Abstract
Objective: MicroRNAs have been found to be deregulated in lung cancers, which play crucial roles in tumorigenesis and progression. FBXW7 and FBXW11, two important F-box proteins of the ubiquitin-proteasome system (UPS), can target multiple substrates for degradation, in order to regulate cell proliferation and survival in cancers. In the present study, we aimed to explore the potential role and regulating mechanism of miR-182 in non-small cell lung cancer (NSCLC). Methods: MiRNA expression was evaluated by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). FBXW7, FBXW11, c-Jun, c-Myc and cyclin D protein levels were detected by western blot. Cell growth was determined using cell counting kit (CCK)-8 reagent and colony formation experiment. Then, cell apoptosis and the cell cycle were analyzed on flow cytometry. The target binding activity of miR-182 with FBXW7 or FBXW11 was evaluated through the Dual-Luciferase Reporter Assay System. Results: It was confirmed that miR-182 was significantly upregulated in tumor tissues, compared with adjacent normal tissues, and this was inversely correlated to the protein levels of FBXW7 and FBXW11. The overexpression of miR-182 in NSCLC cells dramatically promoted cell growth, colony formation capacity and cell cycle progression, and inhibited apoptosis in NSCLC cells. In contrast, the downregulation of miR-182 significantly alleviated these properties in vitro. Furthermore, we demonstrated that miR-182 exerted an oncogenic role in NSCLC by directly targeting FBXW7 and FBXW11. Conclusion: These results bring new insights into the oncogenic role of miR-182 in NSCLC, indicating that miR-182 might be a novel biomarker for the diagnosis and prognosis of NSCLC.
Keywords: miR-182, FBXW7, FBXW11, non-small cell lung cancer
Introduction
Lung cancer is by far the leading cause of cancer-related deaths worldwide, which has a survival rate of <15% [1]. It has been reported that there were 1.8 million newly diagnosed cases and 1.6 million deaths in 2012, worldwide [2]. Non-small cell lung cancer (NSCLC) contributes to 85% of all pulmonary carcinomas, and the high mortality of NSCLC patients has never decreased over the years [3]. To date, surgical resection is the most effective treatment for NSCLC. However, since most cases are diagnosed at an advanced stage, only few patients can be cured by surgical treatment. Therefore, early diagnosis through predictive biomarkers and therapy through prognostic biomarkers are vital to improve the survival rate of NSCLC patients.
MicroRNAs (miRNAs) is a class of small (19-22 nucleotides) non-coding RNAs that can recognize and bind to specific sites in the 3’untranslated region (3’UTR) of target mRNAs, ultimately leading to the degradation of target mRNA or translational repression. MiRNAs play crucial roles in various biological processes, including proliferation, development, differentiation and apoptosis [4]. In recent years, miRNAs have been proven to be involved in the regulation of tumorigenesis and development, and growing evidence has shown that the deregulation of certain miRNAs that regulate oncogenes or tumor-suppressing genes is involved in the pathogenesis of cancers [5]. Moreover, burgeoning amounts of evidence has indicated that an aberrant expression of miRNAs play important roles in NSCLC occurrence and development [6,7]. Therefore, the identification of NSCLC-specific miRNAs and their targets can be used as diagnostic markers or therapeutic targets. This would helpful in designing future clinical trials and better stratifying patients beyond the present standard. Furthermore, this may contribute to the novel drug discovery for NSCLC.
The ubiquitin proteasome system (UPS) is a major regulatory pathway for protein degradation, and plays critical roles in multiple biological processes by targeting key regulators for destruction. E3 ubiquitin ligases is a vital member of UPS, which works with E1 and E2 enzymes to recognize and bind substrates. Ubiquitin molecules are transferred onto the target proteins, and polyubiquitination ultimately leads to the proteolysis of target proteins through the 26S proteasome. As the UPS regulates the degradation of multiple oncogenes and tumor suppressors, the dysregulation of this pathway is implicated in tumor occurrence and progression [8]. FBXW7 and FBXW11, also known as β-transducin repeat-containing protein 2 (β-TrCP2), are two important members of E3 ubiquitin ligases, which recognize various substrates for degradation to regulate cell proliferation, invasion and survival in cancers. Multiple miRNAs have been found to target these two proteins to regulate cancer progression and metastasis [9-11].
In the present study, miR-182 was predicted as a potential regulator of both FBXW7 and FBXW11 by in silico analysis. For the expression of miR-182, FBXW7 and FBXW11 were examined on malignant tissues and adjacent normal tissues obtained from NSCLC patients by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and western blot. The direct inhibition of the expression of FBXW7 and FBXW11 through miR-182 and the potential role of miR-182 as an oncogene in NSCLC tumorigenesis have been confirmed in H460 cells. In addition, FBXW7 and FBXW11 have been validated to be targets of miR-182 by luciferase reporter activity assay. Therefore, the present data demonstrates that miR-182 functions as an oncogene by regulating E3 ubiquitin ligases in NSCLC. This would contribute to the development of novel therapeutic targets for NSCLC.
Materials and methods
Patient samples and cell culture
Fresh malignant tissues and adjacent normal tissues were collected from 11 NSCLC patients who underwent surgery in the Department of Thoracic Surgery at the First Affiliated Hospital of Harbin Medical University. The experiment was performed after explaining the procedure to the patient and a written consent was obtained. This study conforms to the Code of Ethics of the World Medical Association (Declaration of Helsinki) printed in the British Medical Journal (July 18, 1964). None of these patients received treatment for NSCLC before surgery. Normal lung tissues adjacent to the tumor were taken 3 cm away from the tumor tissues. The clinical and pathological profiles of these patients are shown in Table 1. Patients were histopathologically diagnosed and verified by experienced pathologists. The specimens were immediately snap-frozen in liquid nitrogen after surgery, and stored at -80°C.
