Abstract
Therapies designed to reduce androgen production or receptor activation are effective in limiting prostate tumor growth. However, prolonged treatment with anti-androgen therapies results in the progression of prostate cancers into an androgen refractory state. Neuroendocrine differentiation (NED) has been associated with the progression of prostate cancers to an androgen resistant phenotype. In this work we investigated the effect of disrupting androgen receptor signaling in promoting NED of prostate carcinoma cells and whether it is accompanied by an increase in T-type Ca2+ channel expression. The effect of disrupting androgen signaling was assessed in LNCaP and 22Rv1 prostate cancer cells following treatment with the androgen receptor blocker, bicalutamide, or hormone-depleted media. Treatment of LNCaP cells with bicalutamide or hormone-depleted media for 4-10 d evoked considerable morphological and biochemical changes consistent with NED including the development of long neurite-like processes and the expression of the neuronal marker, tubulin IIIβ. PCR analysis of bicalutamide-stimulated cells revealed no significant changes in Cav3.2 mRNA. However, stimulation of LNCaP cells with bicalutamide or hormone-depleted media for 10 d evoked a significant increase in Cav3.2 protein expression and the appearance of functional T-type Ca2+ channels. Inhibition of T-type Ca2+ channel function with various pharmacological blockers disrupted the morphological differentiation of LNCaP cells. Bicalutamide-evoked expression of functional T-type Ca2+ channels in LNCaP cells promoted chemoresistance to docetaxel. These findings indicate that disruption of androgen receptor signaling in prostate cancer cells evokes increased expression of functional T-type Ca2+ channels, which may result in significant morphological and biochemical changes.
Keywords: Prostate cancer, bicalutamide, neuroendocrine differentiation, T-type calcium channel
Introduction
Prostate cancer is the second most commonly diagnosed cancer in males, leading to significant mortality [1]. Androgen receptor activation is an important factor regulating the growth and progression of prostate cancer. Treatment of prostate cancers with androgen-deprivation therapies, including androgen production blockers, castration, or inhibitors of androgen receptor function, reduce cancer growth [2,3]. The androgen receptor blocker bicalutamide (Casodex®) is widely used to treat androgen-dependent prostate cancers because of its high affinity for the androgen receptor and effectiveness in reducing tumor growth [3]. However, prolonged treatment with androgen-deprivation therapies leads to the development of androgen-independent (or castration-resistant) cancer with increased incidence of metastasis [4].
A critical step in the progression of prostate cancers to an androgen independent phenotype is the appearance of neuroendocrine differentiated cells [5]. Neuroendocrine differentiation (NED) has been correlated with tumor progression and increased mortality in prostate cancer patients [6,7]. As a result of NED, localized populations of prostate cancer cells develop longer neurite-like processes and a rounder cell body relative to those cells that are undifferentiated. Furthermore, these cells exhibit increased expression of several secretory and neuronal markers, including chromogranin-A, neuron-specific enolase, and tubulin IIIβ, as well as increased secretion of mitogenic neurochemicals including neurotensin, somatostatin, bombesin, serotonin and parathyroid hormone-related peptide [8-12]. We and others have shown that increased expression of T-type Ca2+ channels promotes the morphological and biochemical differentiation of prostate cancer cells under certain conditions [13-15]. Changes in Ca2+ homeostasis coupled to secretion of mitogenic factors in differentiated cells can potentially stimulate androgen-independent cell growth in prostate cancers. Several factors including androgen depletion, radiation treatment, increased expression of interleukin-6 (IL-6), or exposure to cAMP-activating agents promote the differentiation of prostate cancer cells in vivo and in vitro [11,16-18]. However, there is some controversy regarding the role of NED as a clinical predictor of androgen-independent prostate cancer [19].
Increased intracellular Ca2+ regulates several signaling pathways that promote cell proliferation, migration, and differentiation of cancer cells [20]. Influx from the extracellular space via ion channels and release from intracellular stores can promote increased cytosolic Ca2+ concentration. Thus, changes in the expression of voltage-gated Ca2+ channels can alter intracellular Ca2+ concentration and promote Ca2+-dependent cellular processes such as cell differentiation. Our previous research indicates that induction of NED in LNCaP cells treated with interleukin-6 (IL-6) + forskolin (FSK) or sodium butyrate (NaBu) evokes increased expression of T-type Ca2+ channels [14,15]. Although bicalutamide evokes biochemical changes consistent with NED [21], its role in promoting T-type Ca2+ channel expression has yet to be explored. In the present work we assessed the ability of bicalutamide and hormone-depleted media to promote the NED of LNCaP cells, an androgen-dependent cell line that serves as the primary model of NED in vitro, as evidenced by morphological and biochemical changes and whether NED is accompanied by changes in the expression of T-type Ca2+ channels.
Methods
Cell culture
LNCaP cells were obtained from the American Type Culture Collection (Lot#: 59722254, ATCC Manassas, VA) and cultured as previously described [14,15]. 22Rv1 cells were purchased from Sigma (Lot#: 15A027, St. Louis, MO). Cells were grown at 37°C in RPMI media containing Glutamax (Invitrogen, Grand Island, NY) and supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin and 50 µg/mL streptomycin in a 5% CO2/95% air humidified atmosphere. Hormone-depleted media consisted of phenol red-free RPMI media, supplemented with 10% charcoal-stripped fetal bovine serum (cs-FBS, depleted of endogenous steroids and hormones), 50 U/mL penicillin and 50 µg/mL streptomycin. Previous studies identified a four-fold difference in the testosterone content between FBS and cs-FBS [22]. Cells passaged no more than 20 times were used in this study. Cells were grown on plastic well plates (for morphological or molecular assays) or poly-D-lysine-coated glass coverslips (for whole cell recordings). Cell cultures were stimulated with bicalutamide (10-20 µM, R&D, Minneapolis, MN) or exposed to hormone-depleted media for ≥ 4 days.
Morphometric analysis
A Nikon Eclipse Ti microscope equipped with a 20X inverted objective and Photometrics Coolsnap EZ cooled camera was utilized to capture images of the LNCaP cells. The neurite-like morphology of LNCaP cells was analyzed in at least 20 random fields of view per condition (from ≥ 2 cell cultures). Images were collected blindly regarding treatment conditions. The following parameters were measured, per cell, using the Nikon NIS-Elements imaging software: total neurite-like length (μm) and the average number of primary neurite-like processes emanating from the cell body.
Immunohistochemistry
Immunohistochemical analysis was conducted as described by Weaver et al. [15] with the following modifications: 1) An Alexa 546-conjugated secondary antibody (ThermoFisher, Cat.# A-11010) replaced the Alexa 555-conjugated secondary antibody; and 2) Cells were mounted with anti-fading media containing Hoechst 33342 (Invitrogen, Grand Island, NY) rather than DAPI. Fluorescence was detected using a Nikon Eclipse Ti microscope equipped with TRIC and DAPI filters. All images were collected under the same experimental conditions including exposure time, brightness and resolution.
PCR analysis
RNA extraction and cDNA synthesis were completed as previously described in detailed by Weaver et al. [15]. Amplification of Cav3.2 and GAPDH utilized primers identical to those noted in Weaver et al. [15]. Amplification conditions were optimized at: one cycle of denaturation at 95°C for 30 s followed by 40 cycles of amplification (95°C/15 s, 61.5°C/1 min). Neurotensin amplification followed the same amplification protocol as stated above with the following neurotensin-specific primers: forward primer: 5’-GCA TGC TAC TCC TGG CTT TC-3’; reverse: 5’-CCA AGA GGG AAC ATG TGC TT-3’. Reaction control, message production, and Cav3.2 normalization was determined as previously reported by Weaver et al. [15].
