Abstract
High genome copy number (viral load) of human papillomavirus (HPV) is being discussed as a risk factor for high-grade cervical lesions. However, conflicting data about the integration status or viral load of the virus as risk factors for prevalent high-grade squamous intraepithelial lesions (HSIL) are found in the literature. To investigate whether viral load and/or integration status are indicative for prevalent ASCUS/LSIL or HSIL, we determined the HPV16 viral load and the physical state of the genome in 644 women with single HPV16 infections stratified by their cytology results from a large Danish population-based cohort consisting of 40,399 women. Cervical smear samples were tested using a multiplex quantitative real-time PCR (qPCR) with primers specific for HPV16 E2, E6 and beta actin, allowing simultaneous determination of the genome’s physical state and the viral copy number per cell. The associations of viral load and physical state with cervical abnormalities were assessed using multinomial logistic regression. We found that a 10-fold increase in viral load was significantly associated with the presence of ASCUS/LSIL (OR=3.91; 95% CI, 2.49-6.13) and HSIL (OR=4.1; 95% CI, 2.45-6.68). A significant association with HSIL was observed for primarily integrated genomes (OR=6.68; 95% CI, 1.45-30.8). Among women with integrated viral genomes, we observed a trend towards increased risk of ASCUS/LSIL (OR=1.32; 95% CI -2.90-3.44) and HSIL (OR=5.10; 95% CI -0.67-38.9) per 10-fold increase in viral load, although not statistically significant. In conclusion, increasing viral load and integrated viral genomes were significantly associated with prevalent HSIL, thus indicating that viral load and physical state may potentially be useful triage markers for HPV16-positive women during cervical screening.
Keywords: HPV, cervical lesions, viral load, physical state, triage marker
Introduction
High-risk human papillomavirus (HR-HPV) testing as a primary tool for cervical cancer screening is recommended by national guidelines in an increasing number of countries [1,2]. These recommendations are based on results from randomized controlled trials showing that HR-HPV testing significantly reduces the number of incident cervical cancer cases in women above age 30 after three or five year screening intervals compared to cytology only [3,4]. Longer screening intervals of 3-5 years will lead to a reduction in overtreatment of precursor lesions, which may spontaneously regress in between subsequent examinations in the majority of cases [1,5]. In addition, testing for markers indicating a prevalent high-grade squamous intraepithelial lesion (HSIL) would be beneficial for efficient and safe screening algorithms.
HPV16 viral load has been discussed as a potential risk factor for prevalent lesions [6-10]. While some studies found an association between viral load and prevalent high-grade lesions [8,11], others could not confirm this association [12,13].
In addition, integration of the virus as an indicator for HSIL was also described previously [7,14-17]. A mixture of episomal and integrated genomes can be found in normal epithelium as well as in low grade cervical abnormalities, while integrated HPV DNA is more frequent in but not exclusive to high-grade cervical lesions [14-18].
The aim of the present study was to evaluate the risk of prevalent low-grade or high-grade cervical abnormalities according to HPV viral load and physical state separately and according to an interplay between viral load and physical state. The study was based on liquid-based cytology (LBC) samples from 644 women with a single HPV16 infection from a Danish population-based cohort of 40,399 women.
Material and methods
The Danish LBC cohort
The data collection procedures of this study have previously been described in detail [19,20]. From 2002 to 2005, 42,854 consecutive liquid-based cytology (SurePath) samples from the Department of Pathology at Copenhagen University Hospital, Hvidovre, Denmark, were collected. The samples were sent to the pathology department for cytological examination. Residual material was sent to the Division of Experimental Virology, University Hospital Tübingen, Germany for HPV DNA testing (Hybrid Capture2, Qiagen, Hilden, Germany) and genotyping (INNO LiPA v2, Innogenetics, Gent, Belgium). Samples inadequate for HPV testing, or with missing/equivocal identification on the cell sample (n=167) were excluded. In addition, we excluded 2,288 samples as they were duplicate samples from the same women within the observation period. Consequently, the population-based cohort study included 40,399 women.
