Abstract
Chronic inflammation plays a decisive role at different stages of cancer development. Inflammasomes are oligomeric protein complexes activated in response to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs and DAMPs are released from infected cells, tumors and damaged tissues. Inflammasomes activate and release inflammatory cytokines such as IL-1β and IL-18. The various inflammasomes and inflammatory cytokines and chemokines play contrasting roles in cancer development and progression. In this review, we describe the roles of different inflammasomes in lung, breast, gastric, liver, colon, and prostate cancers and in glioblastomas.
Keywords: Inflammasomes, cancer, tumor microenvironment, immune system
Introduction
Inflammasomes are oligomeric protein complexes that are activated upon recognition of diverse pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). They serve as platforms for caspase-1 activation and maturation of inflammatory cytokines, IL-1β and IL-18. Caspase1-associated cell death or pyroptosis is involved in inflammation and tissue repair [1,2]. Cleavage of gasdermin D (GSDMD) during pyroptosis [3] promotes the release of IL-1β and IL-18 [4] through the membrane pores [5].
Inflammasomes are classified as canonical and non-canonical types. The canonical inflammsomes include the NLR present in the complex such as the NLRP3-, NLRC4-, NLRP1-, or NLRP6-inflammasome and absent in melanoma 2 (AIM2), one of the PYHIN family members [1]. The non-canonical includes, and a complex that consists of CARD9, Malt1, Bcl-10, caspase-8 and ASC [6]. As shown in Figure 1A, canonical inflammasomes are activated in two steps [7], namely (1) NF-κB mediated transcription of pro-IL-1β and pro-IL-18 upon activation of toll-like receptors (TLRs), NOD1 or NOD2 by DAMPS, PAMPS or tumor necrosis factor-α (TNF-α) [8] and (2) inflammasomes also recognize DAMPs and PAMPs assembled with ASC and recruit procaspase-1 through its N-terminal caspase recruitment (CARD) domain followed by its autocatalytic cleavage. The assembly of different inflammasomes involves distinct proteins. NLRP1 inflammasome is independent of ASC [1]. NLRC4 inflammasomes are assembled when NAIP proteins sense bacterial proteins and recruit NLRC4 [1]. LPS-stimulated CD14 monocytes exhibit a distinct one-step pathway of inflammasome activation by regulating caspases-4 and -5, a process that requires Syk activity and Ca2+ flux [9]. AIM2 inflammasome is activated by binding to double-stranded DNA (dsDNA) resulting in structural changes [1]. However the mechanisms of activation of different inflammasomes by various signals are not fully understood.
Figure 1.
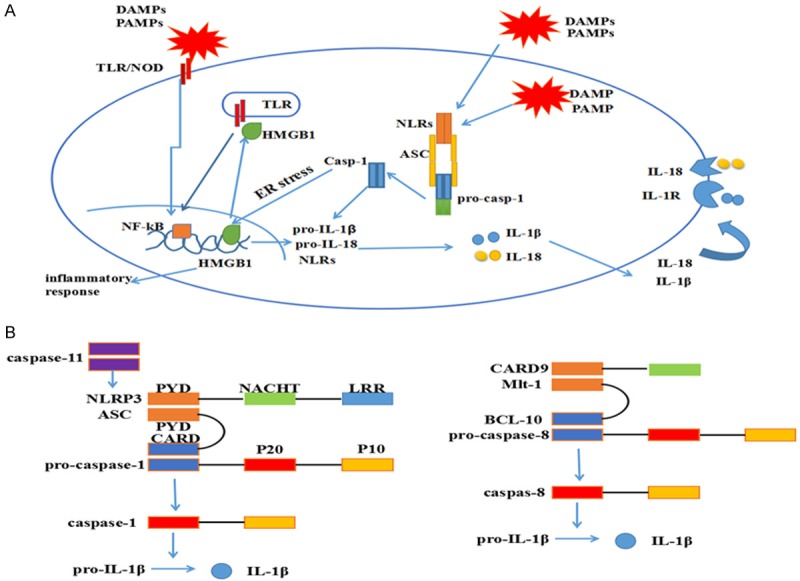
Activation of canonical and non-canonical inflammasomes. A: Canonical inflammasomes are activated in two steps. First, TLR or NOD1/2 sense PAMPs and DAMPS, which activate NF-κB and induce the expression of pro-IL1β and pro-IL18. Second step involves sensing of PAMPs and DAMPs by NLRs or AIM2 through mechanisms that are not fully understood. NLRP3 and NLRC4 interact with pro-caspase-1 through ASC, whereas NLRP1 interacts with caspase-1 directly. Activated NLRs promote conversion of pro-caspase-1 into caspase-1, which further catalyzes the proteolytic cleavage of pro-IL1β and pro-IL18 resulting in active IL-1β and IL-18. Caspase-1 also induces pyroptotic cell death. B: Non-canonical inflammasomes Malt1 activate caspase-8 and caspase-11, which induce pyroptosis, apoptosis and activation of IL-1β.
As shown in Figure 1B, noncanonical inflammasomes are activated by caspases-8 and -11 [7]. Caspase-11 cleaves gasdermin D and induces pyroptosis and activates IL-1β via NLRP3-dependent caspase-1 activation [10]. Caspase-8 is an IL-1β-converting enzyme during NLRP3 inflammasome activation in caspase1-deficient bone marrow-derived dendritic cells (BMDC) [11], dendritic cells (DCs) [12] and macrophages [13].
Inflammation plays an important role in many cancers. However, the role of inflammasomes in cancers is controversial. This is probably due to heterogeneity of cancer cells and the different cell types associated with cancer such as cancer-associated fibroblasts, tumor-infiltrating immune cells, endothelial cells, adipocytes and pericytes as well as various chemokines and cytokines [14,15]. Moreover, hypoxic microenvironment in cancer affects the function of inflammasomes in cancer. In this review, we describe the role of different inflammasomes in various malignancies.
Inflammasomes in lung cancer
Lung cancer is the predominant cause of cancer death worldwide with a 5 year survival rate of 16.6% after diagnosis [16]. In non-small-cell lung cancer (NSCLC) patients, serum levels of IL-18 [17] and IL-1β [18] were higher than healthy controls. Moreover, caspase-1, IL-1β, and IL-18 are overexpressed in lung cancer tissues and AIM2 inflammasome is upregulated in NSCLC, whereas high levels of NLRP3 inflammasome are reported in small-cell lung cancer (SCLC) [19]. Therefore, inflammasomes are associated with the biological features of lung cancer. Besides, different inflammasomes are expressed in distinct cell lines and tumor tissues suggesting that their transcription was cell and tissue specific. Therefore, inflammasomes in different subtypes of lung cancer are associated with their histological classification, grading, tumor invasion and chemoresistance [19].
Further, inflammasomes play critical roles in lung cancer. IL-1β represses microRNA-101 (miR-101) expression through the COX2-HIF1α pathway, thereby promoting the proliferation and migration of NSCLC cells [20]. This implies that inflammasomes regulate hypoxia in lung cancer. The NLRP3 inflammasome enhances human lung adenocarcinoma A549 cell proliferation via IL-1β/ERK/CREB and IL-18/AKT/CREK signaling pathways [21]. Moreover, activated NLRP3 inflammasome promotes the metastasis by decreasing E-cadherin and increasing Snail via IL1β [21]. In human NSCLC patients, decreased E-cadherin expression is associated with lymph node metastasis [22] and reduced overall survival [23]. On the other hand, Snail overexpression is associated with poor prognosis and NSCLC progression [24].
Immune response is critical in lung cancer progression. Plasmacytoid dendritic cells (pDCs) activate AIM2 that induces calcium efflux and mitochondrial reactive oxygen species (ROS), which results in activation of calpain and IL-1β that facilitate lung cancer cell proliferation [25]. However, a work report that pDCs play a dual role in lung tumor regression in mice model which was dependent on the different dose of LPS [26]. These diverse outcomes may be due to differences in the lung tumor phenotypes and models used for the study. Hence, inflammasomes are potential biomarkers as they modulate the biological behavior of different subtypes of lung cancer.
IL-1β gene polymorphism is associated with lung cancer risk. The IL-1β-31T/T genotype gene is associated with increased NSCLC risk [27], but, IL-1β-511C/C genotype is not associated with NSCLC [28]. However, both IL-1β-31T/T and IL-1β-511C/C genotypes are associated with greater risk of NSCLC in smokers and alcohol drinkers [28]. IL-1β-31 gene polymorphism was not associated with lung cancer risk in female non-smokers [29].
