Abstract
This study was performed to evaluate the cardioprotective effects of osthole (OST) in a rat model of myocardial ischemia/reperfusion injury (MI/RI) and the underlying mechanism. We exposed rat hearts to left anterior descending coronary artery ligation for 30 min followed by 24 h of reperfusion. The results showed that pretreatment with OST ameliorated MI/RI as evidenced by histopathological examination. Moreover, the terminal deoxynucleotidyl transferase dUTP nick end-labeling assay demonstrated that OST suppressed myocardial apoptosis, which may be related to an increase in the Bcl-2/Bax ratio and inhibition of caspase-3 and caspase-9 activation. Furthermore, we determined that OST ameliorated impaired mitochondrial morphology and the oxidation system; OST also attenuated levels of pro-inflammatory cytokines, including tumor necrosis factor α and interleukins 6 and 1β. In conclusion, OST exerted a strong favorable cardioprotective effect on MI/RI, possibly by suppressing the inflammatory response and inhibiting cell apoptosis.
Keywords: Osthole, myocardial ischemia, inflammatory, apoptosis
Introduction
Myocardial ischemia/reperfusion injury (MI/RI), which refers to restoration of blood flow and reperfusion of ischemic myocardium, aggravates structural damage causing cell death and leading to expansion of the infarction [1]. MI/RI results in further damage to cardiac function and affects the prognosis of patients who have suffered a myocardial infarction [2]. Although the pathogenesis of MI/RI is more complex, many studies have shown that oxygen free radicals [3], calcium overload [4], and inflammatory mediators [5] are involved in the mechanism. Further research is needed to fully understand the mechanisms of ischemia reperfusion (I/R) injury and to identify novel therapeutic strategies.
Ischemia/reperfusion injury (I/R injury) is an intricate process involving numerous mechanisms. Apoptosis, inflammation, and oxidative injury all play a core role in I/R injury [6]. Monocytes, leukocytes, and other inflammatory cells infiltrate the injured area soon after ischemia [7]. Activated macrophages secrete pro-inflammatory cytokines that recruit neutrophils and promote myocardial damage. Many studies have shown that post-I/R inflammation aggravates myocardial damage [8]. In addition, MI/RI induces the production of oxygen free radicals, which may further trigger apoptosis [9]. Cardiomyocyte apoptosis and inflammation have been identified as characteristics of MI/RI. There is growing evidence that ischemia triggers apoptosis of cardiomyocytes and then expands and partially contributes to cardiac death through reperfusion [10]. Blocking apoptosis can prevent the reduction of contractile cells, minimizing I/R-induced cardiac injury and slowing down myocardial stunning and heart failure [11].
The natural coumarin derivative 7-methoxy-8-isopentenoxycou marin, also known as osthole (OST), was isolated from Cnidium monnieri (L.) Cusson [12]. Accumulating evidence suggests that OST has anti-inflammatory [13], anti-apoptotic [14], anti-oxidative stress [15], and neurotrophic properties [16] that make it promising for therapeutic applications. Moreover, OST has protective effects against MI/RI in rats [17]. However, the underlying mechanisms of the therapeutic effects of MI/RI by OST in the rat model remain unknown. Thus, in the present study, we investigated the potential role of OST against MI/RI in rats and explored the possible mechanisms through which OST mediates these effects.
Materials and methods
Animals
Adult Sprague-Dawley rats (male, 220-250 g) were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, P.R. China). The animals were housed in a room at 23 ± 2°C under 55 ± 5% relative humidity and a 12 h light/12 h dark cycle. All procedures were approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University.
MI/RI procedure
The MI/RI model was induced by ligating the left anterior descending coronary artery (LAD) for 30 min followed by 24 h of reperfusion. The rats were anesthetized by administering sodium pentobarbital (50 mg/kg) intraperitoneally. Following tracheal intubation, 6-0 silk was used to ligate the LAD for a 30-min ischemic period. Reperfusion was then allowed for 24 h by releasing the LAD ligation. Rats in the Sham control group were treated with the same surgical procedures except that ligation of the LAD was not performed.
