Abstract
European Respiratory Society (ERS) guidelines recommend the assessment of patients with interstitial lung disease (ILD) and severe pulmonary hypertension (PH), as defined by a mean pulmonary artery pressure (mPAP) ≥35 mmHg at right heart catheterisation (RHC). We developed and validated a stepwise echocardiographic score to detect severe PH using the tricuspid regurgitant velocity and right atrial pressure (right ventricular systolic pressure (RVSP)) and additional echocardiographic signs.
Consecutive ILD patients with suspected PH underwent RHC between 2005 and 2015. Receiver operating curve analysis tested the ability of components of the score to predict mPAP ≥35 mmHg, and a score devised using a stepwise approach. The score was tested in a contemporaneous validation cohort. The score used “additional PH signs” where RVSP was unavailable, using a bootstrapping technique.
Within the derivation cohort (n=210), a score ≥7 predicted severe PH with 89% sensitivity, 71% specificity, positive predictive value 68% and negative predictive value 90%, with similar performance in the validation cohort (n=61) (area under the curve (AUC) 84.8% versus 83.1%, p=0.8). Although RVSP could be estimated in 92% of studies, reducing this to 60% maintained a fair accuracy (AUC 74.4%).
This simple stepwise echocardiographic PH score can predict severe PH in patients with ILD.
Short abstract
A stepwise echocardiographic score to predict severe group 3 pulmonary hypertension http://ow.ly/9cIC30iGSMj
Introduction
Interstitial lung diseases (ILDs) are commonly associated with the development of pulmonary hypertension (PH-ILD) [1–3]. There appears to be no clear correlation between the severity of ILD and PH according to pulmonary function indices or extent of fibrosis on computed tomography [2, 4], suggesting the presence of an exaggerated vascular phenotype. PH-ILD impacts negatively on symptoms and prognosis [2, 5, 6], including survival after lung transplantation [7, 8]. This is especially true for patients with severe PH, defined as mean pulmonary arterial pressure (mPAP) ≥35 mmHg on right heart catheterisation (RHC), in whom prognosis is particularly poor [9]. Current European Respiratory Society (ERS)/European Society of Cardiology (ESC) PH guidelines recommend that patients with severe PH-ILD should be identified and referred to specialist centres with expertise in both chronic lung disease and PH [10], where they may be considered for pulmonary vasodilator therapy on an individual basis, or entered into clinical trials.
Echocardiography is key in the identification of patients with PH who should be referred for RHC. According to ESC/ERS guidelines, estimation of PH by echocardiography should be based upon assessment of peak tricuspid regurgitation velocity (TRV) in addition to “additional echocardiographic PH signs” [10]. Previous echocardiographic studies in ILD patients highlight that the use of TRV or right ventricular systolic pressure (RVSP) can under- and overestimate PH [11]. Relying on RVSP alone may be unhelpful in ILD patients, as this value could not be obtained in 44–55% of previous studies [11, 12].
Therefore, we sought to evaluate the role of “additional” echocardiographic PH signs in risk-stratifying ILD patients for the presence of severe PH. We developed and validated a stepwise echocardiographic score. In addition, we tested the accuracy of the score when TRV was unavailable.
Methods
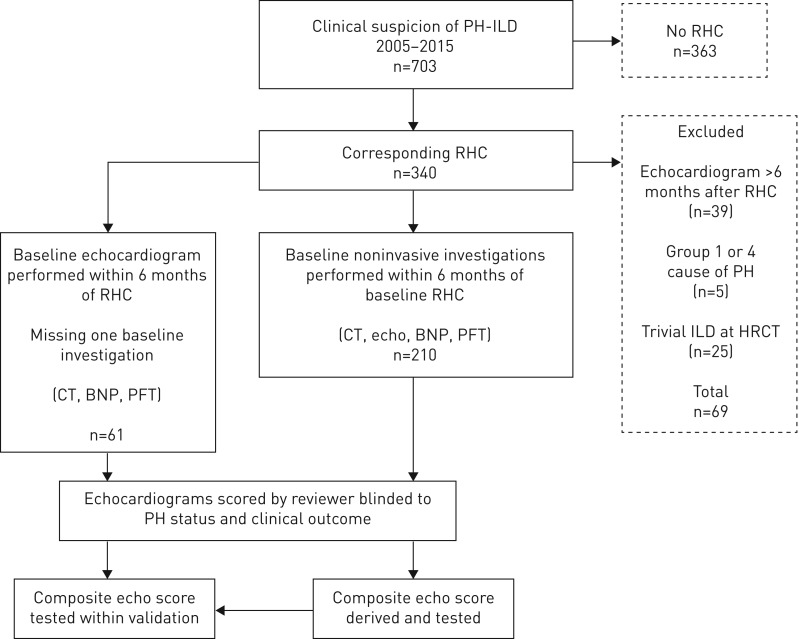
Consecutive ILD patients referred to the Royal Brompton Hospital National Pulmonary Hypertension Service (London, UK) with suspected PH between 2005 and 2015 were reviewed, with demographics and ILD diagnosis collected (figure 1). Patients with echocardiograms performed >6 months from RHC were excluded, as were those with high-resolution computed tomography (HRCT) demonstrating absent or trivial interstitial fibrosis. If patients within the study received PH therapies following the RHC, only the echocardiogram prior to the RHC was used in the analysis. This study had institutional ethics review board approval (Royal Brompton, Harefield reference 2016PH002B).