HPV testing and genotyping
Samples were analyzed with the Hybrid Capture 2 (HC2) test (Qiagen) using the high-and low-risk probe sets as described before [19,20]. DNA was extracted from all HPV positive cervical samples using the QIAsymphony system and was subjected to LiPA HPV Genotyping v2 (Innogenetics, Ghent, Belgium) according to the manufacturer’s instructions and as previously described [19,21,22].
Viral load and physical state determination
Quantification of the HPV16 E2 and E6 genes and the human beta actin gene was performed using a Light Cycler 480 (Roche Diagnostics). Primer and probe sequences for 16E2 and E6 have been described [18,23]. Beta actin was detected using commercially available predesigned primers (#Hs03023880_g1; Applied Biosystems). Multiplex PCR was performed in a final volume of 20 µl containing 1× Taqman Fast Advanced Mastermix (Applied Biosystems), 0.2 µM primer (Invitrogen) and probes (Biomers; or 1× Applied Biosystems beta actin assay) and 2 µl DNA sample. Amplification conditions were 15 min at 95°C, followed by 60 s at 94°C and 90 s at 60°C with single acquisition for 45 cycles.
Beta actin measurement was used to normalize the HPV copy numbers to the number of cells per sample. Cell numbers were calculated assuming a DNA content of 6.6 pg DNA per human diploid cell. The standard curve for beta actin was performed using eleven serial 1:2 dilutions of total genomic DNA isolated from normal human keratinocytes containing 15,151 to 15 genome equivalents, respectively.
We generated standard curves for the quantification of HPV16E6 and E2 using serial 1:10 dilutions of the pBS-HPV16 plasmid.
Physical state was calculated as the E2/E6 ratio for each sample. Samples were categorized in E2/E6<0.15 (low: primarily integrated genomes), E2/E6 between 0.15 and <0.85 (intermediate: episomal and integrated genomes), and E2/E6 ≥0.85 (high: primarily episomal genomes).
Statistical analysis
To investigate the association between viral load, physical state, age and cytology status, we applied a multivariate multinomial logistic regression model with cytology as a categorical, ordered outcome with levels “normal”, “ASCUS (atypical squamous cells of undetermined significance)/LSIL (low grade squamous intraepithelial lesion)” and “HSIL (high grade squamous intraepithelial lesion)”. We adjusted for age, HPV16 viral load, and physical state in the model, and also after determining relevance, included the interaction term between physical state and viral load. HPV16 viral load was log 10-transformed to ensure an approximately normal distribution. Age was kept as a linear variable, and physical state was transformed to a categorical variable with three categories: low, intermediate, and high. We compared the multinomial logistic model with linear terms for age, viral load and physical state to the multinomial logistic model with higher order polynomials for the same covariates to study the possible non-linear effects of viral load, physical state and age on cytology. In addition to the multinomial model, we also analyzed the data using a two-category logistic regression model, comparing samples from women with HSIL vs normal cytology and ASCUS/LSIL vs normal cytology, respectively.
We rejected the proportional odds model by re-categorizing the outcome into two binary logistic regression models, and then comparing the slopes of the two models [24]. Additionally, we also compared the residual deviance of the fitted multinomial logistic model to the corresponding proportional odds model using the chi-square test.
Reporting of effect was done by plotting the estimated probabilities of ASCUS/LSIL and HSIL, respectively, by viral load, physical state (intermediate: 0.15-0.85 and high: ≥0.85) and age. In the graphical display, age was fixed at 25 and 35 years, which corresponds roughly to the first and third quartiles of the study population age distribution (median: 30 years). Relative risks associated with changes in viral load, physical state and age were presented by odds ratios (OR) with 95% confidence intervals (95% CI). All analyses were conducted using the statistical software package R (vs 3.0.2).