Table 1.
Clinical and pathological data of patients in this study
| Patient | Gender | Age (yr) | Pathologic | Type |
|---|---|---|---|---|
| 1 | Female | 51 | T1N0M0 | Adenocarcinoma |
| 2 | Male | 55 | T1N0M0 | Squamous cell carcinoma |
| 3 | Male | 55 | T1N2M0 | Adenocarcinoma |
| 4 | Female | 57 | T1N0M0 | Adenocarcinoma |
| 5 | Female | 54 | T2N0M0 | Adenocarcinoma |
| 6 | Male | 61 | T2N0M0 | Adenocarcinoma |
| 7 | Male | 63 | T2N1M0 | Adenocarcinoma |
| 8 | Female | 65 | T2N2M0 | Adenocarcinoma |
| 9 | Female | 64 | T2N0M0 | Adenocarcinoma |
| 10 | Male | 61 | T1N0M0 | Squamous cell carcinoma |
| 11 | Male | 55 | T1N2M0 | Squamous cell carcinoma |
Human NSCLC H460 cells were kindly provided by the Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). H460 cells were cultured in RPMI 1640 medium (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (Biological Industries, CT, USA), penicillin and streptomycin (100 IU/ml) at 37°C under a 5% CO2 atmosphere in a humidified incubator.
qRT-PCR analysis
MiRNAs were isolated using a miRNA extraction kit (HaiGene, Harbin, China), according to manufacturer’s instructions, and reverse-transcribed to cDNA using the TaqMan miRNA RT primer. For miRNA detection, qRT-PCR was performed using a TaqMan miRNA assay kit (HaiGene, Harbin, China) under the Opticon 2 System (Bio-Rad, CA, USA). Since commercial kits were used, the primer sequences were not disclosed in the datasheet. The relative expression abundance of miRNAs was calculated using the 2-ΔΔCt method, and small nuclear RNA U6b was used for normalization.
Western blot analysis
In order to isolate the total proteins, cells or tissues were lysed using RIPA lysis buffer (Thermo Fisher Scientific, MA, USA) supplemented with 1 mM of PMSF (Sigma-Aldrich, Shanghai, China). The lysates were kept on ice for 30 minutes, centrifuged at 13,000 g for 15 minutes, and the supernatants were collected. Then, the proteins were quantified using a bicinchoninic acid (BCA) protein assay kit. Equal amounts of total protein (50 μg, each lane) were separated on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane (PALL, New York, USA) in 20 mM of Tris-HCl (pH 8.0) containing 150 mM of glycine and 20% (v/v) methanol. Then, the membranes were blocked with 5% non-fat dry milk in 1×TBS containing 0.05% Tween 20 at room temperature for one hour. Next, the membranes were immunoblotted with antibodies against FBXW7 (1:4,000; Abcam), FBXW11 (1:1,000; Proteintech), c-Myc (1:1,000; CST), cdc25A (1:500, CST), or c-Jun (1:1,000) overnight at 4°C, and β-Actin antibody (1:1,000; Santa Cruz Biotechnology) was used as control. Afterwards, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; Santa Cruz Biotechnology) for two hours at room temperature. The signals were developed using an enhanced chemiluminescence reagent (Thermo Fisher Scientific, MA, USA), and exposed on a LAS-4000 CCD camera system. The relative intensity of the bands were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Maryland, USA).
miRNA transfection
MiR-182 mimics, miR-182 negative control (miR-NC), miR-182 inhibitors, and miR-182 inhibitor negative control (anti-miR-NC) were purchased from RiboBio (Guangdong, China). These were individually and transiently transfected with H460 cells at a final concentration of 100 nM using the Lipofectamine 2000 reagent (Invitrogen, CA, USA). The miR-182 inhibitors were modified antisense oligonucleotides designed specifically to bind to and inhibit endogenous miR-182 with a rare off-target effect: miR-NC: 5’-ACAUCUGCGUAAGAUUCGAGUCUA-3’; miR-182 mimics: 5’-UUUGGCAAUGGUAGAACUCACACU-3’; anti-miR-NC: 5’-GCGTAACTAATACATCGGATTCGT-3’; miR182 inhibitors: 5’-AGUGUGAGUUCUACCAUUGCCAAA-3’.
Cell growth assay
Each group of H460 cells were collected at 24, 48 and 72 hours after transfection. Then, cells were incubated with 10 μl of the cell counting kit-8 (CCK-8) reagent (Beyotime, HaiMen, China) for two hours at 37°C. Next, absorbance at 450 nm was measured at each time point using an enzyme immunoassay analyzer. The experiment was conducted in three separate wells for each sample, and performed in triplicate.
Colony formation assay
Each group of H460 cells was collected and trypsinized at 48 hours after transfection. Then, cells were seeded in 6-well plates at 500 cells per well for further culture for 14 days. Cells were subsequently fixed with paraformaldehyde (4% v/v, Sigma) for 20 minutes, and stained with crystal violet (0.2% w/v, Sigma) for five minutes after washing with PBS. The experiment was independently performed in triplicate.