FIGURE 1.
Cohort identification and exclusion. A flow diagram describing the methodology of the study, from patient identification and selection to the development and validation of the echocardiography (echo) score in the derivation (n=210) and validation (n=61) cohorts; the latter derived from the same population over the same time period, but with some missing brain natriuretic peptide (BNP) and pulmonary function testing (PFT) data from the time of right heart catheterisation (RHC). CT: computed tomography; PH: pulmonary hypertension; ILD: interstitial lung disease; HRCT: high-resolution CT.
Echocardiography
The following echocardiographic parameters were evaluated: 1) “pressure domains”: TRV, RVSP, early diastolic pulmonary regurgitation velocity, right atrial pressure (RAP) and pulmonary valve acceleration time; 2) “right ventricle (RV) morphology domains”: RV:left ventricle (LV) basal diameter ratio, right atrial (RA) size and LV eccentricity index (a measure of septal flattening); and 3) “RV function domains”: tricuspid annular plane systolic excursion (TAPSE) and fractional area change (percentage change in RV area between end-diastole and end-systole).
Images were acquired using a 3-MHz frequency harmonic phased-array transducer. Doppler echocardiography was performed as per the American Society of Echocardiography recommendations [13, 14]. The two-dimensional echo datasets were interpreted by cardiologists with advanced echocardiography training. Briefly, the maximum RV to RA pressure difference was derived from the peak velocity of tricuspid regurgitation using the simplifed Bernoulli equation. RA pressure was based on inferior vena cava diameter and respiratory collapse. RVSP was estimated by adding RAP to the RV and RA pressure differential. Further explanation of echocardiographic parameters is provided in the online supplementary material.
Right heart catheterisation
RHC was performed using standard techniques [10] with haemodynamic measurements obtained at rest in all patients. Cardiac output was measured using the indirect Fick method with oxygen consumption estimated using the LaFarge equation. Pulmonary vascular resistance (PVR) was calculated as (mPAP – pulmonary capillary wedge pressure)/cardiac output.
Pulmonary function testing
Pulmonary function testing (PFT) was performed with predicted values calculated using American Thoracic Society/ERS criteria [15]. Measurements performed included spirometric [16] and single-breath transfer factor of the lung for carbon monoxide and transfer coefficient of the lung for carbon monoxide adjusted for alveolar volume [17].
Computed tomography analysis
Computed tomography was performed at full inspiration in the supine position. Images were interpreted by two radiologists blinded to the clinical data. High-resolution reconstructions were reviewed to estimate interstitial disease extent. Severity of fibrosis was scored as limited (<20%) or extensive (>20%) [18]. Conflict of extent of fibrosis by the reviewers of more than one point was resolved by consensus.
Generation of the echocardiographic score
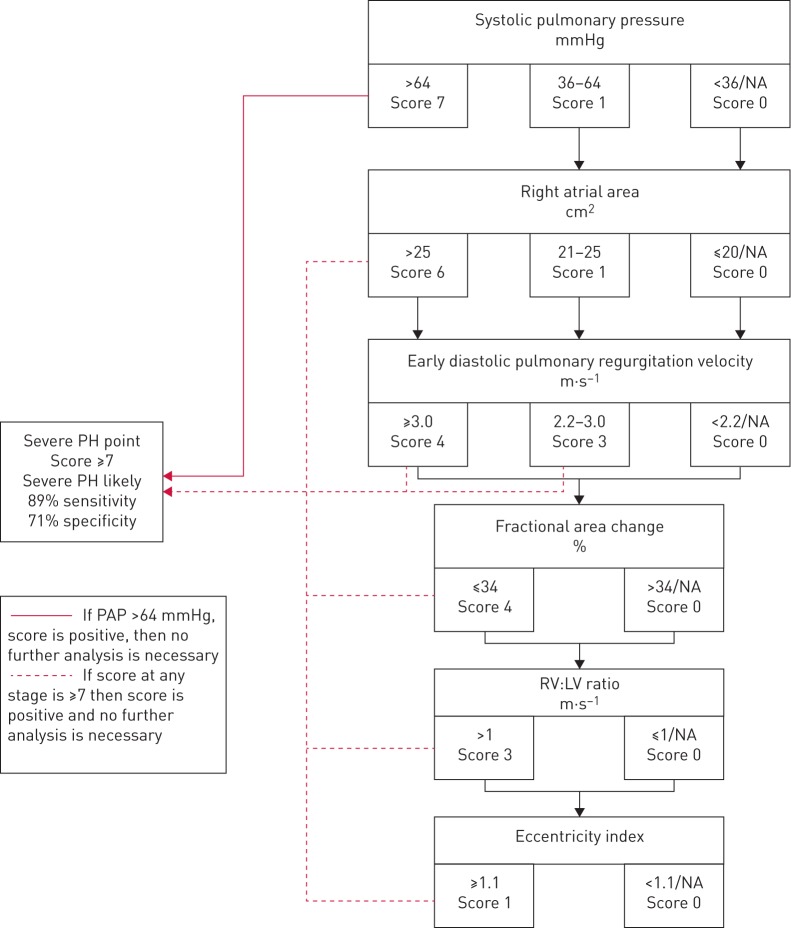
Echocardiographic variables used in the model were selected by expert opinion and according to ESC/ERS guidelines, and the ability to predict severe PH was assessed by receiver operating curve (ROC) analysis. The strongest predictors (area under the curve (AUC) >70%) were incorporated into a contingent stepwise model to predict severe PH. Threshold values of each individual variable were chosen a priori, again based upon expert consensus and guidelines. Each variable was entered into the score as a binary or categorical variable depending on the threshold value. The weighting of each threshold within the score was determined by choosing a model with optimal AUC with 900 000 different score combinations analysed (table 1). A contingent stepwise method was used to allow for missing data, and was designed to be easy to use with the strongest most available predictors used upfront in the score. This was to facilitate rapid staging of severe PH (figure 2). For example, when the strongest predictor of severe PH