Results
A total of 644 women were positive for an HPV16 single infection at the baseline examination. Of these, 469 women had normal cytology, while 175 had an abnormal cytology result. Of those with abnormal cytology, 78 had a low-grade abnormality (ASCUS/LSIL) and 97 women had a high-grade abnormality (HSIL). The crude distributions of viral load and physical state in women with normal cytology, ASCUS/LSIL and HSIL are shown in Figure 1 and Table 1. The median viral load was significantly lower in women with normal cytology than in women with either ASCUS/LSIL (P<0.0001) or HSIL (P<0.0001) (Figure 1A). Viral loads ranged from 0.01 viral DNA copies per cell (c/c) to 2,281 c/c (median: 1.2 c/c) in women with normal cytology and from 0.12 c/c to 2,886 c/c (median: 12.44 c/c) in women with abnormal cytology (ASCUS/LSIL and HSIL). A significantly lower median E2/E6 ratio (P=0.0003) was observed in women with HSIL compared to cytologically normal women, while no significant difference in physical state was found when comparing women with normal cytology and those with a prevalent ASCUS/LSIL (P=0.245) (Figure 1B).
Figure 1.
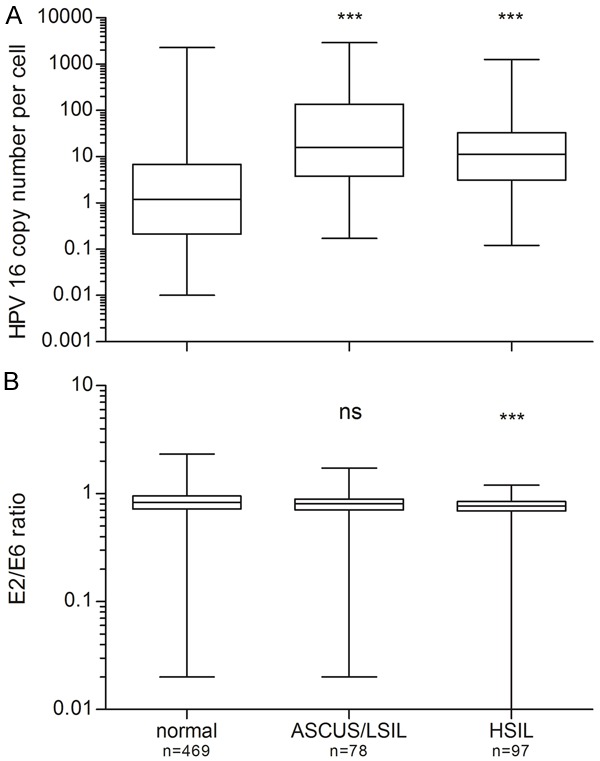
HPV16 viral load (A) and physical status (B) in women with normal cytology, ASCUS/LSIL or HSIL. The upper and lower boundaries of the boxes represent the 75th and 25th percentiles, respectively. The black line within the box indicates the median, and the whiskers represent the range. Significant differences in viral load were observed between women with normal cytology and women with ASCUS/LSIL, as well as between women with normal cytology and women with HSIL. No significant difference (ns) was observed in viral physical state between women with normal cytology and women with ASCUS/LSIL cytology (P=0.245). A highly significant difference was found between women with normal cytology and women with HSIL (***, P<0.001).
Table 1.