Cell cycle analysis
H460 cells were harvested by trypsinization after transfection, and fixed in 75% ice-cold ethanol overnight at 4°C after washing two times with PBS. Then, the cell cycle assay was performed using a cell cycle assay kit (HaiGene, Harbin, China), according to manufacturer’s instructions. Briefly, the fixed cells were first resuspended in PBS at a concentration of 3×105/ml. Then, cells were treated with RNaseA (Sigma-Aldrich, Shanghai, China) at room temperature for 20 minutes, followed by incubation with propidium iodide (PI; Sigma-Aldrich, Shanghai, China) for 15 minutes. Cell cycle analysis was performed using a Guava® easyCyte flow cytometer with GuavaSoft 2.5 (Millipore, MA, USA). Data were presented as the percentage of cells in G1, S and G2 populations. The experiment was performed in triplicate to obtain the standard deviation (SD).
Cell apoptosis analysis
An Annexin V-fluorescein isothiocyanate (FITC)/PI Kit (HaiGene, Harbin, China) was used to analyze cell apoptosis after transfection on the Guava® flow cytometer (Millipore, MA, USA). Cells were harvested by trypsinization and resuspended in binding buffer at a concentration of 1×105/ml. Then, cells were treated with Annexin V-FITC and PI for 15 minutes at room temperature. Finally, cells were counted on a flow cytometer, and the data were analyzed using the FlowJo software. The percentage of cells that were Annexin V positive, but PI negative, was compared among the different treatment groups. Each experiment was performed in triplicate.
Luciferase activity assay
The 3’UTR of the FBXW7 and FBXW11 genes were obtained by gene synthesis, and inserted downstream of the luciferase reporter gene in a pmirGLO vector (Promega, WI, USA). For the luciferase reporter assay, H460 cells were seeded in a 24-well plate and incubated for 24 hours before transfection. Next, firefly luciferase constructs containing the 3’UTR of the potential miR-182 and miR-182 mimics, or miR-182 inhibitors, or the corresponding negative controls were co-transfected into H460 cells using Lipofectamine 2000. Cells were collected at 48 hours after transfection, and measured using the Dual-Luciferase Reporter System (Promega, WI, USA), according to manufacturer’s protocols. The pRL-TK Renilla luciferase activity was used for normalization. Three independent experiments were performed, and data were presented as mean ± SD.
Statistical analysis
All the experiments were performed in triplicate, and the results were presented as mean ± SD. Data among different groups were compared by paired t-test and one-way ANOVA using SPSS software version 13.0 (SPSS; Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Results
Identifying potential miRNAs that target both FBXW7 and FBXW11 in NSCLC
Since each miRNA is capable of regulating multiple target genes, multiple F-box proteins may be suppressed by the same miRNA. Hence, an attempt was made to explore a certain miRNA that regulates both FBXW7 and FBXW11 in NSCLC. The predictive algorithms of TargetScan and microRNA.org were used to identify potential miRNAs that could regulate both FBXW7 and FBXW11, in order to gain a better understanding of the roles of these two F-box proteins in NSCLC. The overlapped 12 miRNAs by these two predictive algorithms were distinguished as potential miRNAs that target these two F-box proteins (Figure 1).
Figure 1.
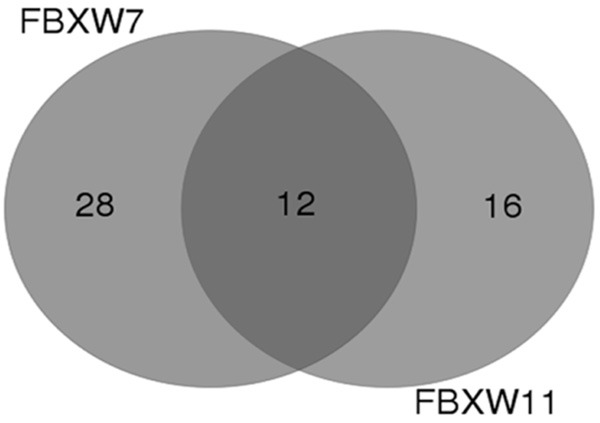
Identification of miRNAs potentially regulating both FBXW7 and FBXW11 by the prediction algorithm of TargetScan and microRNA.org.
MiR-182 was significantly upregulated in NSCLC tissues and reversely correlated with the protein levels of FBXW7 and FBXW11
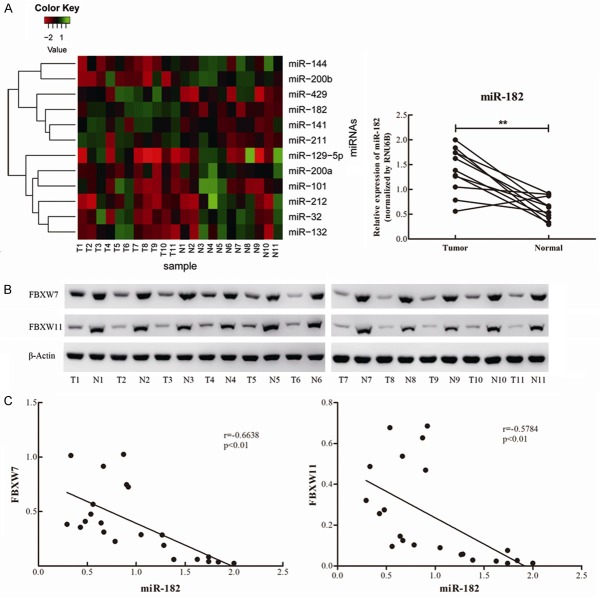
In order to confirm whether these potential 12 miRNAs are involved in NSCLC, their relative expression levels were tested in 11 paired NSCLC tumor tissues and the corresponding adjacent normal tissues using qRT-PCR (refer to Table 1 for patient information). These results revealed that four miRNAs were dramatically upregulated and three miRNAs were significantly downregulated in NSCLC tumor tissues, compared with their corresponding adjacent normal tissues (Table 2), while there was no significant differentiation for the other miRNAs (Figure 2A). Among these four upregulated miRNAs, the increasing-fold of miR-182 levels was the highest (2.27-fold) in NSCLC tissues (Figure 2A). Thus, miR-182 was investigated in the following study.