Experimental groups
Rats were given standard chow ad libitum for the duration of the study and allowed 1 week to adapt to the laboratory environment before the experiment. The rats were randomly divided into five groups (n = 10 per group): (1) Sham; (2) I/R; (3) I/R+OST 20 mg/kg; (4) I/R+OST 40 mg/kg; (5) I/R+OST 80 mg/kg. The rats were administered the drug (once daily, intragastrically) for 7 days before the operation. The rats in the Sham and I/R groups were given equal volumes of water at the same time.
Histopathological analysis of the heart
Rat hearts were removed and fixed in 10% formalin solution. After embedding in paraffin wax, the tissues were sliced to a 4-µm thickness, and the sections were stained with hematoxylin-eosin (HE) solution for the histopathological examination.
Terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay
Apoptosis was carried out using the In Situ Cell Death Detection Kit, POD (Roche, Manheim, Germany) according to the manufacturer’s protocol. TUNEL-positive brown cells were considered apoptotic cells.
Determination of serum creatine kinase (CK), lactate dehydrogenase (LDH), malondialdehyde (MDA), and superoxide dismutase (SOD) levels
After reperfusion, the concentrations of CK, LDH, MDA, and SOD were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). All procedures were performed according to the manufacturer’s instructions. All measurements were performed in duplicate.
Determination of cytokines in serum by enzyme-linked immunosorbent assay (ELISA)
Serum levels of interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α were measured using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from heart tissues and reversed based on the protocol. Quantitative real-time PCR was carried out according to the manufacturer’s instructions. All data were quantified by use of the threshold cycle normalized to β-actin. The primers used in the study were listed: IL-6 forward: 5’-GAC TTC CAG CCA GTT GCC TT-3’; reverse: 5’-TCT CCT CTC CGG ACT TGT GAA-3’; IL-1β forward: 5’-CAG GAT GAG GAC CCA AGC AC-3’; reverse: 5’-CAG GTC GTC ATC ATC CCA CG-3; Bcl-2 forward: 5’-GGG ATG ACT TCT CTC GTC GC-3’; reverse: 5’-CCA CAA TCC TCC CCC AGT TC-3’; Bax forward: 5’-AGG ACG CAT CCA CCA AGA AG-3’; reverse: 5’-CAG TTG AAG TTG CCG TCT GC-3’; β-actin forward: 5’-CCC GCG AGT ACA ACC TTC TT-3’; reverse: 5’-CGC AGC GAT ATC GTC ATC CA-3’.
Western blot analysis
Total protein was extracted from rat heart tissues using RIPA buffer and measured using the BCA method. First, the protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). After blocking with 5% milk, primary antibodies against Bcl-2 (1:1000, ab59348; Abcam, Cambridge, MA, USA), Bax (1:1000, ab53154; Abcam), caspase-3 (1:1000, #9664; Cell Signaling Technology, Danvers, MA, USA), and caspase-9 (1:1000, #9507; Cell Signaling Technology) were incubated with the membranes overnight at 4°C. After incubation with a horseradish peroxidase-conjugated secondary antibody for 1 h at 37°C, immunoreactivity was detected by enhanced chemiluminescent reagents (Nanjing KeyGEN Biotechnology, Najiing, China), and a gel imaging system (Tanon Science & Technology Co., Ltd., Beijing, China) was used to visualize the protein bands.
Statistical analysis
All data are expressed as means ± standard deviation and analyzed by one-way analysis of variance followed by Tukey’s multiple comparison test using Graphpad Prism 5 software (GraphPad Software, La Jolla, CA, USA). A P-value < 0.05 was considered significant.