Viral load and physical state in women with normal cytology, ASCUS/LSIL or HSIL
| Viral DNA load (copies/cell) | Normal cytology | ASCUS/LSIL | HSIL |
|
| |||
| Number of samples (N) | 469 | 78 | 97 |
| Range | 0.01-2280.56 | 0.17-2886.30 | 0.12-1260.68 |
| Interquartile range | 0.21-6.71 | 3.97-130.01 | 3.21-31.76 |
| Median | 1.20 | 15.68 | 11.26 |
|
| |||
| Physical state of the viral genome (E2/E6 ratio) | |||
|
| |||
| Number of samples (N) | 469 | 78 | 97 |
| Range | 0.02-2.33 | 0.02-1.72 | 0.0-1.2 |
| Interquartile range | 0.72-0.95 | 0.71-0.89 | 0.69-0.84 |
| Median | 0.83 | 0.81 | 0.77 |
| ≥0.85 (Primarily episomal) | 210 [44.8%] | 32 [41.0%] | 24 [24.7%] |
| 0.15-0.85 (Episomal and integrated) | 251 [53.5%] | 45 [57.7%] | 70 [72.2%] |
| <0.15 (Primarily integrated) | 8 [1.7%] | 1 [1.3%] | 3 [3.1%] |
Figure 2 shows the estimated odds of ASCUS/LSIL and HSIL, respectively, in women with primarily episomal genomes (E2/E6 ratio ≥0.85) and in those with intermediate E2/E6 ratio (0.15-0.85). Estimates are shown for women aged 25 years (black curve) and 35 years (red curve), respectively, and across a range of viral loads. Generally, the odds of developing ASCUS/LSIL or HSIL increased with increasing viral load for both categories of physical state. We found, that age had no statistically significant impact on the odds off prevalent cervical lesions as shown by the overlap of the two graphs. In addition, the respective estimated ORs for increase in age were very close to 1 (0.98 vs 1 for ASCUS/LSIL and HSIL, respectively) and neither estimates were significant (P=0.22 and P=0.76, respectively).
Figure 2.
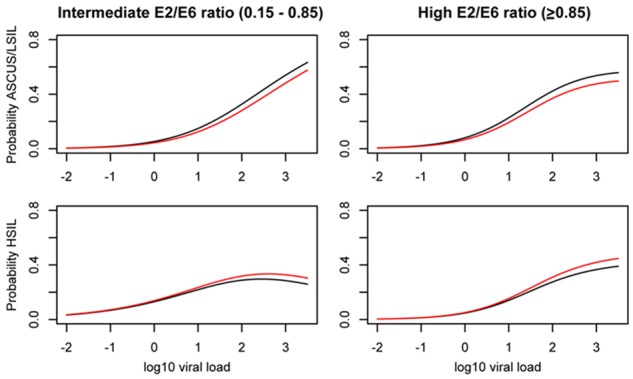
Dependency graphs for estimated probabilities of given cytology result (ASCUS/LSIL and HSIL), in women with high (≥0.85) or intermediate (0.15-0.85) E2/E6 ratios, across a range of viral loads, and exemplified for ages 25 (black), and 35 (red).
As shown in Table 2, a 10-fold increase in viral load of HPV16 significantly increased the odds of prevalent ASCUS/LSIL (OR=3.91; 95% CI, 2.49-6.13) or HSIL (OR=4.10; 95% CI, 2.45-6.68). Women with primarily integrated viral genomes (E2/E6 ratio <0.15) and those with intermediate E2/E6 ratio (0.15-0.85) had an increased risk of HSIL (OR=6.68; 95% CI, 1.45-30.8; OR=2.73; 95% CI, 1.22-6.09, respectively) compared to women with primarily episomal genomes (E2/E6 ratio ≥0.85). In contrast, no increased risk for ASCUS/LSIL was observed according to physical state of the genome. The combination of high viral load and primarily integrated viral genomes (E2/E6 ratio <0.15) yielded a high OR (OR=5.1; 95% CI, -0.67-38.9) for the presence of HSIL, but the estimate was not significant (P=0.12).
Table 2.
Odds ratios (OR) for ASCUS/LSIL or HSIL according to HPV viral load and physical state in women with single HPV16 infection
| ASCUS/LSIL | HSIL | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | OR | (95% CI) | P-value | n | OR | 95% CI | P-value | |
| Viral load per 10 fold increase | 78 | 3.91 | (2.49-6.13) | <0.00001 | 97 | 4.10 | (2.45-6.68) | <0.00001 |
| Physical state | ||||||||
| Intermediate (E2/E6 ratio 0.15-0.85) | 45 | 0.66 | (0.29-1.51) | 0.324 | 70 | 2.73 | (1.22-6.09) | 0.0014 |
| Primarily integrated (E2/E6 ratio <0.15) | 1 | 1.89 | (0.21-16.8) | 0.5699 | 3 | 6.68 | (1.45-30.8) | 0.0148 |
| Viral load per 10 fold increase in women with primarily integrated genomes (E2/E6 ratio <0.15) | 1 | 1.32 | (-2.90-3.44) | 0.86 | 3 | 5.10 | (-0.67-38.9) | 0.12 |
The same analysis was performed using a two-category logistic regression model (Table 3). Results under this model agreed closely with the multinomial model, thus confirming our estimates. However, the small number of women (N=12) with primarily integrated genomes (E2/E6 ratio <0.15) limits our conclusions.