Table 2.
Deregulated miRNAs potentially regulating FBXW7 and FBXW11 between paired NSCLC malignant tissues and adjacent normal tissues
| miRNA | Fold change | P value |
|---|---|---|
| miR-182 | 2.27 | 0.0010 |
| miR-429 | 1.89 | 0.0389 |
| miR-141 | 1.67 | 0.0036 |
| miR-211 | 1.62 | 0.0209 |
| miR-200a | -1.35 | 0.0125 |
| miR-200b | -1.37 | 0.0086 |
| miR-144 | -1.37 | 0.0101 |
The fold-change values were represented by the ratio of means of malignant groups and normal groups by the method of 2-ΔΔCt. Negative Fold-Change values indicate relative high expression in adjacent normal tissues. p-values indicate the significance level for each miRNA analyzed using paired t-test.
Figure 2.
Up-regulation of miR-182 in NSCLC tumor tissues and inverse correlation with FBXW7 and FBXW11. A. The heat map generated by cluster analysis of the predicted 12 miRNAs that potentially targeting both FBXW7 and FBXW11 in NSCLC based on the data of TaqMan quantitive RT-PCR. The paired expression of miR-182 in malignant and normal tissues of NSCLC was individually listed on the right pannel. B. The protein levels of FBXW7 and FBXW11 in 11 paired NSCLC tissues were detected by Western blot, and β-Actin was used for normalization. C. The inverse correlation between miR182 and the protein levels of FBXW7 or FBXW11 in 11 paired NSCLC tissues. **P<0.01.
In order to further investigate the correlation between miR-182 and these two F-box proteins, the protein levels of FBXW7 and FBXW11 in NSCLC tissues and adjacent normal tissues were detected by western blot. Results revealed that the expression of FBXW7 and FBXW11 was dramatically downregulated in NSCLC tissues (Figure 2B), suggesting that miR-182 was inversely correlated with the protein levels of FBXW7 and FBXW11 (Figure 2C). Taken together, it could be speculated that miR-182 might be an oncogene that exerts regulatory effects on the tumorigenesis and progression of NSCLC through the suppression of FBXW7 and FBXW11.
MiR-182 promotes NSCLC cell proliferation in vitro
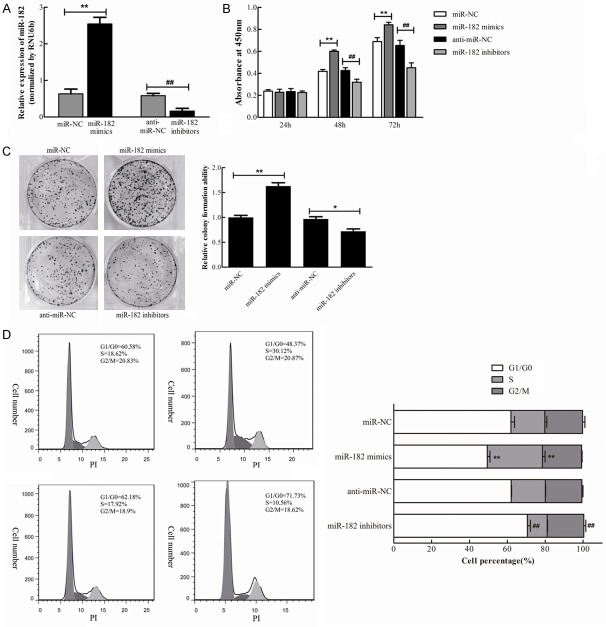
In order to determine the effect of miR-182 on the proliferation of NSCLC cells, H460 cells were employed for subsequent analysis. The miR-182 mimics, miR-182 inhibitors, or corresponding negative controls (miR-NC and anti-miR-NC) were individually and transiently transfected into H460 cells. Then, the relative expression of miR-182 was determined by qRT-PCR assay at 48 hours after transfection. Results revealed that this was dramatically increased by the transfection of miR-182 mimics, and decreased by miR-182 inhibitors (Figure 3A).
Figure 3.
miR-182 promotes NSCLC cells proliferation. miR-182 mimics, miR-182 inhibitors, and corresponding negative controls were transfected into H460 cells individually. A. miR-182 level was determined by qRT-PCR 48 h after transfection. B. The effect of miR-182 overexpression and down-regulation on cell growth was determined by CCK-8 reagent at 24 h, 48 h, and 72 h after transfection. C. Representative micrographs of crystal violet stained colonies formed by four groups of H460 cells. D. The effect of miR-182 on cell cycle progression of H460 cells analyzed by flow cytometry and quantification of cell percentages in G0-G1, S, and G2-M phase. **P<0.01 vs. miR-NC, ##P<0.01 vs. anti-miR-NC.
