had a higher recurrence rate compared with first-stage urethroplasty for LS cases. More recently, Han et al. [83] examined their cohort of urethroplasty patients and showed results of their multivariate analysis and found prior urethroplasty was a predictor of follow-up but critically more strictures were detected on longer term follow-up after urethroplasty. Authors found a mean time to recurrence of 34 months, with recurrences occurring as late at 87 months. On multivariate analysis, if follow-up exceeded 48 months there was a statistically significant increase in recurrence being detected. These results confirm that late recurrences do occur even beyond 5 years of follow-up.
Table 2.
| Factors | Risk ratio (range) |
|---|---|
| Diseases | |
| Smoking | 1.8 (1.0–3.1) |
| Diabetes mellitus | 2.0 (0.8–4.9) |
| Chronic obstructive pulmonary disease | 1.3 (0.4–4.4) |
| Connective tissue disease | 1.3 (0.3–4.7) |
| Coronary artery disease | 1.0 (0.4–2.5) |
| Stricture aetiology | |
| Trauma | 2.6 (0.98–6.9) |
| Iatrogenic | 3.4 (1.2–10) |
| Infectious | 7.3 (2.3–23.7) |
| Lichen sclerosus | 5.9 (2.1–16.5) |
| Radiation | 3.3 (0.8–14) |
| Location | |
| Anterior | 0.49 (0.2–1.2) |
| Posterior | 0.67 (0.3–1.7) |
| Panurethral | 1.4 (0.38–5) |
| Prior treatment | |
| Urethroplast | 6.9 (2.1–22.6) |
| Urethrotomy | 0.8 (0.2–2.8) |
| Dilation | 0.7 (0.3–1.4) |
| Hypospadias | 1.6 (0.7–3.9) |
| Stricture length >5 cm | 2.3 (1.2–4.5) |
| Age | 0.99 (0.98–1.01) |
Fibrosis is an important pathology which can occur in many other tissues—liver cirrhosis, atherosclerosis, Dupuytren's contracture, and glomerulosclerosis. For these diseases, early pathogenesis of fibrosis is related to inflammation and the final pathways of fibrogenesis are similar and stereotypical. Alcoholic liver cirrhosis is a fibrotic disease developing after toxic damage. In this pathology, circulating autoantibodies against acetaldehyde adducts and lipid peroxidation-derived antigens are detected [84]. T cells infiltrating the liver paranchyma are also the first sign of increased extracellular matrix (ECM), particularly type III collagen. As long as type III collagen exists, fibrosis is still reversible, but the appearance of type I collagen heralds an irreversible stage [85], [86]. Atherosclerosis is characterized by sclerosis of the arterial wall due to pathogenic fibrotic changes [87]. Like other fibrotic disorders, an imbalance between profibrotic and antifibrotic cytokines induces fibroblast proliferation and hyper production of ECM [88]. Dupuytren's contracture is characterized by fibrotic nodules with progressive, contraction of the palmar fascia. The aetiology of this condition is still unclear. Pathological responses to traumatic stress factors may affect the microvasculature, leading to localized ischemia and the generation of oxygen-free radicals providing an inflammatory reaction in this disease. This inflammatory response is followed by the production of profibrotic TGF-β1. For this condition, fibroblast transdifferentiation into myofibroblasts are essential cellular components [89]. Another fibrotic pathology is glomerulosclerosis. Here neutrophils are the first cells recruited to glomerula. Neutrophil degranulation, releasing inflammatory and profibrotic cytokines and macrophages are the chief source of TGF-β1, finally resulting in the end-stage of glomerular fibrosis [90]. Unfortunately despite this knowledge of the molecular and cellular mechanisms of fibrosis in other conditions fibrosis remains very difficult to treat. This research has not yet translated into clinically applicable diagnostic, preventive, and therapeutic measures.