Table 3.
Odds ratios (OR) for ASCUS/LSIL or HSIL according to viral load or physical state at baseline calculated by using a two category logistic regression model
| ASCUS/LSIL | HSIL | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | OR | (95% CI) | P-value | n | OR | 95% CI | P-value | |
| Viral load per 10 fold increase | 78 | 3.77 | (2.48-6.18) | <0.00001 | 97 | 4.2 | (2.54-7.74) | <0.00001 |
| Physical state | ||||||||
| Intermediate (E2/E6 ratio 0.15-0.85) | 45 | 0.74 | (0.33-1.71) | 0.47 | 70 | 2.75 | (1.27-6.85) | 0.0168 |
| Primarily integrated (E2/E6 ratio <0.15) | 1 | 1.78 | (0.09-11.38) | 0.61 | 3 | 6.99 | (1.32-31.3) | 0.0128 |
| Viral load per 10 fold increase in women with primarily integrated genomes (E2/E6 ratio <0.15) | 1 | 1.27 | (0.24, 32.8) | 1.0 | 3 | 5.52 | (0.75, 40.7) | 1.0 |
We also investigated whether it is possible to identify women with prevalent HSIL by combining an initial viral load measurement with a subsequent E2/E6 ratio determination. Our results showed, that log10 viral load yields an AUC of 0.78 (P-value <0.001) to classify between normal and abnormal (ASCUS/LSIL + HSIL) cytology. Using the E2/E6 ratio to then specify between ASCUS/LSIL vs HSIL, an AUC of 0.62 (P-value =0.03) was calculated. While a high viral load appears to be a good classifier for the presence of a prevalent lesion (ASCUS/LSIL and HSIL), a subsequent determination of the E2/E6 ratio has a fair effect on the classification of this lesion (ASCUS/LSIL or HSIL).
Taken together, we found that increasing viral load was significantly associated with an increased risk for prevalent ASCUS/LSIL or HSIL. In addition, women with primarily integrated viral genomes, as measured by an E2/E6 ratio <0.15, also had an increased risk for prevalent HSIL.
Discussion
We determined HPV16 viral load and physical state of the viral genome in 644 women with single HPV16 infections and either a normal or an abnormal cytology result (ASCUS/LSIL or HSIL) from a population-based cohort of 40,399 women. Several studies reported an association between high viral load and the presence of high-grade cervical abnormalities in smaller cohorts of women with mixed HR-type infections [6,7,9,25-27]. Here, we extend this observation to a cohort comprising a large number of women with single HPV16 infections. We also demonstrate an association of high viral load with ASCUS/LSIL and thereby challenge earlier conflicting data based on multiple infections including HPV16 [22]. An increased viral load was observed in low-grade as well as high-grade cervical abnormalities as compared to normal cytology results. Hence, while it is possible to distinguish between a normal and an abnormal cytology result by sole determination of the viral load, our results indicate that is not possible to further discriminate between a low- or high-grade squamous intraepithelial lesion (P=0.072). This means that the sole determination of HPV16 DNA load to distinguish between the severity of cervical lesions is not useful. While some studies reported a significant increase of viral load not only between normal and high-grade lesions, but also between low-grade and high-grade lesions [6,7,28,29], others either found a higher viral load in low-grade lesions compared to high-grade lesions [30] or no association between HPV16 DNA load and lesion grade [12,13]. In contrast, our results clearly support that a low viral load might be used as a triage marker for the absence of prevalent abnormal cervical lesions, while a high viral load indicates a prevalent cervical lesion including ASCUS/LSIL or HSIL. Further discrimination between the different grades of cervical lesions requires additional triage markers for the detection of high-grade lesions.