was present and the highest threshold was met, the score was positive. Where data was missing or in lower risk categories, the stepwise score was designed to be performed in sequence until a threshold for severe PH is reached or all available variables analysed. The threshold for the echocardiographic score becoming positive (and severe PH likely) was determined by the score, which offered the best balance of sensitivity and specificity. Once the threshold is met, no further analysis is needed. The derivation cohort included patients with all noninvasive assessments performed at RHC, and the validation cohort were missing either brain natriuretic peptide (BNP) or PFTs at RHC. The composite echocardiographic score was derived and tested within the derivation cohort, and tested in the validation cohort.
TABLE 1.
Threshold values of individual variables within the composite echocardiographic score, and the final score based upon area under the curve (AUC) analysis
| Score | Permutations | Score | ||
| Minimum | Maximum | |||
| RV systolic pressure mmHg | ||||
| >64 | 4 | 8 | 5 | 7 |
| >35 | 1 | 5 | 5 | 1 |
| ≤35 or NA | 0 | 0 | 1 | 0 |
| Right atrial area cm2 | ||||
| >25 | 3 | 7 | 5 | 6 |
| >20 | 1 | 2 | 4 | 1 |
| ≤20 or NA | 0 | 0 | 1 | 0 |
| Early diastolic pulmonary regurgitation velocity mmHg | ||||
| >36 | 4 | 8 | 5 | 4 |
| ≥20 | 1 | 3 | 3 | 3 |
| <20 or NA | 0 | 0 | 1 | 0 |
| RV fractional area change % | ||||
| <35 | 1 | 5 | 5 | 4 |
| ≥35 | 0 | 0 | 1 | 0 |
| RV/LV short axis dimension | ||||
| >1 | 1 | 6 | 6 | 3 |
| ≤1 or NA | 0 | 0 | 0 | 0 |
| Systolic eccentricity index | ||||
| ≥1.1 | 1 | 4 | 4 | 1 |
| <1.1 or NA | 0 | 0 | 1 | 0 |
| Total permutations n | 900 000 | |||
| Maximum score | 25 | |||
Each component of the composite score was selected as that having the highest AUC to predict pulmonary hypertension using receiver operating curve analysis (figure 1 and online supplementary material) and consensus. For example, for right ventricular systolic pressure (RVSP), a minimum score of 4 and a maximum of 8 was set; this was done for each threshold and variable, thereby creating a stepwise score. Different combinations of score components (n=900 000) were then tested, and the model with the best AUC chosen, which is displayed as the best score. The order of analysis using the echo composite score is shown in the third column. For example, if a score of 7 was achieved at the first step (if RVSP >64 mmHg), then the echo score became positive to predict severe PH, and no further analysis was needed. If the RVSP was <64 mmHg, the second factor, right atrial area, was considered, and so on. RV: right ventricular; NA: not available; LV: left ventricular.
FIGURE 2.
Severe pulmonary hypertension (PH) in interstitial lung disease (ILD) stepwise composite echocardiographic score. If an overall score of ≥7 is achieved, then the score is positive for the prediction of severe PH with a mean pulmonary arterial pressure (PAP) ≥35 mmHg. Where the right ventricular systolic pressure (RVSP) is >64 mmHg, the score is positive and no further analysis is necessary. Where RVSP is not available or intermediate then each step is continued until either a score of ≥7 is achieved, or if the final score is <7, in which case the score is negative and severe PH is unlikely. NA: not available; RV: right ventricle; LV: left ventricle.
Finally, the cohorts were combined for a post hoc analysis evaluating the effect of increasing RVSP (or TRV) unavailability (in effect, blinding available data) to check the integrity of the score with unavailable RVSP, as seen in historic cohorts. The echocardiographic score was evaluated using a bootstrapping method, which randomly blinded available RVSP at each percentage point from 8% missing RVSP data (as was found in our cohort) up to 60% (as seen in historic cohorts [11, 12]). 100 iterations were performed at each percentage missing RVSP data between 8% and 60%, with random patient selection for RVSP blinding at each iteration. The same method was used to compare the sensitivity of the stepwise echocardiographic score to using RVSP alone (using a threshold of RVSP 64 mmHg) (figure 2).