Western blot analysis
Immunoblot analysis was conducted as previously described [14,15], using a specific antibody against the Cav3.2 subunit (Cat.# sc25691, Santa Cruz, Dallas, TX). Cultured cells were lysed using a ready-made RIPA buffer (Thermo Fisher, Waltham, MA) supplemented with Halt Protease Inhibitor Cocktail (Thermo Fisher). After assessing the protein concentration using a Pierce BCA protein Assay kit (Thermo Fisher), the same amount of protein was allocated to each sample. Protein samples were combined with Bolt sample buffer supplemented with reducing agent (Thermo Fisher) and boiled for 10 min at 70°C. Samples were separated by SDS-PAGE on 8% precast Bolt gels (Thermo Fisher). Proteins were transferred to nitrocellulose membranes, which were subsequently blocked in SuperBlock blocking buffer (Thermo Fisher), containing 0.1% Tween-20 before overnight incubation with rabbit anti-Cav3.2 (1:500). Blots were analyzed using secondary antibodies conjugated to horseradish peroxidase (anti-rabbit IgG from Jackson ImmunoResearch, Cat.# 111-035-003) and a chemiluminescent substrate (SuperSignal West Pico Chemiluminescence Substrate, Pierce). To control for equal loading of protein in each sample, membranes were treated with stripping buffer (Restore Plus, Thermo Fisher) for 30 min at room temperature and reprobed with an α tubulin-specific antibody (1:2000 dilution, Cat.# 05-829, Upstate) followed by incubation with the corresponding secondary antibody (anti-mouse IgG from Jackson Immuno Research, Cat.# 115-035-146) and immunodetection.
Cell surface protein biotinylation
LNCaP cells were cultured in 60 mm Petri dishes at 80% confluency and cell surface proteins were biotinylated as outlined by Weaver et al. [15] with noted deviations. Succinctly, surface proteins were biotinylated using EZ-link sulfo-NHS-SS biotin (Thermo Fisher, Rockford, IL). A 5 min incubation of the mixture in PBS supplemented with 100 mM glycine terminated the biotinylation reaction. After washing with PBS, proteins were extracted with a Tris-based cell lysis buffer, collected as the supernatant fraction post centrifugation (6000 × g for 10 min at 4°C), and quantitatively assessed using the Pierce BCA protein assay kit (Thermo Fisher). Pull-down of the biotinylated proteins was accomplished by overnight incubation of protein samples with Pierce Streptavidin Agarose Resin (Thermo Fisher). Agarose beads were collected by centrifugation (6000 × g for 5 min) and after three washes with cell lysis buffer the biotinylated proteins were eluted with Bolt sample buffer supplemented with reducing agent (Thermo Fisher). The samples were subjected to immune-detection to assess surface expression of Cav3.2 proteins. To control for equal loading of protein in each sample, biotinylated membrane fractions were treated with stripping buffer (Restore Plus, Thermo Fisher) for 30 min at room temperature and reprobed with a ATPase-specific antibody (1:1000 dilution, Cat.# MA-3-929, Sigma) followed by incubation with the corresponding secondary antibody and immuno-detection.
Electrophysiology
The detailed methodology previously described by Weaver et al. [15], for recordings of Ca2+ currents in the whole cell configuration at room temperature (22-24°C) was followed with one noted exception. Briefly, recordings were conducted on LNCaP cells visualized using a Nikon Eclipse-Ti inverted microscope (Nashua, NH, USA) equipped with Hoffman optics. Recording electrodes were filled with a HEPES-base buffer solution at pH 7.4 and normal external saline for the calcium ion current measurements was also maintained at pH 7.4 in a HEPES-based buffer. Ca2+ currents were generated by applying either a 750 ms-voltage ramp from -100 to +80 mV or a 200 ms depolarizing step, rather than a 400 ms depolarizing step as reported previously, to various potentials from a holding potential of -100 mV. A MultiClamp 700A amplifier and Pclamp software (Axon Instruments, Foster City, CA) recorded voltage commands, data acquisition and analysis. MultiClamp 700B Commander automatically compensated for pipette offset, whole cell capacitance and series resistance. Normalization of cell size was accomplished by dividing current amplitudes by cell capacitance.
Cell viability assay
Cell viability was determined by the XTT colorimetric assay (Biotium, Hayward, CA) according to the manufacturer’s protocol. Cells (5 × 104) were grown as triplicates on 96 well plates and allowed to adhere for 24 h. LNCaP cells were exposed to bicalutamide (20 μM) for 4 or 10 d. After such period, cells were treated with docetaxel (DTX 100-1000 nM), NiCl2 (100 µM), or NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-Benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride hydrate, Sigma] (5 μM) for 2 d. The viability of 22Rv1 cells was assessed 72 h after treatment with IL-6 (50 ng/mL) +FSK (50 µM), bicalutamide (20 µM) or hormone-depleted media. Changes in cell viability were determined by measuring the absorbance at 540 nm using a Multiscan FC microplate reader (Thermo Fisher Scientific). Cell viability was expressed as a percent of control cells (non-treated).
Data analysis
Values are presented as mean ± SEM where indicated. Statistical analyses consisted of t-test for pairwise comparisons or one-way ANOVA followed by post hoc analysis using Tukey’s honest significant difference test for unequal n for comparisons between multiple groups using SigmaStat software. Throughout, P<0.05 was regarded as significant.
Results
Morphological and biochemical changes following disruption of androgen receptor signaling in LNCaP cells
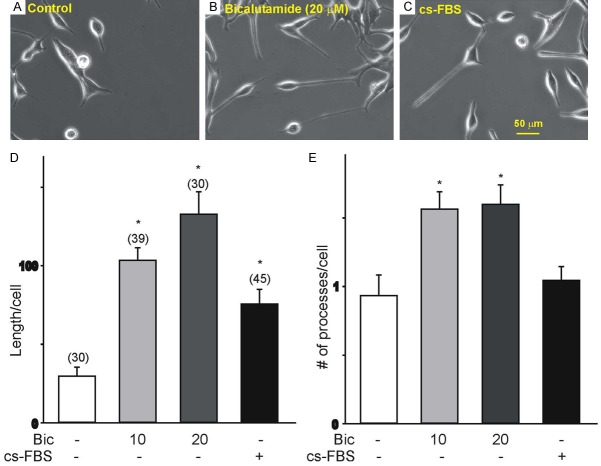
We assessed bicalutamide-evoked morphological and biochemical changes following treatment of LNCaP cells for 4 d. LNCaP cells were treated with 10 or 20 μM bicalutamide since these concentrations induce significant changes in cell proliferation [23]. Bicalutamide-evoked changes were compared with those generated with a hormone-depleted media (cs-FBS). Treatment with hormone-depleted media was used to mimic the condition generated by androgen deprivation [24]. Non-stimulated LNCaP cells presented an epithelial morphology with thin unbranched processes shorter than one length of the cell body (Figure 1A). Stimulation with bicalutamide (10 and 20 μM) for 4 d induced considerable morphological changes consistent with NED comprising the presence of rounded cell bodies and multiple, longer neurite-like processes (Figure 1B) in comparison to the non-stimulated LNCaP cells. Culture of LNCaP cells in hormone-depleted media also caused the development of longer neurite-like processes (Figure 1C) relative to non-stimulated LNCaP cells. Measurements of the total neurite-like processes’ length and the average number of processes per cell signaled that stimulation of LNCaP cells with bicalutamide (10 and 20 μM) for 4 d caused a significant increase in total neurite-like length and average number of processes per cell, compared to non-stimulated LNCaP cells (Figure 1D, 1E). LNCaP cells stimulated with hormone-depleted media (cs-FBS) resulted in a significant increase in the total length of neurite-like processes without a noticeable change in the average number of processes per cell (Figure 1D, 1E).