Integration of human papillomavirus DNA into the host genome could be a prerequisite for a persistent infection and might be an important factor for malignant transformation. Integration causes disruption of the E2/E4 open reading frame, which blocks oncogene inhibition activation due to the loss of the repressor activities of E2 and E8^E2 [31,32]. The continuous expression of the oncogenes E6 and E7 contributes to malignant transformation and is observed after integration of viral DNA into the cellular genome [33,34]. We determined the integration status of the viral genome by quantitative multiplex PCR and found that the majority of samples harbored a mixture of episomal and integrated HPV genomes, which is in line with previous reports [7,22,26,28]. Mixed forms of the HPV16 genome also exist in samples with normal and ASCUS/LSIL cytology, which might indicate that integration of HPV16 DNA into the host genome is an early event [7,14,18,30]. In agreement with previous reports we also demonstrated a significantly higher proportion of integrated viral genomes (E2/E6 ratio <0.15) in women with HSIL compared to women with normal cytology [14,17,26,30,35]. No significant differences between women with normal cytology and ASCUS/LSIL (P=0.25), or between ASCUS/LSIL and HSIL (P=0.08) were observed. In addition, our results indicate an effect of increasing viral load in women with primarily integrated viral genomes (OR=5.1 per 10-fold increase in viral load). However, the finding was not statistically significant, which was likely due to the low number of women with integrated genomes (n=12) and the low number of HSIL-cases in this group (n=3). However, while we confirmed our finding by two different statistical methods, it is important to point out that despite the high number of women participating, the number of samples with primarily integrated viral genomes (E2/E6<0.15) was low (n=12). Thus, future larger studies are required to confirm our findings.
In summary, we confirmed previous reports demonstrating a higher viral load in women with a low or high-grade cervical lesion compared to women with normal cytology. Furthermore, women with integrated HPV16 genomes had significantly higher odds of high-grade cervical abnormalities than women with primarily episomal genomes. However, considering useful triage strategies of positive HPV-test results, the integration parameter (E2/E6<0.15) does not appear to be appropriate for the detection or segregation of ASCUS/LSIL or HSIL cases. In addition, the combination of high viral load and primarily integrated viral genomes was not statistically significant. Estimates for sequential measurements showed that a high viral load result might be indicative for a prevalent lesion. A further classification of those women with a HSIL lesion by E2/E6 ratio determination is questionable. On the other hand, our data show that a high viral load indicates women with ASCUS/LSIL or HSIL lesions. Thus, a combination of a low viral load and an E2/E6 ratio ≥0.85 (mostly episomal) might be indicative for the absence of a prevalent lesion after a positive HPV16 test result, which could be used as a triage strategy.
In conclusion, this is the first report analyzing the interplay of HPV16 viral load, its integration status and the presence of cervical lesions in a large cohort of women with single HPV16 infections suggesting their applicability as immediate triage strategy after a positive HPV16 test result.
Acknowledgements
This work was supported by the Mermaid project (MERMAID-2).
Disclosure of conflict of interest
LTT has received a travel grant from Sanofi Pasteur MSD. CM has received support for conference participation and speaker’s fees from Sanofi Pasteur MSD. SKK has received speaker’s and advisory board fees and research grants through her institution from Sanofi Pasteur MSD and Merck. TI received speaker honoraria from Hologic GmbH, Becton Dickinson Diagnostics GmbH and Sanofi Pasteur MSD; and his institution received an unconditional research grant from Hologic GmbH and Becton Dickinson Diagnostics GmbH.