Statistical analysis
All statistical analysis was performed using R version 3.3.1 (R Foundation for Statistical Computing; www.r-project.org). Data were summarised as n (%) for categorical variables and mean±sd or median (interquartile range) for continuous variables, as appropriate. Continuous variables were compared between groups using ANOVA for parametric data and Kruskal–Wallis test for nonparametric data, or the Wilcoxon signed-rank test or t-test, as appropriate. For categorical variables, a Chi-squared test was used.
Results
Patient demographics and ILD diagnosis
The derivation cohort consisted of 210 patients, with a mean age of 61±11 years, 55% of whom were male. ILD diagnoses included idiopathic pulmonary fibrosis (IPF; n=62), connective tissue disease (CTD)-associated ILD (n=59), sarcoidosis (n=43), chronic hypersensitivity pneumonitis (n=16), idiopathic nonspecific interstitial pneumonitis (n=16) and “other ILD” (n=14), including unclassifiable ILD (four out of 14) and smoking-related ILD (four out of 14). CTD-ILD included patients with scleroderma (36% of all CTDs), undifferentiated CTD, rheumatoid arthritis (14%) and antisynthetase syndrome (14%), mixed CTD (12%), Sjogren's syndrome (5%) and systemic lupus erythematous (5%).
RHC and BNP data
RHC excluded PH (mPAP <25 mmHg) in 46 (22%) patients. PH was mild–moderate in severity (mPAP 25–34 mmHg) in 79 (38%) patients and severe (mPAP ≥35 mmHg) in 85 (40%) patients. BNP increased in a stepwise fashion across the groups (p<0.001 between groups). Patients with severe PH were younger than patients with mild–moderate PH (p<0.001). More patients with sarcoidosis had severe PH (n=25, 58%) in the sarcoid group versus (n=60, 36%) in the nonsarcoid group (p=0.01) (table 2), but this was not explained by sarcoidosis patients having more severe fibrosis at computed tomography or lung function parameters.
TABLE 2.
Baseline right heart catheter and noninvasive variables
| Total derivation cohort | mPAP <25 mmHg | mPAP 25–34 mmHg | mPAP ≥35 mmHg | p-value | |
| Subjects | 210 | 46 | 79 | 85 | |
| Age years | 61±11 | 63±11 | 64±11 | 58±12 | 0.004 |
| Male % | 55 | 52 | 54 | 56 | 0.9 |
| ILD diagnosis | |||||
| CTD | 59 (28) | 15 (25) | 21 (36) | 23 (39) | 0.7 |
| Sarcoidosis | 43 (20) | 4 (9) | 14 (33) | 25 (58) | 0.01 |
| IPF | 62 (29) | 18 (29) | 28 (45) | 16 (26) | 0.02 |
| CHP | 16 (8) | 6 (38) | 2 (12) | 8 (50) | 0.1 |
| NSIP | 16 (8) | 2 (12) | 8 (50) | 6 (38) | 0.5 |
| Other ILD | 14 (7) | 1 (7) | 6 (43) | 7 (50) | 0.4 |
| Right heart catheter | |||||
| mPAP mmHg | 33±11 | 20±4 | 29±3 | 43±7 | <0.001 |
| PVR Wood units | 6.0±3.6 | 2.6±1.5 | 4.6±1.8 | 8.8±3.8 | <0.001 |
| Cardiac output L·min−1·m−2 | 4.3±1.3 | 4.8±1.3 | 4.1±1.3 | 4.1±1.2 | 0.02 |
| PCWP mmHg | 10±5 | 8±5 | 10±5 | 11±5 | 0.008 |
| BNP ng·L−1 | 102 (44–266) | 48 (30–72) | 90 (42–141) | 241 (105–436) | <0.001 |
| Pulmonary function tests | |||||
| FEV1 L | 1.6±0.6 | 1.6±0.6 | 1.5±0.5 | 1.6±0.6 | 0.9 |
| FEV1 % pred | 58±18 | 62±21 | 57±17 | 57±17 | 0.2 |
| FVC L | 2.0±0.8 | 2.0±0.8 | 1.9±0.7 | 2.2±0.9 | 0.2 |
| FVC % pred | 60±20 | 61±22 | 59 ±18 | 62±22 | 0.7 |
| TLCO % pred | 25±10 | 28±10 | 25±9 | 24 ±10 | 0.04 |
| KCO % pred | 52±17 | 59±18 | 54±16 | 48±16 | <0.001 |
| PaO2 kPa | 7.9±1.9 | 8.9±1.9 | 8.1±1.9 | 7.1±1.7 | <0.001 |
| CT scan | |||||
| ILD extent <20%/>20% | 14/86 | 15/85 | 19/81 | 9/91 | 0.2 |
Data are presented as n, mean±sd, n (%) or median (interquartile range), unless otherwise stated. mPAP: mean pulmonary pressure at right heart catheterisation; ILD: interstitial lung disease; CTD: connective tissue disease; IPF idiopathic pulmonary fibrosis; CHP: chronic hypersensitivity pneumonitis; NSIP: nonspecific interstitial pneumonia; PVR: pulmonary vascular resistance; PCWP: pulmonary capillary wedge pressure; BNP: brain natriuretic peptide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLCO: transfer factor of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide; PaO2 : arterial oxygen tension (by capillary blood gas analysis); CT: computed tomography.