Figure 1.
Morphological changes in LNCaP cells treated with bicalutamide or hormone-depleted media for 4 d. (A-C) Cell morphology of untreated LNCaP cells (control, A), following 4 d treatment with 20 µM bicalutamide (B), or cultured in hormone-depleted media (C). Note that treatment with 20 µM bicalutamide or hormone-depleted media causes a significant increase in the total length of neurite-like processes per cell. (D, E) Summary of the changes in the length and the number of processes under various culture conditions (bicalutamide, Bic; hormone-depleted media, cs-FBS). The number of cells analyzed under each condition is represented above each bar from two different cell culture sets (*P<0.05 vs. non-stimulated controls). Stimulation of LNCaP cells with bicalutamide (10 µM and 20 µM) and hormone-depleted media evokes morphological changes, which include rounding of the cell body and a significant increase in the length of neurite-like processes emanating from the cell body. Stimulation with bicalutamide significantly increases the number of neurite-like processes per cell.
We next assessed the effect of bicalutamide treatment on the expression of tubulin IIIβ and neurotensin, specific markers of prostate cancer cells undergoing NED [6,25]. To detect the expression of tubulin IIIβ, we performed immunohistochemistry staining of cultured LNCaP cells with a specific antibody against tubulin IIIβ. Cell nuclei were visualized by Hoechst staining. Typical examples of LNCaP cell cultures showing the level of tubulin IIIβ expression under control conditions or following a 4 d-treatment with bicalutamide (20 μM) or cs-FBS are presented in Figure 2A, 2D and 2G, respectively. Quantification of the level of fluorescence indicates that expression of tubulin IIIβ in non-stimulated LNCaP cells was low in comparison to stimulated cells (Figure 2J). Treatment of LNCaP cells with bicalutamide (20 μM, Figure 2D) or cs-FBS (Figure 2G) for 4 d resulted in a significant increase in tubulin IIIβ immunofluorescence compared to non-stimulated cells (Figure 2A). Quantification of tubulin IIIβ expression demonstrated a significant increase in immunofluorescence in LNCaP cells cultured for 4 d with 20 μM bicalutamide compared with control or 10 μM bicalutamine-treated cells (Figure 2J). Culture of LNCaP cells for 4 d in a hormone-depleted media also caused a significant increase in tubulin IIIβ immunofluorescence (Figure 2J). Changes in the expression of neurotensin were detected by qPCR. Exposure of LNCaP cells to bicalutamide (10 and 20 μM) for 4 d did not result in a significant increase in the expression of neurotensin transcripts (Figure 2K). However, treatment of LNCaP cells with hormone-depleted media evoked a significant increase in neurotensin mRNA (Figure 2K).
Figure 2.
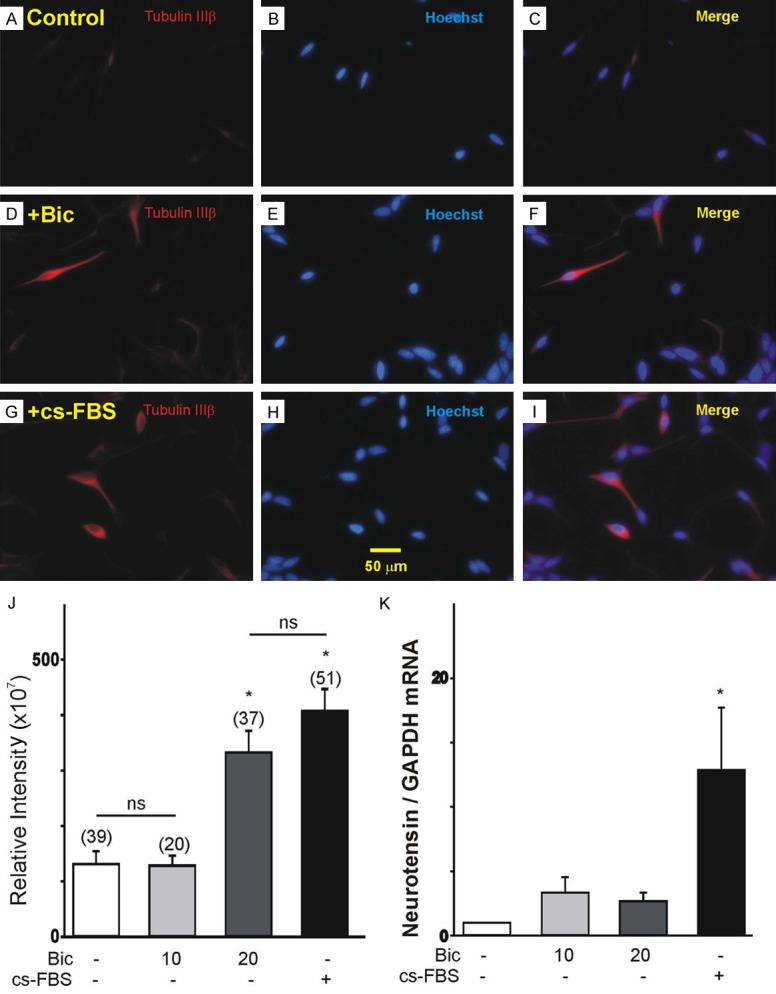
Changes in tubulin IIIβ and neurotensin expression in LNCaP cells treated with bicalutamide or hormone-depleted media. (A, I) Changes in the expression of tubulin IIIβ following treatment with bicalutamide (D, 20 µM, 4 d) or hormone-depleted media (G, 20 µM, 4 d) compared to the control (A). (B, E, H) Images of cell nuclei of the control, bicalutamide and cs-FBS treatments, respectively, stained with Hoechst. (C, F) Merged images of tubulin IIIb expression and cell nuclei in control (C), bicalutamide (F), and cs-FBS (I) treated cells. (J) Summary of the changes in tubulin IIIβ immune-fluorescence under various culture conditions. Treatment of LNCaP cells with bicalutamide (20 μM) or hormone-depleted media (cs-FBS) for 4 d increases the expression of neuronal marker tubulin IIIβ (*P<0.05 vs. control; ns, P>0.05 vs. control or bicalutamide 20 μM-stimulated cells). (K) Changes in the mRNA expression of the neuroendocrine marker, neurotensin following 4 d treatment with bicalutamide (10-20 µM) or hormone-depleted media (cs-FBS, n=3, *P<0.05 vs. control).
Effect of androgen receptor signaling on Cav3.2 T-type Ca2+ channel expression in LNCaP cells
To determine whether stimulation of LNCaP cells with bicalutamide or hormone-depleted media generate changes in Cav3.2 mRNA expression we performed PCR analysis (Figure 3A, 3B). Initially, we measured changes in Cav3.2 mRNA expression following 4 d stimulation with bicalutamide (10 and 20 μM) or hormone-depleted media. We also assessed changes in Cav3.2 mRNA expression after 10 d stimulation of LNCaP cells with bicalutamide (10 and 20 μM) since the effect of bicalutamide may show some delay, resulting in long lasting changes in cell differentiation. As represented in Figure 3A, real time PCR analysis indicates that there are no significant changes in the level of expression of Cav3.2 transcripts between non-stimulated (control) and bicalutamide-stimulated cells after 4 d treatment. Similarly, in samples stimulated with bicalutamide (10 and 20 μM) for 10 d there were no significant changes in Cav3.2 mRNA expression (Figure 3B). However, we observed a significant increase in Cav3.2 mRNA expression in LNCaP cells stimulated with hormone-depleted media at 4 and 10 d (Figure 3A, 3B).
Figure 3.