In summary, urethral strictures are common and management of long segment strictures presents a challenging surgical problem primarily because of stricture recurrence. There are clear predictors of which patients will be most affected—those with comorbidities of diabetes mellitus or coronary artery disease, smoking, stricture aetiology (iatrogenic, infectious or LS), long strictures stricture length, and prior urethroplasty. The use of TEBM offers an additional treatment material but it is not exempt from stricture recurrence. However what it does provide is an in vitro model in which to explore approaches to prevent or reduce tissue contraction. Our recent work looking at inhibitors of collagen crosslinking may offer an approach to reducing its severity once detected but this is an area where much more research is needed to couple the clinical information on which patients are most at risk to early detection and eventually treatment. Currently we conclude that it is an area of unmet clinical need where users of tissue engineered materials will need to be cautious and reported on long-term clinical results.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Santucci R.A., Joyce G.F., Wise M. Male urethral stricture disease. J Urol. 2007;177:1667–1674. doi: 10.1016/j.juro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Warwick R.T. Urethral surgery. In: Mundy A.R., editor. Current operative surgery: urology. Balliere Tindall; London: 1988. pp. 160–218. [Google Scholar]
- 3.Anger J.T., Buckley J.C., Santucci R.A., Elliott S.P., Saigal C.S. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology. 2011;77:481–485. doi: 10.1016/j.urology.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wessells H., Angermeier K.W., Elliott S.P., Gonzalez C.M., Kodama R.T., Peterson A.C. 2016. Male urethral stricture: AUA guideline; pp. 1–32.https://www.researchgate.net/publication/303543755_American_Urological_Association_AUA_Guideline_MALE_URETHRAL_STRICTURE_AUA_GUIDELINE [DOI] [PubMed] [Google Scholar]
- 5.Bhargava S., Patterson J.M., Inman R.D., MacNeil S., Chapple C.R. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–1269. doi: 10.1016/j.eururo.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 6.Osman N.I., Patterson J.M., MacNeil S., Chapple C.R. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol. 2014;66:790–791. doi: 10.1016/j.eururo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcanti A.G., Costa W.S., Baskin L.S., McAninch J.A., Sampaio F.J. A morphometric analysis of bulbar urethral strictures. BJU Int. 2007;100:397–402. doi: 10.1111/j.1464-410X.2007.06904.x. [DOI] [PubMed] [Google Scholar]
- 8.Singh M., Blandy J.P. The pathology of urethral stricture. J Urol. 1976;115:673–676. doi: 10.1016/s0022-5347(17)59331-3. [DOI] [PubMed] [Google Scholar]
- 9.Baskin L.S., Constantinescu S.C., Howard P.S., McAninch J.W., Ewalt D.H., Duckett J.W. Biochemical characterization and quantitation of the collagenous components of urethral stricture tissue. J Urol. 1993;150:642–647. doi: 10.1016/s0022-5347(17)35572-6. [DOI] [PubMed] [Google Scholar]
- 10.Cavalcanti A.G., Yucel S., Deng D.Y., McAninch J.W., Baskin L.S. The distribution of neuronal and inducible nitric oxide synthase in urethral stricture formation. J Urol. 2004;171:1943–1947. doi: 10.1097/01.ju.0000121261.03616.63. [DOI] [PubMed] [Google Scholar]
- 11.Da-Silva E.A., Sampaio F.J., Dornas M.C., Damiao R., Cardoso L.E. Extracellular matrix changes in urethral stricture disease. J Urol. 2002;168:805–807. [PubMed] [Google Scholar]
- 12.Mundy A.R., Andrich D.E. Urethral strictures. BJU Int. 2011;107:6–26. doi: 10.1111/j.1464-410X.2010.09800.x. [DOI] [PubMed] [Google Scholar]
- 13.Lumen N., Hoebeke P., Willemsen P., De Troyer B., Pieters R., Oosterlinck W. Etiology of urethral stricture disease in the 21st century. J Urol. 2009;182:983–987. doi: 10.1016/j.juro.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Fenton A.S., Morey A.F., Aviles R., Garcia C.R. Anterior urethral strictures: etiology and characteristics. Urology. 2005;65:1055–1058. doi: 10.1016/j.urology.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Das S., Tunuguntla H.S. Balanitis xerotica obliterans—a review. World J Urol. 2000;18:382–387. doi: 10.1007/PL00007083. [DOI] [PubMed] [Google Scholar]