Lung function and severity of fibrosis on computed tomography
Analysis of lung function parameters between subgroups of PH severity showed no significant difference in spirometric values between groups, whereas there was a stepwise deterioration in gas transfer coefficient, partial pressure of oxygen (capillary blood gas analysis) and alveolar–arterial gradient (ANOVA p<0.001 for all) as PH increased in severity. The extent of ILD, as scored on HRCT, was >20% in 86% of the cohort, with no difference between the groups according to PH severity (p=0.2) (table 2).
Echocardiographic results
RVSP was detectable in 92% of studies (table 3). RVSP was most likely to be unmeasurable in individuals with an ILD diagnosis of sarcoidosis, with 19% of individuals having inadequate TRV Doppler traces to estimate RVSP, compared to only 5% in the remainder (p=0.002). All direct and indirect echocardiographic measures reflecting elevated pulmonary pressure showed a stepwise increase as PH severity increased across the three groups (table 3).
TABLE 3.
Echocardiographic variables grouped according to severity of pulmonary hypertension at right heart catheterisation (RHC)
| Availability % | Derivation cohort, total | mPAP <25 mmHg | mPAP 25–34 mmHg | mPAP ≥35 mmHg | p-value | |
| Subjects | 228 | 46 | 79 | 85 | ||
| TRVmax m·s−1 | 92 | 3.7±0.6 | 3.3±0.5 | 3.6±0.5 | 4.0±0.6 | <0.001 |
| RVSP mmHg | 92 | 66±19 | 53±13 | 61±18 | 76±17 | <0.001 |
| Pulmonary acceleration time ms | 93 | 77±18 | 82±17 | 80±19 | 70±14 | <0.001 |
| Systolic eccentricity index | 82 | 1.4±0.4 | 1.1±0.2 | 1.2±0.3 | 1.6±0.5 | <0.001 |
| Early PRVmax m·s−1 | 20 | 2.5±0.5 | 2.0±0.3 | 2.3±0.5 | 2.7±0.4 | 0.001 |
| RAP mmHg | 99.5 | 5 (5–10) | 5 (5–10) | 5 (5–10) | 10 (5–10) | 0.008 |
| Fractional area change % | 93 | 37±8 | 41±8 | 39±7 | 34±8 | <0.001 |
| Right atrial area cm2 | 93 | 20±8 | 15±4 | 18±6 | 24±8 | <0.001 |
| TAPSE cm | 92 | 1.8±0.5 | 1.9±0.4 | 1.9±0.5 | 1.7±0.4 | <0.001 |
| RV:LV short axis dimension ratio (systolic) | 81 | 0.9 (0.7–1.4) | 0.7 (0.6–0.9) | 1.0 (0.6–1.1) | 1.3 (0.9–2.0) | <0.001 |
Data are presented as n, mean±sd or median (interquartile range), unless otherwise stated. mPAP: mean pulmonary pressure at RHC; TRVmax: maximum tricuspid regurgitation velocity; RVSP: right ventricular systolic pressure; PRVmax: maximum diastolic pulmonary regurgitation velocity; RAP: right atrial pressure; TAPSE: tricuspid annular plane systolic excursion; RV: right ventricular; LV: left ventricular.
Performance of the composite echocardiographic score
The strongest predictors of severe PH on ROC analysis were selected for the composite stepwise echocardiographic score (the score is outlined in figure 2). These included RVSP (AUC 80.1%), early pulmonary regurgitation gradient (adding RAP, AUC 80.7%; without RAP, AUC 80.8%), right atrial area (AUC 75.5%), TRV (AUC 77.1%), systolic RV:LV diameter on short axis view (AUC 77.5%), LV eccentricity index (AUC 80.6%) and RV fractional area change (AUC 72%). Other parameters, including pulmonary valve acceleration time and TAPSE, performed less well (online supplementary figure S1).
A score ≥7 was identified as the threshold to balance sensitivity and specificity (sensitivity 89%, specificity 71%, PPV 68% and NPV 90%) to predict severe PH. Using this cut-off, the stepwise echocardiographic score was positive in 54% of the derivation cohort. Of the patients who scored positive, 88% achieved this on the first step due to a RVSP of >64 mmHg, such that further analysis was unnecessary. The second step assessed right atrial area, resulting in a further 4.8% of patients reaching the threshold, such that 92.9% of the patients became positive within two steps. The echocardiographic score correctly assigned 78% of individuals to the correct PH status, having incorrectly assigned the absence of severe PH in 5% of the cohort (false negatives) and a falsely assuming severe PH in 17% of the cohort (false positives).