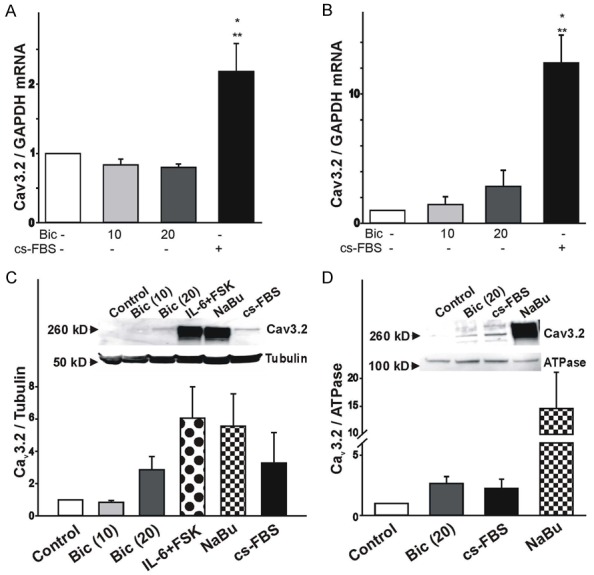
Changes in Cav3.2 mRNA and protein expression following stimulation of LNCaP cells with bicalutamide or hormone-depleted media. (A, B) Changes in the expression of Cav3.2 mRNA in LNCaP cells treated with bicalutamide or hormone-depleted media for 4 days (A, n=4) or over a 10 day-period (B, n=5). Induction of NED with hormone-depleted media but not bicalutamide promotes the expression of Cav3.2 mRNA. (*P<0.05 vs. control, **P<0.05 vs. bicalutamide 20 μM-stimulated cells). (C) Cav3.2 protein expression in whole cell lysates following stimulation with bicalutamide (10-20 μM) or hormone-depleted media (cs-FBS) for 10 d. Co-stimulation with IL-6 (50 ng/mL)+FSK (50 μM) or sodium butyrate (NaBu, 1 mM) for 10 d was used as a positive control. To confirm equal loading, membranes were stripped following Cav3.2 immunoblot and reprobed for tubulin expression. Ratio of Cav3.2 to tubulin expression was determined by densitometry analysis (n=6). Inset: Representative example of immune-detection of Cav3.2 and tubulin collected from LNCaP cells before and after 10 d stimulation with bicalutamide, hormone-depleted media, IL-6+FSK, and NaBu. Note that stimulation of LNCaP cells with bicalutamide or hormone-depleted media promotes Cav3.2 protein expression. (D) Changes in the membrane expression of Cav3.2 proteins following stimulation of LNCaP cells with 20 μM bicalutamide or hormone-depleted media for 10 d. Cav3.2 membrane expression was assessed following biotinylation of membrane proteins. To confirm that equal amounts of proteins were used for the pull down of biotinylated proteins we probed for ATPase. Ratio of biotinylated Cav3.2 to ATPase expression was determined by densitometry analysis (n=4). Inset: Representative example of western blot data of biotinylated samples following stimulation of LNCaP cells with 20 μM bicalutamide, hormone-depleted media, and 1 mM NaBu for 10 d.
To determine whether stimulation of LNCaP cells with bicalutamide or hormone-depleted media alters Cav3.2 protein expression, we performed western blot analysis using a specific antibody against the Cav3.2 channel subunit (see Methods). LNCaP cells were also stimulated with IL-6+FSK or NaBu because these conditions evoke significant expression of Cav3.2 proteins as previously reported [14,15]. First, we tested whether stimulation of LNCaP cells with bicalutamide or hormone-depleted media altered Cav3.2 protein expression in whole cell lysates (Figure 3C). Cav3.2 protein expression following treatment of LNCaP cells with bicalutamide (10 and 20 μM) for 4 d was not detected (results not shown). Therefore, we tested whether long-term exposure to bicalutamide had an effect on Cav3.2 protein expression. As represented in Figure 3C (inset), immunoblot analysis indicates that Cav3.2 expression was absent in non-stimulated cells cultured for 10 d. Treatment of LNCaP cells with 10 μM bicalutamide for 10 d had no effect on Cav3.2 protein expression. However, 10 d-stimulation of LNCaP cells with 20 μM bicalutamide evoked a noticeable increase in the expression of Cav3.2 protein as indicated by the presence of a band with a relative molecular weight of ~260 kD (Figure 3C inset). A similar band was also detected in LNCaP cells stimulated with hormone-depleted media for 10 d. We should point out that co-stimulation of LNCaP cells with IL-6+FSK or exposure to NaBu for 10 d caused a greater increase in Cav3.2 expression compared to that obtained with bicalutamide (20 μM) or hormone-depleted media. These findings are consistent with our previous work indicating that stimulation of LNCaP cells with IL-6+FSK or NaBu for 4 d is sufficient to evoke a significant increase in Cav3.2 protein expression [14,15]. Lack of Cav3.2 protein expression was not due to the lack of protein in the samples as assessed by the presence of tubulin (Figure 3C). The levels of tubulin expression across all treatment conditions was not significantly different compared to non-stimulated controls (results not shown).
To assess whether bicalutamide and hormone-depleted media promote Cav3.2 protein expression in the membrane, we performed immunoblot analysis of biotinylated membrane proteins (Figure 3D). Stimulation of LNCaP cells with bicalutamide (20 μM) or hormone-depleted media for 10 d caused a significant increase in the membrane expression of Cav3.2 proteins (Figure 3D, inset). Lack of Cav3.2 protein expression in non-stimulated cells was not due to the lack of protein in the samples as assessed by the presence of ATPase (Figure 3D, inset). The ratio of Cav3.2 to ATPase indicates that treatment of LNCaP cells with bicalutamide (20 μM) or hormone-depleted media caused a considerable increase in of Cav3.2 membrane protein expression when compared to the non-stimulated controls (Figure 3D). ATPase expression was similar under the relevant conditions (results not shown).
Whole cell recordings (Figure 4) were conducted to evaluate whether increased Cav3.2 protein expression leads to increased functional T-type Ca2+ channels in the membrane. Calcium ion currents were isolated by substitution of sodium ions with external tetraethylammonium, where outward potassium ion currents were blocked with cesium ions in the pipette solution. Macroscopic calcium ion currents were produced by a series of 200 ms depolarizing steps from a holding potential of -100 mV to +80 mV (Figure 4A, stimulation protocol is represented in Figure 4A, lower traces). T-type Ca2+ currents were also prompted by a 750 ms-voltage ramp from -100 mV to +80 mV (0.24 V/s, stimulation protocol is represented in Figure 4B lower trace). Whole cell recordings of LNCaP cells stimulated with 20 μM bicalutamide for ≥ 10 d reveal the presence of transient inward calcium ion currents (Figure 4A, 4B) while no T-type Ca2+ currents were apparent in LNCaP cells stimulated with bicalutamide for 4 d (results not shown). Inward currents underwent rapid inactivation within 100 ms after application of a depolarizing voltage step (Figure 4A). The current-voltage relationship reveals that the transient component was activated at potentials between -30 and -20 mV and reached a peak at -10 mV (Figure 4C). There were no changes in the capacitance values of LNCaP cells treated with bicalutamide or hormone-depleted media between 4 and 10 d (Figure 4D). Typical Ca2+ current densities of LNCaP cells treated with bicalutamide or hormone-depleted media are represented in Figure 4E. Overall, culture of LNCaP cells with bicalutamide or hormone-depleted media resulted in the expression of inward currents in ~20% of all recorded cells (Figure 4E). No inward currents were detected in non-stimulated LNCaP cells (Figure 4E). These results demonstrate that long-term treatment of LNCaP cells with 20 μM bicalutamide results in the functional expression of T-type Ca2+ channels.