Various factors such as cigarette smoking, inhaled particulates, gender, race and ethnicity, age, obesity, infections, and other lung diseases or airway obstruction contribute to lung cancer [30]. Multiple genetic variations are associated with lung cancer suggesting comprehensive analyses [31]. Cigarette smoke (CS) exposure results in activation of IL-1β, a critical inflammatory cytokine. Inflammasome activation by CS promotes lung cancer by releasing CXCL-8 and IL-1β from HBE-14o cells [32]. Immune response to CS involves IL-1 receptor (IL-1R) signaling [33], caspase-1 and P2X7 receptor upregulation and activation [34] and NLRP3/caspase-1 cleavage of IL-1β [35]. Another component of cigarette smoke, α, β-unsaturated aldehyde acrolein represses NF-κB activation, which decreases IL-1β [36]. Besides, inflammasomes also participate in smoke-associated lung carcinogenesis by influencing function of natural killer cells [37]. XWLC-05 lung cancer cell line treated with silica particles secretes IL-1β upregulates enhancer of zeste homolog 2 (EZH2) by downregulating miR-101 and promotes tumor growth and progression [38].
The interaction between the tumor microenvironment and various cytokines and cell types is vital for lung cancer growth and progression. In C57BL/6 mice model with murine Lewis lung carcinoma cell xenograft, secretion of IL-1β from the tumor cells induced abundant vasculature and cytokines such as vascular endothelial growth factor (VEGF), macrophage-inflammatory protein-2 (CXCL2) and hepatocyte growth factor (HGF). This contributes to angiogenesis [39] and chemotactic migration of macrophages [40]. While cultured mouse Lewis lung carcinoma cells do not secrete HGF, co-culturing with stromal fibroblasts and tumor infiltrating cells produces HGF [41]. This suggests that the tumor microenvironment as well as stromal fibroblasts and tumor infiltrating cells is crucial in cancer progression.
In the lung metastasis model, IL-1β promotes the metastasis of breast cancer to lungs by increasing the infiltration of myeloid cells such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) into the tumor microenvironment [42]. NLRP3 promotes the lung metastasis of melanoma cells by suppressing NK cells [43]. Moreover, in the mouse lung metastasis model, the inhibition of NLRP3 suppresses the metastasis of HCC [44]. NLRP3 also promotes the lung metastasis of breast cancer by increasing lymphangiogenesis [45]. All of these studies provide evidence that inflammasomes promote lung metastasis by releasing inflammatory cytokines and suppress immune function. As shown in Figure 2, the role of inflammasomes in lung cancer is controversial and the outcomes depend on the type of stimulating factors and inflammasome components involved.
Figure 2.
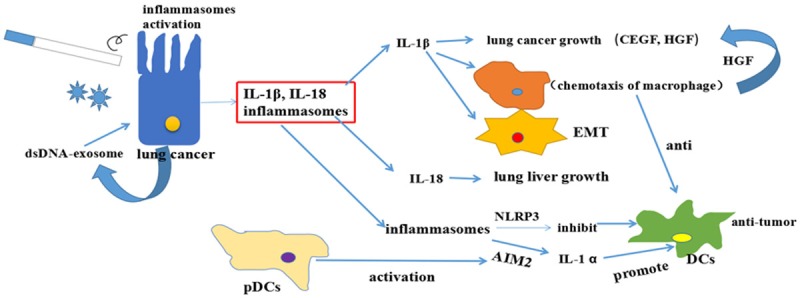
Inflammasomes in lung cancer. Cigarette smoking and inhaled particulate matter stimulates the airway or lung epithelium, which activates inflammasomes that induce the production of IL-1β and IL-18. Both IL-1β and IL-18 promote EMT and secretion of pro-inflammatory cytokines: VEGF, CXCL2 and HGF, which affect the tumor microenvironment and promote lung cancer progression. NLRP3 inflammasome promotes lung cancer by inhibiting natural killer cells (NKs). AIM2 from plasmacytoid dendritic cells (pDCs) suppresses lung cancer progression by activating NKs.
Inflammasomes in breast cancer
Breast cancer is the leading cancer diagnosed in women aged 20-45. The breast cancer risk factors include breast density age, family history and expression of estrogen receptor (ER), progesterone receptor (PR) and HER2 [46]. Moreover, inflammasomes play contrasting roles in breast cancer (Figure 3).
Figure 3.
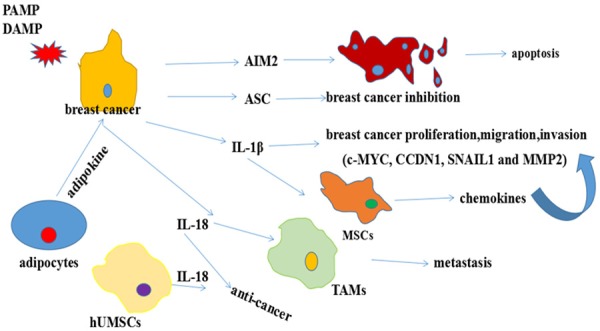
Inflammasomes in breast cancer. IL-1β promotes the proliferation, migration, invasion of breast cancer through the secretion of chemokines: VEGF and HGF. Mesenchymal stem cells (MSCs) that are stimulated by IL-1β also produce chemokines: IL-1β. Adipokines promote breast cancer cell migration and invasion by stimulating the expression and secretion of IL-18 in tumor-associated macrophages (TAMs). However, IL-18 overexpressing MSCs generated from human umbilical cord (hUMSCs) suppress the proliferation, migration and invasion of breast cancer cells. AIM2 and ASC also inhibit breast cancer development through promoting apoptosis.
The AIM2 inflammasome plays a positive role in breast cancer. In breast cancer cell lines MCF-7 cells, IFNγ induces AIM2 expression that promotes apoptosis through the mitochondrial pathway and the expression of pro-apoptotic proteins, Bad and Bax [47]. AIM2 suppresses human breast cancer cell proliferation and mammary tumor growth in a mouse model by repressing NFkB promoter activity [48]. The NLRP3 inflammasome promotes anti-tumor function by stimulating the dendritic cells to release IL-1β [49]. Different inflammasomes play contrasting roles in breast cancer growth and progression. IL-1β is the main effector of inflammasomes and plays a negative role in breast cancer. Increased serum levels of IL-1β are associated with a high rate of recurrence in breast cancer patients and are critical for tumor growth, angiogenesis, invasiveness, relapse and progression [50-53]. Breast cancer proliferation, migration and invasion are enhanced by the IL-1β /IL-1RI/β-catenin signaling pathway that increase expression of c-MYC, CCDN1, SNAIL1 and MMP2 [54]. IL-1β enhances invasiveness of breast ductal cancer cells by activating ERK1/2 [55]. In the tumor microenvironment, a positive feedback exists where in reduction of IL-1β by metastatic breast cancer cells stimulates MSCs to produce chemokine IL-1β that promote aggressiveness of the breast cancer cells [56].
Adipocytes secrete leptin that promotes breast cancer cell migration and invasion by enhancing the expression and secretion of IL-18 through tumor-associated macrophages (TAMs), which are linked to tumor progression and metastasis [57,58]. Moreover, IL-18 gene polymorphisms are associated with increased risk of breast cancer [59,60]. However, multiple studies show that IL-18 plays a positive role in breast cancer therapy. IL-18 overexpressing human mesenchymal stem cells derived from the human umbilical cord (hUMSCs) suppress the proliferation, migration and invasion of breast cancer cells [61,62]. This contradictory role of IL-18 suggests that the context and cell types that generate IL-18 critically modulate cancer progression. Nearly 15% of all breast carcinomas are triple-negative breast cancer (TNBC) subtype. TNBC is associated with poorer prognosis than other breast cancers because of its aggressive and metastatic behavior [63]. ZER is a sequiterpene isolated from Southeast Asian ginger that suppresses IL1β-induced migration and invasion of TNBC cells by inhibiting IL-8 and MMP-3 expression [52]. Therefore, ZER is a promising candidate for treatment of triple-negative breast cancer patients.
However, the role of inflammasomes in breast cancer is not clear-cut. Therefore, further investigations are necessary to determine the relationship of inflammasomes like NLRP1 and NLRP6 with breast cancer.
Inflammasomes in gastric cancer
Gastric cancer (GC) is ranked second among all cancer deaths worldwide [64]. Chronic inflammation is one of the main causes of GC. The etiological connection between gastric tumorigenesis and the chronic inflammation is demonstrated by its association with Helicobacter pylori (H. pylori) infection and chronic gastritis. H. pylori infection induces IL-1β and IL-18 expression through TLR-2/NLRP3/caspase-1 axis, which leads to persistent infection and immune tolerance that ultimately progresses to gastric cancer [65,66]. This process is also dependent on K+ efflux and Ca2+ signaling [67]. IL-18 secretion in response to H. pylori infection re-programs the dendritic cells (DCs) into a tolerance-promoting phenotype [68]. THP-1 cells infected by H. pylori also produce IL-1β [69]. H. pylori-induced expression of IL-1β promotes gastric carcinogenesis by upregulating COX-2 expression and repressing acid secretion [70]. Therefore, H. pylori promote gastric inflammation by inducing the secretion of IL-1β and IL-18, which regulate gastric immunity. Hence, elimination of H. pylori infection is critical to prevent gastric cancer. MUC1 is a mucin protein, which is a critical component of the epithelial barrier and protects against H. pylori-induced pathogenesis by inhibiting NLRP3 activation [71]. Withaferin A is a withanolide obtained from Withania somnifera, which inhibits IL-1β production by H. pylori in dendritic cells by inhibiting NLRP3 inflammasomes [72].