Results
Effect of OST on heart histopathology
As shown in Figure 1, changes in the morphological structures of myocardial tissues were evaluated by HE staining. The I/R group revealed disordered myocardial structures, inflammatory infiltration, and cardiac necrosis compared with the Sham group. The groups pretreated with OST showed markedly reduced inflammatory infiltration and cardiac necrosis compared with the I/R group in a dose-dependent manner.
Figure 1.
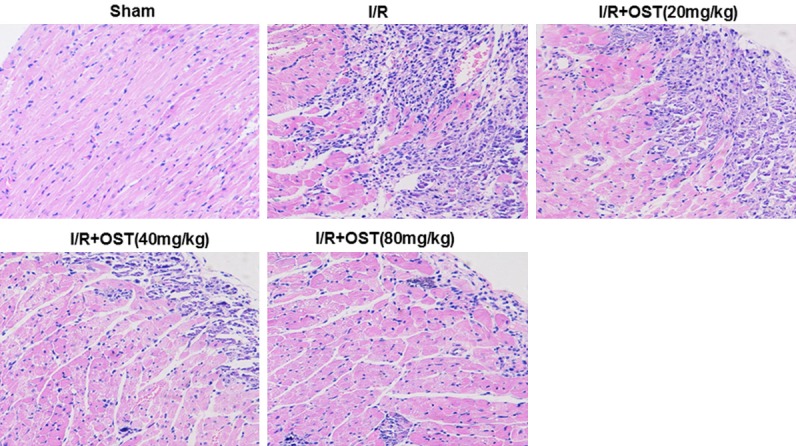
Effects of osthole (OST) on cell morphology and hematoxylin and eosin (HE) staining (×200).
Effect of OST on serum biochemical marker enzymes
Leakage of LDH and CK from myocardial tissues into the blood is an indicator of acute myocardial infarction. As shown in Figure 2, serum CK and LDH levels were significantly higher in the I/R group than in the Sham group (P < 0.05, P < 0.01, respectively). However, levels of CK and LDH were decreased significantly in the high-dose OST (80 mg/kg) group compared with the I/R group (P < 0.05, P < 0.01, respectively).
Figure 2.
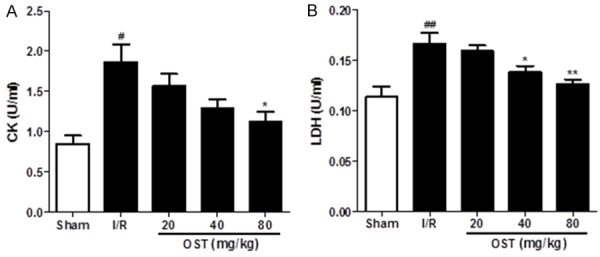
Osthole (OST) reduces creatine kinase (CK) (A) and lactate dehydrogenase (LDH) (B) activity in the serum of rats with ischemia/reperfusion (I/R) injury. Data are means ± standard deviation. #P < 0.05, ##P < 0.01 compared with the Sham group; *P < 0.05, **P < 0.01 compared with the I/R group.
Effect of OST on antioxidant parameters in rat I/R injury
Oxidative stress caused by reperfusion leads to lipid peroxidation. As shown in Figure 3, MDA levels (12.86 ± 2.21 vs. 5.12 ± 0.88, P < 0.01) were increased significantly and SOD activity (42.66 ± 4.16 vs. 61.02 ± 8.45, P < 0.05) was decreased significantly in the I/R versus Sham group. However, pre-treatment with OST decre-ased MDA content and increased SOD activity in serum significantly, especially in the high-dose OST group (80 mg/kg).
Figure 3.