Figure 4.

Functional expression of T-type Ca2+ currents in LNCaP cells treated with bicalutamide or hormone-depleted media for 4-10 days. A. Characteristics of the transient Ca2+ currents generated by a membrane step protocol. The voltage step protocol consisted of voltage steps from a holding potential of -100 mV to +40 mV and is shown below the current trace. B. Representative traces of the inward Ca2+ current generated by a 750 ms-depolarizing voltage ramp from -100 mV to +80 mV. C. Voltage-dependence of the normalized T-type Ca2+ currents generated in LNCaP cells following treatment with bicalutamide (n=4). D. Changes in cell capacitance in LNCaP cells cultured under different conditions and for various periods of time. The number of cells analyzed under each condition is presented above each bar. E. Plots of the number of T-type Ca2+ current expressing cells as a function of cell densities. Note that control cells did not express T-type Ca2+ currents. Approximately 20% of LNCaP cells treated with bicalutamide or hormone-depleted media express T-type Ca2+ currents. Current densities were obtained by dividing current amplitude at -10 mV by cell capacitance.
Effect of T-type Ca2+ channel expression on the morphological differentiation and viability of LNCaP cells
Increased functional expression of T-type Ca2+ channels regulates the morphological differentiation and viability of LNCaP cells undergoing NED [14,15]. Therefore, it was of interest to assess whether bicalutamide (or hormone-depleted media treatment)-evoked expression of T-type Ca2+ channels also regulates the morphological differentiation and viability of LNCaP cells. Changes in the morphological differentiation of LNCaP cells due to increased expression of T-type Ca2+ channels was investigated in LNCaP cells exposed to bicalutamide (20 μM) or hormone-depleted media for 10 d (in order to induce the functional expression of T-type Ca2+ channels), followed by 2 d treatment in the presence or the absence of the T-type Ca2+ channel blockers, NiCl2 (100 µM) or NNC 55-0396 (5 µM). Low concentrations of Ni2+ ions (≤ 100 µM) selectively block T-type Ca2+ currents generated by Cav3.2 subunits [26,27], whereas NNC 55-0396 is also a potent blocker of T-type Ca2+ channels [28]. Changes in the morphological differentiation of LNCaP cells were assessed by measuring the total length and average number of neurite-like processes per cell. Stimulation of LNCaP cells with bicalutamide (20 μM) or hormone-depleted media for 10 d resulted in a significant increase in the total neurite-like length and average number of processes compared to non-stimulated cells (Figure 5A, 5B). Inhibition of T-type Ca2+ channel activity with NiCl2 or NNC 55-0396 for 2 d had a significant effect on the neurite-like outgrowth. Inhibition of T-type Ca2+ channel activity with 100 µM Ni2+ or 5 μM NNC 55-0396 caused a significant reduction in the total length of neurite-like processes of LNCaP cells stimulated with bicalutamide or hormone-depleted media (Figure 5A). NNC 55-0396 also caused a significant reduction in the average number of processes per cell stimulated with bicalutamide or hormone-depleted media, whereas the effect of 100 µM Ni2+ on the average number of processes per LNCaP cell was only observed when cells were stimulated with hormone-depleted media (Figure 5B).
Figure 5.
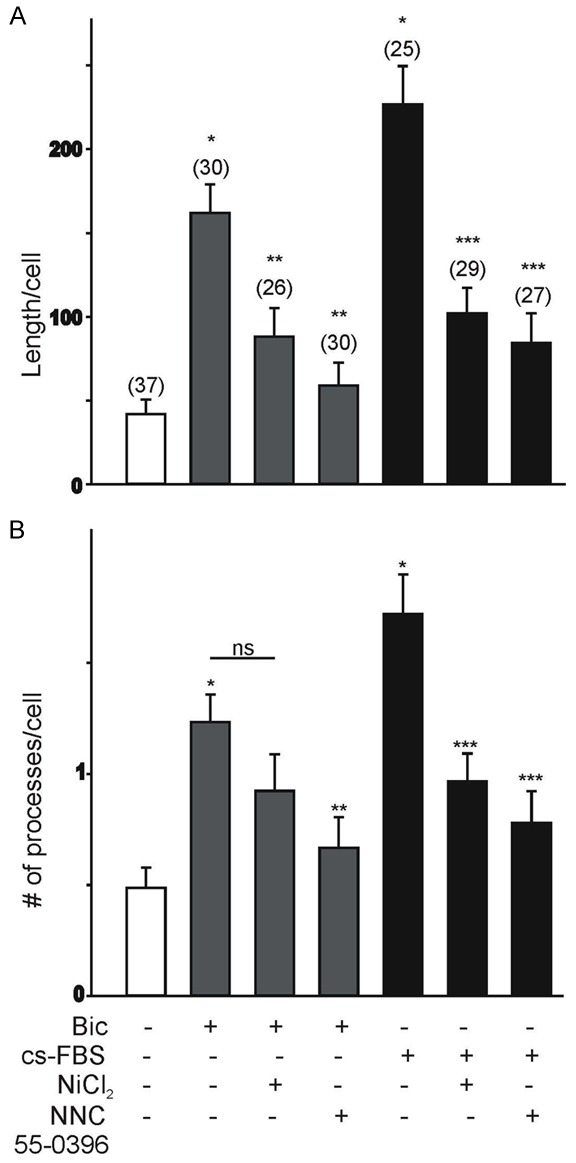
Influence of bicalutamide or hormone-depleted media on T-type Ca2+ channels in regulating the neurite-like morphology of LNCaP cells. (A, B) Effect of Ni2+ ions (NiCl2, 100 µM) or NNC 55-0396 (5 μM) on total neurite-like length (A) and average number of primary processes per cell (B) in LNCaP cells stimulated with bicalutamide or hormone-depleted media for 10 d. Stimulation of LNCaP cells with bicalutamide or hormone-depleted media for 10 d evoked a significant increase in total neurite-like length (A) and average number of primary processes per cell (B) compared to non-stimulated cells. Inhibition of T-type Ca2+ channels with Ni2+ ions or NNC 55-0396 evokes a significant decrease in the total neurite-like length and average number of primary processes per cell. The number of cells analyzed under each condition is represented above each bar (*P<0.05 vs. non-stimulated control cells; **P<0.05 vs. bicalutamide-stimulated cells; ***P<0.05 vs. treatment with hormone-depleted media; ns, P>0.05 vs. bicalutamide-stimulated cells).