There is also increasing evidence that Mycoplasma hyorhinis (M. hyorhinis) plays an important role in many kinds of cancers including gastric cancer. M. hyorhinis-induced NLRP3 inflammasome reduces the release of IL-1β, which promotes gastric cancer metastasis [73].
IL-1β promotes inflammation-induced GC. In the gastric cancer patients, IL-1β levels are significantly higher in the serum and tumor tissues [74,75]. Moreover, IL-1β levels increase with gastric tumor progression [76]. Hence, IL-1β can be used as a prognostic biomarker. Furthermore, IL-1β gene polymorphisms show synergy with H. pylori infection in gastric cancer development [77-79]. IL-1β inhibits gastric acid secretion, induces epigenetic changes, promotes angiogenesis, mobilizes bone marrow cells and adhesive factors to migrate into the site of tumor and induces releases of other inflammatory factors [76]. IL-1β induces methylation of the E-cadherin, which increases the risk of gastric cancer [80]. These results demonstrate that IL-1β promotes proliferation, invasion and metastasis of gastric cancer cells and also affects the tumor microenvironment.
Many studies have investigated the role of individual inflammasome components in GI cancers. Low caspase-1 expression correlates with stage, lymph node metastasis and survival of gastric cancer patients [81]. In heliobacter infections, caspase-1 regulates the balance between Th1 and Th17 cells [82]. ASC/TMS1 mRNA and protein levels are decreased in GC tissues, whereas methylated ASC/TMS1 promoter is an independent prognostic indicator of GC [83].
However, the function of IL-18 and other inflammasomes in GC are not clear and require further investigation.
Inflammasomes in liver cancer
In 2008, liver cancer was the fourth most diagnosed cancer in males and seventh in females; it ranked second among males and fifth among females for cancer-related deaths [84]. In 2012, liver cancer was ranked second in cancer-related deaths worldwide [85]. These data reflect the poor prognosis of liver cancer. As shown in Figure 4, the inflammasomes play conflicting roles in liver cancer.
Figure 4.
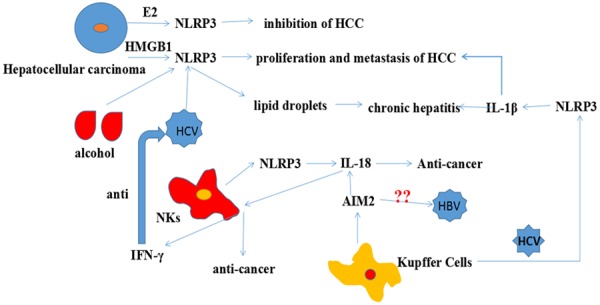
Inflammasomes in liver cancer. HMGB1, alcohol and HCV promote HCC proliferation and metastasis by activating the NLRP3 inflammasome. However, NLRP3 produced NKs can suppress HCC and 17β-estradiol (E2) also can inhibit the HCC. IL-18 stimulates NKs to secrete IFN-γ in response to HCV. Kupffer Cells stimulated by HBV also contribute to HCC.
Sex hormones play a protective role in hepatocellular carcinoma (HCC). The 17β-estradiol (E2) decreased HCC progression by increasing NLRP3 inflammasome via E2/ERβ/MAPK signaling pathway [86]. Moreover, NLRP3 inflammasome components are significantly downregulated in human HCC than inflamed and normal hepatic tissues [87]. These studies indicate that the NLRP3 inflammasome plays a positive role in HCC. However, many other studies suggest a contrary role of NLRP3 inflammasome in HCC. In hypoxia, HMGB1 enhances caspase-1, IL-1β and IL-18 levels through NLRP3 and promotes HCC invasion, whereas stable knockdown of HMGB1 suppresses HCC metastasis [88]. These data suggest that more comprehensive investigations are necessary to decipher the role of the NLRP3 inflammasome in HCC.
The interplay between NK cells and inflammasomes is critical in HCC progression. IL-18 induction due to NLRP3 inflammasome activation results in NK cell-mediated suppression of colorectal cancer metastasis to the liver [89]. AIM2 activation induces IL-18 expression in human Kupffer cells, which upon coculturing with NK cells produce IFNγ that regulates innate immunity [90]. IL-1β increases major histocompatibility complex class I-related chain A (MICA) levels, which antagonizes NKG2D-mediated immune-surveillance [91].
The major risk factor for HCC is cirrhosis, which is caused by viral infections alcohol and metabolic factors [92,93]. Inflammasomes are differentially regulated by various hepatitis B virus (HBV) antigens. In chronic hepatitis B (CHB) patients, high AIM2 expression is observed in the high HBV replication group than the low HBV replication group; moreover, high AIM2 levels are positively associated with IL-1β and IL-18 expression in CHB patients [94]. This observation suggests that AIM2 activation may eliminate hepatitis B virus. However, another study demonstrated that AIM2 prevents the recognition of dsDNA expressed by the HBV and therefore assists immune evasion [90]. NLRP3, which is activated by hepatitis C virus (HCV) accumulates in lipid droplets and coordinates with sterol regulatory element-binding proteins (SREBPs) to promote liver disease pathogenesis associated with chronic HCV [95]. In alcoholic liver disease (ALD), NLRP3 plays a protective role, whereas NLRC4 promotes ALD [96]. Ethanol induces the activation of HSCs and promotes production of pro-inflammatory cytokines such as IL-1β, which contribute to liver fibrosis [97]. IL-1β recruits and activates hepatic iNKT cells that promote liver inflammation and neutrophil infiltration, and induce alcohol-related liver injury [98]. Inflammasomes are also upregulated in the non-alcoholic steatohepatitis (NASH) [99]. DAMPs trigger endoplasmic reticulum (ER) stress, which activates inflammasomes leading to inflammation steatosis [100]. Thus, inflammasomes play an important role in the progression of hepatitis. However, the role of other inflammasomes like NLRP1 and NLRC4 requires in-depth investigation.
IL-1β and IL-18 are also important in hepatitis and HCC progression. IL-1β is involved in the pathology of viral fulminant hepatitis [101] and alcoholic steatohepatitis [102]. Hepatic macrophages induced by HCV produce IL-1β through the NLRP3 inflammasome, which contributes to the liver disease [103]. However, there are contrasting reports regarding IL-1β inhibiting HBV infection in liver cells. Watashi et al. reported that priming with IL-1β reduced host cell susceptibility to HBV infection via activation-induced cytidine deaminase (AID) [104] and oxidative stress [105]. HCV-infected monocytes induce IL-18 via inflammasomes that activate NK cells, which produce IFN-γ [106]. However, patients with chronic HCV infection show reduced monocytes function and low IFN-γ levels [106]. This may be due to changes in membrane protein composition on monocytes derived from chronic HCV patients that show depleted levels of IFN-γ due to decreased numbers of CD14+ monocytes [106]. These data show that inflammasomes derived from monocytes, NKs and macrophages perform different functions in hepatitis. Moreover, further investigations are necessary to decipher the roles of NLRP1, NLRP4 and NLRP6 in liver cancer.
Inflammasomes in colorectal cancer
Colorectal cancer (CRC) is one of the major causes of morbidity and mortality in developed countries. Recent data show that the incidence rates of CRC have rapidly increased in China. CRC is frequently associated with inflammatory bowel disease (IBD). Many studies have reported the role of individual inflammasome components in CRC. However, their specific roles may be context dependent. AIM2 reduces Akt activation and tumor burden in murine colorectal cancer models [107]. Reduced AIM2 expression is associated with advanced cancer stages [108] and poor outcomes for CRC patients [109]. However, AIM2 restoration promotes invasiveness in AIM2-deficient colon cancer cells [110]. AIM2-containing microsatellites in the coding region are associated with colorectal cancer progression [111].
NLRP3 induces epithelial-mesenchymal transition (EMT) in colon cancer cells, which contributes to metastasis [112]. Dietary cholesterol promotes AOM-induced colorectal cancer through the activation of NLRP3 by AMPKα/mitochondrial ROS signaling pathway [113]. NLRP3 inflammasome-mediated maturation of IL-1β and IL-18 is necessary for DSS-induced colitis [114]. NLRP3 depletion suppresses colitis [115]. However, some studies show that NLRP3 inflammasome protects against DSS-induced colitis [116] and colitis-associated tumorigenesis in hematopoietic cells [117]. NLRP3 inflammasome promotes IL-18 expression, which suppresses metastatic colorectal cancer growth in the liver by promoting tumoricidal activity of NK cells [89]. This implicates NLRP3 in promoting liver metastasis.