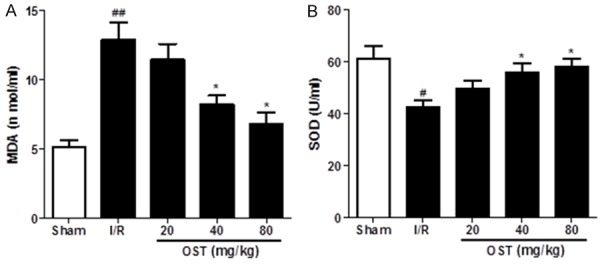
Osthole (OST) reduces the lipid peroxidation product malondialdehyde (MDA) (A) and enhances the antioxidant enzyme superoxide dismutase (SOD) (B) in serum of ischemia/reperfusion (I/R) injured rats. Data are mean ± standard deviation. #P < 0.05, ##P < 0.01 compared with Sham group; *P < 0.05 compared with the I/R group.
Effect of OST on pro-inflammatory cytokines and expression of inflammation-related proteins
Inflammation is one of the important causes of MI/RI. Thus, we investigated the serum levels of pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α by ELISA. As shown in Figure 4, serum IL-1β, IL-6, and TNF-α levels were increased significantly in the I/R group compared with the Sham group (P < 0.01), whereas the levels decreased dose-dependently in the groups treated with OST, especially 40 and 80 mg/kg OST. Taken together, these data suggest that OST decreased the release of cytokines in rats with myocardial I/R injury.
Figure 4.
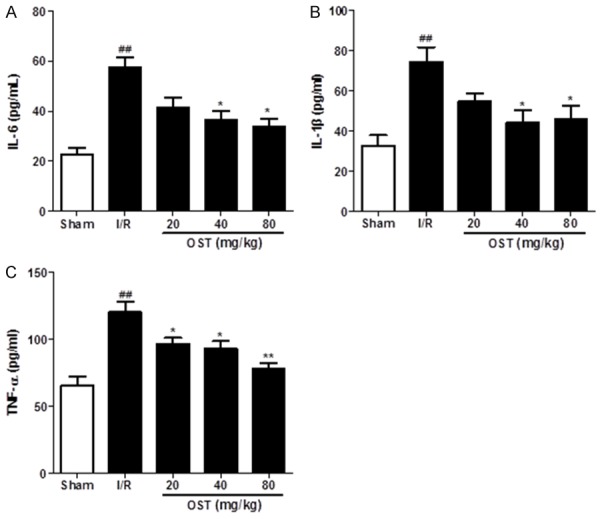
Osthole (OST) reduces the levels of the pro-inflammatory cytokines interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α as detected by enzyme-linked immunosorbent assay (A-C). Data are means ± standard deviation. ##P < 0.01 compared with the Sham group; *P < 0.05, **P < 0.01 compared with the I/R group.
Effect of OST on myocardial apoptosis and expression of apoptosis-related proteins
Many studies have reported that apoptosis contributes to myocardial cell death. The effects of myocardial I/R on cardiomyocyte apoptosis were detected by TUNEL assay (Figure 5). Apoptotic cells appear brown in color after TUNEL staining. In contrast to the Sham group, the number of apoptotic myocardial cells was increased significantly in the I/R group (P < 0.01). However, pretreatment with OST markedly reduced the number of apoptotic cells, but no obvious changes were detected in the 40 and 80 mg/kg groups.
Figure 5.
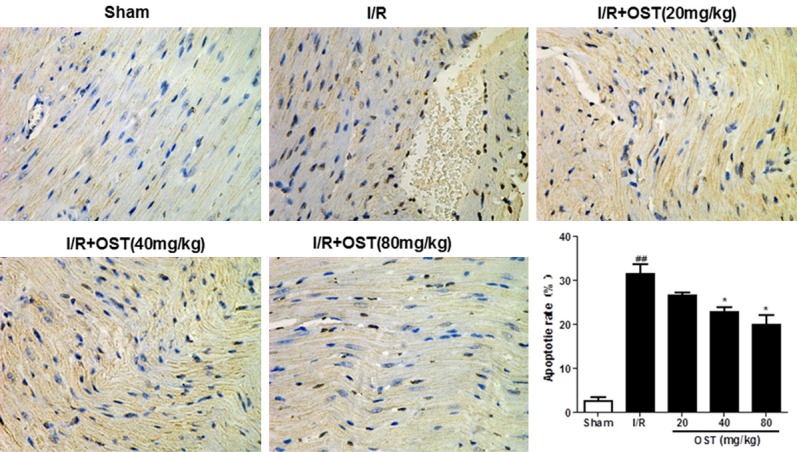
Effects of suppressing osthole (OST) on cardiomyocyte apoptosis (×400). Data are means ± standard deviation. ##P < 0.01 compared with the Sham group; *P < 0.05 compared with the ischemia/reperfusion (I/R) group.