- 16.Miller K., Posh M., Brandes S.B. Urethral stricture and urethroplasy in the pelvic irradiated patient. In: Brandes S.B., editor. Urethral reconstructive surgery. Springer; Saint Louis: 2008. pp. 241–249. [Google Scholar]
- 17.McAninch J.W., Laing F.C., Jeffrey R.B., Jr. Sonourethrography in the evaluation of urethral strictures: a preliminary report. J Urol. 1988;139:294–297. doi: 10.1016/s0022-5347(17)42391-3. [DOI] [PubMed] [Google Scholar]
- 18.Steenkamp J.W., Heyns C.F., de Kock M.L. Outpatient treatment for male urethral strictures—dilatation versus internal urethrotomy. S Afr J Surg. 1997;35:125–130. [PubMed] [Google Scholar]
- 19.Steenkamp J.W., Heyns C.F., de Kock M.L. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol. 1997;157:98–101. [PubMed] [Google Scholar]
- 20.Tonkin J.B., Jordan G.H. Management of distal anterior urethral strictures. Nat Rev Urol. 2009;6:533–538. doi: 10.1038/nrurol.2009.181. [DOI] [PubMed] [Google Scholar]
- 21.Albers P., Fichtner J., Bruhl P., Muller S.C. Long-term results of internal urethrotomy. J Urol. 1996;156:1611–1614. [PubMed] [Google Scholar]
- 22.Pansadoro V., Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol. 1996;156:73–75. [PubMed] [Google Scholar]
- 23.Heyns C.F., Steenkamp J.W., De Kock M.L., Whitaker P. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol. 1998;160:356–358. doi: 10.1016/s0022-5347(01)62894-5. [DOI] [PubMed] [Google Scholar]
- 24.Naude A.M., Heyns C.F. What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat Clin Pract Urol. 2005;2:538–545. doi: 10.1038/ncpuro0320. [DOI] [PubMed] [Google Scholar]
- 25.Vanni A.J., Zinman L.N., Buckley J.C. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol. 2011;186:156–160. doi: 10.1016/j.juro.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Mazdak H., Meshki I., Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol. 2007;51:1089–1092. doi: 10.1016/j.eururo.2006.11.038. [discussion 92] [DOI] [PubMed] [Google Scholar]
- 27.Tavakkoli Tabassi K., Yarmohamadi A., Mohammadi S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol J. 2011;8:132–136. [PubMed] [Google Scholar]
- 28.Mazdak H., Izadpanahi M.H., Ghalamkari A., Kabiri M., Khorrami M.H., Nouri-Mahdavi K. Internal urethrotomy and intraurethral submucosal injection of triamcinolone in short bulbar urethral strictures. Int Urol Nephrol. 2010;42:565–568. doi: 10.1007/s11255-009-9663-5. [DOI] [PubMed] [Google Scholar]
- 29.Jin T., Li H., Jiang L.H., Wang L., Wang K.J. Safety and efficacy of laser and cold knife urethrotomy for urethral stricture. Chin Med J. 2010;123:1589–1595. [PubMed] [Google Scholar]
- 30.McDermott D.W., Bates R.J., Heney N.M., Althausen A. Erectile impotence as complication of direct vision cold knife urethrotomy. Urology. 1981;18:467–469. doi: 10.1016/0090-4295(81)90291-0. [DOI] [PubMed] [Google Scholar]
- 31.Graversen P.H., Rosenkilde P., Colstrup H. Erectile dysfunction following direct vision internal urethrotomy. Scand J Urol Nephrol. 1991;25:175–178. doi: 10.3109/00365599109107943. [DOI] [PubMed] [Google Scholar]
- 32.Dutkiewicz S.A., Wroblewski M. Comparison of treatment results between holmium laser endourethrotomy and optical internal urethrotomy for urethral stricture. Int Urol Nephrol. 2012;44:717–724. doi: 10.1007/s11255-011-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atak M., Tokgoz H., Akduman B., Erol B., Donmez I., Hanci V. Low-power holmium:YAG laser urethrotomy for urethral stricture disease: comparison of outcomes with the cold-knife technique. Kaohsiung J Med Sci. 2011;27:503–507. doi: 10.1016/j.kjms.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Hussain M., Lal M., Askari S.H., Hashmi A., Rizvi S.A. Holmium laser urethrotomy for treatment of traumatic stricture urethra: a review of 78 patients. JPMA. J Pak Med Assoc. 2010;60:829–832. [PubMed] [Google Scholar]