To test the potential influence of disease subgroup on the score, the IPF, CTD-ILD and sarcoidosis patient subgroups were individually removed from the total cohort, and the composite echocardiographic score retested. The AUC of the score remained similar between disease subgroups (online supplementary figure S2).
The characteristics of the derivation and validation cohorts were similar in terms of haemodynamic, BNP and lung function parameters (table 4). The performance of the score to predict severe PH remained similar in the derivation and validation cohort (AUC 84.8% versus 83.1%, p=0.8).
TABLE 4.
Comparison of the derivation and validation cohorts
| Derivation cohort | Validation cohort | p-value | |
| Subjects | 210 | 61 | |
| Age years | 61±11 | 61±13 | 0.9 |
| Male % | 55 | 39 | 0.03 |
| ILD diagnosis | |||
| CTD | 59 (28) | 33 (54) | <0.001 |
| Sarcoidosis | 43 (20) | 6 (10) | 0.06 |
| IPF | 62 (29) | 5 (8) | 0.007 |
| CHP | 16 (8) | 6 (10) | 0.5 |
| NSIP | 16 (8) | 6 (10) | 0.5 |
| Other ILD | 14 (7) | 5 (8) | 0.7 |
| RHC | |||
| mPAP mmHg | 33±11 | 33±12 | 0.8 |
| PVR Wood units | 6.0±3.6 | 6.9±5.6 | 0.3 |
| Cardiac output L·min-1 | 4.3±1.3 | 4.1±1.4 | 0.6 |
| PCWP mmHg | 10±5 | 10±5 | 0.9 |
| BNP ng·L−1 | 102 (44–266) | 103 (42–306) | 0.7 |
| Pulmonary function tests | |||
| FEV1 L | 1.6±0.6 | 1.6±0.8 | 0.5 |
| FEV1 % pred | 58±18 | 62±21 | 0.3 |
| FVC L | 2.0±0.8 | 2.0±0.9 | 0.7 |
| FVC % pred | 60±20 | 65±22 | 0.2 |
| TLCO % pred | 25±10 | 27±10 | 0.2 |
| KCO % pred | 52±17 | 54±17 | 0.7 |
| PaO2 kPa | 7.9±1.9 | 8.5±2.1 | 0.1 |
| CT scan | |||
| ILD extent# 20%/>20% | 14/86 | 19/81 | 0.5 |
Data are presented as n, mean±sd or median (interquartile range), unless otherwise stated. ILD: interstital lung disease; CTD: connective tissue disease; IPF idiopathic pulmonary fibrosis; CHP: chronic hypersensitivity pneumonitis; NSIP: nonspecific interstitial pneumonia; RHC: right heart catheterisation; mPAP: mean pulmonary pressure; PVR: pulmonary vascular resistance; PCWP: pulmonary capillary wedge pressure; BNP: brain natriuretic peptide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLCO: transfer factor of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide; PaO2: arterial oxygen tension (by capillary blood gas analysis); CT: computed tomography. #: formal scoring of severity on CT (see methods section).
Utility of the echocardiographic score when RVSP is unavailable
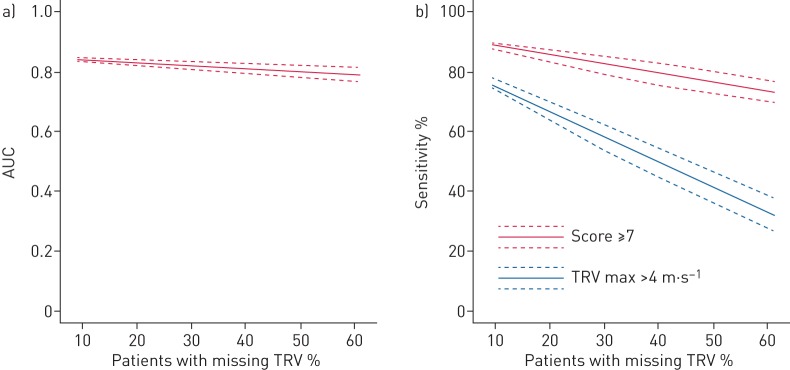
As TRV had been historically more difficult to measure compared to our cohort, we assessed the effect of increasingly unmeasurable TRV (RVSP) on the performance of the score. The effect of inability to record TRV on the echocardiographic score was modelled for prevalence of missing data (figure 3a). With increasing number of patients missing TRV (8% to 60%), AUC did not drop significantly (84% as seen in the original cohort to 79% with 60% unavailable). Moreover, there was a clear advantage in using the composite echocardiographic score to predict severe PH, as opposed to TRV alone, especially with increasing TRV unavailability (figure 3b).
FIGURE 3.