To further demonstrate the anti-apoptotic mechanism of OST, we evaluated the expression of apoptosis-related proteins by western blot analysis (Figure 6). According to our results, the expression levels of Bax (P < 0.01), caspase-3 (P < 0.01), and caspase-9 (P < 0.01) were upregulated by I/R injury, whereas the anti-apoptotic protein Bcl-2 (P < 0.01) was downregulated. OST significantly reversed the apoptosis-related protein expression induced by I/R. Consistent with the TUNEL assay results, OST demonstrated an anti-apoptotic effect by reducing the expression of pro-apoptotic proteins, including Bax, caspase-3, and caspase-9, and increasing the expression of the anti-apoptotic protein Bcl-2.
Figure 6.
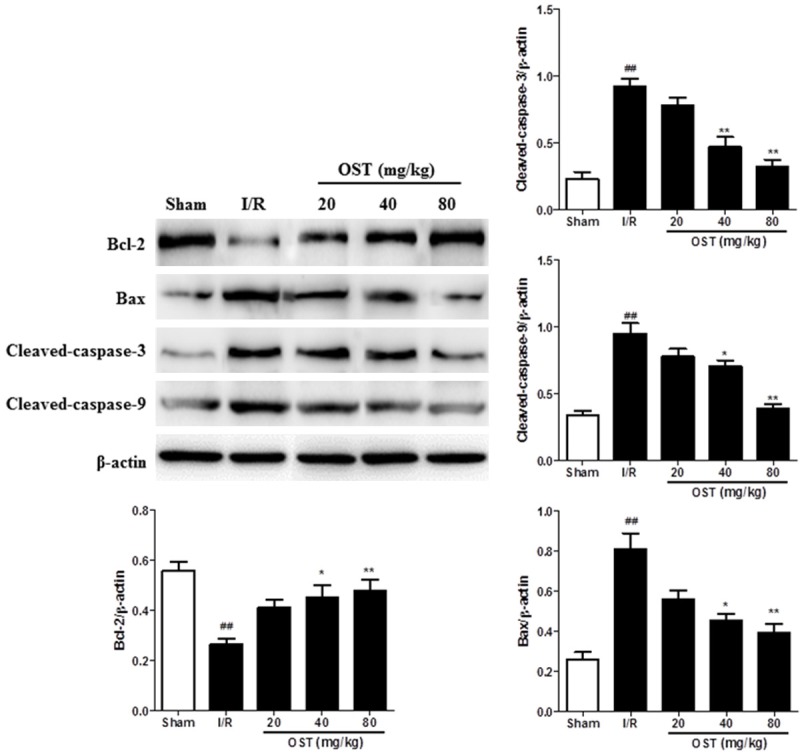
The apoptosis-related proteins Bcl-2, Bax, caspase-3, and caspase-9 were measured by Western blot analysis. ##P < 0.01 compared with the Sham group; *P < 0.05, **P < 0.01 compared with the ischemia/reperfusion (I/R) group.