In order to elucidate the effect of miR-182 on cell growth, the CCK-8 reagent was used to determine cell viability at 24, 48 and 72 hours after transfection. As shown in Figure 3B, there was a significant increase in growth rate in miR-182 mimic-transfected cells at 48 and 72 hours, compared with miR-NC cells, while there was significant decrease in growth rate in miR-182 inhibitor-transfected cells, compared with anti-miR-NC cells. These results indicate that the overexpression of miR-182 promoted the growth of NSCLC cells. Next, colony formation assay was performed in NSCLC cells to further prove the pro-proliferating effect of miR-182. Results revealed that the overexpression of miR-182 increased the colony number of H460 cells, and these colonies were much larger than that in the negative control, while the downregulation of miR-182 depressed the colony formation (Figure 3C). Enhanced cell growth in cancer cells is closely correlated with cell cycle progression. Therefore, it was investigated whether cell cycle progression contributes to the promotion of cell growth for miR-182 overexpressed cells. As shown in Figure 3C, there was a dramatic decrease in the percentage of cells in the G1/G0 phase (12.5%) and an increase in cells in the S phase (11.3%) in miR-182 overexpressed H460 cells, compared with negative control cells. In contrast, there was a significant increase in the percentage of cells in the G1/G0 phase (8.6%) and a decrease in the percentage of cells in the S phase (7.6%) in miR-182 downregulated H460 cells, compared with anti-miR-NC cells (Figure 3D). Taken together, the overexpression of miR-182 promotes the proliferation and cell cycle progression of NSCLC cells in vitro.
MiR-182 inhibits the apoptosis of NSCLC cells in vitro
In order to further confirm the oncogenic role of miR-182 in NSCLC cells, Annexin V-FITC/PI staining was used to evaluate its involvement in apoptosis. As shown in Figure 4, the apoptosis rate of H460 cells transfected with miR-182 mimics was lower than miR-NC cells (3.3% vs. 7.9%), while the apoptosis rate of H460 cells transfected with miR-182 inhibitors was much higher than anti-miR-NC cells (12.05% vs. 8.03%). Therefore, these results indicate that the overexpression of miR-182 inhibited the apoptosis of NSCLC cells, while the inhibition of miR-182 promoted NSCLC cell apoptosis.
Figure 4.
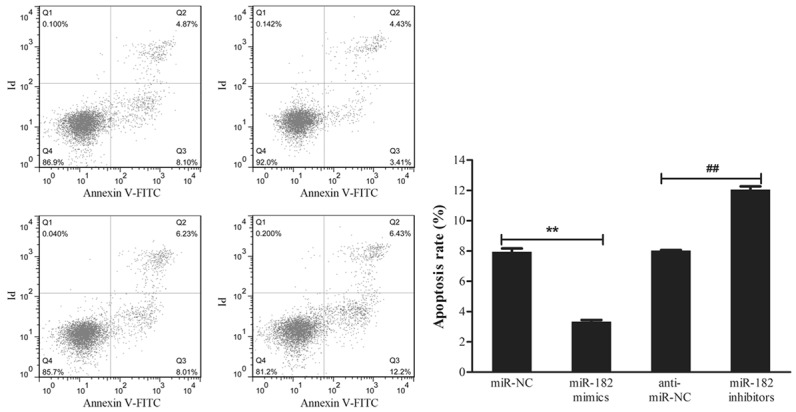
miR-182 inhibits the apoptosis of NSCLC cells. H460 cells were transfected with miR-182 mimics, miR-182 inhibitors, or corresponding negative controls for 48 h individually. Cell apoptosis was detected using flow cytometry by Annexin V-FITC/PI staining. The experiment was performed in triplicate. **P<0.01 vs. miR-NC, ##P<0.01 vs. anti-miR-NC.
MiR182 regulates the protein levels of FBXW7 and FBXW11 and their substrates
As it is known, the UPS pathway plays an important role in various cancers by degrading a variety of oncogenes or tumor suppressors, and it was proven that miR-182 is inversely correlated with the protein levels of FBXW7 and FBXW11 in 11 paired NSCLC tissues. Based on these evidences, it was explored whether miR-182 influenced the expression of FBXW7 and FBXW11. Western blot results revealed that the protein levels of FBXW7 and FBXW11 were significantly downregulated in H460 cells transfected with miR-182 mimics, compared with miR-NC cells, while these were dramatically upregulated in cells transfected with miR-182 inhibitors, compared with anti-miR-NC cells (Figure 5). Furthermore, the protein levels of the well-known substrates of FBXW7 and FBXW11 (c-Jun, c-Myc and cyclin D [9]) were also tested. Similarly, it was found that the protein levels of these corresponding substrates significantly increased in miR-182 overexpressed cells, but decreased in miR-182 downregulated cells (Figure 5). These results suggest that miR-182 might function as an oncogene by negatively regulating FBXW7 and FBXW11 to suppress the degradation of their substrates.
Figure 5.
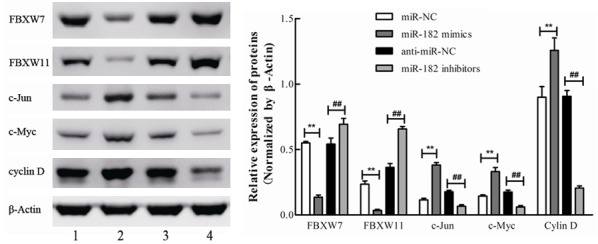
The expression patterns of FBXW7, FBXW11 and corresponding substrates regulated by miR-182. H460 cells were transfected with miR-182 mimics, miR-182 inhibitors, or corresponding negative controls for 48 h individually. The protein levels of FBXW7, FBXW11, c-Jun, c-Myc, and cyclin D were determined by Western blot. The relative band intensities were analyzed by Image-Pro Plus 6.0, β-Actin was used for normalization. **P<0.01 vs. miR-NC, ##P<0.01 vs. anti-miR-NC.