- 35.Webster G.D., Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach: experience with 74 cases. J Urol. 1991;145:744–748. doi: 10.1016/s0022-5347(17)38442-2. [DOI] [PubMed] [Google Scholar]
- 36.Eltahawy E.A., Virasoro R., Schlossberg S.M., McCammon K.A., Jordan G.H. Long-term followup for excision and primary anastomosis for anterior urethral strictures. J Urol. 2007;177:1803–1806. doi: 10.1016/j.juro.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Park S., McAninch J.W. Straddle injuries to the bulbar urethra: management and outcomes in 78 patients. J Urol. 2004;171:722–725. doi: 10.1097/01.ju.0000108894.09050.c0. [DOI] [PubMed] [Google Scholar]
- 38.Cooperberg M.R., McAninch J.W., Alsikafi N.F., Elliott S.P. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006–2010. doi: 10.1016/j.juro.2007.07.020. [discussion 10] [DOI] [PubMed] [Google Scholar]
- 39.Morey A.F., Kizer W.S. Proximal bulbar urethroplasty via extended anastomotic approach—what are the limits? J Urol. 2006;175:2145–2149. doi: 10.1016/S0022-5347(06)00259-X. [discussion 9] [DOI] [PubMed] [Google Scholar]
- 40.Erickson B.A., Granieri M.A., Meeks J.J., Cashy J.P., Gonzalez C.M. Prospective analysis of erectile dysfunction after anterior urethroplasty: incidence and recovery of function. J Urol. 2010;183:657–661. doi: 10.1016/j.juro.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Patterson J.M., Chapple C.R. Surgical techniques in substitution urethroplasty using buccal mucosa for the treatment of anterior urethral strictures. Eur Urol. 2008;53:1162–1171. doi: 10.1016/j.eururo.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Greenwell T.J., Venn S.N., Mundy A.R. Changing practice in anterior urethroplasty. BJU Int. 1999;83:631–635. doi: 10.1046/j.1464-410x.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 43.Wessells H., McAninch J.W. Current controversies in anterior urethral stricture repair: free-graft versus pedicled skin-flap reconstruction. World J Urol. 1998;16:175–180. doi: 10.1007/s003450050048. [DOI] [PubMed] [Google Scholar]
- 44.Harrison C.A., MacNeil S. The mechanism of skin graft contraction: an update on current research and potential future therapies. Burns. 2008;34:153–163. doi: 10.1016/j.burns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Lumen N., Oosterlinck W., Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int. 2012;89:387–394. doi: 10.1159/000341138. [DOI] [PubMed] [Google Scholar]
- 46.Barbagli G., Sansalone S., Djinovic R., Romano G., Lazzeri M. Current controversies in reconstructive surgery of the anterior urethra: a clinical overview. Int Braz J Urol. 2012;38:307–316. doi: 10.1590/s1677-55382012000300003. [discussion 16] [DOI] [PubMed] [Google Scholar]
- 47.Barbagli G. When and how to use buccal mucosa grafts in penile and bulbar urethroplasty. Minerva Urol Nefrol. 2004;56:189–203. [PubMed] [Google Scholar]
- 48.Markiewicz M.R., Lukose M.A., Margarone J.E., 3rd, Barbagli G., Miller K.S., Chuang S.K. The oral mucosa graft: a systematic review. J Urol. 2007;178:387–394. doi: 10.1016/j.juro.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 49.Barbagli G., Vallasciani S., Romano G., Fabbri F., Guazzoni G., Lazzeri M. Morbidity of oral mucosa graft harvesting from a single cheek. Eur Urol. 2010;58:33–41. doi: 10.1016/j.eururo.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Mangera A., Patterson J.M., Chapple C.R. A systematic review of graft augmentation urethroplasty techniques for the treatment of anterior urethral strictures. Eur Urol. 2011;59:797–814. doi: 10.1016/j.eururo.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Barbagli G., Palminteri E., Guazzoni G., Montorsi F., Turini D., Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005;174:955–957. doi: 10.1097/01.ju.0000169422.46721.d7. [discussion 7–8] [DOI] [PubMed] [Google Scholar]
- 52.Barbagli G., De Angelis M., Palminteri E., Lazzeri M. Failed hypospadias repair presenting in adults. Eur Urol. 2006;49:887–894. doi: 10.1016/j.eururo.2006.01.027. [discussion 95] [DOI] [PubMed] [Google Scholar]