References
- 1.Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L. European guidelines for quality assurance in cervical cancer screening. Second edition--summary document. Ann Oncol. 2010;21:448–458. doi: 10.1093/annonc/mdp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, Kinney WK, Massad LS, Mayeaux EJ, Saslow D, Schiffman M, Wentzensen N, Lawson HW, Einstein MH. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015;125:330–7. doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 3.Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJ International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687–97. W214–5. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 6.Hesselink AT, Berkhof J, Heideman DA, Bulkmans NW, van Tellingen JE, Meijer CJ, Snijders PJ. High-risk human papillomavirus DNA load in a population-based cervical screening cohort in relation to the detection of high-grade cervical intraepithelial neoplasia and cervical cancer. Int J Cancer. 2009;124:381–386. doi: 10.1002/ijc.23940. [DOI] [PubMed] [Google Scholar]
- 7.Saunier M, Monnier-Benoit S, Mauny F, Dalstein V, Briolat J, Riethmuller D, Kantelip B, Schwarz E, Mougin C, Pretet JL. Analysis of human papillomavirus type 16 (HPV16) DNA load and physical state for identification of HPV16-infected women with high-grade lesions or cervical carcinoma. J Clin Microbiol. 2008;46:3678–3685. doi: 10.1128/JCM.01212-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman M, Hildesheim A, Herrero R, Bratti C. Human papillomavirus testing as a screening tool for cervical cancer. JAMA. 2000;283:2525–2526. [PubMed] [Google Scholar]
- 9.Snijders PJ, Hogewoning CJ, Hesselink AT, Berkhof J, Voorhorst FJ, Bleeker MC, Meijer CJ. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33-positive women with normal cytology. Int J Cancer. 2006;119:1102–1107. doi: 10.1002/ijc.21956. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Hildesheim A. Chapter 5: viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr. 2003:35–40. doi: 10.1093/oxfordjournals.jncimonographs.a003480. [DOI] [PubMed] [Google Scholar]
- 11.Swan DC, Tucker RA, Tortolero-Luna G, Mitchell MF, Wideroff L, Unger ER, Nisenbaum RA, Reeves WC, Icenogle JP. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J Clin Microbiol. 1999;37:1030–1034. doi: 10.1128/jcm.37.4.1030-1034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson S, Safari H, Mints M, Lewensohn-Fuchs I, Gyllensten U, Johansson B. Type distribution, viral load and integration status of high-risk human papillomaviruses in pre-stages of cervical cancer (CIN) Br J Cancer. 2005;92:2195–2200. doi: 10.1038/sj.bjc.6602648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung JL, Lo KW, Cheung TH, Tang JW, Chan PK. Viral load, E2 gene disruption status, and lineage of human papillomavirus type 16 infection in cervical neoplasia. J Infect Dis. 2006;194:1706–1712. doi: 10.1086/509622. [DOI] [PubMed] [Google Scholar]
- 14.Briolat J, Dalstein V, Saunier M, Joseph K, Caudroy S, Pretet JL, Birembaut P, Clavel C. HPV prevalence, viral load and physical state of HPV-16 in cervical smears of patients with different grades of CIN. Int J Cancer. 2007;121:2198–2204. doi: 10.1002/ijc.22959. [DOI] [PubMed] [Google Scholar]
- 15.Cricca M, Venturoli S, Leo E, Costa S, Musiani M, Zerbini M. Molecular analysis of HPV 16 E6I/E6II spliced mRNAs and correlation with the viral physical state and the grade of the cervical lesion. J Med Virol. 2009;81:1276–1282. doi: 10.1002/jmv.21496. [DOI] [PubMed] [Google Scholar]
- 16.Hudelist G, Manavi M, Pischinger KI, Watkins-Riedel T, Singer CF, Kubista E, Czerwenka KF. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol. 2004;92:873–880. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Tonon SA, Picconi MA, Bos PD, Zinovich JB, Galuppo J, Alonio LV, Teyssie AR. Physical status of the E2 human papilloma virus 16 viral gene in cervical preneoplastic and neoplastic lesions. J Clin Virol. 2001;21:129–134. doi: 10.1016/s1386-6532(01)00155-x. [DOI] [PubMed] [Google Scholar]