FBXW7 and FBXW11 are direct targets of miR-182 in NSCLC cells
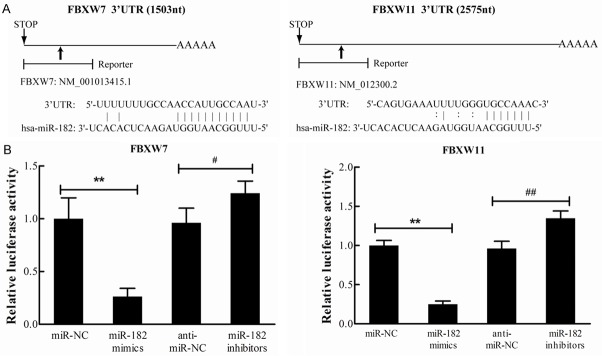
It has been proven in the present study that the overexpression of miR-182 decreased and the downregulation of miR-182 increased the protein levels of FBXW7 and FBXW11 in H460 cells. In addition, these predictive algorithms demonstrate that there were putative miR-182-binding seed sequences within the 3’UTRs of FBXW7 and FBXW11 mRNAs (Figure 6A). Based on these evidences, it could be hypothesized that these two F-box proteins might be the direct targets of miR-182. In order to confirm this, the FBXW7-3’UTR and FBXW11-3’UTR reporter plasmids (pmirGLO luciferase plasmid) were constructed and transfected into H460 cells, along with miR-182 mimics or miR-182 inhibitors for luciferase assay evaluation. Results revealed that miR-182 mimics decreased luciferase activity, while miR-182 inhibitors exhibited a significant increase in luciferase activity (Figure 6B), compared with the corresponding negative controls. Furthermore, these results indicate that FBXW7 and FBXW11 are the direct targets of miR-182 in NSCLC.
Figure 6.
miR-182 direct targets FBXW7 and FBXW11 in NSCLC cells. A. Predicted miR-182 binding sites in 3’-UTR of FBXW7 and FBXW11 mRNA. B. Relative luciferase activity was determined 48 h after cotransfection with pmirGLO reporter plasmid and miR-182 mimics/mi-182 inhibitors. Renilla luciferase activity was used for normalization. **P<0.01 vs. miR-NC, #P<0.05 vs. anti-miR-NC, ##P<0.01 vs. anti-miR-NC.
Discussion
MiR-182 was first identified as a retina-specific miRNA that regulates sensory organ development [12]. In recent years, the deregulation of miR-182 has been demonstrated to be involved in tumorigenesis and progression in various types of cancers [13-22]. In the present study, miR-182 was proven to be significantly upregulated in NSCLC tissues, compared with adjacent normal tissues. Moreover, the overexpression of miR-182 through the transfection of mimics into NSCLC cells dramatically promoted cell growth, colony formation and cell cycle progression, but inhibited apoptosis. Furthermore, miR-182 inhibitors potentially attenuated these oncogenic properties, which were consistent with other studies on miR-182 for lung cancer [23,24]. However, Sun et al. reported that miR-182 functioned as a tumor suppressor, inhibiting cell proliferation by targeting RGS17 [25]. Meanwhile, Zhu et al. demonstrated that an apoptosis-related gene, RASA1, could be suppressed by miR-182 to support its tumor-suppressing role [26], which sounds a little farfetched. RASA1 is a negative regulator of the RAS-MPK-ERK pathway, which is constitutively activated in various cancers [27]. Hence, the suppression of RASA1 might lead to the ectopic activation of this pathway, and further enhance the aggressive phenotype of cancers. In fact, in the controversial role of miR-182 in lung cancers, it is reasonable to speculate that miR-182 may be mediated by an already diversified and still expanding number of target genes, and the molecular routes through which it exerts its regulatory effects are largely context-dependent. Moreover, a single target is not sufficient to explain the pluripotency of this miRNA in cancer.
FBXW7 (also known as CDC4) and FBXW11 (also known as β-TrCP2) have been implicated in various human cancers [8,28]. These two F-box proteins have similar structures, contains an F-box motif at its N-terminus and seven substrate-binding WD-40 repeats at its C-terminus, and are recognized consensus DSGXXS degrons in most of its target substrates. FBXW7 plays a central role in cell cycle progression, cell growth and survival by targeting oncogenic proteins, including Cyclin E, c-Myc, c-Jun, and Notch-1, in a variety of human tumors [29,30]. Consequently, FBXW7 has been recognized as a tumor suppressor, in which its mutations have been found in various neoplasms, including breast cancer, colon cancer and leukemia [31-33]. In addition, FBXW7 mRNA has been found to be reduced in breast cancer patients, and was significantly associated with poor prognosis [34]. FBXW7 expression was also found to be decreased in hepatocellular carcinoma tissues, which was correlated with poor clinical pathological features, including large tumor size, high pathological grading and advanced TNM staging [35]. All the above evidence suggests that FBXW7 is a bona fide tumor suppressor, and multiple miRNAs have been confirmed to exert regulatory effects in various cancers by targeting FBXW7, such as miR-25, miR-92a, miR-155 and miR-27a [10,36-38]. In the present study, we confirmed that miR-182 promotes cell growth, cell cycle progression and colony formation by suppressing FBXW7, which is consistent with its tumor-suppressing role in other cancers. Unlike FBXW7, the role of FBXW11 in tumorigenesis remains debatable. β-TrCP1, which functions similarly with FBXW11, has been intensively studied in a variety of cancers. It was found that its target includes both oncogenes (Mcl-1, MYC and cyclin D) and tumor suppressors (p53, PDCD4 and FOXO3a), further highlighting the context-dependent functions of β-TrCP1 proteins in human cancer [39,40]. Notably, β-TrCP1 was found to negatively regulate the cell growth and motility of lung cancer cells, and the expression of FBXW11 was found to be negative in the distal metastasis of lung cancer [41]. Similarly, FBXW11 has been proven to be downregulated by the miR-106b-25 cluster to enhance cell migration and invasion in NSCLC cells [11]. In concert with the above referred study, the present study was able to prove that FBXW11 is downregulated by miR-182 in NSCLC cells to promote cell growth and cell cycle progression, indicating its tumor-suppressing role in NSCLC. Therefore, we speculate that the dual roles of β-TrCP may be due to their multiple substrates for degradation, and it is likely that an individual substrate may only have defined roles in specific types of cancers.