The role of NLRC4 is also controversial. Allen et al. reported that NLRP3-/- rather than NlRP4-/- mice exhibit increased colitis and tumorigenesis [117]. However, Hu et al. reported that Casp1-/- and Nlrc4-/- mice showed greater tumor load than Nlrp3-/- and wild-type mice and NLRC4 expression induces apoptosis and decreases the proliferation of epithelial cells [118]. Meanwhile, NLRP6 activity is necessary to regulate self-renewal of the intestinal epithelium and maintain intestinal homeostasis [119]. NLRP6 is critical in hematopoietic cells for protection against inflammation-related colon tumorigenesis [120]. NLRP6 suppresses tumorigenesis by promoting secretion of IL-18 from epithelial cells [121]. NLRP1 functions similar to NLRP6 in the colon epithelial cell compartment to repress tumorigenesis [122]. Moreover, NLRP1 attenuates CRC through IL-8 and IL-1β, similar to NLRP3 [122]. NLRP12 represses the noncanonical NF-κB pathway through NIK and TRAF3, resulting in suppression of colon inflammation and tumorigenesis [123].
IL-1β promotes CRC cell growth through IL-1β/NF-Kb/miR-181a/PTEN signaling pathway [124]. Complement system is involved in colorectal cancer through the IL-β/IL-17A axis through promoting the inflammation and complement-activation product C5a represented a potent inducer for IL-1β in neutrophil [125]. IL-1β promotes tumor growth and invasion by inducing epithelial to mesenchymal transition (EMT) and stem cell phenotype via Zeb1 [126]. In fibroblasts, IL-1β stimulates the production of COX-2, which is a major inflammatory mediator that promotes the proliferation and invasiveness of colon cancer cells [127]. IL-1β produced by neutrophils promotes colitis-associated tumorigenesis through IL-6 [128]. Thus, IL-1β plays a major role in CRC, CRC-associated microenvironment and CRC stem cells. Therefore, IL-1β inhibition is a potential therapeutic strategy to treat CRC. Inositol hexaphosphate inhibits the IL-1β-stimulated colon cancer by degrading MMPs [129].
However, IL-18 plays a tumor-suppressive role in CRC. Eosinophil promote the death of colon cancer cell colo-205 through IL-18 [130]. In Nlrp3 inflammasome-deficient mice, enhanced tumorigenesis is associated with deregulated IL-18 production and increased macrophage infiltration and IL-18-/- mice contained significantly more tumors than those of treated wild-type mice. IL-18 suppresses CRC through release IFN-γ and the IFN-γ promote the production of tumor suppressor STAT1 [131]. Moreover, IL-18 suppresses metastasis of CRC to lung cancer by activating T cells [132] and increasing the number of NK cells [89]. Thus, IL-18 can be used as an effective therapy for CRC.
Extrusion maintains homeostatic epithelial cell numbers and protects against infections. IL-18 secreted by the intestinal epithelial cells upon inflammasome activation recruit immune cells that prevent dissemination of bacteria traversing the epithelium [133,134]. However extrusion upregulates survival signaling in metastatic tumor cells and helps them to escape from the epithelium [135]. Moreover, persistent IL-18 may play a positive role in colitis [136] and colorectal cancer [137]. These studies demonstrate that extrusion plays a role in epithelium-derived tumor metastasis. However, it is not clear if cancer cells originating from the parenchyma cells induce metastasis through extrusion like hepatocellular carcinoma.
The gut microbiota also regulates susceptibility to multiple human diseases. Changes in intestinal microbial composition are associated with multiple human diseases like IBD and colon cancer [138]. Low fiber diet exacerbates colitis development, whereas very high intake of dietary fiber or short-chain fatty acids (SCFA) acetate protects against colitis by stimulating K+ efflux and hyperpolarization of colonic epithelial cells leading to NLRP3 inflammasome activation [139]. NLRP3 and AIM2 sense the microbial DNA and regulate the gut microbiota, especially in the context of colitis and colorectal cancer [140]. Aim2-/- mice are highly susceptible to colon tumor development and DSS-induced colitis due to perturbations in gut microbiota [141,142]. The activation of the NLRP6-ASC inflammasome does not change the gut microbiota composition [143]. Thus different inflammasomes play a complex role in CRC, depending on their source, context of cancer development, downstream signaling and gastrointestinal microflora.
Inflammasomes in prostate cancer
Prostate cancer is a male-specific cancer that especially affects older men. The initiation of prostate cancer has been linked with multiple factors such as age, race, diet, heredity, and environment [144]. In recent years, there has been a surge in understanding the role of inflammation in prostate cancer.
Hypoxia is a common feature of prostate cancer. Hypoxia contributes to prostate cancer by priming cells for the activation of NLRP3 and AIM2 inflammasomes [145]. Several stimuli such as uric acid crystals, urine reflux, bacteria, or fungi cause injury or infection within the prostate activates inflammasome-mediated proinflammatory cytokines and drive tumor development. For example, Propionibacterium acnes strongly activate inflammasomes in the neutrophils residing in the prostate gland, resulting in prostatitis and prostate cancer [146]. AIM2 inflammasome plays a role in the development of human prostate hyperplasia and prostate cancer [147].
IL-1β and IL-18 serum levels correlate with the risk of carcinoma and the prognosis of established prostate cancer. High serum IL-18 levels have been observed in locally advanced prostate cancer patients [148]. Besides, IL-1β and IL-18 exert immunosuppressive effects and support tumor promoting microenvironment [148]. IL-1β drives prostate cancer progression [149] and also promotes skeletal colonization and progression of metastatic prostate cancer cells [150]. Moreover, IL-1β polymorphisms such as IL-1β-511 (rs16944) and IL-1β-31 (rs1143627) are associated with prostate cancer risk [151,152].
Anakinra, a IL-1 receptor antagonist inhibits both IL-1α and IL-1β and is the most widely used therapeutic agent for prostate cancer [153]. Glucosamine attenuates prostate cancer cell proliferation and migration by targeting IL-1β [154]. Inflammasome-associated studies in prostate cancer require further detailed investigation.
Inflammasomes in glioblastoma
Glioblastoma is one of the most aggressive and fatal primary brain tumors in adults. Malignant gliomas are highly invasive with a dismal prognosis despite progress in early diagnosis and aggressive therapeutic interventions [155].
In patients with glioblastoma, the expression of NLRP3 inflammasome predicts poor survival in patients that have undergone radiotherapy [156]. NLRP3 inhibition reduces tumor growth and prolongs the survival of glioblastoma model mice following IR treatment [156]. Caspase-1 is increased in both glioblastoma tissues and glioma cell lines, U87 and T98G, whereas miR-214 inhibits glioblastoma cell proliferation and migration by suppressing caspase-1 [157].
However, the role of IL-1β in regulating glioblastoma progression is still controversial. IL-1β promotes proliferation, migration, and invasion of human glioma cells [158] and increases the cancer stem cell phenotype in murine and human proneural glioma stem cells [159]. IL-1β activates the p38 MAPK pathway and enhances IL-6 that promotes glioblastoma progression [160]. IL-1β also stimulates the secretion of exosomes containing the small heat shock protein, CRYAB that promotes glioblastoma progression [161]. However, IL-1β inhibits the transactivation activity of hypoxia-inducible factor 1 (HIF-1), thereby downregulating adrenomedullin (AM) expression and inducing glioma cell apoptosis [162]. Therefore, cancer microenvironment such as degree of hypoxia can influence cancer outcomes. IL-1β promotes the brain metastasis of breast cancer stem-like cells (CSCs) by activating astrocytes, which are critical for CSC survival [163].
IL-18 plays a controversial role in malignant glioma. IL-18 induces IL-2, which demonstrates significant antitumor activity in glioma models [164]. IL18-expressing BMSCs effectively inhibit intracranial glioma in rats through bone marrow-derived mesenchymal stem cells [165]. In the tumor microenvironment, IL-18 secreted by the microglia promotes migration of glioma cells [166]. However, IL-18 also promotes Fas-mediated apoptosis in Fas-transduced C6 glioma cells [167]. Moreover, IL-18 stimulates macrophages, T lymphocytes and NK cells to produce IFN γ that displays antitumor effects [168]. Moreover, intraperitoneal rIL-18 substantially delays the growth of subcutaneously inoculated gliomas in collaboration with natural killer cells [169].
Hence, different inflammasomes play distinct roles in glioma based on their origin. Moreover, the roles of NLRP1, NLRP6 and AIM2 in glioblastoma are still unknown.
Crosstalk between exosomes and inflammasomes in cancers
Exosomes are nano-sized membrane vesicles that are secreted by both normal and cancer cells, which mediate various biological processes such as immune response and tumorigenesis. Inflammasome-derived exosomes directly activate NF-κB signaling pathway in macrophages [170] which promotes gastric cancer progression [171].