- 18.Peitsaro P, Johansson B, Syrjanen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol. 2002;40:886–891. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaer SK, Munk C, Junge J, Iftner T. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179–189. doi: 10.1007/s10552-013-0320-z. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen LT, Frederiksen K, Munk C, Junge J, Castle PE, Iftner T, Kjaer SK. High-risk and low-risk human papillomavirus and the absolute risk of cervical intraepithelial neoplasia or cancer. Obstet Gynecol. 2014;123:57–64. doi: 10.1097/AOG.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer. 2015;137:193–203. doi: 10.1002/ijc.29374. [DOI] [PubMed] [Google Scholar]
- 22.Manawapat A, Stubenrauch F, Russ R, Munk C, Kjaer SK, Iftner T. Physical state and viral load as predictive biomarkersfor persistence and progression of HPV16-positive cervical lesions: results from a population based long-term prospective cohort study. Am J Cancer Res. 2012;2:192–203. [PMC free article] [PubMed] [Google Scholar]
- 23.Pretet JL, Dalstein V, Monnier-Benoit S, Delpeut S, Mougin C. High risk HPV load estimated by Hybrid Capture II correlates with HPV16 load measured by real-time PCR in cervical smears of HPV16-infected women. J Clin Virol. 2004;31:140–147. doi: 10.1016/j.jcv.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE. Regression modeling strategies. Springer; 2001. [Google Scholar]
- 25.Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, Kantelip B, Schaal JP, Mougin C. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, He X, Yu G, Wu Y. Effectiveness of HPV 16 viral load and the E2/E6 ratio for the prediction of cervical cancer risk among Chinese women. J Med Virol. 2013;85:646–654. doi: 10.1002/jmv.23490. [DOI] [PubMed] [Google Scholar]
- 27.Josefsson AM, Magnusson PK, Ylitalo N, Sorensen P, Qwarforth-Tubbin P, Andersen PK, Melbye M, Adami HO, Gyllensten UB. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet. 2000;355:2189–2193. doi: 10.1016/S0140-6736(00)02401-6. [DOI] [PubMed] [Google Scholar]
- 28.Cricca M, Morselli-Labate AM, Venturoli S, Ambretti S, Gentilomi GA, Gallinella G, Costa S, Musiani M, Zerbini M. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol Oncol. 2007;106:549–557. doi: 10.1016/j.ygyno.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S, Mahata S, Shishodia G, Pande S, Verma G, Hedau S, Bhambhani S, Kumari A, Batra S, Basir SF, Das BC, Bharti AC. Physical state & copy number of high risk human papillomavirus type 16 DNA in progression of cervical cancer. Indian J Med Res. 2014;139:531–543. [PMC free article] [PubMed] [Google Scholar]
- 30.Kulmala SM, Syrjanen SM, Gyllensten UB, Shabalova IP, Petrovichev N, Tosi P, Syrjanen KJ, Johansson BC. Early integration of high copy HPV16 detectable in women with normal and low grade cervical cytology and histology. J Clin Pathol. 2006;59:513–517. doi: 10.1136/jcp.2004.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desaintes C, Demeret C, Goyat S, Yaniv M, Thierry F. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 1997;16:504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubenrauch F, Straub E, Fertey J, Iftner T. The E8 repression domain can replace the E2 transactivation domain for growth inhibition of HeLa cells by papillomavirus E2 proteins. Int J Cancer. 2007;121:2284–2292. doi: 10.1002/ijc.22907. [DOI] [PubMed] [Google Scholar]
- 33.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Knebel Doeberitz M, Bauknecht T, Bartsch D, zur Hausen H. Influence of chromosomal integration on glucocorticoid-regulated transcription of growth-stimulating papillomavirus genes E6 and E7 in cervical carcinoma cells. Proc Natl Acad Sci U S A. 1991;88:1411–1415. doi: 10.1073/pnas.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahla S, Kochbati L, Chanoufi MB, Maalej M, Oueslati R. HPV-16 E2 physical status and molecular evolution in vivo in cervical carcinomas. Int J Biol Markers. 2014;29:e78–85. doi: 10.5301/jbm.5000051. [DOI] [PubMed] [Google Scholar]