- 53.Barbagli G., Perovic S., Djinovic R., Sansalone S., Lazzeri M. Retrospective descriptive analysis of 1,176 patients with failed hypospadias repair. J Urol. 2010;183:207–211. doi: 10.1016/j.juro.2009.08.153. [DOI] [PubMed] [Google Scholar]
- 54.Davis J.S., Kitlowski E.A. The Immediate contraction of cutaneous grafts and its cause. Arch Surg. 1931;23:954–965. [Google Scholar]
- 55.Hinshaw J.R., Miller E.R. Histology of healing split-thickness, full-thickness autogenous skin grafts and donor sites. Arch Surg. 1965;91:658–670. doi: 10.1001/archsurg.1965.01320160112027. [DOI] [PubMed] [Google Scholar]
- 56.Corps B.V. The effect of graft thickness, donor site and graft bed on graft shrinkage in the hooded rat. Br J Plast Surg. 1969;22:125–133. doi: 10.1016/s0007-1226(69)80053-6. [DOI] [PubMed] [Google Scholar]
- 57.Rudolph R. The effect of skin graft preparation on wound contraction. Surg Gynecol Obstet. 1976;142:49–56. [PubMed] [Google Scholar]
- 58.Davies D.M. Plastic and reconstructive surgery. Scars, hypertrophic scars, and keloids. Br Med J (Clin Res Ed) 1985;290:1056–1058. doi: 10.1136/bmj.290.6474.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudolph R. Contraction and the control of contraction. World J Surg. 1980;4:279–287. doi: 10.1007/BF02393383. [DOI] [PubMed] [Google Scholar]
- 60.Gabbiani G., Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972;66:131–146. [PMC free article] [PubMed] [Google Scholar]
- 61.Tomasek J.J., Haaksma C.J. Fibronectin filaments and actin microfilaments are organized into a fibronexus in Dupuytren's diseased tissue. Anat Rec. 1991;230:175–182. doi: 10.1002/ar.1092300205. [DOI] [PubMed] [Google Scholar]
- 62.Welch M.P., Odland G.F., Clark R.A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osborne C.S., Reid W.H., Grant M.H. Investigation into the biological stability of collagen/chondroitin-6-sulphate gels and their contraction by fibroblasts and keratinocytes: the effect of crosslinking agents and diamines. Biomaterials. 1999;20:283–290. doi: 10.1016/s0142-9612(98)00179-3. [DOI] [PubMed] [Google Scholar]
- 64.Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denefle J.P., Lechaire J.P., Zhu Q.L. Cultured epidermis influences the fibril organization of purified type I collagen gels. Tissue Cell. 1987;19:469–478. doi: 10.1016/0040-8166(87)90041-3. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh M.M., Boyce S., Layton C., Freedlander E., Mac Neil S. A comparison of methodologies for the preparation of human epidermal-dermal composites. Ann Plast Surg. 1997;39:390–404. doi: 10.1097/00000637-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Harrison C.A., Gossiel F., Layton C.M., Bullock A.J., Johnson T., Blumsohn A. Use of an in vitro model of tissue-engineered skin to investigate the mechanism of skin graft contraction. Tissue Eng. 2006;12:3119–3133. doi: 10.1089/ten.2006.12.3119. [DOI] [PubMed] [Google Scholar]
- 68.Harrison C.A., Heaton M.J., Layton C.M., Mac Neil S. Use of an in vitro model of tissue-engineered human skin to study keratinocyte attachment and migration in the process of reepithelialization. Wound Repair Regen. 2006;14:203–209. doi: 10.1111/j.1743-6109.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 69.Ralston D.R., Layton C., Dalley A.J., Boyce S.G., Freedlander E., MacNeil S. Keratinocytes contract human dermal extracellular matrix and reduce soluble fibronectin production by fibroblasts in a skin composite model. Br J Plast Surg. 1997;50:408–415. doi: 10.1016/s0007-1226(97)90327-1. [DOI] [PubMed] [Google Scholar]
- 70.Chakrabarty K.H., Heaton M., Dalley A.J., Dawson R.A., Freedlander E., Khaw P.T. Keratinocyte-driven contraction of reconstructed human skin. Wound Repair Regen. 2001;9:95–106. doi: 10.1046/j.1524-475x.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 71.Redden R.A., Doolin E.J. Collagen crosslinking and cell density have distinct effects on fibroblast-mediated contraction of collagen gels. Skin Res Technol. 2003;9:290–293. doi: 10.1034/j.1600-0846.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 72.Raya-Rivera A., Esquiliano D.R., Yoo J.J., Lopez-Bayghen E., Soker S., Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee T.Y., Chin G.S., Kim W.J., Chau D., Gittes G.K., Longaker M.T. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–184. [PubMed] [Google Scholar]