AIM2 is activated by dsDNA from dead and cancer cells, which are sensed by AIM2 [1]. Exosomes containing dsDNA from intact lung cancer cells activate AIM2 in adjacent cells, leading to the production of inflammatory cytokines [172]. The pro-inflammatory cytokines and chemokines recruit stromal cells and induce angiogenesis leading to tumor progression [173]. Although chemotherapies are critical for cancer treatment, severe gastrointestinal tract toxicity restricts their application. The cytotoxicity is due to exosomes containing “self-DNA” that activates the AIM2 inflammasome, resulting in secretion of IL-1β and IL-18 that induces intestinal mucositis and late-onset diarrhea [174].
Tumor-derived exosomes serve as vehicles of intercellular communication and are emerging as mediators of tumorigenesis and immune escape. Exosomes from the serum of patients with nasopharyngeal carcinoma (NPC) or the supernatant of TW03 cells increase the production of proinflammatory cytokines such as IL-1β, IL-6, and IL-10, which correlates with advanced lymph node stage and poor prognosis in NPC patients [175].
Liver fibrosis is the main cause of hepatocellular carcinoma. Exosomes derived from CCl4-treated hepatocytes induce the expression of IL-1β and IL-23 in HSCs [176] and contribute to liver fibrosis. In non-alcoholic steatohepatitis (NASH), lipids induce release of hepatocyte exosomes, which activates the macrophages that release IL-1β and IL-6 [177].
These studies indicate that exosomes serve as vehicles to activate inflammasomes and promote the production of inflammatory factors, which are critical for tumor progression. However, the connection of exosomes and NLRP1, NLRP3, NLRP6 inflammasomes is unknown. Moreover, it is unclear if exosomes containing inflammasomes form a metastasis niche. More investigations are necessary to unravel these unanswered questions.
Perspectives
In recent years, the complex roles of activated inflammasomes have gained attention in cancer development and therapy. Many factors trigger activation of inflammasomes. Moreover, different inflammasomes play diverse roles within the same tissue. Therefore, given their complex roles, it is important to explore their function in context of tissue or cell specificity and cancer stages. Compounds that target inflammasomes such as IL-1β neutralizing antibodies and IL-18 binding protein have been developed. However, their clinical application in cancer therapy needs to be determined. In the future, the mechanism of the activation of the various inflammasomes and their effect on the immune system needs to be addressed in greater detail. The various signaling mechanisms that regulate the activation of different inflammasomes also need to be defined in greater detail. While pyroptosis is vital in anti-cancer treatment, oxidative stress, mitochondrial dysfunction and release of inflammatory factors are also involved in carcinogenesis. Therefore, their role in tumor cell apoptosis, necrosis and ferroptosis needs to be explored.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81472311, No. 81772607).
Disclosure of conflict of interest
None.
References
- 1.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 3.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 4.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–54. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 7.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a doubleedged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. 2015;6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 11.Antonopoulos C, Russo HM, El SC, Martin BN, Li X, Kaiser WJ, Mocarski ES, Dubyak GR. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem. 2015;290:20167–84. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonopoulos C, El SC, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurung P, Anand PK, Malireddi RK, Vande WL, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–46. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y, Zang A, Jiao S, Chen S, Yan F. The interleukin-18 gene promoter -607 A/C polymorphism contributes to non-small-cell lung cancer risk in a Chinese population. Onco Targets Ther. 2016;9:1715–9. doi: 10.2147/OTT.S99581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zhao W, Zhao Z, Wu J, Chen L, Ma Y, Li Q, Lu D, Jin L, Wang J. IL1B gene polymorphisms, age and the risk of non-small cell lung cancer in a Chinese population. Lung Cancer. 2015;89:232–7. doi: 10.1016/j.lungcan.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36:7501–13. doi: 10.1007/s13277-015-3473-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, Lou JT, Liu MF. IL-1beta-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. 2014;74:4720–30. doi: 10.1158/0008-5472.CAN-14-0960. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan X, Wang H, Xie W. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol Rep. 2016;35:2053–64. doi: 10.3892/or.2016.4569. [DOI] [PubMed] [Google Scholar]
- 22.Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–96. [PubMed] [Google Scholar]
- 23.Yan B, Zhang W, Jiang LY, Qin WX, Wang X. Reduced E-Cadherin expression is a prognostic biomarker of non-small cell lung cancer: a metaanalysis based on 2395 subjects. Int J Clin Exp Med. 2014;7:4352–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Grant JL, Fishbein MC, Hong LS, Krysan K, Minna JD, Shay JW, Walser TC, Dubinett SM. A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev Res (Phila) 2014;7:150–60. doi: 10.1158/1940-6207.CAPR-13-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorrentino R, Terlizzi M, Di Crescenzo VG, Popolo A, Pecoraro M, Perillo G, Galderisi A, Pinto A. Human lung cancer-derived immunosuppressive plasmacytoid dendritic cells release IL-1alpha in an AIM2 inflammasome-dependent manner. Am J Pathol. 2015;185:3115–24. doi: 10.1016/j.ajpath.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Rega A, Terlizzi M, Luciano A, Forte G, Crother TR, Arra C, Arditi M, Pinto A, Sorrentino R. Plasmacytoid dendritic cells play a key role in tumor progression in lipopolysaccharide-stimulated lung tumor-bearing mice. J Immunol. 2013;190:2391–402. doi: 10.4049/jimmunol.1202086. [DOI] [PubMed] [Google Scholar]
- 27.Bhat IA, Naykoo NA, Qasim I, Ganie FA, Yousuf Q, Bhat BA, Rasool R, Aziz SA, Shah ZA. Association of interleukin 1 beta (IL-1beta) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene. 2014;2:123–33. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu KS, Zhou X, Zheng F, Xu XQ, Lin YH, Yang J. Influence of interleukin-1 beta genetic polymorphism, smoking and alcohol drinking on the risk of non-small cell lung cancer. Clin Chim Acta. 2010;411:1441–6. doi: 10.1016/j.cca.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Su M, Zhou B. [Association of genetic polymorphisms in IL-6 and IL-1beta gene with risk of lung cancer in female non-smokers] . Zhongguo Fei Ai Za Zhi. 2014;17:612–7. doi: 10.3779/j.issn.1009-3419.2014.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen JE, Cascone T, Gerber DE, Heymach JV, Minna JD. Targeted therapies for lung cancer: clinical experience and novel agents. Cancer J. 2011;17:512–27. doi: 10.1097/PPO.0b013e31823e701a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall AL, Christiani DC. Genetic susceptibility to lung cancer--light at the end of the tunnel? Carcinogenesis. 2013;34:487–502. doi: 10.1093/carcin/bgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, Folkerts G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim Biophys Acta. 2011;1812:1104–10. doi: 10.1016/j.bbadis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–78. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 34.Eltom S, Stevenson CS, Rastrick J, Dale N, Raemdonck K, Wong S, Catley MC, Belvisi MG, Birrell MA. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One. 2011;6:e24097. doi: 10.1371/journal.pone.0024097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden BT, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–28. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Hamilton RJ, Taylor DE, Holian A. Acroleininduced cell death in human alveolar macrophages. Toxicol Appl Pharmacol. 1997;145:331–9. doi: 10.1006/taap.1997.8189. [DOI] [PubMed] [Google Scholar]
- 37.Ather JL, Martin RA, Ckless K, Poynter ME. Inflammasome activity in non-microbial lung Inflammation. J Environ Immunol Toxicol. 2014;1:108–117. [PMC free article] [PubMed] [Google Scholar]
- 38.Lei YM, Zu YF, Wang J, Bai S, Shi YF, Shi R, Duan J, Cui D, Chen J, Xiang Y, Dong J. Interleukin-1beta-mediated suppression of microRNA-101 and upregulation of enhancer of zeste homolog 2 is involved in particle-induced lung cancer. Med Oncol. 2015;32:387. doi: 10.1007/s12032-014-0387-8. [DOI] [PubMed] [Google Scholar]
- 39.Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1–19. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherry B, Horii Y, Manogue KR, Widmer U, Cerami A. Macrophage inflammatory proteins 1 and 2: an overview. Cytokines. 1992;4:117–30. [PubMed] [Google Scholar]