To assess whether bicalutamide-induced expression of T-type Ca2+ channels has a significant effect on cell proliferation, changes in cell viability were assessed using the XTT assay (see Methods). We also sought to determine whether bicalutamide-induced expression of T-type Ca2+ channels would increase the resistance of LNCaP cells to treatment with the microtubule-disrupting agent docetaxel (DTX). LNCaP cells were treated with bicalutamide (20 μM) for 4 or 10 d in order to evoke the expression of T-type Ca2+ channels (Figure 6A, 6B). After a 4 d- or 10 d-exposure to bicalutamide, cells were treated with docetaxel (DTX 100-1000 nM) for an additional 2 d. In non-stimulated or cells treated with 20 μM bicalutamide for 4 d, DTX caused a significant reduction in cell viability, especially at concentrations ≥ 500 nM (Figure 6A). However, in cells treated with 20 μM bicalutamide for 10 d, DTX exposure did not evoke any further effect on cell viability, whereas non-stimulated cells cultured for 10 d continued to respond to DTX treatment (Figure 6B). These findings suggest that long-term bicalutamide treatment increases the resistance of LNCaP cells undergoing NED to DTX. To investigate the possibility that the expression of functional T-type Ca2+ channels contribute to the viability of bicalutamide-treated LNCaP cells they were treated with bicalutamide (20 μM) for 4 or 10 d (Figure 6C, 6D). After this period, cells were treated for 2 d with 100 µM Ni2+ or 5 μM NNC 55-0396 in the presence or absence of docetaxel (DTX, 500 nM). As represented in Figure 6C, inhibition of T-type Ca2+ channels with Ni2+ or NNC 55-0396 did not have an effect on the viability of LNCaP cells pre-treated with bicalutamide for 4 d, consistent with the lack of Cav3.2 protein and functional T-type Ca2+ channels at this stage. However, co-treatment with the DTX and the T-type Ca2+ channel inhibitors causes a significant reduction in cell viability. The opposite findings were observed in cells cultures treated with 20 μM bicalutamide for 10 d. Under this condition, inhibition of T-type Ca2+ channels with Ni2+ or NNC 55-0396 caused a significant reduction in the viability of LNCaP cells exposed to 20 μM bicalutamide for 10 d (Figure 6D). However, co-treatment with the DTX and the T-type Ca2+ channel inhibitors had no further effect on cell viability. These findings suggest that increased T-type Ca2+ channel expression after long term exposure to bicalutamide regulates the viability of LNCaP cells.
Figure 6.
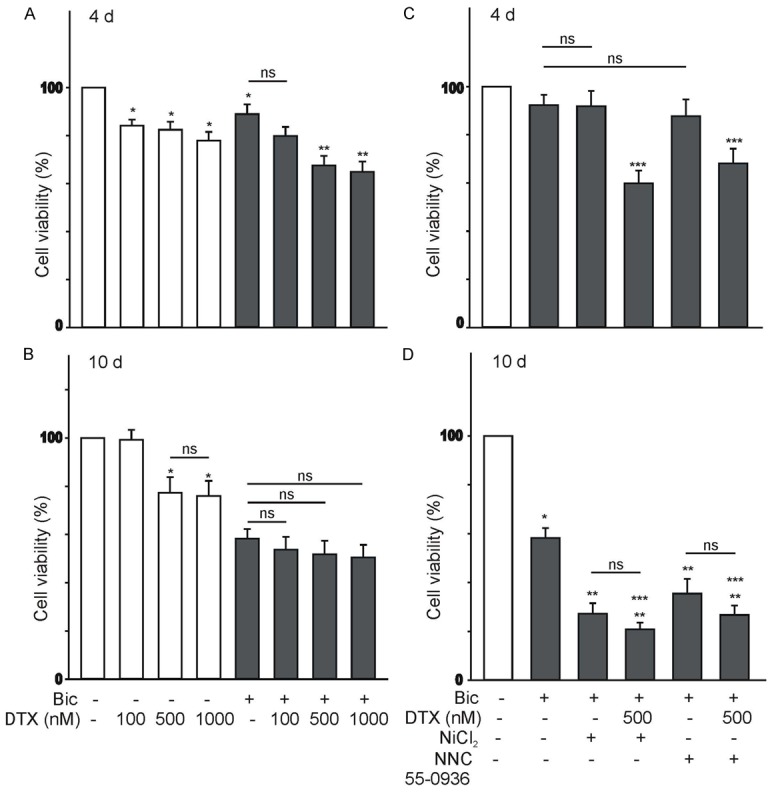
Effect of the microtubules-disrupting drug docetaxel (DTX) on the LNCaP cell viability following bicalutamide treatment for 4-10 d. (A, B) DTX evokes a significant reduction in the viability of control and bicalutamide-treated cells (4 d, A). In cultures treated with bicalutamide for 10 d, increasing concentrations of DTX had no effect on cell viability (B). In these experiments, LNCaP cells were treated with 20 μM bicalutamide for 4 or 10 d (A and B, respectively). After this period, cells were exposed to DXT (100-1000 nM) for 2 d. Cell viability was determined by the XTT assay at the end of the 6 or 12 d treatment period [ns=no significant, *P<0.05 vs. control cells (not treated with bicalutamide), **P<0.05 vs. bicalutamide 20 μM-treated cells, n=16]. (C, D) Inhibition of T-type Ca2+ channels causes a significant reduction in LNCaP cell viability when stimulated with bicalutamide for 10 d, but not after 4 d treatment. In these experiments, LNCaP cells were treated with 20 μM bicalutamide for a period of 4 or 10 d in order to evoke T-type Ca2+ channel expression (C and D, respectively). After this period, cells were exposed to 100 μM Ni2+ ions or 5 μM NNC 55-0396 DXT with or without DTX (500 μM). Inhibition of T-type Ca2+ channels with 100 μM Ni2+ ions or 5 μM NNC 55-0396 causes a significant reduction in the viability of bicalutamide-treated cell cultures for 10 d [ns=no significant, *P<0.05 vs. control cells (not treated with bicalutamide), **P<0.05 vs. bicalutamide-treated cells, ***P<0.05 vs. cells co-treated with bicalutamide and 100 μM Ni2+ ions or 5 μM NNC 55-0396, n=12].
Effect of androgen receptor signaling on Cav3.2 T-type Ca2+ channel expression in 22Rv1 cells
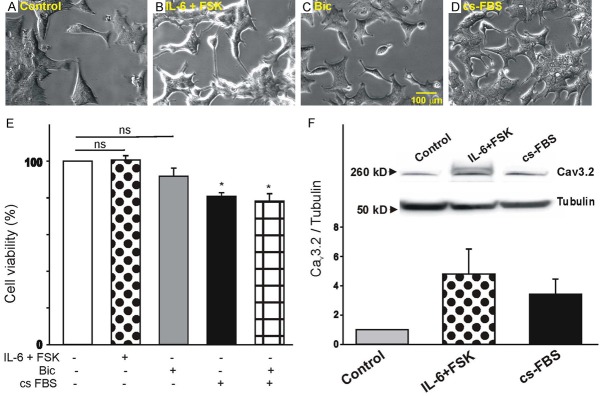
To assess whether disruption of androgen-receptor signaling also alter the properties of other prostate cancer cells we use the 22Rv1 cell line, derived from a primary prostate tumor [29]. Although 22Rv1 cells do not require androgens for growth, they can exhibit increased proliferation in the presence of androgens [29]. Like LNCaP cells, 22Rv1 cells also express androgen receptors at the protein level [29]. Since little is known regarding the effect of neuroendocrine factors in inducing morphological changes in 22Rv1 cells, cultures were treated with IL-6+FSK, bicalutamide (20 µM) or hormone-depleted media for 4 d. As represented in Figure 7A-D, treatment of 22Rv1 cells with IL-6+FSK, bicalutamide (20 µM) or hormone-depleted media has a minimal effect on cell shape with the majority of cells still possessing a polygonal morphology. To further characterize how these treatments may affect cell proliferation we performed the XTT assay (Figure 7E). Treatment of 22Rv1 cells with IL-6+FSK or bicalutamide (20 µM) had no effect of cell viability. However, exposure of 22Rv1 cells to hormone-depleted media caused a significant reduction in cell proliferation that was not further enhanced by treatment with bicalutamide. Furthermore, treatment of 22Rv1 with IL-6+FSK or hormone-depleted media evoked a significant increase in the expression of Cav3.2 channel protein.
Figure 7.