Line plots demonstrating the effect of increasing unavailability of tricuspid regurgitation on the echocardiographic score area under the curve (AUC) for severe pulmonary hypertension (PH) analysis and its effect on sensitivity. Given the frequent “real-life” difficulty assessing tricuspid regurgitant velocity (TRV) at echocardiography in interstitial lung disease (ILD) patients, models were created to demonstrate the effect of increasing unavailability of TRV on a) the AUC of the echocardiographic score and b) the sensitivity of the score in predicting severe PH. We simulated an increasing level of unavailable TRV by randomly blinding available TRV values, with 100 iterations (by bootstrapping), and calculated the AUC and sensitivity following each iteration. We tested levels of TRV unavailability ranging between 8% (observed in the original cohort) and 60% (by 1% increments). The line plots show that AUC for the echo score is preserved across a wide spectrum of TRV unavailability (a). In addition, there was a minor reduction in the sensitivity of the score, despite a dramatic reduction in TRV availability from 93% to 40%, as opposed to TRV alone (using a cut-off of 4 m·s−1, missing values considered ≤4 m·s−1) with a sensitivity that is halved.
Discussion
This validated stepwise echocardiographic score combines traditional assessment of RVSP and additional echocardiographic PH variables to identify severe PH in ILD. We have shown that a contingent stepwise echocardiographic score, using both RVSP as well as other PH echocardiographic signs of RV dysfunction, can predict severe PH within a large cohort of ILD patients, with a high sensitivity and specificity. The score performs well in a large representative validation cohort, and remains sensitive even when TRV is unavailable.
Our cohort had a much higher rate of TRV availability (92%) compared to previous studies (discussed later). However, we recognise that the detection of TRV may be lower in other centres, therefore we demonstrated that the score remains accurate even when TRV is unavailable, using statistical modelling. We showed that the use of additional PH parameters beyond TRV (RVSP) made the score even more reliable than when only using TRV alone, and is in keeping with our clinical experience in other subgroups of PH patients. A panel of PH physicians and echocardiologists decided upon the best parameters to include in the stepwise echocardiographic score, based on a clinical and pathophysiological approach, and consideration of the latest ERS/ESC guidelines of echocardiographic assessment of PH [10]. The scores for each parameter were then tested in the derivation cohort using bootstrapping to derive the weighting of each variable within the score. Although the score has six steps, 93% of the cohort scored positively by the second step. The additional steps within the score are necessary to identify individuals with a missing TRV and are the reason for preserved sensitivity despite a reducing availability of RVSP, which is a unique property of this score.
We have demonstrated that this score is valid for broad ILD populations in wider settings. This relates firstly to the used of standardised echocardiographic PH and RV parameters, which can be widely utilised. Secondly, the cohort of ILD patients in which the score was derived and tested in a heterogeneous group, and we show that the score performs similarly with individual ILD groups excluded, which make it applicable as a screening tool for severe PH in any ILD cohort. The ILD diagnoses within the validation cohort were slightly different to those within the derivation ILD diagnoses; however, the AUC for predicting severe PH was similar in both (p=0.8), which strengthens these findings of applicability for all ILD subpopulations.
The clinical utility of an echocardiographic score to predict the presence of severe group 3 PH in ILD patients is appealing, especially as RHC is now performed less often in patients with severe ILD, mainly due to the potential risk of invasive haemodynamic testing in patients with severe lung disease, as well as current therapeutic limitations in these patients. In parallel, the information available from detailed PH parameters on echocardiographic assessment is increasing. This is the largest validated score using echocardiographic assessment of additional PH variables to identify severe PH in ILD, and has major clinical applicability. The onset of PH in patients with lung disease (group 3 PH) [19] is adversely prognostic, especially with the onset of severe PH and RV dysfunction [9], and is important to detect for prognostic reasons, triaging listing for lung transplantation, and for consideration of potential targeted PH therapies.
Previous echocardiographic studies in ILD-PH have demonstrated that TRV is often unmeasurable [11, 12, 20, 21]. This is believed to relate to an alteration in the orientation of the heart due to changes in intrathoracic pressure and suboptimal echocardiographic windows in ILD patients. In addition, a recent echocardiographic study evaluated RHC measurements in 192 patients with advanced lung disease, 54% of whom had ILD, where RVSP could be measured in only 52% of the cohort [22]. The authors concluded with a good tricuspid regurgitation envelope available, TRV did reliably detect PH in advanced lung disease, but with TRV present, the integration of other right heart abnormalities did not add to the prediction of PH. However, 47% of patients without a measurable TRV had PH, and the presence of two or more abnormal right heart measurements did discriminate between patients with and without PH, especially when PH was severe (defined as mPAP ≥35 mmHg) [22]. Our study support and extends these findings, that TRV or RVSP is the strongest predictor of severe PH, and the integration of other PH signs improves the detection of severe PH when TRV is not measurable.
In our study, we demonstrated that the patients with severe PH had no difference in spirometry or severity of fibrosis seen at computed tomography compared to patients with nonsevere PH, which confirms previous studies [2, 4], and supports the hypothesis that patients develop severe PH due to additional factors other than the severity of parenchymal fibrosis alone. It is this observation, as well as the previous observation that patients with severe PH have worse outcomes than patients with mild–moderate PH [9]. We believe this phenotype of PH-ILD should be studied in future trials of pulmonary vascular therapies, making the validation of such a score useful for both clinical practice and as a research tool.