- 41.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, Nukiwa T. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–75. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 42.Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep. 2016;6:36107. doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, Moller A, Smyth MJ. NLRP3 suppresses NK cell-mediated responses to carcinogeninduced tumors and metastases. Cancer Res. 2012;72:5721–32. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 44.Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Li MQ, Hu B, Zhang ZF, Cheng W, Shan Q. Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One. 2014;9:e89961. doi: 10.1371/journal.pone.0089961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, Frank AC, Scholich K, Pierre S, Syed SN, Olesch C, Ringleb J, Ören B, Döring C, Savai R, Jung M, von Knethen A, Levkau B, Fleming I, Weigert A, Brüne B. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1beta. J Exp Med. 2017;214:2695–2713. doi: 10.1084/jem.20160392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westbrook K, Stearns V. Pharmacogenomics of breast cancer therapy: an update. Pharmacol Ther. 2013;139:1–11. doi: 10.1016/j.pharmthera.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu ZY, Yi J, Liu FE. The molecular mechanism of breast cancer cell apoptosis induction by absent in melanoma (AIM2) Int J Clin Exp Med. 2015;8:14750–8. [PMC free article] [PubMed] [Google Scholar]
- 48.Chen IF, Ou-Yang F, Hung JY, Liu JC, Wang H, Wang SC, Hou MF, Hortobagyi GN, Hung MC. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5:1–7. doi: 10.1158/1535-7163.MCT-05-0310. [DOI] [PubMed] [Google Scholar]
- 49.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 50.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKeown HF. Maurice Berman Prize 2003. J Orthod. 2004;31:279–87. doi: 10.1179/146531204225020562. [DOI] [PubMed] [Google Scholar]
- 52.Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee HC, Lee SK, Kil WH, Kim SW, Nam SJ, Kim S, Lee JE. Zerumbone suppresses IL-1beta-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytother Res. 2014;28:1654–60. doi: 10.1002/ptr.5178. [DOI] [PubMed] [Google Scholar]
- 53.Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, BenBaruch A. Inflammatory mediators in breast cancer: coordinated expression of TNFalpha & IL-1beta with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;11:130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Yepez EA, Ayala-Sumuano JT, Lezama R, Meza I. A novel beta-catenin signaling pathway activated by IL-1beta leads to the onset of epithelialmesenchymal transition in breast cancer cells. Cancer Lett. 2014;354:164–71. doi: 10.1016/j.canlet.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Lan F, Zheng Z, Xie F, Wang L, Liu W, Han J, Zheng F, Xie Y, Huang Q. Epidermal growth factor (EGF) and interleukin (IL)-1beta synergistically promote ERK1/2-mediated invasive breast ductal cancer cell migration and invasion. Mol Cancer. 2012;11:79. doi: 10.1186/1476-4598-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escobar P, Bouclier C, Serret J, Bieche I, Brigitte M, Caicedo A, Sanchez E, Vacher S, Vignais ML, Bourin P, Genevieve D, Molina F, Jorgensen C, Lazennec G. IL-1beta produced by aggressive breast cancer cells is one of the factors that dictate their interactions with mesenchymal stem cells through chemokine production. Oncotarget. 2015;6:29034–47. doi: 10.18632/oncotarget.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li K, Wei L, Huang Y, Wu Y, Su M, Pang X, Wang N, Ji F, Zhong C, Chen T. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol. 2016;48:2479–87. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 58.Chanmee T, Ontong P, Konno K, Itano N. Tumorassociated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Ren D, Li Y, Xu J, Liu C, Zhao Y. Increased cancer risk associated with the -607C/A polymorphism in interleukin-18 gene promoter: an updated meta-analysis including 12,502 subjects. J BUON. 2015;20:902–17. [PubMed] [Google Scholar]
- 60.Back LK, Farias TD, Da CP, Muniz YC, Ribeiro MC, Fernandes BL, Fernandes CK, de Souza IR. Functional polymorphisms of interleukin-18 gene and risk of breast cancer in a Brazilian population. Tissue Antigens. 2014;84:229–33. doi: 10.1111/tan.12367. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Hu J, Sun S, Li F, Cao W, Wang YU, Ma Z, Yu Z. Mesenchymal stem cells expressing interleukin-18 suppress breast cancer cells in vitro. Exp Ther Med. 2015;9:1192–1200. doi: 10.3892/etm.2015.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun S, Liu X, Jiang D, Lu Z, Li F. [Effect of interleukin-18 gene modified human umbilical cord mesenchymal stem cells on proliferation of breast cancer cell] . Zhonghua Yi Xue Za Zhi. 2014;94:2013–7. [PubMed] [Google Scholar]
- 63.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 64.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 65.Pachathundikandi SK, Muller A, Backert S. Inflammasome activation by helicobacter pylori and its implications for persistence and immunity. Curr Top Microbiol Immunol. 2016;397:117–31. doi: 10.1007/978-3-319-41171-2_6. [DOI] [PubMed] [Google Scholar]
- 66.Koch KN, Muller A. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes. 2015;6:382–7. doi: 10.1080/19490976.2015.1105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kameoka S, Kameyama T, Hayashi T, Sato S, Ohnishi N, Hayashi T, Murata-Kamiya N, Higashi H, Hatakeyama M, Takaoka A. Helicobacter pylori induces IL-1beta protein through the inflammasome activation in differentiated macrophagic cells. Biomed Res. 2016;37:21–7. doi: 10.2220/biomedres.37.21. [DOI] [PubMed] [Google Scholar]
- 68.Oertli M, Muller A. Helicobacter pylori targets dendritic cells to induce immune tolerance, promote persistence and confer protection against allergic asthma. Gut Microbes. 2012;3:566–71. doi: 10.4161/gmic.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kameoka S, Kameyama T, Hayashi T, Sato S, Ohnishi N, Hayashi T, Murata-Kamiya N, Higashi H, Hatakeyama M, Takaoka A. Helicobacter pylori induces IL-1beta protein through the inflammasome activation in differentiated macrophagic cells. Biomed Res. 2016;37:21–7. doi: 10.2220/biomedres.37.21. [DOI] [PubMed] [Google Scholar]
- 70.Guo T, Qian JM, Zhao YQ, Li XB, Zhang JZ. Effects of IL-1beta on the proliferation and apoptosis of gastric epithelial cells and acid secretion from isolated rabbit parietal cells. Mol Med Rep. 2013;7:299–305. doi: 10.3892/mmr.2012.1165. [DOI] [PubMed] [Google Scholar]
- 71.Ng GZ, Menheniott TR, Every AL, Stent A, Judd LM, Chionh YT, Dhar P, Komen JC, Giraud AS, Wang TC, McGuckin MA, Sutton P. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut. 2016;65:1087–99. doi: 10.1136/gutjnl-2014-307175. [DOI] [PubMed] [Google Scholar]
- 72.Kim JE, Lee JY, Kang MJ, Jeong YJ, Choi JA, Oh SM, Lee KB, Park JH. Withaferin A inhibits helicobacter pylori-induced production of IL-1beta in dendritic cells by regulating NF-kappaB and NLRP3 inflammasome activation. Immune Netw. 2015;15:269–77. doi: 10.4110/in.2015.15.6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J, Meng G. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One. 2013;8:e77955. doi: 10.1371/journal.pone.0077955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deans DA, Wigmore SJ, Gilmour H, PatersonBrown S, Ross JA, Fearon KC. Elevated tumour interleukin-1beta is associated with systemic inflammation: a marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006;95:1568–75. doi: 10.1038/sj.bjc.6603446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macri A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C. Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184–93. doi: 10.1080/13547500600565677. [DOI] [PubMed] [Google Scholar]
- 76.Yin S, Lan C, Pei H, Zhu Z. Expression of interleukin 1beta in gastric cancer tissue and its effects on gastric cancer. Onco Targets Ther. 2016;9:31–5. doi: 10.2147/OTT.S94277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B, Luo MX, Zhou X, Lv Y, Su GQ. Correlation between interleukin-1beta-511 C/T polymorphism and gastric cancer in Chinese populations: a meta-analysis. Med Sci Monit. 2016;22:1742–50. doi: 10.12659/MSM.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hong JB, Zuo W, Wang AJ, Lu NH. Helicobacter pylori infection synergistic with IL-1beta gene polymorphisms potentially contributes to the carcinogenesis of gastric cancer. Int J Med Sci. 2016;13:298–303. doi: 10.7150/ijms.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park MJ, Hyun MH, Yang JP, Yoon JM, Park S. Effects of the interleukin-1beta-511 C/T gene polymorphism on the risk of gastric cancer in the context of the relationship between race and H. pylori infection: a meta-analysis of 20,000 subjects. Mol Biol Rep. 2015;42:119–34. doi: 10.1007/s11033-014-3748-7. [DOI] [PubMed] [Google Scholar]
- 80.Huang FY, Chan AO, Rashid A, Wong DK, Seto WK, Cho CH, Lai CL, Yuen MF. Interleukin-1beta increases the risk of gastric cancer through induction of aberrant DNA methylation in a mouse model. Oncol Lett. 2016;11:2919–2924. doi: 10.3892/ol.2016.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jee CD, Lee HS, Bae SI, Yang HK, Lee YM, Rho MS, Kim WH. Loss of caspase-1 gene expression in human gastric carcinomas and cell lines. Int J Oncol. 2005;26:1265–71. [PubMed] [Google Scholar]