Effect of OST on myocardial inflammation and apoptosis-related mRNA levels
To determine the protective effects of OST on apoptosis and inflammation in myocardial tissues, the mRNA levels of IL-6, IL-1β, Bcl-2, and Bad were detected by qPCR. As shown in Figure 7, the expression levels of IL-6 (P < 0.01), IL-1β (P < 0.01), and Bax (P < 0.01) were upregulated by I/R injury, whereas the anti-apoptotic protein Bcl-2 was decreased significantly (P < 0.01). The groups pretreated with OST significantly reversed inflammation and apoptosis-related mRNA expression. These results suggest that OST attenuated MI/RI in rats by inhibiting apoptosis and inflammation.
Figure 7.
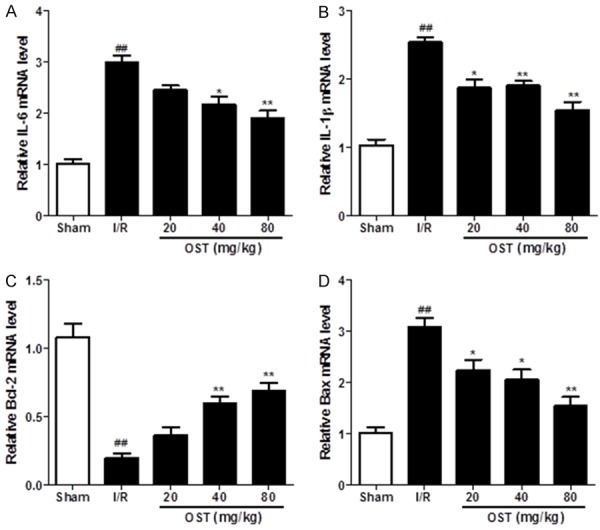
Inflammation- and apoptosis-related IL-6, IL-1β, Bcl-2, and Bax mRNA levels were measured by quantitative polymerase chain reaction. ##P < 0.01 compared with the Sham group; *P < 0.05, **P < 0.01 compared with the ischemia/reperfusion (I/R) group.
Discussion
As one of the most serious problems in many countries worldwide, ischemic heart disease, characterized by a reduced blood supply to the heart, remains the most common cause of death in the industrialized world [18]. However, although the mechanisms of I/R injury are fully understood, few effective strategies for this problem exist. Therefore, it is essential to identify a novel drug to treat myocardial I/R damage.
Reperfusion injury is associated with an inflammatory cascade that perpetuates further damage to cardiac tissue after a period of ischemia. MI/RI promotes free radical generation and triggers the release of TNF-α from ischemic tissue [19]. TNF-α further stimulates the release of other pro-inflammatory cytokines that contribute to cardiac dysfunction [20]. The pro-inflammatory cytokine IL-6 is a downstream target of IL-1β; therefore, its upregulation indicates the presence of inflammation [21]. These cytokines not only cause inflammatory injury but also stimulate neutrophils to migrate into myocardial tissues, resulting in damage to the heart [22]. The results of this study showed that OST reduced the release of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) into serum during MI/RI in a dose-dependent manner. Therefore, the OST mechanism that protects against MI/RI may involve suppression of the inflammatory reaction.
Accumulation of neutrophils may be involved in the pathogenesis of cardiomyocyte apoptosis, as neutrophils release various pro-inflammatory cytokines [23]. Apoptosis leads to tissue damage secondary to reperfusion injury after ischemia, which is a vital pathophysiological mechanism associated with MI/RI. In our study, the TUNEL assay (Figure 5) was performed to examine myocardial apoptosis. In contrast to the Sham group, myocardial apoptosis was increased significantly in the I/R group, while pretreatment with OST significantly decreased apoptosis in myocardial tissue. Furthermore, recent evidence suggests that apoptosis contributes, in part, to overall myocyte death during the reperfusion period [24]. As an important mitochondrial regulator during myocardial apoptosis, Bcl-2 exerts anti-apoptotic effects by blocking the release of cytochrome c and downregulating caspase activity [25]. Apoptosis-related proteins, such as Bax, caspase-3, and caspase-9, also play pivotal roles in apoptosis. The caspase-independent apoptotic pathway responds to death signals by releasing apoptosis-inducing factor from the mitochondrial inner membrane space, which is then translocated to the nucleus [26]. In addition, consistent with those results, pretreatment with OST significantly decreased myocardial apoptosis through expression of apoptosis-related proteins, including Bax, caspase-3, caspase-9, and Bcl-2, indicating that OST plays an anti-apoptotic role by regulating Bax/Bcl and caspase-3/caspase-9.