As the leading cause of cancer-related death worldwide, lung cancer is often diagnosed at the late stage with poor prognosis. Thus, the identification of useful predictive and diagnostic biomarkers is indeed vital to improve the outcomes of lung cancer patients. As promising biomarker candidates, miRNAs have been intensively studied in lung cancer, and some of these have been proven to be able to effectively separate healthy samples from malignant samples. For example, Song et al. reported that the combined use of expression levels of miR-98 and miR-205 can discriminate cancerous lung tissue samples from normal tissue samples with a specificity and sensitivity of 100% for both [42]. In the present study, it was found that miR-182 was significantly upregulated in malignant lung tissues, and promoted the carcinoma phenotype in NSCLC cells, indicating its potential role in regulating tumorigenesis and progression. Notably, the combination of miR-182 with miR-126 and miR-205 has been demonstrated to be credible biomarkers for discriminating malignant samples from healthy controls at an accuracy of 84.49%, and with a sensitivity and specificity of 91.40% and 77.14%, respectively [42]. In addition, the combined application of miRNAs with protein biomarkers may also help better predict treatment outcomes. Thus, miR-182 and FBXW7 or FBXW11 might cooperate to predict the outcomes of NSCLC patients, which certainly requires more clinical data support. However, the application of these exciting results in clinical practice still needs proper practical validation, even though these have already been proven to be statistically significant in laboratory experiments.
In summary, the results of the present study demonstrate that elevated miR-182 in NSCLC cells may be involved in carcinogenesis and progression by suppressing FBXW7 and FBXW11. This finding suggests that inhibiting miR-182 or enhancing FBXW7/FBXW11 may be a useful therapeutic strategy for NSCLC treatment.
Acknowledgements
This study was supported in part by the Overseas Scholars Research Fund of Heilongjiang Province. An amount of 50,000 RMB was granted to the first author, Hao Chang, under Grant no. 1152hq30. The first author, Hao Chang, was also supported in part by the Returning Overseas Students Fund of Heilongjiang Province, who was granted an amount of 50,000 RMB, under Grant no. LC07C19. The corresponding author, Shengfa Wang, was supported in part by the Introduction of Talents Fund of the First Hospital of Harbin, and was granted an amount of 500,000 RMB, under Grant no. 2013SYYRCYJ04.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 6.Skrzypski M, Dziadziuszko R, Jassem J. MicroRNA in lung cancer diagnostics and treatment. Mutat Res. 2011;717:25–31. doi: 10.1016/j.mrfmmm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Ren P, Gong F, Zhang Y, Jiang J, Zhang H. MicroRNA-92a promotes growth, metastasis, and chemoresistance in non-small cell lung cancer cells by targeting PTEN. Tumour Biol. 2016;37:3215–3225. doi: 10.1007/s13277-015-4150-3. [DOI] [PubMed] [Google Scholar]
- 8.Lau AW, Fukushima H, Wei W. The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci (Landmark Ed) 2012;17:2197–2212. doi: 10.2741/4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu ZH, Pfeffer LM. MicroRNA regulation of F-box proteins and its role in cancer. Semin Cancer Biol. 2016;36:80–87. doi: 10.1016/j.semcancer.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C, Shen L, Mao L, Wang B, Li Y, Yu H. miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem Biophys Res Commun. 2015;458:63–69. doi: 10.1016/j.bbrc.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 11.Savita U, Karunagaran D. MicroRNA-106b-25 cluster targets beta-TRCP2, increases the expression of Snail and enhances cell migration and invasion in H1299 (non small cell lung cancer) cells. Biochem Biophys Res Commun. 2013;434:841–847. doi: 10.1016/j.bbrc.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 13.Wei Q, Lei R, Hu G. Roles of miR-182 in sensory organ development and cancer. Thorac Cancer. 2015;6:2–9. doi: 10.1111/1759-7714.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Sheng C, Huang L, Zhang H, Huang L, Cheng Z, Zhu Q. MiR-183/-96/-182 cluster is up-regulated in most breast cancers and increases cell proliferation and migration. Breast Cancer Res. 2014;16:473. doi: 10.1186/s13058-014-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei R, Tang J, Zhuang X, Deng R, Li G, Yu J, Liang Y, Xiao J, Wang HY, Yang Q, Hu G. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene. 2014;33:1287–1296. doi: 10.1038/onc.2013.65. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Du L, Wen Z, Yang Y, Li J, Wang L, Zhang X, Liu Y, Dong Z, Li W, Zheng G, Wang C. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. Int J Colorectal Dis. 2013;28:697–703. doi: 10.1007/s00384-013-1674-0. [DOI] [PubMed] [Google Scholar]