- 82.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol. 2012;188:3594–602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 83.Wu L, Zhang C, Wang X, Ding X, Deng J, Liang H. Methylation of ASC/TMS1 promoter is associated with poor prognosis of patients with gastric cancer. Clin Transl Oncol. 2016;18:296–303. doi: 10.1007/s12094-015-1367-y. [DOI] [PubMed] [Google Scholar]
- 84.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 85.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–24. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 86.Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Chen X, Han L. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804–16. doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- 87.Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, Han L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52–62. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 88.Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–75. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, RodrigueGervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity. 2015;43:751–63. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Zannetti C, Roblot G, Charrier E, Ainouze M, Tout I, Briat F, Isorce N, Faure-Dupuy S, Michelet M, Marotel M, Kati S, Schulz TF, Rivoire M, Traverse-Glehen A, Luangsay S, Alatiff O, Henry T, Walzer T, Durantel D, Hasan U. Characterization of the inflammasome in human kupffer cells in response to synthetic agonists and pathogens. J Immunol. 2016;197:356–67. doi: 10.4049/jimmunol.1502301. [DOI] [PubMed] [Google Scholar]
- 91.Kohga K, Tatsumi T, Tsunematsu H, Aono S, Shimizu S, Kodama T, Hikita H, Yamamoto M, Oze T, Aketa H, Hosui A, Miyagi T, Ishida H, Hiramatsu N, Kanto T, Hayashi N, Takehara T. Interleukin-1beta enhances the production of soluble MICA in human hepatocellular carcinoma. Cancer Immunol Immunother. 2012;61:1425–32. doi: 10.1007/s00262-012-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 93.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han Y, Chen Z, Hou R, Yan D, Liu C, Chen S, Li X, Du W. Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients. Virol J. 2015;12:129. doi: 10.1186/s12985-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McRae S, Iqbal J, Sarkar-Dutta M, Lane S, Nagaraj A, Ali N, Waris G. The hepatitis C virus-induced NLRP3 inflammasome activates the sterol regulatory element-binding protein (SREBP) and regulates lipid metabolism. J Biol Chem. 2016;291:3254–67. doi: 10.1074/jbc.M115.694059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.DeSantis DA, Ko CW, Liu Y, Liu X, Hise AG, Nunez G, Croniger CM. Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm. 2013;2013:751374. doi: 10.1155/2013/751374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955–65. doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 98.Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, Bai L, Lian Z, Wei H, Sun R, Tian Z. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J Hepatol. 2015;62:1311–8. doi: 10.1016/j.jhep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 99.Szabo G, Iracheta-Vellve A. Inflammasome activation in the liver: focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S18–23. doi: 10.1016/j.clinre.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Zhang K, Li Z, Guo B. ER Stress-induced inflammasome activation contributes to hepatic inflammation and steatosis. J Clin Cell Immunol. 2016:7. doi: 10.4172/2155-9899.1000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo S, Yang C, Diao B, Huang X, Jin M, Chen L, Yan W, Ning Q, Zheng L, Wu Y, Chen Y. The NLRP3 inflammasome and IL-1beta accelerate immunologically mediated pathology in experimental viral fulminant hepatitis. PLoS Pathog. 2015;11:e1005155. doi: 10.1371/journal.ppat.1005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iracheta-Vellve A, Petrasek J, Satishchandran A, Gyongyosi B, Saha B, Kodys K, Fitzgerald KA, Kurt-Jones EA, Szabo G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol. 2015;63:1147–55. doi: 10.1016/j.jhep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale MJ. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watashi K, Liang G, Iwamoto M, Marusawa H, Uchida N, Daito T, Kitamura K, Muramatsu M, Ohashi H, Kiyohara T, Suzuki R, Li J, Tong S, Tanaka Y, Murata K, Aizaki H, Wakita T. Interleukin-1 and tumor necrosis factor-alpha trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID) J Biol Chem. 2013;288:31715–27. doi: 10.1074/jbc.M113.501122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Togashi H, Ohno S, Matsuo T, Watanabe H, Saito T, Shinzawa H, Takahashi T. Interferongamma, tumor necrosis factor-alpha, and interleukin 1-beta suppress the replication of hepatitis B virus through oxidative stress. Res Commun Mol Pathol Pharmacol. 2000;107:407–17. [PubMed] [Google Scholar]
- 106.Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147:209–220. doi: 10.1053/j.gastro.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:90613. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu R, Truax AD, Chen L, Hu P, Li Z, Chen J, Song C, Chen L, Ting JP. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget. 2015;6:33456–69. doi: 10.18632/oncotarget.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135:2387–96. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 110.Patsos G, Germann A, Gebert J, Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer. 2010;126:1838–1849. doi: 10.1002/ijc.24905. [DOI] [PubMed] [Google Scholar]
- 111.Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz M, Gebert J, Dihlmann S. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer. 2007;46:1080–9. doi: 10.1002/gcc.20493. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, Wang Y, Du Q, Lu P, Fan H, Lu J, Hu R. Inflammasome-independent NLRP3 is required for epithelial-mesenchymal transition in colon cancer cells. Exp Cell Res. 2016;342:184–92. doi: 10.1016/j.yexcr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Du Q, Wang Q, Fan H, Wang J, Liu X, Wang H, Wang Y, Hu R. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem Pharmacol. 2016;105:42–54. doi: 10.1016/j.bcp.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 114.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–9. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 115.Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors. Dig Dis. 2012;30(Suppl 1):82–90. doi: 10.1159/000341681. [DOI] [PubMed] [Google Scholar]
- 116.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–91. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–40. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci U S A. 2011;108:9601–6. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187–94. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Williams TM, Leeth RA, Rothschild DE, Coutermarsh-Ott SL, McDaniel DK, Simmons AE, Heid B, Cecere TE, Allen IC. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J Immunol. 2015;194:3369–80. doi: 10.4049/jimmunol.1402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–54. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hai PP, Feng BT, Li L, Nan HY, Hong Z. IL-1beta/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys. 2016;604:20–6. doi: 10.1016/j.abb.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Ning C, Li YY, Wang Y, Han GC, Wang RX, Xiao H, Li XY, Hou CM, Ma YF, Sheng DS, Shen BF, Feng JN, Guo RF, Li Y, Chen GJ. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1beta/IL-17A axis. Mucosal Immunol. 2015;8:1275–84. doi: 10.1038/mi.2015.18. [DOI] [PubMed] [Google Scholar]
- 126.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu Y, Zhu M, Lance P. IL1beta-mediated Stromal COX-2 signaling mediates proliferation and invasiveness of colonic epithelial cancer cells. Exp Cell Res. 2012;318:2520–30. doi: 10.1016/j.yexcr.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Wang K, Han GC, Wang RX, Xiao H, Hou CM, Guo RF, Dou Y, Shen BF, Li Y, Chen GJ. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014;7:1106–15. doi: 10.1038/mi.2013.126. [DOI] [PubMed] [Google Scholar]
- 129.Kapral M, Wawszczyk J, Jurzak M, Hollek A, Weglarz L. The effect of inositol hexaphosphate on the expression of selected metalloproteinases and their tissue inhibitors in IL-1beta-stimulated colon cancer cells. Int J Colorectal Dis. 2012;27:1419–28. doi: 10.1007/s00384-012-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, Capron M. IL-18 Is Involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J Immunol. 2015;195:2483–92. doi: 10.4049/jimmunol.1402914. [DOI] [PubMed] [Google Scholar]