In conclusion, our present results demonstrate that OST exhibited significant cardioprotective effects against I/R injury, which was associated with its antioxidant, anti-apoptotic, and anti-inflammatory activities. Thus, OST deserves additional experimental and clinical study in cardiovascular research.
Acknowledgements
This work was supported by Guangdong Provincial Science and Technology Project (2016A020215054) and Crosswise Tasks Fund of the Third Military Medical University (SKLKF201003).
Disclosure of conflict of interest
None.
References
- 1.Xia KP, Ca HM, Shao CZ. Protective effect of notoginsenoside R1 in a rat model of myocardial ischemia reperfusion injury by regulation of Vitamin D3 upregulated protein 1/NF-kappaB pathway. Pharmazie. 2015;70:740–744. [PubMed] [Google Scholar]
- 2.Paelestik KB, Jespersen NR, Jensen RV, Johnsen J, Botker HE, Kristiansen SB. Effects of hypoglycemia on myocardial susceptibility to ischemia-reperfusion injury and preconditioning in hearts from rats with and without type 2 diabetes. Cardiovasc Diabetol. 2017;16:148. doi: 10.1186/s12933-017-0628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Zhao Y, Xia Z, Du H, Gao Y, Xue D, Zhu Z, Chai Y. Metabolic profiles revealed anti-ischemia-reperfusion injury of Yangxinshi tablet in Rats. J Ethnopharmacol. 2018;214:124–133. doi: 10.1016/j.jep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran S, Boovarahan SR, Shanmugam K, Vedarathinam RC, Kurian GA. Sodium thiosulfate preconditioning ameliorates ischemia/reperfusion injury in rat hearts via reduction of oxidative stress and apoptosis. Cardiovasc Drugs Ther. 2017;31:511–524. doi: 10.1007/s10557-017-6751-0. [DOI] [PubMed] [Google Scholar]
- 5.Badalzadeh R, Baradaran B, Alihemmati A, Yousefi B, Abbaszadeh A. Troxerutin preconditioning and ischemic postconditioning modulate inflammatory response after myocardial ischemia/reperfusion injury in rat model. Inflammation. 2017;40:136–143. doi: 10.1007/s10753-016-0462-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Wei T, Gao J, Chang X, He H, Luo F, Zhou R, Ma C, Liu Y, Yan T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis. 2015;20:1433–1443. doi: 10.1007/s10495-015-1174-5. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadipoor A, Lee RH, Prockop DJ, Bartosh TJ. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl Res. 2016;177:127–142. doi: 10.1016/j.trsl.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CM, Shen SW, Wang T, Zhang XH. Myocardial ischemic post-conditioning attenuates ischemia reperfusion injury via PTEN/Akt signal pathway. Int J Clin Exp Med. 2015;8:15801–15807. [PMC free article] [PubMed] [Google Scholar]
- 9.Hooshdaran B, Kolpakov MA, Guo X, Miller SA, Wang T, Tilley DG, Rafiq K, Sabri A. Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res Cardiol. 2017;112:62. doi: 10.1007/s00395-017-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan H, Chen L, Ma J. Penehyclidine hydrochloride post-conditioning reduces ischemia/reperfusion-induced cardiomyocyte apoptosis in rats. Exp Ther Med. 2017;14:4272–4278. doi: 10.3892/etm.2017.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J, Guo N, Liang J, Yuan P, Shi Q, Tang X, Yu L. DNA microarray gene expression profile of Mycobacterium tuberculosis when exposed to osthole. Pol J Microbiol. 2013;62:23–30. [PubMed] [Google Scholar]