Effect of androgen receptor signaling on the morphology and T-type Ca2+ channel expression of 22Rv1 cells. (A-D) Cell morphology of untreated 22Rv1 cells (control, A), following 4 d treatment with IL-6+FSK (B), bicalutamide (20 µM, C), or hormone-depleted media (D). Note that treatment with IL-6+FSK, bicalutamide or hormone-depleted media has minimal effect on cell morphology. (E) Effect of IL-6+FSK, bicalutamide or hormone-depleted media on the viability of 22Rv1 cells (n=8, *P<0.05 vs. non-stimulated controls). (F) Changes in Cav3.2 protein expression in whole cell lysates following 22Rv1 stimulation with IL-6+FSK or hormone-depleted media (n=5). Inset: Representative example of immune-detection of Cav3.2 and tubulin in 22Rv1 cells before and after 10 d stimulation with IL-6+FSK or hormone-depleted media. Note that stimulation of 22Rv1 cells with IL-6+FSK or hormone-depleted media promotes Cav3.2 protein expression.
Discussion
This work was undertaken to assess whether disruption of androgen signaling promotes the NED of LNCaP cells and whether this effect is correlated with increased expression of T-type Ca2+ channels. LNCaP cells have been extensively used as a model to investigate the role of various factors in the induction of NED [24,30,31]. In vivo experiments have also demonstrated that LNCaP cells share similar characteristics to those of adenocarcinomas, including growth inhibition by androgen depletion therapies [32]. Although the present work demonstrates that disruption of androgen receptor signaling evokes the molecular and functional expression of T-type Ca2+ channels in the androgen-dependent LNCaP cell line, the overall clinical significance of this finding in the progression of prostate cancer requires further studies, particularly in patients undergoing androgen-ablation therapy.
Our present results indicate that disruption of androgen receptor signaling in LNCaP cells with bicalutamide or hormone-depleted media for 4 or 10 d evoke considerable morphological and biochemical changes consistent with the induction of NED including the development of neurite-like processes and the expression of several neuroendocrine markers including tubulin IIIβ and neurotensin. These results are consistent with previous findings demonstrating increased expression of neurotensin following long-term exposure of LNCaP cells to bicalutamide [21]. Interestingly, our present results reveal significant differences in the differentiation program triggered by treatment of LNCaP cells with 10 and 20 µM biculatamide. The lower concertation of biculatamide evokes morphological changes without any molecular changes, including no change in tubulin IIIβ expression. On the contrary, treatment of LNCaP cells with 20 µM biculatamide evokes both morphological changes and increased expression of tubulin IIIβ during a 4 d-stimulation period. It is possible that morphological differentiation occurs at a faster rate than molecular differentiation. Thus, molecular differentiation may require longer exposure time in order to develop under our experimental conditions. We should also mention, that the expression of some differentiation features is time-dependent. For example, morphological differentiation and tubulin IIIβ expression occurs within 4 d stimulation with bicalutamide or hormone-depleted media, whereas increased expression of neurotensin transcripts only occurs in hormone-depleted media. Furthermore, there were some differences in the temporal pattern of expression of neurotensin and morphological changes following treatments with bicalutamide or hormone-depleted. Treatment of LNCaP cells with hormone-depleted media, but not bicalutamide, results in significant morphological and biochemical changes consistent with NED, including the development and lengthening of neurite-like processes and the expression of differentiation markers [present results; 12, 18, 33]. These findings indicate that bicalutamide and hormone-depleted media do not trigger identical differentiation programs.
Disruption of androgen receptor signaling with bicalutamide or hormone-depleted treatments evokes the expression of T-type Ca2+ channels in LNCaP cells undergoing NED. Thus, stimulation of LNCaP cells for ≥ 10 d with bicalutamide or hormone-depleted media induces a significant expression of the Cav3.2 T-type Ca2+ channel subunit, resulting in functional channels in the membrane as determined by whole cell recordings. However, the level of protein expression of T-type Ca2+ channels produced in response to disrupting androgen receptor function is significantly less than that produced by IL-6+FSK or NaBu-treatments. Similarly, treatment of 22Rv1 cells for 10 d with IL-6+FSK or hormone-depleted media induces a significant increase in Cav3.2 protein expression. However, differently from LNCaP cells, treatment with IL-6+FSK or hormone-depleted media had a minimal effect on the morphological differentiation of 22Rv1 cells. Although stimulation of LNCaP cells with bicalutamide or hormone-depleted media induces a significant increase in Cav3.2 protein expression, functional T-type Ca2+ channels were only detected in ~20% of recorded cells. We previously reported that approximately 40-50% of LNCaP cells stimulated with IL-6+FSK or NaBu express functional T-type Ca2+ channels after 4 d treatment [14,15]. We should mention that one limitation of our whole cell recordings is incomplete clamping of distal neurite-like processes. Thus, it is possible that we underestimated the extent of differentiated LNCaP cells expressing functional T-type Ca2+ channels, which may be preferentially localized in distal processes, farther away from the cell body.
It appears that the protein expression of the Cav3.2 T-type Ca2+ channel subunit is regulated post-transcriptionally in LNCaP cells treated with bicalutamide, since no changes in Cav3.2 mRNA were detected within a 4-10 d stimulation period. On the contrary, hormone-depletion evokes a significant increase in Cav3.2 mRNA after 4 d treatment, which is consistent with previous findings [34]. Thus, different from the effect of bicalutamide, treatment of LNCaP cells with hormone-depleted media appears to upregulate Cav3.2 expression at the transcriptional level. Therefore, our data indicate that Cav3.2 T-type Ca2+ channel subunit expression can be regulated by transcriptional or post-transcriptional mechanisms in LNCaP cells undergoing NED. We have also described different regulatory mechanisms in Cav3.2 protein expression following stimulation with IL-6 and NaBu [14,15]. Furthermore, functional expression of T-type Ca2+ channels in the membrane is also influenced by cAMP levels in LNCaP cells [15]. Intrinsic factors such as endogenous production of hydrogen sulfide (H2S) can also regulate the functional expression of T-type Ca2+ channels in LNCaP cells undergoing NED [34]. Future work is required to explore the synergistic effect of other factors in promoting T-type Ca2+ channel expression following bicalutamide induction of NED.
Increased T-type Ca2+ channel expression regulates the morphological differentiation of LNCaP cells undergoing NED. Thus, our present data indicates that blockade of functional T-type Ca2+ channels with NiCl2 or NNC 55-0936 evokes a considerable reduction in the neurite-like length and morphology of differentiated LNCaP cells. We should point out that morphological differentiation occurred following 4 d stimulation with bicalutamide or hormone-depleted media, when no functional T-type Ca2+ channels can be detected on the membrane. However, after long-term exposure to bicalutamide, inhibition of T-type Ca2+ channels causes a significant reduction in neurite-like outgrowth. Thus, it appears that functional expression of T-type Ca2+ channels sustains the morphological differentiation of prostate cancer cells. However, morphological differentiation during NED can be initiated independently of T-type Ca2+ channel expression. Furthermore, in 22Rv1 cells, disruption of androgen receptor signaling results in a significant increase in the expression of T-type Ca2+ channel subunits without any noticeable effect on differentiation.
Functional expression of T-type Ca2+ channels also regulates the viability and DTX resistance of LNCaP cells treated with bicalutamide for 10 d. Our present results demonstrate that long term treatment with bicalutamide increases the resistance of prostate cancer cells to DTX. On the contrary, 4 d exposure to bicalutamide evoked a considerable reduction in the cell viability following DTX treatment. Furthermore, inhibition of T-type Ca2+ channels with NiCl2 or NNC 05-0936 caused a significant reduction in bicalutamide long-term treated cell cultures. These findings indicate that functional expression of T-type Ca2+ channels promote cell viability, which can be reduced by inhibition of functional T-type Ca2+ channels. Previous work has shown the importance of T-type Ca2+ channel function and subsequence changes in intracellular Ca2+ in the regulation of cell differentiation and survival [34-36]. Because of their lower threshold for activation, T-type Ca2+ channel activity can be significant at membrane potentials close to rest, resulting in a “window current” that regulates various Ca2+-dependent processes [34-36]. Other than a direct effect on gene expression, increased intracellular Ca2+ can also regulate exocytosis of mitogenic factors such as neurotensin, which can further promote cell survival [8].