- 74.Steenfos H., Spencer E.M., Hunt T. Insulin-like growth factor I has a major role in wound healing. Surg Forum. 1989;40:68. [Google Scholar]
- 75.Scharffetter K., Heckmann M., Hatamochi A., Mauch C., Stein B., Riethmuller G. Synergistic effect of tumor necrosis factor-alpha and interferon-gamma on collagen synthesis of human skin fibroblasts in vitro. Exp Cell Res. 1989;181:409–419. doi: 10.1016/0014-4827(89)90098-0. [DOI] [PubMed] [Google Scholar]
- 76.Ghahary A., Shen Y.J., Nedelec B., Scott P.G., Tredget E.E. Enhanced expression of mRNA for insulin-like growth factor-1 in post-burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mol Cell Biochem. 1995;148:25–32. doi: 10.1007/BF00929499. [DOI] [PubMed] [Google Scholar]
- 77.El-Kassaby A., Abou Shwareb T., Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179:1432–1436. doi: 10.1016/j.juro.2007.11.101. [DOI] [PubMed] [Google Scholar]
- 78.Fossum M., Skikuniene J., Orrego A., Nordenskjold A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 2012;101:755–760. doi: 10.1111/j.1651-2227.2012.02659.x. [DOI] [PubMed] [Google Scholar]
- 79.Patterson J.M., Bullock A.J., MacNeil S., Chapple C.R. Methods to reduce the contraction of tissue-engineered buccal mucosa for use in substitution urethroplasty. Eur Urol. 2011;60:856–861. doi: 10.1016/j.eururo.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 80.Breyer B.N., McAninch J.W., Whitson J.M., Eisenberg M.L., Mehdizadeh J.F., Myers J.B. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol. 2010;183:613–617. doi: 10.1016/j.juro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Kinnaird A.S., Levine M.A., Ambati D., Zorn J.D., Rourke K.F. Stricture length and etiology as preoperative independent predictors of recurrence after urethroplasty: a multivariate analysis of 604 urethroplasties. Can Urol Assoc J. 2014;8:E296–E300. doi: 10.5489/cuaj.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warner J.N., Malkawi I., Dhradkeh M., Joshi P.M., Kulkarni S.B., Lazzeri M. A multi-institutional evaluation of the management and outcomes of long-segment urethral strictures. Urology. 2015;85:1483–1487. doi: 10.1016/j.urology.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 83.Han J.S., Liu J., Hofer M.D., Fuchs A., Chi A., Stein D. Risk of urethral stricture recurrence increases over time after urethroplasty. Int J Urol. 2015;22:695–699. doi: 10.1111/iju.12781. [DOI] [PubMed] [Google Scholar]
- 84.Worrall S., de Jersey J., Nicholls R., Wilce P. Acetaldehyde/protein interactions: are they involved in the pathogenesis of alcoholic liver disease? Dig Dis. 1993;11:265–277. doi: 10.1159/000171418. [DOI] [PubMed] [Google Scholar]
- 85.Miller Y.I., Choi S.H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wick G., Brunner H., Penner E., Timpl R. The diagnostic application of specific antiprocollagen sera. II. Analysis of liver biopsies. Int Arch Allergy Appl Immunol. 1978;56:316–324. doi: 10.1159/000232037. [DOI] [PubMed] [Google Scholar]
- 87.Wick G., Grundtman C., Mayerl C., Wimpissinger T.F., Feichtinger J., Zelger B. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 88.Grenier S., Sandig M., Mequanint K. Smooth muscle alpha-actin and calponin expression and extracellular matrix production of human coronary artery smooth muscle cells in 3D scaffolds. Tissue Eng Part A. 2009;15:3001–3011. doi: 10.1089/ten.TEA.2009.0057. [DOI] [PubMed] [Google Scholar]
- 89.Shih B., Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. doi: 10.1038/nrrheum.2010.180. [DOI] [PubMed] [Google Scholar]
- 90.Strutz F., Neilson E.G. New insights into mechanisms of fibrosis in immune renal injury. Springer Semin Immunopathol. 2003;24:459–476. doi: 10.1007/s00281-003-0123-5. [DOI] [PubMed] [Google Scholar]