- 13.Huang T, Dong Z. Osthole protects against inflammation in a rat model of chronic kidney failure via suppression of nuclear factor-kappaB, transforming growth factor-beta1 and activation of phosphoinositide 3-kinase/protein kinase B/nuclear factor (erythroid-derived 2)-like 2 signaling. Mol Med Rep. 2017;16:4915–4921. doi: 10.3892/mmr.2017.7125. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Ding D, Zhang M. Neuroprotection of osthole against cerebral ischemia/reperfusion injury through an anti-apoptotic pathway in rats. Biol Pharm Bull. 2016;39:336–342. doi: 10.1248/bpb.b15-00699. [DOI] [PubMed] [Google Scholar]
- 15.Huang WC, Liao PC, Huang CH, Hu S, Huang SC, Wu SJ. Osthole attenuates lipid accumulation, regulates the expression of inflammatory mediators, and increases antioxidants in FL83B cells. Biomed Pharmacother. 2017;91:78–87. doi: 10.1016/j.biopha.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 16.Chao X, Zhou J, Chen T, Liu W, Dong W, Qu Y, Jiang X, Ji X, Zhen H, Fei Z. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010;1363:206–211. doi: 10.1016/j.brainres.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 17.Wang XY, Dong WP, Bi SH, Pan ZG, Yu H, Wang XW, Ma T, Wang J, Zhang WD. Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. Int J Mol Med. 2013;32:365–372. doi: 10.3892/ijmm.2013.1386. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Sun Z, Meng F. Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation. 2017;40:1903–1911. doi: 10.1007/s10753-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 19.Das A, Salloum FN, Durrant D, Ockaili R, Kukreja RC. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J Mol Cell Cardiol. 2012;53:858–869. doi: 10.1016/j.yjmcc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Z, Liu J, Bian H, Cai J, Zhu G. Suppression of P2X7/NF-kappaB pathways by Schisandrin B contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Arch Pharm Res. 2016;39:499–507. doi: 10.1007/s12272-016-0713-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang BF, Yoshioka J. The Emerging Role of Thioredoxin-Interacting Protein in Myocardial Ischemia/Reperfusion Injury. J Cardiovasc Pharmacol Ther. 2017;22:219–229. doi: 10.1177/1074248416675731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trachtenberg BH, Hare JM. Inflammatory cardiomyopathic syndromes. Circ Res. 2017;121:803–818. doi: 10.1161/CIRCRESAHA.117.310221. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Xu X, Li Y, Kou J, Huang F, Liu B, Liu K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Jeddi S, Ghasemi A, Asgari A, Nezami-Asl A. Role of inducible nitric oxide synthase in myocardial ischemia-reperfusion injury in sleep-deprived rats. Sleep Breath. 2017 doi: 10.1007/s11325-017-1573-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Bai F, Huang Q, Nie J, Lu S, Lu C, Zhu X, Wang Y, Zhuo L, Lu Z, Lin X. Trolline ameliorates liver fibrosis by inhibiting the NF-kappaB pathway, promoting HSC apoptosis and suppressing autophagy. Cell Physiol Biochem. 2017;44:436–446. doi: 10.1159/000485009. [DOI] [PubMed] [Google Scholar]
- 26.He X, Li S, Liu B, Susperreguy S, Formoso K, Yao J, Kang J, Shi A, Birnbaumer L, Liao Y. Major contribution of the 3/6/7 class of TRPC channels to myocardial ischemia/reperfusion and cellular hypoxia/reoxygenation injuries. Proc Natl Acad Sci U S A. 2017;114:E4582–e4591. doi: 10.1073/pnas.1621384114. [DOI] [PMC free article] [PubMed] [Google Scholar]