- 18.Rapti SM, Kontos CK, Papadopoulos IN, Scorilas A. Enhanced miR-182 transcription is a predictor of poor overall survival in colorectal adenocarcinoma patients. Clin Chem Lab Med. 2014;52:1217–1227. doi: 10.1515/cclm-2013-0950. [DOI] [PubMed] [Google Scholar]
- 19.Segura MF, Hanniford D, Menendez S, Reavie L, Zou X, Alvarez-Diaz S, Zakrzewski J, Blochin E, Rose A, Bogunovic D, Polsky D, Wei J, Lee P, Belitskaya-Levy I, Bhardwaj N, Osman I, Hernando E. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci U S A. 2009;106:1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Mao P, Song L, Wu J, Huang J, Lin C, Yuan J, Qu L, Cheng SY, Li J. miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol. 2010;177:29–38. doi: 10.2353/ajpath.2010.090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Liu J, Segura MF, Shao C, Lee P, Gong Y, Hernando E, Wei JJ. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012;228:204–215. doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 22.Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013;8:e55502. doi: 10.1371/journal.pone.0055502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang WB, Chen PH, Hsu T 1st, Fu TF, Su WC, Liaw H, Chang WC, Hung JJ. Sp1-mediated microRNA-182 expression regulates lung cancer progression. Oncotarget. 2014;5:740–753. doi: 10.18632/oncotarget.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning FL, Wang F, Li ML, Yu ZS, Hao YZ, Chen SS. MicroRNA-182 modulates chemosensitivity of human non-small cell lung cancer to cisplatin by targeting PDCD4. Diagn Pathol. 2014;9:143. doi: 10.1186/1746-1596-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Fang R, Li C, Li L, Li F, Ye X, Chen H. Hsa-mir-182 suppresses lung tumorigenesis through down regulation of RGS17 expression in vitro. Biochem Biophys Res Commun. 2010;396:501–507. doi: 10.1016/j.bbrc.2010.04.127. [DOI] [PubMed] [Google Scholar]
- 26.Zhu YJ, Xu B, Xia W. Hsa-mir-182 downregulates RASA1 and suppresses lung squamous cell carcinoma cell proliferation. Clin Lab. 2014;60:155–159. doi: 10.7754/clin.lab.2013.121131. [DOI] [PubMed] [Google Scholar]
- 27.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 28.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 30.Minella AC, Clurman BE. Mechanisms of tumor suppression by the SCF(Fbw7) Cell Cycle. 2005;4:1356–1359. doi: 10.4161/cc.4.10.2058. [DOI] [PubMed] [Google Scholar]
- 31.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–322. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 32.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, Nordgren H, Grander D, Reed SI, Widschwendter M, Sangfelt O, Spruck C. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 33.Malyukova A, Dohda T, von der Lehr N, Akhoondi S, Corcoran M, Heyman M, Spruck C, Grandér D, Lendahl U, Sangfelt O. The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signaling. Cancer Res. 2007;67:5611–5616. doi: 10.1158/0008-5472.CAN-06-4381. [DOI] [PubMed] [Google Scholar]
- 34.Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci. 2011;102:439–445. doi: 10.1111/j.1349-7006.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- 35.Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, Yao Y, Liu Q. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13:110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong J, Cui Z, Li L, Ma Q, Wang Q, Gao Y, Sun H. MicroRNA-25 promotes gastric cancer proliferation, invasion, and migration by directly targeting F-box and WD-40 Domain protein 7, FBXW7. Tumour Biol. 2015;36:7831–7840. doi: 10.1007/s13277-015-3510-3. [DOI] [PubMed] [Google Scholar]
- 37.Tang B, Lei B, Qi G, Liang X, Tang F, Yuan S, Wang Z, Yu S, He S. MicroRNA-155-3p promotes hepatocellular carcinoma formation by suppressing FBXW7 expression. J Exp Clin Cancer Res. 2016;35:93. doi: 10.1186/s13046-016-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lerner M, Lundgren J, Akhoondi S, Jahn A, Ng HF, Akbari Moqadam F, Oude Vrielink JA, Agami R, Den Boer ML, Grandér D, Sangfelt O. MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle. 2011;10:2172–2183. doi: 10.4161/cc.10.13.16248. [DOI] [PubMed] [Google Scholar]
- 39.Skaar JR, Pagan JK, Pagano M. SCF ubiquitin ligase-targeted therapies. Nat Rev Drug Discov. 2014;13:889–903. doi: 10.1038/nrd4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–247. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He N, Li C, Zhang X, Sheng T, Chi S, Chen K, Wang Q, Vertrees R, Logrono R, Xie J. Regulation of lung cancer cell growth and invasiveness by beta-TRCP. Mol Carcinog. 2005;42:18–28. doi: 10.1002/mc.20063. [DOI] [PubMed] [Google Scholar]
- 42.Song R, Liu Q, Hutvagner G, Nguyen H, Ramamohanarao K, Wong L, Li J. Rule discovery and distance separation to detect reliable miRNA biomarkers for the diagnosis of lung squamous cell carcinoma. BMC Genomics. 2014;15(Suppl 9):S16. doi: 10.1186/1471-2164-15-S9-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]