- 131.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–20. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang CY, Lee J, Kim EY, Park HJ, Kwon CH, Joh JW, Kim SJ. Intratumoral delivery of IL-18 naked DNA induces T-cell activation and Th1 response in a mouse hepatic cancer model. BMC Cancer. 2007;7:87. doi: 10.1186/1471-2407-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity. 2017;46:649–659. doi: 10.1016/j.immuni.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Broz P. Getting rid of the bad apple: inflammasomeinduced extrusion of Salmonella-infected enterocytes. Cell Host Microbe. 2014;16:153–155. doi: 10.1016/j.chom.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 135.Slattum GM, Rosenblatt J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer. 2014;14:495–501. doi: 10.1038/nrc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelialderived IL-18 regulates Th17 cell differentiation and Foxp3(+) Treg cell function in the intestine. Mucosal Immunol. 2015;8:1226–36. doi: 10.1038/mi.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, Flavell RA. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110:9862–7. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 139.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian MC, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 140.Man SM, Karki R, Kanneganti TD. DNA-sensing inflammasomes: regulation of bacterial host defense and the gut microbiota. Pathog Dis. 2016;74:ftw028. doi: 10.1093/femspd/ftw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD. Critical role for the DNA Sensor AIM2 in Stem cell proliferation and cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu S, Peng L, Kwak YT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH. The DNA Sensor AIM2 maintains intestinal homeostasis via regulation of epithelial antimicrobial host defense. Cell Rep. 2015;13:1922–36. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mamantopoulos M, Ronchi F, Van Hauwermeiren F, Vieira-Silva S, Yilmaz B, Martens L, Saeys Y, Drexler SK, Yazdi AS, Raes J, Lamkanfi M, McCoy KD, Wullaert A. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity. 2017;47:339–348. doi: 10.1016/j.immuni.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 144.Karan D, Thrasher JB, Lubaroff D. Prostate cancer: genes, environment, immunity and the use of immunotherapy. Prostate Cancer Prostatic Dis. 2008;11:230–6. doi: 10.1038/pcan.2008.3. [DOI] [PubMed] [Google Scholar]
- 145.Panchanathan R, Liu H, Choubey D. Hypoxia primes human normal prostate epithelial cells and cancer cell lines for the NLRP3 and AIM2 inflammasome activation. Oncotarget. 2016;7:28183–94. doi: 10.18632/oncotarget.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sahdo B, Sarndahl E, Elgh F, Soderquist B. Propionibacterium acnes activates caspase-1 in human neutrophils. APMIS. 2013;121:652–63. doi: 10.1111/apm.12035. [DOI] [PubMed] [Google Scholar]
- 147.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11:1193–202. doi: 10.1158/1541-7786.MCR-13-0145. [DOI] [PubMed] [Google Scholar]
- 148.Dwivedi S, Goel A, Natu SM, Mandhani A, Khattri S, Pant KK. Diagnostic and prognostic significance of prostate specific antigen and serum interleukin 18 and 10 in patients with locally advanced prostate cancer: a prospective study. Asian Pac J Cancer Prev. 2011;12:1843–8. [PubMed] [Google Scholar]
- 149.Chang MA, Patel V, Gwede M, Morgado M, Tomasevich K, Fong EL, Farach-Carson MC, Delk NA. IL-1beta induces p62/SQSTM1 and represses androgen receptor expression in prostate cancer cells. J Cell Biochem. 2014;115:2188–97. doi: 10.1002/jcb.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Q, Russell MR, Shahriari K, Jernigan DL, Lioni MI, Garcia FU, Fatatis A. Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res. 2013;73:3297–305. doi: 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 151.Xu H, Ding Q, Jiang HW. Genetic polymorphism of interleukin-1A (IL-1A), IL-1B, and IL-1 receptor antagonist (IL-1RN) and prostate cancer risk. Asian Pac J Cancer Prev. 2014;15:8741–7. doi: 10.7314/apjcp.2014.15.20.8741. [DOI] [PubMed] [Google Scholar]
- 152.Yencilek F, Yildirim A, Yilmaz SG, Altinkilic EM, Dalan AB, Bastug Y, Isbir T. Investigation of Interleukin-1beta polymorphisms in prostate cancer. Anticancer Res. 2015;35:6057–61. [PubMed] [Google Scholar]
- 153.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai CY, Lee TS, Kou YR, Wu YL. Glucosamine inhibits IL-1beta-mediated IL-8 production in prostate cancer cells by MAPK attenuation. J Cell Biochem. 2009;108:489–98. doi: 10.1002/jcb.22278. [DOI] [PubMed] [Google Scholar]
- 155.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li L, Liu Y. Aging-related gene signature regulated by Nlrp3 predicts glioma progression. Am J Cancer Res. 2015;5:442–9. [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang Z, Yao L, Ma H, Xu P, Li Z, Guo M, Chen J, Bao H, Qiao S, Zhao Y, Shen J, Zhu M, Meyers C, Ma G, Xie C, Liu L, Wang H, Zhang W, Dong Q, Shen H, Lin Z. miRNA-214 inhibits cellular proliferation and migration in glioma cells targeting caspase 1 involved in pyroptosis. Oncol Res. 2017;25:1009–1019. doi: 10.3727/096504016X14813859905646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fathima HK, Ramaswamy P, Nandakumar DN. IL-1beta microenvironment promotes proliferation, migration, and invasion of human glioma cells. Cell Biol Int. 2014;38:1415–22. doi: 10.1002/cbin.10353. [DOI] [PubMed] [Google Scholar]
- 159.Feng X, Szulzewsky F, Yerevanian A, Chen Z, Heinzmann D, Rasmussen RD, Alvarez-Garcia V, Kim Y, Wang B, Tamagno I, Zhou H, Li X, Kettenmann H, Ransohoff RM, Hambardzumyan D. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–94. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gurgis FM, Yeung YT, Tang MX, Heng B, Buckland M, Ammit AJ, Haapasalo J, Haapasalo H, Guillemin GJ, Grewal T, Munoz L. The p38-MK2-HuR pathway potentiates EGFRvIII-IL-1beta-driven IL-6 secretion in glioblastoma cells. Oncogene. 2015;34:2934–42. doi: 10.1038/onc.2014.225. [DOI] [PubMed] [Google Scholar]
- 161.Kore RA, Abraham EC. Inflammatory cytokines, interleukin-1 beta and tumor necrosis factoralpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochem Biophys Res Commun. 2014;453:326–31. doi: 10.1016/j.bbrc.2014.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun W, Depping R, Jelkmann W. Interleukin-1beta promotes hypoxia-induced apoptosis of glioblastoma cells by inhibiting hypoxia-inducible factor-1 mediated adrenomedullin production. Cell Death Dis. 2014;5:e1020. doi: 10.1038/cddis.2013.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. Embo Mol Med. 2013;5:384–96. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu G, Guo Y, Seng Z, Cui G, Qu J. Bone marrowderived mesenchymal stem cells co-expressing interleukin-18 and interferon-beta exhibit potent antitumor effect against intracranial glioma in rats. Oncol Rep. 2015;34:1915–22. doi: 10.3892/or.2015.4174. [DOI] [PubMed] [Google Scholar]
- 165.Xu G, Jiang XD, Xu Y, Zhang J, Huang FH, Chen ZZ, Zhou DX, Shang JH, Zou YX, Cai YQ, Kou SB, Chen YZ, Xu RX, Zeng YJ. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int. 2009;33:466–74. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 166.Yeh WL, Lu DY, Liou HC, Fu WM. A forward loop between glioma and microglia: glioma-derived extracellular matrix-activated microglia secrete IL-18 to enhance the migration of glioma cells. J Cell Physiol. 2012;227:558–68. doi: 10.1002/jcp.22746. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Y, Wang C, Zhang Y, Sun M. C6 glioma cells retrovirally engineered to express IL-18 and Fas exert FasL-dependent cytotoxicity against glioma formation. Biochem Biophys Res Commun. 2004;325:1240–5. doi: 10.1016/j.bbrc.2004.10.165. [DOI] [PubMed] [Google Scholar]
- 168.Kito T, Kuroda E, Yokota A, Yamashita U. Cytotoxicity in glioma cells due to interleukin-12 and interleukin-18-stimulated macrophages mediated by interferon-gamma-regulated nitric oxide. J Neurosurg. 2003;98:385–92. doi: 10.3171/jns.2003.98.2.0385. [DOI] [PubMed] [Google Scholar]
- 169.Kikuchi T, Akasaki Y, Joki T, Abe T, Kurimoto M, Ohno T. Antitumor activity of interleukin-18 on mouse glioma cells. J Immunother. 2000;23:184–9. doi: 10.1097/00002371-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y, Liu F, Yuan Y, Jin C, Chang C, Zhu Y, Zhang X, Tian C, He F, Wang J. Inflammasomederived exosomes activate NF-kappaB signaling in macrophages. J Proteome Res. 2017;16:170–178. doi: 10.1021/acs.jproteome.6b00599. [DOI] [PubMed] [Google Scholar]
- 171.Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun Y, Pan Z, Qian H, Xu W. Exosomes derived from gastric cancer cells activate NF-kappaB pathway in macrophages to promote cancer progression. Tumour Biol. 2016;37:12169–12180. doi: 10.1007/s13277-016-5071-5. [DOI] [PubMed] [Google Scholar]
- 172.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–9. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carrascal MT, Mendoza L, Valcarcel M, Salado C, Egilegor E, Telleria N, Vidal-Vanaclocha F, Dinarello CA. Interleukin-18 binding protein reduces b16 melanoma hepatic metastasis by neutralizing adhesiveness and growth factors of sinusoidal endothelium. Cancer Res. 2003;63:491–7. [PubMed] [Google Scholar]
- 174.Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X, Bi A, Ding J, Sun B, Geng M. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of doublestrand DNA via AIM2 inflammasome activation. Cell Res. 2017;27:784–800. doi: 10.1038/cr.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and Tcell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–52. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, Jang MJ, Jo E, Kim SC, Han YM, Park KG, Jeong WI. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin17 production by gammadelta T cells in liver fibrosis. Hepatology. 2016;64:616–31. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- 177.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–67. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