Limitations
Our study was retrospective, but subjects were consecutive and were derived from our large tertiary population of ILD patients. Inevitably, the cohort had a high pretest probability of PH, and patients with severe PH were relatively over-represented. The high pretest probability of PH and high proportion of severe PH might explain the observed high rate of TRV availability (92%), which is much higher than other published series. However, given the similar populations in previous series, we feel this is most likely to reflect the detailed modern echocardiography studies now available. Therefore, the findings of this study apply broadly to patients with ILD who have a clinical suspicion of PH. A large proportion of our patients had either CTD-ILD or sarcoidosis, such that a limitation to consider is that the overall cohort might be considered to be a less “pure” group 3 PH. However, only patients with significant fibrotic lung disease were included. Furthermore, analysis removing the sarcoid and CTD-ILD populations in turn showed the score behaved similarly without these subgroups. A final limitation is that the usual definition of severe PH in lung disease not only considers the mPAP ≥35 mmHg, but also low cardiac output; however, the RHC data did not have reliable cardiac output measurements from every study. We therefore felt that using just mPAP ≥35 mmHg in this retrospective setting, although not ideal, would be reasonable.
In summary, a RVSP >64 mmHg or a composite echocardiographic score of ≥7 predicts severe PH with a high sensitivity and specificity in ILD patients. The score can predict severe PH-ILD even in patients without available TRV or RVSP. The use of this noninvasive tool should guide onward assessment at PH and ILD centres, and help stratify patients with severe PH-ILD into much needed clinical trials. While the stepwise echocardiographic score identifies individuals with suspected severe PH, RHC will remain essential for confirmation.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00124-2017_supp (319.8KB, pdf)
Figure S1 00124-2017_figureS1 (76.5KB, jpg)
Figure S2 00124-2017_figureS2 (43.3KB, jpg)
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: A. Kempny reports receiving grants from Actelion Global, outside the submitted work.
Conflict of interest: K. Dimopoulos reports receiving grants from Actelion, GSK, Pfizer and Bayer, outside the submitted work.
Conflict of interest: J. Jacob reports receiving personal fees from Boehringer Ingelheim, outside the submitted work.
Conflict of interest: A. Wells reports receiving personal fees from Intermune, Boehringer Inlgeheim, Gilead, MSD, Roche, Bayer and Chiesi, outside the submitted work.
Conflict of interest: S.J. Wort reports receiving grants from Actelion, GSK, Pfizer and Bayer, outside the submitted work.
Conflict of interest: L.C. Price reports educational grants from Actelion and GSK, during the conduct of the study.
Conflict of interest: Dr. George reports personal fees from Roche, personal fees from Boeringer Ingelheim, outside the submitted work.
References
- 1.Handa T, Nagai S, Miki S, et al. . Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006; 129: 1246–1252. [DOI] [PubMed] [Google Scholar]
- 2.Lettieri CJ, Nathan SD, Barnett SD, et al. . Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. [DOI] [PubMed] [Google Scholar]
- 3.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J 2008; 31: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 4.Zisman DA, Karlamangla AS, Ross DJ, et al. . High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest 2007; 132: 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadrous HF, Pellikka PA, Krowka MJ, et al. . Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005; 128: 2393–2399. [DOI] [PubMed] [Google Scholar]
- 6.Mejía M, Carrillo G, Rojas-Serrano J, et al. . Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest 2009; 136: 10–15. [DOI] [PubMed] [Google Scholar]
- 7.Whelan TP, Dunitz JM, Kelly RF, et al. . Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant 2005; 24: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 8.Fang A, Studer S, Kawut SM, et al. . Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest 2011; 139: 782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes D Jr, Black SM, Tobias JD, et al. . Influence of pulmonary hypertension on survival in advanced lung disease. Lung 2015; 193: 213–221. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 11.Arcasoy SM, Christie JD, Ferrari VA, et al. . Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167: 735–740. [DOI] [PubMed] [Google Scholar]
- 12.Nathan SD, Shlobin OA, Barnett SD, et al. . Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2008; 102: 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, et al. . Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 14.Douglas PS, DeCara JM, Devereux RB, et al. . Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr 2009; 22: 755–765. [DOI] [PubMed] [Google Scholar]
- 15.Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J 1993; 6: Suppl. 16, 1–100. [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, et al. . Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26: 720–735. [DOI] [PubMed] [Google Scholar]
- 18.Goh NS, Desai SR, Veeraraghavan S, et al. . Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008; 177: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 19.Oudiz RJ. Classification of pulmonary hypertension. Cardiol Clin 2016; 34: 359–361. [DOI] [PubMed] [Google Scholar]
- 20.Modrykamien AM, Gudavalli R McCarthy K, et al. . Echocardiography, 6-minute walk distance, and distance-saturation product as predictors of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Respir Care 2010; 55: 584–588. [PubMed] [Google Scholar]
- 21.Alkukhun L, Wang XF, Ahmed MK, et al. . Non-invasive screening for pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2016; 117: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsallem M, Boulate D, Kooreman Z, et al. . Investigating the value of right heart echocardiographic metrics for detection of pulmonary hypertension in patients with advanced lung disease. Int J Cardiovasc Imaging 2017; 33: 825–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00124-2017_supp (319.8KB, pdf)
Figure S1 00124-2017_figureS1 (76.5KB, jpg)
Figure S2 00124-2017_figureS2 (43.3KB, jpg)