NED is a typical trait in the development of androgen-resistant prostate cancers. However, its prognostic significance is still debated, suggesting significant heterogeneity among prostate cancer patients undergoing similar treatments [19,37]. We propose that some of this heterogeneity may arise from differences in the differentiation pathway triggered by anti-androgen therapies and changes in intracellular Ca2+ [38]. As our present results indicate, treatment of prostate cancer cells with an androgen receptor blocker or hormone-depleted media results in slightly different differentiation pathways. These differences may be enhanced in vivo by extrinsic factors in the microenvironment such as inflammation (leading to increased production of pro-inflammatory cytokines, e.g. IL-6) and cAMP-stimulating hormones [39,40]. Thus, understanding the progression of neuroendocrine cells will have critical importance in developing more personalized treatments for prostate cancers.
Acknowledgements
This work was supported by funds provided by the UMES School of Pharmacy and grant P031B090209 from the US Department of Education.
Disclosure of conflict of interest
None.
Abbreviations
- DTX
docetaxel
- IL-6
interleukin-6
- LNCaP
lymph node carcinoma of the prostate
- NaBu
sodium butyrate
- NED
neuroendocrine differentiation
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 3.Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F. Androgen receptor antagonists for prostate cancer therapy. Endocr Relat Cancer. 2014;21:T105–18. doi: 10.1530/ERC-13-0545. [DOI] [PubMed] [Google Scholar]
- 4.Chuu CP, Kokontis JM, Hiipakka RA, Fukuchi J, Lin HP, Lin CY, Huo C, Su LC. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J Biomed Sci. 2011;18:63. doi: 10.1186/1423-0127-18-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipianskaya P, Cohen A, Chen C, Hsia E, Squires J, Li Z, Zhang Y, Li W, Chen X, Xu H, Huang J. Androgen-deprivation therapy-induced aggressive prostate cancer with neuroendocrine differentiation. Asian J Androl. 2014;16:541–544. doi: 10.4103/1008-682X.123669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grobholz R, Griebe M, Sauer CG, Michel MS, Trojan L, Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36:562–70. doi: 10.1016/j.humpath.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26:745–56. doi: 10.1038/sj.onc.1209814. [DOI] [PubMed] [Google Scholar]
- 9.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, Ha MJ, Tsokos M, Sheahan MD, Nguyen P, Niklinski WT, Myers CE, Trepel JB. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci U S A. 1994;91:5330–4. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21:8471–82. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–30. [PubMed] [Google Scholar]
- 12.Huang J, Yao J, Di Sant’Agnese P, Yang Q, Bourne P, Na Y. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. 2006;66:1399–1406. doi: 10.1002/pros.20434. [DOI] [PubMed] [Google Scholar]
- 13.Mariot P, Vanoverberghe K, Lalevee N, Rossier MF, Prevarskaya N. Overexpression of an alpha 1H (Cav3.2) T-type calcium channel during neuroendocrine differentiation of human prostate cancer cells. J Biol Chem. 2002;277:10824–33. doi: 10.1074/jbc.M108754200. [DOI] [PubMed] [Google Scholar]
- 14.Weaver EM, Zamora FJ, Puplampu-Dove YA, Kiessu E, Hearne JL, Martin-Caraballo M. Regulation of T-type calcium channel expression by sodium butyrate in prostate cancer cells. Eur J Pharmacol. 2015;749:20–31. doi: 10.1016/j.ejphar.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Weaver EM, Zamora FJ, Hearne JL, Martin-Caraballo M. Posttranscriptional regulation of T-type Ca2+ channel expression by interleukin-6 in prostate cancer cells. Cytokine. 2015;76:309–320. doi: 10.1016/j.cyto.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Alberti C. Neuroendocrine differentiation in prostate carcinoma: focusing on its pathophysiologic mechanisms and pathological features. G Chir. 2010;31:568–74. [PubMed] [Google Scholar]
- 17.Kim J, Adam RM, Freeman MR. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62:1549–54. [PubMed] [Google Scholar]
- 18.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, Karan D, Batra SK, Lin MF. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–67. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 19.Jeetle SS, Fisher G, Yang ZH, Stankiewicz E, Møller H, Cooper CS, Cuzick J, Berney DM Trans-Atlantic Prostate Group. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch. 2012;461:103–7. doi: 10.1007/s00428-012-1259-2. [DOI] [PubMed] [Google Scholar]
- 20.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–18. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 21.Vias M, Burtt G, Culig Z, Veerakumarasivam A, Neal DE, Mills IG. A role for neurotensin in bicalutamide resistant prostate cancer cells. Prostate. 2007;67:190–202. doi: 10.1002/pros.20518. [DOI] [PubMed] [Google Scholar]
- 22.Sedelaar JP, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69:1724–9. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–5. [PubMed] [Google Scholar]
- 24.Marchiani S, Tamburrino L, Nesi G, Paglierani M, Gelmini S, Orlando C, Maggi M, Forti G, Baldi E. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl. 2010;33:784–93. doi: 10.1111/j.1365-2605.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 25.Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–37. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- 26.Kang HW, Park JY, Jeong SW, Kim JA, Moon HJ, Perez-Reyes E, Lee JH. A molecular determinant of nickel inhibition in Cav3.2 T-type calcium channels. J Biol Chem. 2006;281:4823–30. doi: 10.1074/jbc.M510197200. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–42. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl] -N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride] : a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309:193–9. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- 29.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:226–44. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 30.Zelivianski S, Verni M, Moore C, Kondrikov D, Taylor R, Lin MF. Multipathways for transdifferentiation of human prostate cancer cells into neuroendocrine-like phenotype. Biochim Biophys Acta. 2001;1539:28–43. doi: 10.1016/s0167-4889(01)00087-8. [DOI] [PubMed] [Google Scholar]
- 31.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–47. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 32.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–79. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen R, Dorai T, Szaboles M, Katz AE, Olsson CA, Buttyan R. Transdifferentiation of cultured human prostate cancer cells to a neuroendocrine cell phenotype in a hormone-depleted medium. Urol Oncol. 1997;3:67–75. doi: 10.1016/s1078-1439(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 34.Gackière F, Bidaux G, Delcourt P, Van Coppenolle F, Katsogiannou M, Dewailly E, Bavencoffe A, Van Chuoï-Mariot MT, Mauroy B, Prevarskaya N, Mariot P. Cav3.2 T-type calcium channels are involved in calcium-dependent secretion of neuroendocrine prostate cancer cells. J Biol Chem. 2008;283:10162–73. doi: 10.1074/jbc.M707159200. [DOI] [PubMed] [Google Scholar]
- 35.Fukami K, Sekiguchi F, Yasukawa M, Asano E, Kasamatsu R, Ueda M, Yoshida S, Kawabata A. Functional upregulation of the H2S/Cav3.2 channel pathway accelerates secretory function in neuroendocrine-differentiated human prostate cancer cells. Biochem Pharmacol. 2015;97:300–9. doi: 10.1016/j.bcp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Weiss N, Zamponi GW, De Waard M. How do T-type calcium channels control low-threshold exocytosis? Commun Integr Biol. 2012;5:377–80. doi: 10.4161/cib.19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin Genitourin Cancer. 2011;9:73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–9. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol. 2014;2:231–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Merkle D, Hoffmann R. Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell Signal. 2011;23:507–15. doi: 10.1016/j.cellsig.2010.08.017. [DOI] [PubMed] [Google Scholar]